Abstract
STUDY QUESTION
What are the trends and developments in pre-implantation genetic testing (PGT) in 2016–2017 as compared to previous years?
SUMMARY ANSWER
The main trends observed in this 19th and 20th data set on PGT are that trophectoderm biopsy has become the main biopsy stage for PGT for aneuploidies (PGT-A) and that the implementation of comprehensive testing technologies is the most advanced with PGT-A.
WHAT IS KNOWN ALREADY
Since it was established in 1997, the ESHRE PGT Consortium has been collecting and analysing data from mainly European PGT centres. To date, 18 data sets and an overview of the first 10 years of data collections have been published.
STUDY DESIGN, SIZE, DURATION
The data for PGT analyses performed between 1 January 2016 and 31 December 2017 with a 2-year follow-up after analysis were provided by participating centres on a voluntary basis. Data were collected using a new online platform, which is based on genetic analysis as opposed to the former cycle-based format.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Data on biopsy method, diagnostic technology and clinical outcome were submitted by 61 centres. Records with analyses for more than one PGT for monogenic/single gene defects (PGT-M) and/or PGT for chromosomal structural rearrangements (PGT-SR) indication or with inconsistent data regarding the PGT modality were excluded. All transfers performed within 2 years after the analysis were included enabling the calculation of cumulative pregnancy rates. Data analysis, calculations, figures and tables were made by expert co-authors.
MAIN RESULTS AND THE ROLE OF CHANCE
The current data collection from 2016 to 2017 covers a total of 3098 analyses for PGT-M, 1018 analyses for PGT-SR, 4033 analyses for PGT-A and 654 analyses for concurrent PGT-M/SR with PGT-A.
The application of blastocyst biopsy is gradually rising for PGT-M (from 8–12% in 2013–2015 to 19% in 2016–2017), is status quo for PGT-R (from 22–36% in 2013–2015 to 30% in 2016–2017) and has become the preferential biopsy stage for PGT-A (from 23–36% in 2013–2015 to 87% in 2016–2017). For concurrent PGT-M/SR with PGT-A, biopsy was primarily performed at the blastocyst stage (93%). The use of comprehensive diagnostic technology showed a similar trend with a small increased use for PGT-M (from 9–12% in 2013–2015 to 15% in 2016–2017) and a status quo for PGT-SR (from 36–58% in 2013–2015 to 50% in 2016–2017). Comprehensive testing was the main technology for PGT-A (from 66–75% in 2013–2015 to 93% in 2016–2017) and for concurrent PGT-M/SR with PGT-A (93%).
LIMITATIONS, REASONS FOR CAUTION
The findings apply to the data submitted by 61 participating centres and do not represent worldwide trends in PGT. Details on the health of babies born were not provided in this manuscript.
WIDER IMPLICATIONS OF THE FINDINGS
Being the largest data collection on PGT in Europe/worldwide, the data sets provide a valuable resource for following trends in PGT practice.
STUDY FUNDING/COMPETING INTEREST(S)
The study has no external funding and all costs are covered by ESHRE. There are no competing interests declared.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: PGT, PGD, structural rearrangements, monogenic disorders, aneuploidy, human embryo, registry, data collection, pre-implantation genetic testing
Introduction
The main objectives of the ESHRE PGT Consortium, established in 1997, are to provide guidance and network opportunities to pre-implantation genetic testing (PGT) centres, to promote best practice and to collect data on PGT treatments and their outcome. Eighteen sets of data on PGT cycles, pregnancies, deliveries and children have been published to date (Geraedts et al., 1999, 2000; ESHRE PGD Consortium Steering Committee, 2002; Sermon et al., 2005, 2007; Harper et al., 2006, 2008, 2010; Goossens et al., 2008, 2009, 2012; Moutou et al., 2014; De Rycke et al., 2015, 2017; Coonen et al., 2020). An overview has been presented after 10 years of data collection (Harper et al., 2012). Overall, the data collections provide a valuable resource for data mining and for following trends in PGT practice.
Until data collection XVI (2013), data submission from the participating centres has been retrospective, relying on pre-designed Excel and later on FileMakerPro files (Apple Inc, Cupertino, CA, USA). A new online data registration platform was used from data collection XIX (2016) onwards. Summary data were collected to cover the transition period in the years 2013–2015 (Coonen et al., 2020). The online database offers data registration in real time and has been adapted to accommodate the increased complexity and changes in the overall timeline of PGT treatments. Embryo biopsy, genetic analysis and embryo transfer may occur in completely separate time frames. Data from these different segments of a PGT treatment are registered in connected modules, with genetic analysis as the central module to which multiple oocyte collection modules and multiple embryo transfer modules can be linked. The new database structure is therefore analysis-based as opposed to the former cycle-based database in which one oocyte collection was followed by one analysis and one embryo transfer. The implementation of the online database in 2016 was followed by a participant’s meeting, during which representatives of many centres, the database developers and the steering committee discussed any remaining issues of the platform.
Following a revision of definitions used in infertility care, the previous terms of pre-implantation genetic diagnosis (PGD) and pre-implantation genetic screening (PGS) have been replaced by the term PGT, including PGT for aneuploidies (PGT-A), PGT for monogenic/single gene defects (PGT-M) and PGT for chromosomal structural rearrangements (PGT-SR) (Zegers-Hochschild et al., 2017). From the first Consortium data collection onwards, PGT cycles for chromosomal numerical aberrations of high genetic risk have been combined with cycles for chromosomal structural aberrations (the current PGT-SR indication group) and this classification has been maintained when developing the new database structure in 2015. PGT cycles with sexing for X-linked diseases were no longer presented as a separate category but were grouped within the PGT-SR indication group. The implementation of comprehensive genetic testing has enabled concurrent PGT-M/SR and PGT-A, and data from such double indications were considered as a distinct category.
This paper is the first to present data from PGT treatments registered in the online database.
Materials and methods
The report includes PGT analyses conducted between 1 January 2016 and 31 December 2017 and covers data on PGT indication, biopsy method, diagnostic technology, the efficiency of the different procedures and (cumulative) clinical PGT outcome in terms of positive hCG and live births of all fresh and frozen embryo transfers reported up until 2 years after the analysis date.
Data on PGT treatments were provided by 61 Consortium members, mainly based in European countries (54/61), covering the following treatment modalities: PGT-M, PGT-SR, PGT-A and concurrent PGT-M or PGT-SR with PGT-A. Centres used a unique login account to upload the data in an anonymized format onto an online platform designed for the specific requirements of this data collection (Dynamic Solutions, Barcelona, Spain). The database was exported to SQL Server Management Studio Version 15 (Microsoft Corporation, Redmond, WA, USA) and Structured Query Language (SQL) was used to retrieve the data per analysis from the database. These data were exported to Excel 2016 (Microsoft Corporation, Redmond, WA, USA) where the tables with the numbers and percentages were generated. Prism version 5 (Graphpad, San Diego, CA, USA) was used to generate the figures. A total of 9520 genetic analyses conducted within 2016 and 2017 were entered into the database. Of these, 153 (1.6%) were analysed for more than one PGT-M/PGT-SR indication, 57 (0.6%) concerned HLA and 15 (0.2%) a mitochondrial mutation load analysis. Regarding the low numbers, details on these analyses are left out of the report. Another 331 (3.5%) were excluded because no indication for PGT was filled in and 161 (1.7%) analyses had to be excluded because of inconsistent data regarding the PGT modality or the number of indications. Within the included PGT analyses (n = 8803), missing data were left blank and are reported as ‘not reported’. Following curation, in-depth data analysis was carried out by expert members of the ESHRE PGT Consortium Steering Committee.
The data from 2016 to 2017 are compared to the data from 2013 to 2015 (Coonen et al., 2020) or to means from earlier datasets.
The terminology used in this report was based on the revised glossary for infertility care (Zegers-Hochschild et al., 2017). A clinical pregnancy was defined as the presence of at least one positive heartbeat. An ongoing pregnancy was defined as the presence of at least one positive heart beat at 12 weeks of gestation and a live birth was defined as a liveborn child after 20 weeks of gestation.
Results
The current data collection covers a total of 8803 analyses initiated in 2016–2017 with PGT-M accounting for 35%, PGT-SR for 12%, PGT-A for 46% and concurrent PGT-M/SR with PGT-A for 7% of analyses. Overall data per PGT modality are presented in Figs 1–3, Tables I and II and accompanying text, while detailed results, per modality and per sub-indication, can be found in Supplementary Tables SI–SXII.
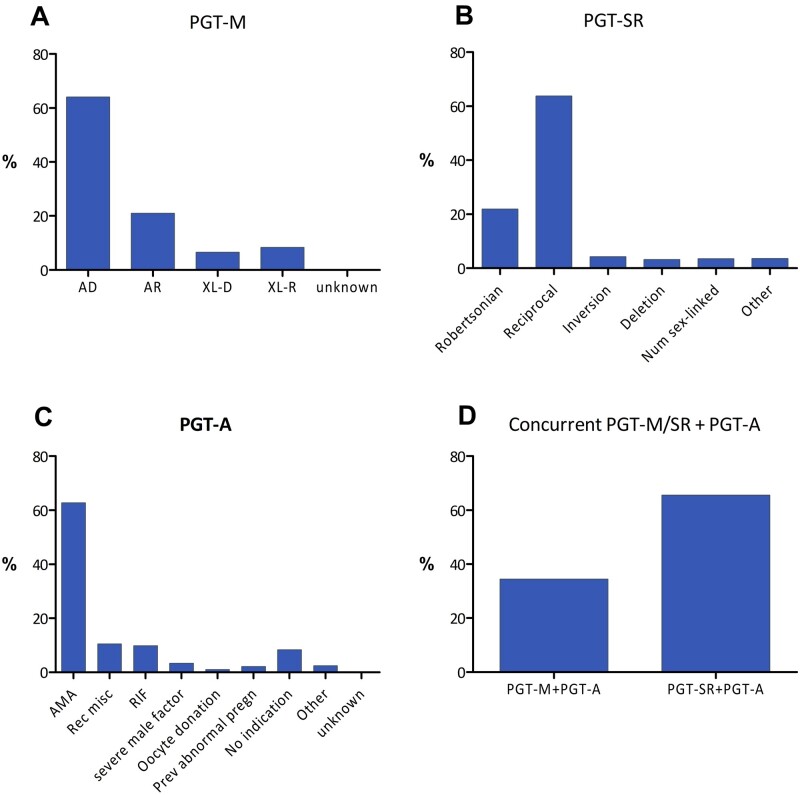
Figure 1.
Distribution of PGT indications in 2016–2017. (A) Pre-implantation testing for monogenic/single gene defects (PGT-M), (B) PGT for chromosomal structural rearrangements (PGT-SR), (C) PGT for aneuploidies (PGT-A) and (D) concurrent PGT-M/SR with PGT-A. AD, autosomal dominant; AR, autosomal recessive; XL-D, X-linked dominant; XL-R, X-linked recessive; AMA, advanced maternal age; Rec misc, recurrent miscarriage; RIF, repeated implantation failure; Prev abnormal pregn, previous abnormal pregnancy.
Figure 3.
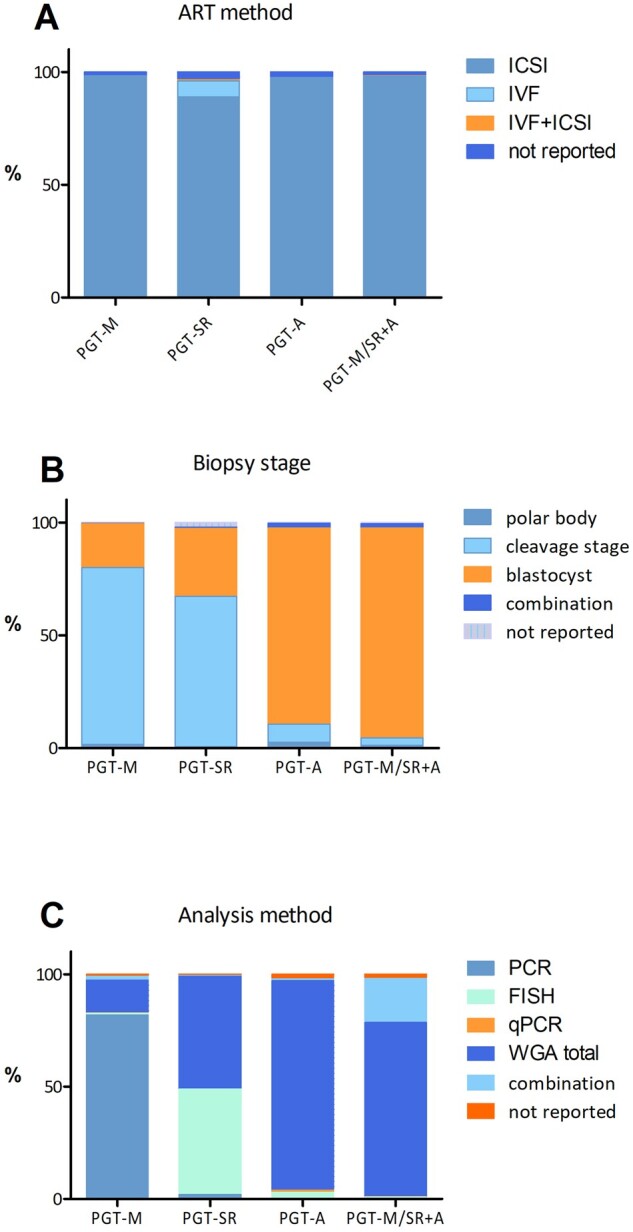
Distribution of methods used among the different PGT modalities in 2016–2017. (A) ART method, (B) biopsy stage and (C) analysis method.
Table I.
Data on biopsy and analysis at embryo level.
| PGT-M | PGT-SR | PGT-A | PGT-M/SR+PGT-A | Total | Total/no. of biopsied embryos | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Embryos biopsied (initial) | 18 126 | 100% | 4928 | 100% | 14 851 | 100% | 3218 | 100% | 41 123 | 100% | 100% | |
| Fresh embryos biopsied | 17 360 | 96% | 4511 | 92% | 14 117 | 95% | 3145 | 98% | 39 133 | 95% | 95% | |
| Thawed/warmed embryos biopsied | 66 | 4% | 417 | 8% | 734 | 5% | 73 | 2% | 1990 | 5% | 5% | |
|
| ||||||||||||
| Embryos analysed | 17 527 | 100% | 4894 | 100% | 14 559 | 100% | 3227 | 100% | 40 207 | 100% | 98% | |
| Embryos without diagnosis | 2109 | 12% | 317 | 6% | 454 | 3% | 162 | 5% | 3042 | 8% | 7% | |
| embryos with failed diagnosis | 1235 | 7% | 215 | 4% | 199 | 1% | 102 | 3% | 1751 | 4% | 4% | |
| embryos with inconclusive results | 874 | 5% | 102 | 2% | 255 | 2% | 60 | 2% | 1291 | 3% | 3% | |
| Embryos with diagnosis | 15 418 | 88% | 4577 | 94% | 14 105 | 97% | 3065 | 95% | 37 165 | 92% | 90% | |
|
| ||||||||||||
| Embryos for immediate transfer | 1764 | 547 | 428 | 60 | 2799 | 7% | ||||||
| Embryos for freezing | 3902 | 689 | 6630 | 1500 | 12 721 | 31% | ||||||
PGT-M, pre-implantation genetic testing for monogenic/single gene defects; PGT-SR, PGT for chromosomal structural rearrangements; PGT-A, PGT for aneuploidies.
Table II.
Data on data transfer and pregnancy outcome (% are related to number of transfers).
| PGT-M | PGT-SR | PGT-A | PGT-M/SR+PGT-A | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyses | 3098 | 1018 | 4033 | 654 | 8803 | ||||||
|
| |||||||||||
| Analyses without transfer* | 800 | 26% | 406 | 40% | 1787 | 44% | 229 | 35% | 3222 | 37% | |
| Analyses with transfer* | 2298 | 74% | 612 | 60% | 2246 | 56% | 425 | 65% | 5581 | 63% | |
|
| |||||||||||
| Transfers | 3182 | 100% | 773 | 100% | 2684 | 0% | 540 | 100% | 7179 | 100% | |
| Transfers with fresh embryos | 1322 | 42% | 355 | 46% | 241 | 9% | 34 | 6% | 1952 | 27% | |
| Transfers with thawed/warmed embryos | 1850 | 58% | 415 | 54% | 2440 | 91% | 505 | 94% | 5210 | 73% | |
| Transfers with fresh and thawed/warmed embryos | 9 | 0% | 3 | 0% | 2 | 0% | 1 | 0% | 15 | 0% | |
| Transfers not reported | 1 | 0% | 0 | 0% | 1 | 0% | 0 | 0% | 2 | 0% | |
| SET | 2784 | 87% | 702 | 91% | 2364 | 88% | 501 | 93% | 6351 | 88% | |
| DET | 388 | 12% | 68 | 9% | 309 | 12% | 39 | 7% | 804 | 11% | |
| >DET | 10 | 0% | 3 | 0% | 11 | 0% | 0 | 0% | 24 | 0% | |
| SET_unknown | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |
|
| |||||||||||
| Positive hCG | 1364 | 43% | 297 | 38% | 1621 | 60% | 315 | 58% | 3597 | 50% | |
|
| |||||||||||
| Analyses lost to FU after positive hCG test | 26 | 1% | 2 | 0% | 30 | 1% | 7 | 1% | 65 | 1% | |
| PUL | 121 | 4% | 46 | 6% | 123 | 5% | 29 | 5% | 319 | 4% | |
| Blighted ovum | 104 | 3% | 15 | 2% | 50 | 2% | 7 | 1% | 176 | 2% | |
| Ectopic pregnancies | 10 | 0% | 0 | 0% | 12 | 0% | 2 | 0% | 24 | 0% | |
| Clinical pregnancies | 1103 | 35% | 234 | 30% | 1406 | 52% | 270 | 50% | 3013 | 42% | |
|
| |||||||||||
| Analyses lost to FU after clinical pregnancies | 280 | 9% | 57 | 7% | 278 | 10% | 39 | 7% | 654 | 9% | |
| Pregnancy losses <12 weeks | 57 | 2% | 10 | 1% | 89 | 3% | 16 | 3% | 172 | 2% | |
| Ongoing pregnancies (>12 weeks) | 766 | 24% | 167 | 22% | 1039 | 39% | 215 | 40% | 2187 | 30% | |
| Singletons | 724 | 23% | 158 | 20% | 972 | 36% | 205 | 38% | 2059 | 29% | |
| Multiples | 42 | 1% | 9 | 1% | 67 | 2% | 10 | 2% | 128 | 2% | |
|
| |||||||||||
| Analyses lost to FU after ongoing pregnancy | 2 | 0% | 0 | 0% | 3 | 0% | 0 | 0% | 5 | 0% | |
| Pregnancy losses 12–20 weeks | 11 | 0% | 3 | 0% | 9 | 0% | 9 | 2% | 32 | 0% | |
| Pregnancies with no live birth | 10 | 0% | 0 | 0% | 13 | 0% | 2 | 0% | 25 | 0% | |
| Pregnancies with at least 1 live birth | 743 | 23% | 164 | 21% | 1014 | 38% | 204 | 38% | 2125 | 30% | |
|
| |||||||||||
| Live born children | 787 | 175 | 1084 | 215 | 2261 | ||||||
Per cent (%) expressed per number of analyses.
SET, single embryo transfer; DET, double embryo transfer; PUL, pregnancy of unknown location; FU, follow up.
PGT-M
For 2016–2017, 3098 PGT-M analyses were reported, the majority of which were linked with a single oocyte collection (96%) or biopsy event (98%). The mean female age was 32.9 years (Supplementary Table SI). Nearly two-thirds of PGT-M analyses were performed for an autosomal dominant disease (64%), followed by autosomal recessive and X-linked indications, which accounted for 21% and 15%, respectively (Fig. 1A). The top 10 of the indications for which PGT-M is applied can be seen in Fig. 2. Nine per cent of the analyses are performed for Huntington's disease, followed by cystic fibrosis (4.6%) and neurofibromatosis, hereditary breast cancer type 1 and myotonic dystrophy type 1 (each 4.3%). Similar to previous data sets, ICSI was the main fertilization method (98%) and cleavage-stage biopsy was the most widely used biopsy stage (78%) (Fig. 3). PCR was still the most widely used first-line method of DNA amplification (82%). In comparison to other modalities, the application of blastocyst biopsy is still limited and gradually rising (8% in 2013, 12% in 2015 and 19% in 2016–2017). The implementation of comprehensive diagnostic technology (9% in 2013, 12% in 2015 and 15% in 2016–2017) showed a similar slowly increasing trend. Further detailed results on ART method, biopsy stage and analysis method for PGT-M analyses can be found in Supplementary Table SI.
Figure 2.
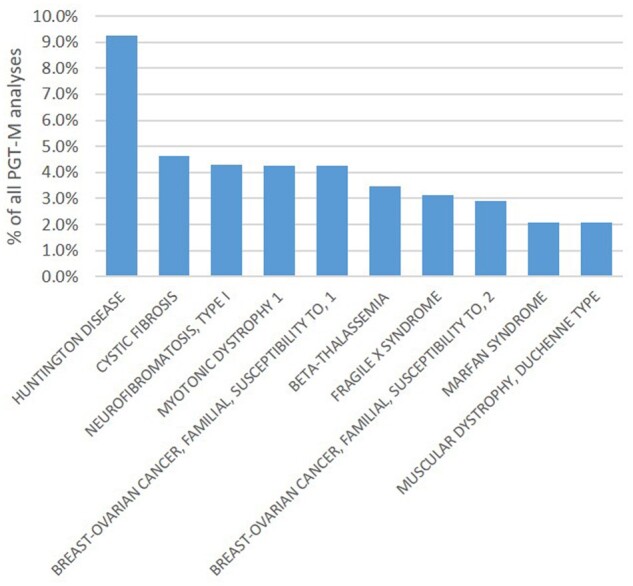
Top 10 of the indications for which PGT-M was applied in 2016–2017.
Data on biopsy and analysis at embryo level for PGT-M are presented in Table I and Supplementary Table SII. Of the 18 126 embryos for biopsy, 4% were thawed/warmed embryos. The majority of the embryos analysed (88%) gave a diagnostic result of which 47% were genetically transferable. This is in line with previous data sets (88–90% of analysed embryos gave a diagnostic result and on average 44% was genetically transferable in data set XVI–XVIII). Of the 12% embryos without diagnosis, 5% showed an inconclusive result while in 7% the analysis failed. Of all transferable embryos, 24% were freshly transferred, 53% were cryopreserved and 22% could not be used for the patient, most likely because of poor embryo development between biopsy and transfer stage. When expressed per biopsied embryo, 40% was genetically transferable, and 10% and 22% were transferred and cryopreserved, respectively.
From the 2-year total of 3098 analyses, 74% were coupled with at least one embryo transfer procedure performed within the 2 years following the analysis. In total, 3182 transfers were performed within this period, either in a fresh (42%) or a frozen (58%) cycle (Table II, Supplementary Table SIII). The majority of transfers (87%) involved a single embryo. Overall, a positive hCG was obtained in 43% of transfers, yielding a clinical pregnancy rate of 35% per embryo transfer procedure. This is in line with the clinical outcome reported in the 2013–2015 data set (34% per embryo transfer). When expressed per analysis (n = 3098) and per analysis with at least one transfer (n = 2298) the cumulative positive hCG and clinical pregnancy rates 2 years after the analysis were 44%/59% (positive hCG) and 36%/48% (clinical pregnancy), respectively. In 9% of the transfers, the pregnancy was lost to follow up after the clinical pregnancy. Of the remaining 823 clinical pregnancies, 766 (93%) went on to an ongoing pregnancy and 743 (90%) ended in at least one live birth. The number of reported live born children in the remaining transfers was 787.
PGT-SR
For 2016–2017, 1018 PGT-SR analyses were reported, the majority of which were linked with a single oocyte collection (94%) or biopsy event (98%). The mean age of women undergoing PGT-SR was 33.5 years (Supplementary Table SIV). PGT for reciprocal translocations was performed more often than for any other type of structural rearrangement (64%) while Robertsonian translocations accounted for 22% of PGT-SR analyses (Fig. 1B). Both figures are similar to those of previous data sets. ICSI was the preferred fertilization method (89%) (Fig. 3). Cleavage-stage biopsy was most commonly applied (67%). This figure indicates a status quo, compared to the figures of the previous data set (75% in 2013, 73% in 2014 and 63% in 2015). Biopsy at the blastocyst stage was performed in 30% of cases. After an increase from 36% to 58% between 2013 and 2015, the use of comprehensive, whole-genome amplification (WGA)-based technology stagnated at 50% (35% array comparative genomic hybridization (array CGH) and 14% next-generation sequencing (NGS)) for the years 2016–2017. In nearly half of the analyses (47%), FISH was still the preferred methodology. Further detailed results on ART method, biopsy stage and analysis method for PGT-SR analyses can be found in Supplementary Table SIV.
Data on biopsy and analysis at embryo level for PGT-SR are presented in Table I and Supplementary Table SV. Eight per cent of the 4928 embryos for biopsy were thawed/warmed embryos. A diagnosis was assigned in 94% of analysed embryos. Of all transferable embryos, 39% were freshly transferred, 49% were cryopreserved and 12% could not be used for the patient because of insufficient embryo morphology.
Of all analyses, 60% were linked with at least one embryo transfer procedure within the 2 years following the analysis, either in a fresh (46%) or a frozen (54%) cycle (Table II and Supplementary Table SVI). A single embryo was transferred in 91% of embryo transfers.
A positive hCG was obtained in 38% of transfers, with a clinical pregnancy rate of 30% per embryo transfer, which is slightly lower than the outcome reported in the 2013–2015 data set (34% per embryo transfer). Per analysis, the cumulative positive hCG and clinical pregnancy rates were 29% and 23%, respectively, while per analysis with transfer the rates were 49% and 38%. Seven per cent of the transfers with a clinical pregnancy were lost to follow up. The remaining 177 clinical pregnancies resulted in 167 (94%) ongoing pregnancies and 164 (93%) pregnancies with at least one live birth.
PGT for aneuploidies
For 2016–2017, 4033 PGT-A analyses were reported, the majority of which were linked with a single oocyte collection (95%) or biopsy event (93%). The average female age of women applying for PGT-A was 39.1 years. As expected, the age of women referred for advanced maternal age (AMA) (41.1, range 25.2–51.2 years) and oocyte donation (42.6 years) were highest (Supplementary Table SVII). The most common indication for PGT-A was AMA (63%), followed by recurrent miscarriage and repeated implantation failure (each 10%) (Fig. 1C). In 8% of analyses, PGT-A was performed without a reported medical indication. The trend of performing PGT-A as a common IVF add-on was noted for the first time in the data sets 2013–2015 (5–9% without indication).
In 97% of all analyses, ICSI was used for fertilization (Fig. 3). The majority of biopsies were performed at the blastocyst stage (87%). Cleavage-stage biopsy, which was still the preferred biopsy stage in 2015 (47%), only accounted for 8% of cases in 2016–2017.
For the genetic analysis, WGA-based methodologies, coupled with either array CGH (39%) or with NGS (54%), were the preferred methods. Together, these comprehensive approaches were applied in 93% of analyses, which was a further increase from the 75% figure from 2015. Although FISH was not recommended for PGT-A (ESHRE PGT-SR/PGT-A Working Group et al., 2020), the 2016–2017 data showed that it was still used in 3% of analyses. Further detailed results on ART method, biopsy stage and analysis method for PGT-A analyses can be found in Supplementary Table SVII.
Data on biopsy and analysis at embryo level for PGT-A are presented in Table I and Supplementary Table SVIII. Only a minority of the 14 851 embryos had been cryopreserved prior to biopsy (5%). Of the embryos analysed, 97% gave a diagnosis: 62% of these embryos were aneuploid, 34% were euploid and 4% of embryos were diagnosed as mosaic embryos. Most of the euploid and mosaic embryos were cryopreserved (89% and 92%, respectively). This was expected, given the frequent use of blastocyst biopsy with comprehensive analysis, which is usually coupled with embryo cryopreservation and transfer in a later cycle. The majority of the aneuploid embryos were discarded (79%) and 21% were cryopreserved.
At least one embryo transfer procedure was carried out for 56% of analyses, the largest part (91%) being a frozen embryo transfer and involving a single embryo (88%), which was either euploid (95%) or mosaic (4%) (Table II and Supplementary Table SIX). A positive hCG was obtained in 60% of transfers, leading to a clinical pregnancy rate of 52% per embryo transfer. The 2013–2015 data set showed a clinical pregnancy rate of 47% per embryo transfer, indicating a better outcome for the current data set. Per analysis, a cumulative positive hCG rate of 40% and a clinical pregnancy rate of 35% was reached, while after including only the analyses with a transfer, these rates were 72% and 63%, respectively. With a lost to follow up after a clinical pregnancy of 10% of the transfers, a total of 1084 live born children were reported.
Concurrent PGT-M/PGT-SR and PGT-A
For 2016–2017, 654 analyses for this ‘double’ indication group were reported. One-third concerned PGT-M with PGT-A (34%) and two-thirds involved PGT-SR with PGT-A (66%) (Fig. 1D). Although the majority of analyses are again linked with a single oocyte collection (89%) or biopsy event (86%), it is clear that the practice of accumulation before analysis is more applied for this modality. Since only 2% of the biopsied embryos were thawed/warmed before biopsy, the embryos are biopsied before cryopreservation and the biopsied samples are stored and, later on, pooled into one analysis. Pooling of more than a single cohort of embryos likely yields a larger series of samples for testing and ensures a better chance that a genetically transferable embryo ensues after selection for both PGT-M/SR and PGT-A. Accumulation at the level of analysis circumvents multiple vitrification-warming of embryos compared to the practice of accumulation at the level of biopsy.
The mean female age of women applying for concurrent PGT-M/PGT-SR and PGT-A was 34.3 years (Supplementary Table SX). As holds true for the other PGT modalities, the majority of cycles were performed with ICSI (98%) (Fig. 3). Biopsy was primarily performed at the blastocyst stage (93%). For the PGT-SR/PGT-A subgroup, comprehensive WGA methods were applied in 96% of analyses (60% array CGH and 36% NGS). For the PGT-M/PGT-A subgroup, the most frequent methods were WGA followed by SNP array (20%), WGA and NGS (16%) and combined methods (56%). Further detailed results on ART method, biopsy stage and analysis method for concurrent PGT-M/SR and PGT-A analyses can be found in Supplementary Table SX.
Data on biopsy and analysis at embryo level for PGT-M/SR with PGT-A are presented in Table II and Supplementary Table SXI. Here again, the majority of embryos for biopsy were fresh embryos (98%). The genetic diagnosis was successful for 95% of analysed embryos.
Within the group of embryos that are genetically transferable for PGT-M/PGT-SR (n = 1353), many embryos were cryopreserved: 92% of euploid embryos, 61% of mosaic embryos and even 23% of aneuploid embryos. As can be expected, the majority of the latter embryo group is discarded (77%), and so are 38% of mosaic embryos.
Within the group of non-genetically transferable embryos (n = 1156), the majority is discarded: 71% of euploid embryos, 63% of mosaic embryos, 84% of aneuploid embryos and 67% of embryos without a diagnosis for PGT-A. Remarkably, a part of the non-genetically transferable embryos is still cryopreserved (29% euploid embryos, 38% mosaic embryos, 16% aneuploid embryos and 33% of embryos without a diagnosis for PGT-A). In 556 embryos (18%) only a PGT-A diagnosis was entered into the database instead of a combined PGT-M/SR and PGT-A diagnosis. These were therefore labelled as missing in Supplementary Table SXI.
At least one embryo transfer procedure was feasible for 65% of analyses (Table II and Supplementary Table SXII). Nearly all transfers involved a frozen embryo transfer (94%) and a single embryo (93%). Embryos effectively transferred were usually genetically transferable plus euploid (74%), mosaic (1%) or diagnosis for PGT-A was not reported (4%). Three per cent of the transferred embryos were euploid, genetically non-transferable and 18% was euploid with unreported genetic transferability.
A positive hCG was obtained in 58% of transfers leading to a clinical pregnancy rate of 50% per embryo transfer. Per analysis, the cumulative positive hCG rate was 48% and the clinical pregnancy rate 41%. Per analysis with transfer, these rates are 74% and 64%. About 215 live born children have been reported.
Discussion
This data report of the ESHRE PGT Consortium involves data from two calendar years, 2016 and 2017. The relative contributions of PGT-M (35%), PGT-SR (13%), PGT-A (47%) and concurrent PGT-M/SR/A (7%) do not differ from previous data sets. The major indication of each PGT modality (AMA for PGT-A, reciprocal translocation for PGT-SR and autosomal dominant disease for PGT-M) has not changed either. Compared to the overview of the first 10 years of data collection (Harper et al., 2012), the top five of PGT-M indications have evolved: cystic fibrosis, Huntington's disease and myotonic dystrophy type 1 remain frequent indications, while testing for hereditary breast cancer syndromes and neurofibromatosis has become more frequent than analyses for hereditary haemoglobinopathies and fragile X.
Trophectoderm biopsy has replaced cleavage-stage biopsy as the preferential method for biopsy for PGT-A and for concurrent PGT-M/SR/A. This shift in the biopsy method is linked to the implementation of comprehensive genetic testing. The turn from Day 3 to Day 5/6 biopsy, as well as the use of comprehensive diagnostics, is slower for PGT-SR and is the slowest for PGT-M. Linkage-based haplotyping for PGT-M can be carried out with targeted multiplex PCR or with genome-wide methods (SNP array or NGS-based). The latter generic methods circumvent the need for locus-specific preclinical workup, thereby reducing the workload as well as the waiting time for the couples, but the high cost of equipment and consumables may constitute an insurmountable barrier for many centres and this could explain why the implementation of generic methods is slow for the PGT-M indication group.
The efficiency of diagnostic testing is high (over 94%) for PGT-SR, PGT-A and concurrent PGT-M/SR and PGT-A, whereas a slightly poorer efficiency (88%) is obtained for PGT-M. These diagnostic efficiencies are in line with previous datasets (2011–2012 and 2013–2015) and it remains to be seen whether in the coming years of efficiency can be improved with new comprehensive techniques.
After PGT-A, 4% of the analysed embryos were reported to be mosaic, which seems lower than currently reported percentages. Only 12 out of 61 centres reported mosaic embryos. Before the first paper on the transfer of mosaic embryos leading to healthy babies in 2015 (Greco et al., 2015), mosaic embryos were not transferred (Viotti, 2020). It is likely that PGT centres were still adapting their transfer policy in 2016–2017. Moreover, array CGH, which is less sensitive in detecting mosaicism, was used in 37% of analyses.
The historical mean clinical pregnancy rate is stable at around 30% per embryo transfer for all PGT modalities, but points to better outcomes for PGT-M (35%) and PGT-A (52%) in the current data set. Enhanced outcomes were observed already in the 2013–2015 data set with clinical pregnancy rates per embryo transfer of 34% for PGT-M and PGT-SR, and 47% for PGT-A, indicating that further improvement was mainly obtained for PGT-A (52%). As the implementation of trophectoderm biopsy (87%), comprehensive testing (93%) and frozen embryo transfer (91%) is the most advanced for this indication group, the question arises of whether these practices are somehow associated with improved clinical outcomes. A similar good clinical pregnancy rate (50% per embryo transfer) was found for concurrent PGT-M/SR and PGT-A, a modality with similar practices of mainly trophectoderm biopsy (93%), comprehensive testing (78%) and frozen embryo transfer (94%). There was no change for PGT-M or PGT-SR without PGT-A, neither for the clinical outcomes nor the practices (trophectoderm biopsy 19% and 30%, respectively, comprehensive testing 15% and 50%). It remains to be seen whether further advances in ART practices for PGT-M and PGT-SR lead to better clinical outcomes.
This report presents the first data set from the online platform. A particular benefit of this new database is that its structure allows calculating cumulative outcome rates from the multiple fresh/frozen transfers following an analysis. This is an asset, given that since the implementation of vitrification, and the introduction of a freeze-all strategy related to the introduction of trophectoderm biopsies and comprehensive testing, ART/PGT outcome measurements have shifted to cumulative success rates.
However, with the implementation of the new online platform, the number of registered treatments has decreased (8803 analyses in 2016–2017 versus 11 120 cycles to oocyte retrieval in 2015), although the number of participating centres is in line with previous years (61 as compared to the average number of 62 from 2010 to 2015). This trend does not reflect the true activity in the PGT field. It is clear from other registries, such as the ESHRE European IVF-Monitoring Consortium, that the use of PGT is increasing (Wyns et al., 2020). Data submission to the PGT Consortium is done on a voluntary basis (compared to IVF/ICSI data submission, which is compulsory in some countries and linked with reimbursement of IVF/ICSI cycles). Data registration is time-consuming as it involves detailed data and this may particularly form a burden for large PGT centres. Therefore, it was accepted that some centres registered partial data. In this way, by focusing on data quality and not on quantity, the data presented are reliable and still reflect the major trends in these PGT centres. The ESHRE PGT Consortium Steering Committee greatly acknowledges the effort of all centres for their contribution.
The new online platform has a specific module for follow up of children born, these data were not included in the current data report. It was decided to collect these data over a longer period and report them later in a separate manuscript. Data on PGT with HLA matching were not included either. PGT for this indication is offered in a limited number of PGT centres (11/61 (18%) of Consortium members) and the reported data make up <4% (116/3098) of PGT-M cycles in the current dataset. Therefore, these data may be combined over a longer time period to generate a large and more specific dataset, as was done before in the multi-centre ESHRE study for PGT with HLA matching (Kakourou et al., 2018).
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Supplementary Material
Acknowledgements
The Steering Committee greatly acknowledges the effort of all contributing centres.
List of the centres participating in one or more data collections discussed in this report:
Argentina: Fecunditas, Department of Genetics and IVF, Buenos Aires; Austria: Landes-Frauen und Kinderklinik Linz, Human Genetics; Belgium: Brussels Free University, Centre of Medical Genetics, Brussels; Ghent University Hospital, Infertility Centre, Ghent; LIFE (Leuven Institute for Fertility and Embryology); Leuven University Fertility Center, Campus Gasthuisberg, Leuven; Brazil: Fertility—Centro de Fertilzação Assis, Sao Paoloa; Bulgaria: ObGyn Hospital ‘Dr.Shterev’, IVF unit; Cyprus: The Cyprus Institute of Neurology and Genetics, Molecular Genetics Thalassemia Department; Czech Republic: Institute Pronatal, Genetics, Praha; Denmark: Fertility Unit and Centre for Preimplantation Genetic Diagnosis; Finland: Helsinki University Central Hospital/IVF Unit, Dept of Obst&Gyn; France: C.M.C.O.-SIHCUS, CECOS Alsace, Unité de Diagnostic pré-implantatoire, Service de Biologie de la Reproduction, Strasbourg; Institut Universitaire de Recherche Clinique, Laboratoire de Génétique Moléculaire, Montpellier; Germany: Women’s Hospital University Kiel, Reproductive Medicine; Zentrum Für Humangenetik, Humangenetisches Labor, Regensburg; Medizinische Universitaet zu Luebeck, Klinik für Frauenheilkunde und Geburtshilfe; Kinderwunsch centrum München, IVF Labor, Munich; Kinderwunschzentrum an der Gedächteniskirche; University of Heidelberg, Molecular Genetics Unit of Department of Gynaecological Endocrinology and Reproductive Medicine, Heidelberg; CERF, Dr Thiemann, Dr Wetzka, Dr Wolk, Dr Hanjalic-Beck, Dr Friebel, Freiburg; Greece: University of Athens, St. Sophia’s Children’s Hospital, Department of Medical Genetics, Athens; EMBRYOGENESIS, Genetics, Thessaloniki; PGD laboratory, Genesis Athens Clinic, Centre for Human Reproduction, Athens; Embryolab, Thessaloniki; Hungary: Versys Clinics Human Reproduction Institute; Istenhegyi Gene Diagnostic Center; Ireland: Cork Fertility centre, Fernhurst House, Cork; Israel: Lis Maternity Hospital, Dept. of IVF, Tel Aviv; Italy: S.I.S.M.E.R. s.r.l. Bologna; European Hospital, Medicina della Riproduzione, Rome; Genoma, Molecular Genetics laboratories, Rome; FertiClinic, Rome; GENERA, reproductive medicine centres/Igenomix Italy; Fondazione IRCCS, Ca’ Granda Ospedale, Maggiore Policlinico, Milano; IRCCS Ospedale San Raffaele, centro Scienze della Natalita; Japan: St. Mother Hospital, Kitakyushu; Takeuchi Ladies Clinic/Infertility Center, Aira-shi, Kagoshima; Poland: INVICTA, Gdansk; Portugal: University of Porto Faculty of Medicine, Dept. Genetics, Faculty of Medicine, Porto; Russia: Center for Reproductive Medicine MAMA; Scotland: Glasgow Royal Infirmary, Assisted conception services, Glasgow; Royal Edinburgh Infirmary, Edinburgh Fertility and Reproductive Endocrine Centre, Edinburgh; Singapore: Singapore General Hospital, Centre for Assisted Reproduction (CARE), Singapore; Spain: Institut Universitari Dexeus, Obstetrícia, Ginecologia i Reproducció, Barcelona; Instituto Valenciano de Infertilidad (IVI); Instituto Marques, Infertility department, Barcelona; Sistemas Genomicos SL, Reproductive Medicine, Valencia; U.R.H. Garcia del real; Hospital Quiron Madrid, Laboratorio de Reproduccion Asistida; Fundacion Puigvert, Seminologia i Reproduccio, Barcelona; Sweden: Karolinska University Hospital, Department of Clinical Genetics; Sahlgrenska University Hospital, Department of Ob/Gyn; Switzerland: Procrea; Taiwan: Chang Gung Memorial Hospital, Obstetrics and Gynecology, Taoyuan County; The Netherlands: Maastricht University Medical Center, Obstetrics and Gynecology, Maastricht; University Medical Center Groningen, IVF/Fertility Laboratory, Groningen; University Medical Center Utrecht, Utrecht; Turkey: Istanbul Memorial Hospital, Reproductive endocrinology & ART centre, Istanbul; USA: Reproductive Biology associates.
Contributor Information
A van Montfoort, Department of Obstetrics & Gynaecology, GROW School for Oncology and Developmental Biology, Maastricht University Medical Centre, Maastricht, The Netherlands.
F Carvalho, Genetics—Department of Pathology, Faculty of Medicine, University of Porto, Porto, Portugal; i3s—Instituto de Investigacao e Inovacao em Saude, University of Porto, Porto, Portugal.
E Coonen, Departments of Clinical Genetics and Obstetrics & Gynaecology, GROW School for Oncology and Developmental Biology, Maastricht University Medical Centre, Maastricht, The Netherlands.
G Kokkali, Reproductive Medicine Unit, Genesis Athens Clinic, Chalandri, Athens, Greece.
C Moutou, Laboratoire de Diagnostic préimplantatoire, Université de Strasbourg, Hôpitaux Universitaires de Strasbourg, CMCO, Schiltigheim, France.
C Rubio, PGT-A Research, Igenomix, Valencia, Spain.
V Goossens, ESHRE Central Office, Grimbergen, Belgium.
M De Rycke, Centre for Medical Genetics, UZ Brussel, Brussels, Belgium.
Authors’ roles
M.D.R. drafted the manuscript, contributed to the tables, designed the figures and was responsible for the final editing of the manuscript. A.v.M. was responsible for raw data curation, contributed to the tables, designed and drafted the figures and edited the manuscript. F.C., G.K., C.M. and C.R. edited the manuscript. V.G. was responsible for the data collection and edited the manuscript. All authors revised and approved the final manuscript.
Data availability
All data are incorporated into the article and its online supplementary material.
Funding
The study has no external funding and all costs are covered by the European Society of Human Reproduction and Embryology.
Conflict of interest
There are no competing interests to declare.
References
- Coonen E, van Montfoort A, Carvalho F, Kokkali G, Moutou C, Rubio C, De Rycke M, Goossens V.. ESHRE PGT Consortium data collection XVI-XVIII: cycles from 2013 to 2015. Hum Reprod Open 2020;2020:hoaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rycke M, Belva F, Goossens V, Moutou C, SenGupta SB, Traeger-Synodinos J, Coonen E.. ESHRE PGD Consortium data collection XIII: cycles from January to December 2010 with pregnancy follow-up to October 2011. Hum Reprod 2015;30:1763–1789. [DOI] [PubMed] [Google Scholar]
- De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C.. ESHRE PGD Consortium data collection XIV-XV: cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013. Hum Reprod 2017;32:1974–1994. [DOI] [PubMed] [Google Scholar]
- ESHRE PGD Consortium Steering Committee. ESHRE Preimplantation Genetic Diagnosis Consortium: data collection III (May 2001). Hum Reprod 2002;17:233–246. [DOI] [PubMed] [Google Scholar]
- ESHRE PGT-SR/PGT-A Working Group, Coonen E, Rubio C, Christopikou D, Dimitriadou E, Gontar J, Goossens V, Maurer M, Spinella F, Vermeulen N.. et al. ESHRE PGT Consortium good practice recommendations for the detection of structural and numerical chromosomal aberrations. Hum Reprod Open 2020;2020:hoaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraedts J, Handyside A, Harper J, Liebaers I, Sermon K, Staessen C, Thornhill A, Vanderfaeillie A, Viville S.. ESHRE Preimplantation Genetic Diagnosis (PGD) Consortium: preliminary assessment of data from January 1997 to September 1998. ESHRE PGD Consortium Steering Committee. Hum Reprod 1999;14:3138–3148. [DOI] [PubMed] [Google Scholar]
- Geraedts J, Handyside A, Harper J, Liebaers I, Sermon K, Staessen C, Thornhill A, Viville S, Wilton L;. European Society of Human Reproduction and Embryology Preimplantation Genetic Diagnosis Consortium Steering Committee. ESHRE preimplantation genetic diagnosis (PGD) consortium: data collection II (May 2000). Hum Reprod 2000;15:2673–2683. [DOI] [PubMed] [Google Scholar]
- Goossens V, Harton G, Moutou C, Scriven PN, Traeger-Synodinos J, Sermon K, Harper JC;. European Society of Human Reproduction and Embryology PGD Consortium. ESHRE PGD Consortium data collection VIII: cycles from January to December 2005 with pregnancy follow-up to October 2006. Hum Reprod 2008;23:2629–2645. [DOI] [PubMed] [Google Scholar]
- Goossens V, Harton G, Moutou C, Traeger-Synodinos J, Van Rij M, Harper JC.. ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod 2009;24:1786–1810. [DOI] [PubMed] [Google Scholar]
- Goossens V, Traeger-Synodinos J, Coonen E, De Rycke M, Moutou C, Pehlivan T, Derks-Smeets IA, Harton G.. ESHRE PGD Consortium data collection XI: cycles from January to December 2008 with pregnancy follow-up to October 2009. Hum Reprod 2012;27:1887–1911. [DOI] [PubMed] [Google Scholar]
- Greco E, Minasi MG, Fiorentino F.. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med 2015;373:2089–2090. [DOI] [PubMed] [Google Scholar]
- Harper JC, Boelaert K, Geraedts J, Harton G, Kearns WG, Moutou C, Muntjewerff N, Repping S, SenGupta S, Scriven PN.. et al. ESHRE PGD Consortium data collection V: cycles from January to December 2002 with pregnancy follow-up to October 2003. Hum Reprod 2006;21:3–21. [DOI] [PubMed] [Google Scholar]
- Harper JC, Coonen E, De Rycke M, Harton G, Moutou C, Pehlivan T, Traeger-Synodinos J, Van Rij MC, Goossens V.. ESHRE PGD Consortium data collection X: cycles from January to December 2007 with pregnancy follow-up to October 2008. Hum Reprod 2010;25:2685–2707. [DOI] [PubMed] [Google Scholar]
- Harper JC, de Die-Smulders C, Goossens V, Harton G, Moutou C, Repping S, Scriven PN, SenGupta S, Traeger-Synodinos J, Van Rij MC.. et al. ESHRE PGD consortium data collection VII: cycles from January to December 2004 with pregnancy follow-up to October 2005. Hum Reprod 2008;23:741–755. [DOI] [PubMed] [Google Scholar]
- Harper JC, Wilton L, Traeger-Synodinos J, Goossens V, Moutou C, SenGupta SB, Pehlivan Budak T, Renwick P, De Rycke M, Geraedts JP.. et al. The ESHRE PGD Consortium: 10 years of data collection. Hum Reprod Update 2012;18:234–247. [DOI] [PubMed] [Google Scholar]
- Kakourou G, Kahraman S, Ekmekci GC, Tac HA, Kourlaba G, Kourkouni E, Sanz AC, Martin J, Malmgren H, Gimenez C.. et al. The clinical utility of PGD with HLA matching: a collaborative multi-centre ESHRE study. Hum Reprod 2018;33:520–530. [DOI] [PubMed] [Google Scholar]
- Moutou C, Goossens V, Coonen E, De Rycke M, Kokkali G, Renwick P, SenGupta SB, Vesela K, Traeger-Synodinos J.. ESHRE PGD Consortium data collection XII: cycles from January to December 2009 with pregnancy follow-up to October 2010. Hum Reprod 2014;29:880–903. [DOI] [PubMed] [Google Scholar]
- Sermon K, Moutou C, Harper J, Geraedts J, Scriven P, Wilton L, Magli MC, Michiels A, Viville S, De Die C.. ESHRE PGD Consortium data collection IV: May-December 2001. Hum Reprod 2005;20:19–34. [DOI] [PubMed] [Google Scholar]
- Sermon KD, Michiels A, Harton G, Moutou C, Repping S, Scriven PN, SenGupta S, Traeger-Synodinos J, Vesela K, Viville S.. et al. ESHRE PGD Consortium data collection VI: cycles from January to December 2003 with pregnancy follow-up to October 2004. Hum Reprod 2007;22:323–336. [DOI] [PubMed] [Google Scholar]
- Viotti M. Preimplantation genetic testing for chromosomal abnormalities: aneuploidy, mosaicism, and structural rearrangements. Genes (Basel) 2020;11:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, , Bergh C, , Calhaz-Jorge C, , De Geyter C, , Kupka MS, , Motrenko T, , Rugescu I, , Smeenk J, , Tandler-Schneider A, , Vidakovic S, et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoaa032. 10.1093/hropen/hoaa032PMC: 32760812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID. et al. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.