Abstract
BACKGROUND/OBJECTIVES
This study aimed to analyze the association between dietary omega-3 fatty acid intake and depression in postmenopausal women using data from the Korea National Health and Nutrition Examination Survey (KNHANES) VI.
SUBJECTS/METHODS
The KNHANES is a cross-sectional nationwide health and nutrition survey. Dietary data, including omega-3 fatty acids, were assessed using the 24-h recall method. Depression was evaluated using a survey questionnaire. The association between dietary omega-3 fatty acids and depression was evaluated using multivariate logistic regression analysis. Depression, according to the dietary omega-3 fatty acid intake, was expressed as the odds ratio (OR) with a 95% confidence interval (CI). A total of 4,150 postmenopausal women were included in the analysis.
RESULTS
In the fully-adjusted model, the group with the highest dietary omega-3 fatty acid intake significantly showed lower prevalence of depression than the group with the lowest intake (OR, 0.52; 95% CI, 0.33–0.83); a significant linear trend was detected (P for trend = 0.04). According to the dose-response analysis using cubic restricted spline regression, this association was linear and monotonic (P for non-linearity = 0.32).
CONCLUSIONS
In this study, the dietary omega-3 fatty acid intake in postmenopausal women was inversely proportional to depression in a dose-response manner. Large cohort studies are needed to verify the causality between omega-3 fatty acids and depression in Korean postmenopausal women.
Keywords: Omega-3 fatty acids, menopause, depression, women, Korea
INTRODUCTION
Menopause is a physiological state in which estrogen secretion is reduced due to ovarian failure, resulting in the termination of ovulatory cycles and the permanent cessation of menstruation [1]. During this period, women may experience certain health problems, such as facial flushing, chills, headaches, memory loss, depression, and arthralgia [2,3]. These symptoms may lead to a depressed mood or clinical depression [4]. Indeed, the prevalence of depression during menopause is 2-fold higher than that in the premenopausal state [5]. Depression is a mood disorder caused by complex interactions between physical, psychological, and situational factors.
Recent studies have reported that dietary omega-3 fatty acids may play a role in depression [6]. A meta-analysis including nine cross-sectional and seven cohorts showed a significantly decreased risk of depression with increased levels of omega-3 fatty acids [7]. In addition, a pooled analysis of nine clinical trials conducted in China, Europe, and the United States on adults aged 60 years and older consistently showed that the symptoms of depression were significantly lower in individuals who were taking omega-3 fatty acid supplements at doses of more than 1.5 g/day [8]. However, few studies have analyzed the association between dietary omega-3 fatty acids and depression in postmenopausal Korean women.
Omega-3 fatty acids may play a major role in preventing depression as the metabolites of omega-3 fatty acids can exert anti-inflammatory action and have a protective effect against depression by increasing the serotonin levels [9]. Indeed, postmenopausal women who have deficient levels of estrogen tend to be at a greater risk of depressive disorders and mood symptoms [10,11,12]. Estrogen plays a critical role in promoting the growth and survival of neurons [13] and the prefrontal cortex [14] and is involved in the serotonergic system in several brain areas [15]. Since estrogen can stimulate the conversion of essential fatty acids into long-chain metabolites [16], an estrogen-deficit-associated omega-3 fatty acid deficiency may exacerbate the risks of depression. Therefore, it is important to determine whether omega-3 fatty acids can be an alternative therapy (with other cardiometabolic health benefits) for postmenopausal women, who are at a higher risk of depression.
Previous studies that investigated the role of omega-3 fatty acids in depression among postmenopausal women were mostly clinical studies reported from the United States and Iran [17,18] and only scant epidemiologic data are available for Korean postmenopausal women [19,20]. Previous Korean studies did not consider the possible errors in the measurement of dietary assessments (for instance, participants with implausible total energy intake were not screened) or dose-response relationships on menopause. Therefore, it is pertinent to investigate whether a higher intake of omega-3 fatty acids is associated with lower rates of depression in a dose-dependent manner, considering various potential confounding factors and measurement errors in Korean postmenopausal women who are at a high risk of depression due to menopause and the presence of climacteric symptoms.
The purpose of this study was to analyze the association between dietary omega-3 fatty acid intake and depression and to determine the dose-response patterns in postmenopausal women using representative data from the Korean population.
SUBJECTS AND METHODS
Study population
The Korea National Health and Nutrition Examination Survey (KNHANES) is a cross-sectional nationwide health and nutrition survey of non-institutionalized Korean individuals aged over 1 year and residing in South Korea. To facilitate the representability of the sample and accuracy of the estimation, a complex sampling method of two-stage stratified cluster sampling was used. The detailed KNHANES survey methods and protocols have been described elsewhere [21]. Briefly, various demographic, lifestyle, dietary, and health-related data were obtained through health interviews, health examinations, and nutrition surveys. KNHANES was first started in 1998 and was conducted every 3–4 years in the early part of its history. The rolling sample survey method was implemented subsequently and has been conducted every year since 2007. This study used the data from KNHANES VI, the most recently completed survey period; the dietary omega-3 fatty acid intake variable was available at the time of analysis.
For the purposes of this study, both natural and artificial menopause were categorized as menopause; this was based on amenorrhea or menstruation-related items in the women's health questionnaire. A total of 22,948 respondents were registered in KNHANES VI (2013–2015). Individuals who met the following criteria were excluded from the analysis: 1) men (n = 10,599), 2) pregnant, lactating, or premenopausal women (n = 7,818), 3) women with a total energy intake of less than 500 kcal or more than 5,000 kcal per day [22] (n = 380), and 4) women with no data on depression (n = 1). Finally, 4,150 postmenopausal women were included in the analysis. The study followed the guidelines of the Declaration of Helsinki. All participants signed an informed consent form, and the study was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (approval No. 2013-07CON-03-4C, 2013-12EXP-03-5C).
Demographic and lifestyle information
The demographic, lifestyle, and health information were collected using self-reported questionnaires. The household income levels were classified into four categories: low, mid-low, mid-high, and high. Education levels were categorized as less than high school graduation and high school graduation or higher. The body mass index (BMI) was calculated and categorized based on the BMI criteria of the World Health Organization for Asian populations: normal or less (< 23 kg/m2) and overweight or obesity (≥ 23 kg/m2) [23]. Based on their smoking and drinking status, they were classified as smokers or non-smokers and drinkers or non-drinkers, respectively. The metabolic equivalents of task (METs-h/week) [24] were calculated as the weekly physical activity time and through the application of weights by exercise intensity. The survey data on the days and hours spent performing intense/moderate/walking physical activity were obtained, and a weight value according to each exercise intensity was applied. In the 2014 and 2015 surveys, high- and moderate-intensity physical activities were further categorized into work and leisure activities, and the average value of each item was used to calculate the METs-h/week. The calculated values of METs-h/week were classified into tertiles for the analysis. The responses for the use of unspecified dietary supplements were classified as “yes” or “no”.
Dietary assessment
The dietary information was obtained using a 24-h recall method, which contains information regarding every consumed food item, in addition to the meal times, places of eating, and the quantity of food consumed during the 24 h before the survey. Assisting aids were used to enhance the validity of the recall data. These data were converted to individual foods using the food recipe database developed by the Korea Health Industry Development Institute [25], and the nutrient intake was calculated using the food composition table published by the Rural Development Administration [26]. In this study, we used the daily energy intake (kcal) and dietary omega-3 fatty acid intake (g) as variables for the analysis. The first dietary fatty acid database was established using 24-h recall data from the KNHANES VI-1(2013) [27] and has been updated thereafter [28]. The fatty acid levels of foods were obtained from the food composition tables of national institutions (Rural Development Administration, National Fisheries Research & Development Institute) [29,30], the US Department of Agriculture [31], and the Japan Ministry of Education, Culture, Sports, Science, and Technology [32]. The detailed methods for developing the fatty acid composition table have been presented elsewhere [27].
Criteria for diagnosis of depression
Depression was defined based on the data obtained from a self-reported mental health questionnaire under the supervision of an investigator [33]. Depression was defined based on the following factors: 1) diagnosed with depression by a physician, 2) having depression currently, or 3) under treatment for depression. Those who provided a response of “yes” for any of these questions were included in the depression group.
Statistical analysis
The analysis of this study was conducted considering the sampling weight, stratification variables, and cluster variables related to the complex sampling of the KNHANES [21]. The participants were divided into quintiles according to the intake levels of dietary omega-3 fatty acids. To compare the general characteristics between groups, the chi-square test and general linear regression analysis were used for categorical and continuous variables, respectively. The residual adjustment method was used to calculate the levels of energy-adjusted nutritional intake [34]. Multivariate logistic regression analysis was performed to calculate the odds ratios (ORs) and their 95% confidence intervals (CIs). The potential confounding factors and effect modifiers that could affect the association between the dietary omega-3 fatty acid intake levels and depression in the analysis were selected based on a review of the literature [7,19,35,36] and preliminary analysis, determining whether key demographic, lifestyle, or dietary variables were associated with omega-3 fatty acid intake and depression and whether the identified potential confounding variables were not on the causal pathway between omega-3 fatty acid intake and depression in women.
The interactions were tested using multiplicative terms in logistic regression models, and no significant interaction was found. The models, with step-by-step adjustment of confounding factors, were as follows: model 1, unadjusted; model 2, adjusted for age, household income, obesity status, education level, alcohol consumption, smoking status, physical activity, use of dietary supplements, energy-adjusted intake level of dietary fiber, and vitamin C. The P-value for the trend was calculated using the median of the quintiles of dietary omega-3 fatty acid intake as a continuous variable. The dose-response relationship between dietary omega-3 fatty acids and depression was examined semi-parametrically using restricted cubic splines with three knots, excluding values beyond the 5th and 95th percentiles. All analyses in this study were performed using the Statistical Analysis System (SAS version 9.4; SAS Institute, Cary, NC, USA). A significance level of α = 0.05, using two-tailed tests, was considered statistically significant.
RESULTS
General characteristics of participants by intake levels of dietary omega-3 fatty acid
The 4,150 postmenopausal women were divided into quintiles according to their dietary omega-3 fatty acid intake. The median values for the 1st, 2nd, 3rd, 4th, and 5th quintiles were 0.3, 0.6, 0.9, 1.4, and 2.7 g/day, respectively; their general characteristics are presented in Table 1. A higher intake of dietary omega-3 fatty acid was observed among younger women (P < 0.001), alcohol drinkers (P = 0.01), and those with a higher level of education (P < 0.001), higher household income (P < 0.001), and higher physical activity level (P < 0.001), but no significant differences were observed in terms of obesity and smoking status.
Table 1. Characteristics of postmenopausal women according to quintiles of energy-adjusted intake levels of dietary omega-3 fatty acid.
| Characteristics | Dietary omega-3 fatty acids | P-value1) | |||||
|---|---|---|---|---|---|---|---|
| Q1 (n = 830) | Q2 (n = 830) | Q3 (n = 830) | Q4 (n = 830) | Q5 (n = 830) | |||
| Dietary omega-3 fatty acid intake (g/day), median | 0.3 | 0.6 | 0.9 | 1.4 | 2.7 | ||
| Age (yrs) | 67.1 ± 0.3 | 65.0 ± 0.3 | 63.6 ± 0.3 | 62.8 ± 0.3 | 62.9 ± 0.3 | < 0.001 | |
| Education level | < 0.001 | ||||||
| Less than high school graduation | 683 (82.5) | 601 (72.6) | 558 (67.2) | 537 (64.7) | 517 (62.4) | ||
| High school graduation or higher | 145 (17.5) | 227 (27.4) | 272 (32.8) | 293 (35.3) | 312 (37.6) | ||
| Obesity status2) | 0.05 | ||||||
| Normal or less | 297 (35.8) | 296 (35.7) | 293 (35.4) | 341 (41.1) | 324 (39.1) | ||
| Overweight or obesity | 533 (64.2) | 534 (64.3) | 535 (64.6) | 489 (58.9) | 504 (60.9) | ||
| Smoking status | 0.72 | ||||||
| Smokers | 30 (3.7) | 21 (2.6) | 27 (3.3) | 27 (3.3) | 23 (2.8) | ||
| Non-smokers | 781 (96.3) | 794 (97.4) | 792 (96.7) | 797 (96.7) | 795 (97.2) | ||
| Household income | < 0.001 | ||||||
| Low | 222 (26.9) | 218 (26.3) | 196 (23.7) | 181 (22.0) | 175 (21.2) | ||
| Mid-low | 263 (31.8) | 197 (23.8) | 214 (25.9) | 195 (23.7) | 194 (23.5) | ||
| Mid-high | 184 (22.3) | 210 (25.3) | 191 (23.1) | 221 (26.8) | 211 (25.6) | ||
| High | 157 (19.0) | 204 (24.6) | 226 (27.3) | 227 (27.6) | 245 (29.7) | ||
| Physical activity level (METs-h/week)3) | < 0.001 | ||||||
| Low | 332 (40.1) | 287 (34.6) | 287 (34.6) | 251 (30.2) | 242 (29.2) | ||
| Middle | 280 (33.8) | 282 (34.0) | 282 (34.0) | 266 (32.1) | 257 (31.0) | ||
| High | 217 (26.2) | 260 (31.4) | 261 (31.5) | 313 (37.7) | 331 (39.9) | ||
| Alcohol consumption | 0.01 | ||||||
| Non-drinkers | 449 (55.2) | 454 (55.4) | 412 (50.2) | 401 (48.7) | 402 (49.1) | ||
| Drinkers | 364 (44.8) | 365 (44.6) | 408 (49.8) | 423 (51.3) | 417 (50.9) | ||
| Dietary supplement use (yes) | 441 (53.5) | 379 (45.9) | 367 (44.4) | 352 (42.5) | 324 (39.2) | < 0.001 | |
| Dietary fiber intake (g/day)4) | 19.7 ± 0.4 | 23.7 ± 0.4 | 25.6 ± 0.4 | 25.7 ± 0.4 | 28.1 ± 0.4 | < 0.001 | |
| Dietary vitamin C intake (g/day)4) | 109.0 ± 4.4 | 113.6 ± 4.4 | 119.1 ± 4.4 | 116.1 ± 4.4 | 131.3 ± 4.4 | < 0.001 | |
Values are mean ± SE or number (%).
Q, quintile; METs, metabolic equivalents of tasks.1) P-values were derived from the χ2 test for categorical variables and a general linear regression for continuous variables.
2)Obesity status was categorized as follows: normal or less < 23 kg/m2 and overweight or obesity ≥ 23 kg/m2.
3)Physical activity level was calculated as metabolic equivalent time hours per week (METs-h/week) and categorized into tertiles.
4)Values were adjusted for total energy intake.
Association between dietary omega-3 fatty acid intake and depression
The ORs of depression, according to quintiles of dietary omega-3 fatty acid intake, are shown in Table 2. In the unadjusted model (model 1), higher levels of dietary omega-3 fatty acid intake were associated with lower depression (P for trend = 0.02). After adjusting for multiple confounding variables (model 2), this inverse association remained significant (P for trend = 0.04). Compared to women in the lowest quintile of dietary omega-3 fatty acid intake, depression was 1.72-fold less likely in those in the highest quintile (OR, 0.52; 95% CI, 0.33–0.83; model 2).
Table 2. Odds ratio (95% confidence interval) for depression according to quintiles of energy-adjusted intake levels of dietary omega-3 fatty acid in postmenopausal women.
| Dietary omega-3 fatty acids | P for trend1) | |||||
|---|---|---|---|---|---|---|
| Q1 (n = 830) | Q2 (n = 830) | Q3 (n = 830) | Q4 (n = 830) | Q5 (n = 830) | ||
| Dietary omega-3 fatty acid intake (g/day), median | 0.3 | 0.6 | 0.9 | 1.4 | 2.7 | |
| Case | 86 | 70 | 78 | 70 | 61 | |
| Model 12) | 1 | 0.56 (0.39–0.80) | 0.84 (0.58–1.21) | 0.65 (0.43–0.97) | 0.54 (0.36–0.81) | 0.02 |
| Model 23) | 1 | 0.52 (0.36–0.77) | 0.76 (0.51–1.14) | 0.64 (0.41–0.98) | 0.52 (0.33–0.83) | 0.04 |
Q, quintile.
1)P for trend across quintiles of dietary omega-3 fatty acids was calculated using a general linear regression.
2)Model 1: crude.
3)Model 2: adjusted for age, household income, obesity status, education level, alcohol consumption, smoking status, physical activity, use of dietary supplements, and energy-adjusted intake levels of dietary fiber and vitamin C.
Dose-response relationship between dietary omega-3 fatty acid intake and depression
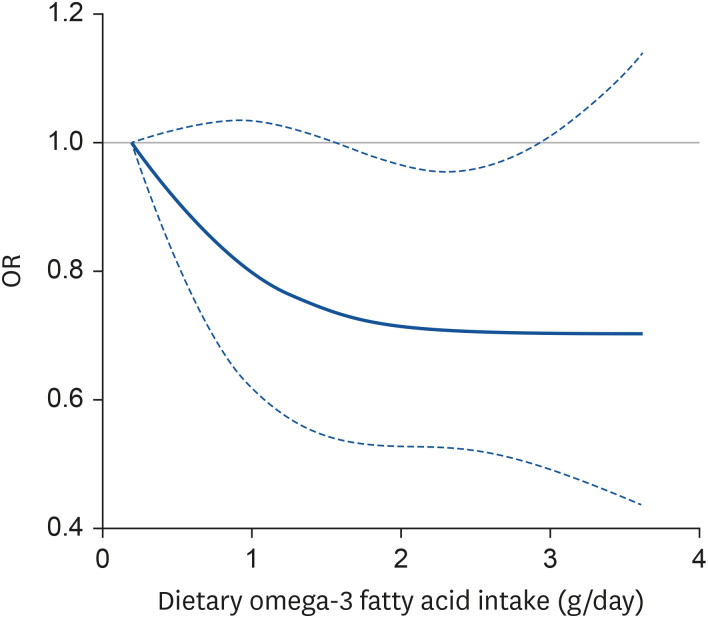
Spline analysis suggested a monotonic dose-response relationship between dietary omega-3 fatty acid intake and depression (P for non-linearity=0.32; Fig. 1). Depression tended to decrease with an increase in dietary omega-3 fatty acid intake; however, further benefits were not evident as the intake of dietary omega-3 fatty acids increased beyond 1.8 g/day.
Fig. 1. ORs of the prevalence of depression by energy-adjusted intake levels of dietary omega-3 fatty acid via restricted cubic spline regression. Dashed lines represent the 95% confidence intervals. The models were adjusted for age, household income, obesity status, education level, alcohol consumption, smoking status, physical activity, use of dietary supplements, and energy-adjusted intake levels of dietary fiber and vitamin C (P for non-linearity = 0.32).
OR, odds ratio.
DISCUSSION
In this nationwide survey in Korea, postmenopausal women with a higher intake of dietary omega-3 fatty acids demonstrated a lower depression than their counterparts, and this association was linear. Omega-3 fatty acids play an important role in brain cell function by directly maintaining cell membrane fluidity in the central nervous system and by regulating ion channels and the cyclic adenosine monophosphate (cAMP) cascade [37,38,39]. In particular, they impact the expression of brain-derived neurotrophic factor and cAMP response element-binding protein, which may lead to improved neurogenesis and neuroplasticity [38,40,41]. Previous studies have also reported that the depletion of omega-3 fatty acids is associated with a lack of neuronal membrane stability, with decreased levels of serotonin, norepinephrine, and dopamine, suggesting lower cognitive function in depression [42].
In this study, depression was lower in postmenopausal women with higher dietary omega-3 fatty acid intake. This may be related to several mechanisms. Owing to the imbalance of eicosanoids, the rapid decrease in estrogen during menopause increases the levels of prostaglandin E2 (PGE2), an inflammation-promoting factor [39,43,44]. This subsequently induces sickness behavior and affects neuronal function, causing cognitive and impulse control disorders [42]. Indeed, a significant association was observed between inflammatory markers and depression in adults in the Netherlands; those with higher levels of inflammatory biomarkers, such as interleukin-6, had increased risks of major depressive disorders [45]. Omega-3 fatty acids act as estrogens, inhibiting the production of PGE2, which is an eicosanoid that consequently inhibits the production of inflammatory cytokines [39,46]. In an animal study, lifetime exposure to omega -3 polyunsaturated fatty acid (PUFA)-rich diets restored the Aβ-induced depressive-like profile [47]. Therefore, the intake of omega-3 fatty acids is believed to have a beneficial effect on depression in postmenopausal women; it relieves the hypersensitive immune system and has an anti-inflammatory effect [48].
A meta-analysis including nine cross-sectional and seven cohort studies reported that a high dietary omega-3 fatty acid intake decreases the risk of depression with a J-shaped association [7]. When divided by study design, depression was 21% lower in cross-sectional studies and 15% decreased in cohort studies [7]. This study explained that there was a high variation in intake levels in the Japanese population due to the very high intake of omega-3 PUFAs. A recent Japanese cohort study published after this meta-analysis also confirmed that Japanese adults with high omega-3 fatty acid intake had a low risk of depressive symptoms [49]. A recent meta-analysis of 12 randomized controlled trials demonstrated that supplementary intake of eicosapentaenoic acid (EPA)-pure (= 100% EPA) and EPA-major formulations (≥ 60% EPA) conferred clinical benefits for depression [50]. However, no benefits were found with supplements containing docosahexaenoic acid (DHA)-pure and DHA-major formulations. However, a recent multi-national multi-disciplinary Delphi study for the application of omega-3 PUFAs in the treatment of major depressive disorders found that current evidence is insufficient to conclude that “omega-3 PUFAs are one of the potential monotherapies for adult major depressive disorder” [51].
The average intake level of omega-3 fatty acids in these postmenopausal women was 1.31 g/day, which seemed to be slightly lower than that in premenopausal women (1.46 g/day) and men (1.76 g/day) in the KNHANES. We observed a monotonic dose-response relationship between dietary omega-3 fatty acid intake and depression at these dietary intake levels; however, this decreasing trend became flatter at more than approximately 1.9 g/day of dietary omega-3 fatty acids. A similar pattern was observed in a pooled recent meta-analysis of nine cross-sectional and seven cohorts, showing a dose-response relationship with a peak decrease in depression risk at approximately 1.8 g/day of omega-3 fatty acids [7]. Although data regarding the effect of omega-3 fatty acids on depression may not be univocal, there is a general agreement on the fact that supplementation with omega-3 fatty acids is useful in improving depressive symptoms, especially in elderly women and that dosages of 2–4 g/day may provide the maximum benefit [17,52,53,54].
Our study has some limitations. Dietary information in this study may not have accurately reflected the usual dietary intake as it was calculated using a 1-day 24-h recall survey, and we could not exclude the possibility of reverse causality between the intake of dietary omega-3 fatty acid and depression in postmenopausal women in this cross-sectional study; two additional concerns are confounding and measurement errors. Although we selected potential confounding factors based on a review of the literature and preliminary analysis, we could not exclude the possibility of residual unknown or unmeasured confounding factors that may have affected these associations. For instance, the total antioxidant capacity, which may inhibit oxidative stress, may influence the association between omega-3 fatty acids and depression. In addition, we did not consider information on the use of hormone therapy because of the lack of data. This is because those with high intake of omega-3 fatty acids may tend to be health-conscious; therefore, they can, in addition, eat more fruits and vegetables, which are rich in antioxidants, and may be a protective dietary factor for depression [55]. In addition, there is the possibility of non-differential misclassification regarding the identification of depression, because we used self-reported information for diagnosing depression. Therefore, in addition to underestimation, overestimation may have occurred due to inaccuracies in the recognition of depression by physicians, recall errors by participants, or under-treatment of depression. To minimize this error and maximize the specificity of the case definition, we used a combination of responses on disease diagnosis or treatment taken. Despite these limitations, our results may be generalized to the Korean population as the KNHANES data are representative of this population. These findings suggest that omega-3 fatty acids may be an alternative therapeutic option for relieving depression in postmenopausal women. Since we investigated mental health during menopause, our findings also provide basic data on the prevention and management of health problems in women. Previous research in this area has largely been inadequate.
In conclusion, our findings suggest that a higher intake of dietary omega-3 fatty acids is associated with lower depression in Korean postmenopausal women. Large-scale prospective cohort studies of Korean women are warranted to verify this association, using omega-3 fatty acids in both dietary and supplement forms.
Footnotes
Funding: This research was supported by the 2020 Yeungnam University Research Grant.
Conflict of Interest: The authors declare no potential conflicts of interests.
- Conceptualization: Park K, Chae M.
- Data curation: Chae M.
- Formal analysis: Chae M.
- Methodology: Park K, Chae M.
- Software: Chae M.
- Supervision: Park K.
- Validation: Park K.
- Visualization: Park K.
- Writing - original draft: Chae M.
- Writing - review & editing: Park K.
References
- 1.World Health Organization. Research on the Menopause in the 1990s. Geneva: World Health Organization; 1996. pp. 12–14. [Google Scholar]
- 2.Oppermann K, Fuchs SC, Donato G, Bastos CA, Spritzer PM. Physical, psychological, and menopause-related symptoms and minor psychiatric disorders in a community-based sample of Brazilian premenopausal, perimenopausal, and postmenopausal women. Menopause. 2012;19:355–360. doi: 10.1097/gme.0b013e31822ba026. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, Sheng L. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110:230–240. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 4.Joffe H, Hall JE, Soares CN, Hennen J, Reilly CJ, Carlson K, Cohen LS. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392–398. doi: 10.1097/00042192-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 5.de Kruif M, Spijker AT, Molendijk ML. Depression during the perimenopause: a meta-analysis. J Affect Disord. 2016;206:174–180. doi: 10.1016/j.jad.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. 2018;226:346–354. doi: 10.1016/j.jad.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Grosso G, Micek A, Marventano S, Castellano S, Mistretta A, Pajak A, Galvano F. Dietary n-3 PUFA, fish consumption and depression: a systematic review and meta-analysis of observational studies. J Affect Disord. 2016;205:269–281. doi: 10.1016/j.jad.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Bai ZG, Bo A, Wu SJ, Gai QY, Chi I. Omega-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: a systematic review and meta-analysis. J Affect Disord. 2018;241:241–248. doi: 10.1016/j.jad.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 9.Giacobbe J, Benoiton B, Zunszain P, Pariante CM, Borsini A. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front Psychiatry. 2020;11:122. doi: 10.3389/fpsyt.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 11.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 12.Bromberger JT, Assmann SF, Avis NE, Schocken M, Kravitz HM, Cordal A. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol. 2003;158:347–356. doi: 10.1093/aje/kwg155. [DOI] [PubMed] [Google Scholar]
- 13.Brinton RD, Tran J, Proffitt P, Montoya M. 17 Beta-estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochem Res. 1997;22:1339–1351. doi: 10.1023/a:1022015005508. [DOI] [PubMed] [Google Scholar]
- 14.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 15.Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13–25. doi: 10.1016/j.pnpbp.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011;94:1914S–1919S. doi: 10.3945/ajcn.110.000893. [DOI] [PubMed] [Google Scholar]
- 17.Freeman MP, Hibbeln JR, Silver M, Hirschberg AM, Wang B, Yule AM, Petrillo LF, Pascuillo E, Economou NI, Joffe H, et al. Omega-3 fatty acids for major depressive disorder associated with the menopausal transition: a preliminary open trial. Menopause. 2011;18:279–284. doi: 10.1097/gme.0b013e3181f2ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masoumi SZ, Kazemi F, Tavakolian S, Rahimi A, Oshvandi K, Soltanian A, Shobeiri F. Effect of citalopram in combination with omega-3 on depression in post-menopausal women: a triple blind randomized controlled trial. J Clin Diagn Res. 2016;10:QC01–05. doi: 10.7860/JCDR/2016/19487.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Je Y. Fish consumption and depression in Korean adults: the Korea National Health and Nutrition Examination Survey, 2013–2015. Eur J Clin Nutr. 2018;72:1142–1149. doi: 10.1038/s41430-017-0083-9. [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Lee DK, Kim B, Na KS, Lee CH, Son YD, Lee HJ. The association between omega-3 fatty acid intake and human brain connectivity in middle-aged depressed women. Nutrients. 2020;12:2191. doi: 10.3390/nu12082191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, Chun C, Khang YH, Oh K. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia; 2000. pp. 15–21. [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Korea Health Industry Development Institute. Development of Nutrient Database. Cheongju: Korea Health Industry Development Institute; 2000. pp. 26–164. [Google Scholar]
- 26.National Institute of Agricultural Sciences. Food Composition Table. 8th ed. Suwon: National Institute of Agricultural Sciences; 2011. [Google Scholar]
- 27.Yoon MO, Kim K, Hwang JY, Lee HS, Son TY, Moon HK, Shim JE. Development of a fatty acids database using the Korea National Health and Nutrition Examination Survey data. J Nutr Health. 2014;47:435–442. [Google Scholar]
- 28.Korea Centers for Disease Control and Prevention. Guidebook for Data Users of the Sixth Korea National Health and Nutriton Examination Survey (KNHANES VI), 2013–2015. Cheongju: Korea Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 29.National Fisheries Research & Development Institute (KR) Fatty Acid Composition of Fisheries Products in Korea. Busan: National Fisheries Research & Development Institute; 2012. pp. 2–123. [Google Scholar]
- 30.Rural Development Administration. Food Composition Table. 7th ed. Suwon: Rural Development Administration; 2006. Fatty acid and cholesterol content of foods; pp. 277–380. [Google Scholar]
- 31.United States Development of Agriculture, Agricultural Research Service. USDA national nutrient database for standard reference. release 25 [Internet] Washington D.C.: United States Development of Agriculture; 2012. [cited 2013 August 19]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/sr11-sr28. [Google Scholar]
- 32.Ministry of Education, Culture, Sports, Science and Technology. The Council for Science and Technology, Subdivision on Resources. Standard Tables of Food Composition in Japan (Fifth Revised and Enlarged Edition) Tokyo: Ministry of Education, Culture, Sports, Science and Technology; 2005. [Google Scholar]
- 33.Korea Centers for Disease Control and Prevention. Korea National Health and Nutrition Survey VI-1 (2013) Health Examination Guidelines. Cheongju: Ministry of Health and Welfare of Korea; 2014. pp. 46–48. [Google Scholar]
- 34.Willett W. Nutritional Epidemiology. 3rd ed. New York (NY): Oxford University Press, USA; 2012. pp. 274–275. [Google Scholar]
- 35.Persons JE, Robinson JG, Ammann EM, Coryell WH, Espeland MA, Harris WS, Manson JE, Fiedorowicz JG. Omega-3 fatty acid biomarkers and subsequent depressive symptoms. Int J Geriatr Psychiatry. 2014;29:747–757. doi: 10.1002/gps.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skelly AC, Dettori JR, Brodt ED. Assessing bias: the importance of considering confounding. Evid Based Spine Care J. 2012;3:9–12. doi: 10.1055/s-0031-1298595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 38.Logan AC. Neurobehavioral aspects of omega-3 fatty acids: possible mechanisms and therapeutic value in major depression. Altern Med Rev. 2003;8:410–425. [PubMed] [Google Scholar]
- 39.Owen C, Rees AM, Parker G. The role of fatty acids in the development and treatment of mood disorders. Curr Opin Psychiatry. 2008;21:19–24. doi: 10.1097/YCO.0b013e3282f29841. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]
- 43.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 44.Saldeen P, Saldeen T. Women and omega-3 fatty acids. Obstet Gynecol Surv. 2004;59:722–730. doi: 10.1097/01.ogx.0000140038.70473.96. [DOI] [PubMed] [Google Scholar]
- 45.Lamers F, Milaneschi Y, Smit JH, Schoevers RA, Wittenberg G, Penninx BW. Longitudinal association between depression and inflammatory markers: results from the Netherlands study of depression and anxiety. Biol Psychiatry. 2019;85:829–837. doi: 10.1016/j.biopsych.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 46.Chamani S, Bianconi V, Tasbandi A, Pirro M, Barreto GE, Jamialahmadi T, Sahebkar A. Resolution of Inflammation in neurodegenerative diseases: the role of resolvins. Mediators Inflamm. 2020;2020:3267172. doi: 10.1155/2020/3267172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bove M, Mhillaj E, Tucci P, Giardino I, Schiavone S, Morgese MG, Trabace L. Effects of n-3 PUFA enriched and n-3 PUFA deficient diets in naïve and Aβ-treated female rats. Biochem Pharmacol. 2018;155:326–335. doi: 10.1016/j.bcp.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Turkmen S, Hernández-Cruz CM, Zamorano MJ, Fernández-Palacios H, Montero D, Afonso JM, Izquierdo M. Long-chain PUFA profiles in parental diets induce long-term effects on growth, fatty acid profiles, expression of fatty acid desaturase 2 and selected immune system-related genes in the offspring of gilthead seabream. Br J Nutr. 2019;122:25–38. doi: 10.1017/S0007114519000977. [DOI] [PubMed] [Google Scholar]
- 49.Horikawa C, Otsuka R, Kato Y, Nishita Y, Tange C, Rogi T, Kawashima H, Shibata H, Ando F, Shimokata H. Longitudinal association between n-3 long-chain polyunsaturated fatty acid intake and depressive symptoms: a population-based cohort study in Japan. Nutrients. 2018;10:1655. doi: 10.3390/nu10111655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, Fan B, Lu C, Mclntyer RS. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9:190. doi: 10.1038/s41398-019-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guu TW, Mischoulon D, Sarris J, Hibbeln J, McNamara RK, Hamazaki K, Freeman MP, Maes M, Matsuoka YJ, Belmaker RH, et al. A multi-national, multi-disciplinary Delphi consensus study on using omega-3 polyunsaturated fatty acids (n-3 PUFAs) for the treatment of major depressive disorder. J Affect Disord. 2020;265:233–238. doi: 10.1016/j.jad.2020.01.050. [DOI] [PubMed] [Google Scholar]
- 52.Bozzatello P, Rocca P, Mantelli E, Bellino S. Polyunsaturated fatty acids: what is their role in treatment of psychiatric disorders? Int J Mol Sci. 2019;20:5257. doi: 10.3390/ijms20215257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wani AL, Bhat SA, Ara A. Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integr Med Res. 2015;4:132–141. doi: 10.1016/j.imr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Logan AC. Omega-3 fatty acids and major depression: a primer for the mental health professional. Lipids Health Dis. 2004;3:25. doi: 10.1186/1476-511X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milajerdi A, Keshteli AH, Afshar H, Esmaillzadeh A, Adibi P. Dietary total antioxidant capacity in relation to depression and anxiety in Iranian adults. Nutrition. 2019;65:85–90. doi: 10.1016/j.nut.2018.11.017. [DOI] [PubMed] [Google Scholar]