Abstract
Novel coronavirus 2019 (COVID-19) is a zoonosis that revised the global economic and societal progress since early 2020. The SARS-CoV-2 has been recognized as the responsible pathogen for COVID-19 with high infection and mortality rate potential. It has spread in 192 countries and infected about 1.5% of the world population, and still, a proper therapeutic approach is not unveiled. COVID-19 indication starts with fever to shortness of breathing, leading to ICU admission with the ventilation support in severe conditions. Besides the symptomatic mainstay clinical therapeutic approach, only Remdesivir has been approved by the FDA. Several pharmaceutical companies claimed different vaccines with exceptionally high efficacy (90–95%) against COVID-19; how long these vaccines can protect and long-term safety with the new variants are unpredictable. After the worldwide spread of the COVID-19 pandemic, numerous clinical trials with different phases are being performed to find the most appropriate solution to this condition. Some of these trials with old FDA-approved drugs showed promising results. In this review, we have precisely compiled the efforts to curb the disease and discussed the clinical findings of Ivermectin, Doxycycline, Vitamin-D, Vitamin-C, Zinc, and cannabidiol and their combinations. Additionally, the correlation of these molecules on the prophylactic and diseased ministration against COVID-19 has been explored.
Keywords: COVID-19, Ivermectin, Doxycycline, Zinc, Vitamin-D, Vitamin-C
Graphical Abstract
1. Introduction
Coronaviruses (CoVs) are single-stranded RNA viruses that consist of spike glycoproteins on the envelope, which look like a crown (coronam as Latin term of the crown) [1]. CoVs are subdivided into four groups: Alpha-coronavirus (α-CoV), Beta-coronavirus (β-CoV), Delta-coronavirus (Δ-CoV), and Gamma-coronavirus (γ-CoV). Severe-Acute-Respiratory-Syndrome CoV-2 (SARS-CoV-2) is belonging to the β-CoV [2]. SARS-CoV-2 genome has 96% similarity of β-CoV RaTG13 of bats though the origin is still unknown [3]. S2 of (one of the spike proteins) SARS-CoV-2 has an amino acid site that allows it to bind with the Angiotensin-Converting Enzyme 2 (ACE2) receptor and infuse its RNA to the host cells. Host cells replicate this RNA rapidly and produce an excessive immune reaction along with redox imbalance in the host which, finally causes the novel coronavirus disease at the end of 2019, renowned as COVID-19 [4], [5]. Fever, dry cough, fatigue, loss of taste or smell, nasal congestion, conjunctivitis (also known as red eyes), sore throat, headache, muscle or joint pain, different types of skin rash, nausea or vomiting, diarrhea, and chills or dizziness are the symptoms developed in COVID-19 [6].
The most susceptible to the COVID-19 are the persons with low immunity system, elderly aged people with different health concerns like cancer, chronic kidney diseases, chronic obstructive pulmonary diseases, a heart condition, pregnancy, and type 2 diabetics mellitus [7]. Interestingly, there might be a role of an individual's blood group to be at risk of SARS-CoV-2 infection [8].
The current therapeutic approach is mostly symptomatic, but many clinical trials are being performed to figure out preventive management. Lately, Remdesivir got approval from the FDA (Food and Drug Administration) to treat hospitalized COVID-19 patients [9]. Different pharmaceutical companies are trying to develop vaccines employing versatile methodologies. Companies like Pfizer/BioNtech, Moderna claimed their mRNA (messenger RNA) vaccines are 94–95% effective against SARS-CoV-2 [10]. Viral vector vaccines (ChAdOx1 nCoV-19) of AstraZeneca and the University of Oxford were studied in 23,848 participants in UK, Brazil, and South Africa. Here, a low dose followed by a standard dose was 90% effective against COVID-19 [11]. Even though, all the vaccines are promising, they are yet to be approved by the WHO for mass usage. Side by side, how long these vaccenic can provide protection and their long-term consequences are still unpredictable.
Due to these panic circumstances, peoples from all groups are eager to get information about the viral cause and cure irrespective of their area. Therefore, in this study, we would like to discuss some FDA-approved drugs and supplements studied against COVID-19 separately or as adjuvant. These drugs were used mostly to treat COVID-19, but some of them were used as prophylaxis. Hence, this document will provide comprehensive information about pharmaceutical efforts against COVID-19 in a precise way.
2. Current COVID-19 epidemic status
Since the arrival of novel coronavirus-2019, it has spread around 192 countries and infected over 162 million people (2.1% of the total world population) around the world [12]. At the time of writing (May 2021) according to Johns Hopkins University, the total recovery from COVID-19 is 91.25 million with more than 3.4 million fatalities [13]. According to the WHO as of 18th May 2021, 40% cumulative cases are accumulated in the north and south Americas, 33% in Europe, 17% in South-East Asia, 6% in Eastern Mediterranean, 2% in Africa, and 2% in Western Pacific region [12].
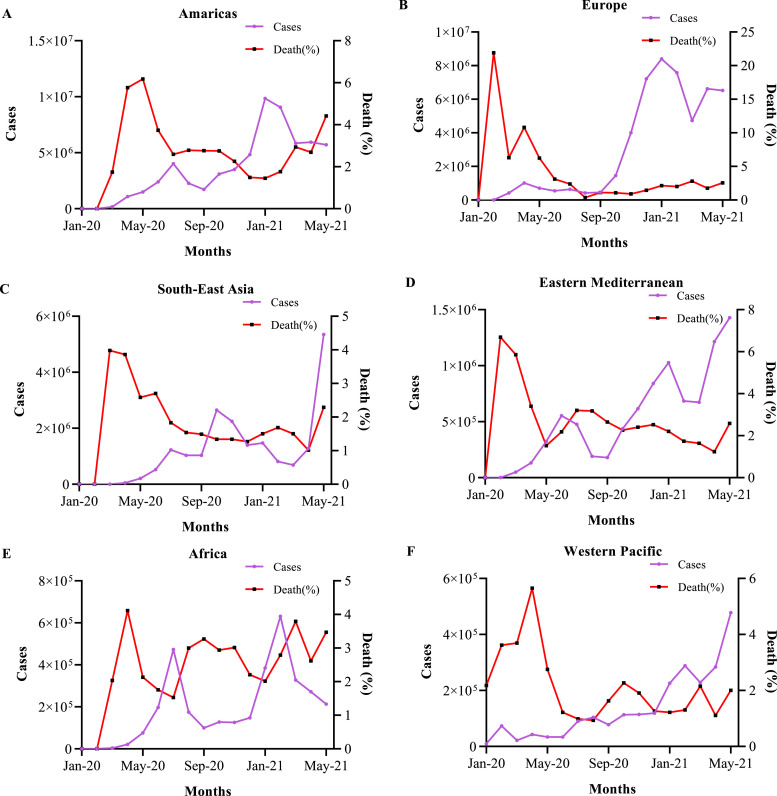
From the 31st January 2020 to 18th May 2021 (17 months) the cases and deaths per month were extracted from the data provided by WHO (Supplementary data) [14]. Those data were plotted in Fig. 1, Fig. 2 where the whole world was divided into six regions (Americas, Europe, South-East Asia, Eastern Mediterranean, Africa, Western Pacific). In Fig. 1A and B, the number of cases and deaths were arranged for all the six regions, and as cases per month were changing in these regions with the months and created a wave shape. The first highest peak was considered as the first wave and the second highest peak as the second wave. The number of cases in the 2nd wave was much greater than the 1st wave (Fig. 1C) which was predicted earlier by Atangana et al. [15]. Cases per month were highest in the Americas but the highest deaths were observed in Europe region although, infection rate and fatality are coming down gradually. But in South-East Asia especially India now reached a critical situation and about to face the most devastating hit by COVID-19.
Fig. 1.
COVID-19 epidemiological status till 2nd May 2021; these graphs explain the condition of the COVID-19 situation in the world. A. Illustrate the total cases that have been reported in different regions (1 million = 1 × 107). B. States the cumulative deaths in different regions. C. Cumulative cases comparison between the 1st wave and 2nd wave of COVID-19.
Fig. 2.
Comparison of the number of cases and percentage of death per month; all the data are plotted in these graphs from January 2020 to 18th May 2021 (17 months). A, B, C, D, E, and F are cumulative cases vs percentage of death per month in Americas, Europe, South-East Asia, Eastern Mediterranean, Africa, and Western Pacific regions respectively.
3. Evolution of COVID-19 around the world for the last 17 months
Though the number of cases and deaths are related to each other the ratio between cases and death is behaving differently in certain regions (Fig. 2). In the Americas region the total case has come down but the death rate is still going up especially in Brazil, U.S.A, Argentina, and Colombia (Fig. 2A). In Europe, death is steady with a fall down in the number of cases per month (Fig. 2B). South-East Asia has now become the burning ground of COVID-19 with the maximum number of cases and death especially in India (Fig. 2C). In Eastern Mediterranean the number of cases has become worst of all time particularly in Iran (Fig. 2D). Interestingly, in Africa, the number of cases has fallen but the death rate is spiking up due to the new range of cases in South Africa (Fig. 2E). The Western Pacific which was meant to the most controlled place also had its highest peak of cases per month lately especially for Japan, the Philippines, and Malaysia (Fig. 2F).
The most probable reasons for these fluctuations of cases and death rates might be related to the application of lockdown protocol, maintaining social distancing, vaccination, and adequate healthcare facility. However, these data are collected based on of regions where different countries followed different approaches. The most of the regions where the condition is worsening, the most common reasons are the saturation of heath care scope and vaccination. At present, the death percentage of south-east Asia is raising sharply against the number of cases which might be the influence of new variants and poor pandemic management in this region.
Nevertheless, the mutation in a virus is inevitable and can change its contagious profile. SARS-CoV-2 has also mutated as it jumped through lots of individuals. Particular four variants (B.1.1.7, B.1.351, B.1.1.28.1, and B.1.617) of SARS-CoV-2 have raised certain concerns due to their greater transmissibility than the native virus [16]. Around 152 countries have reported with B.1.1.7 variant patients followed by 106 countries with B.1.351, 61 countries with B.1.1.28.1, and 62 countries with new variant B.1.617. Among these four variants B.1.1.7 is the most alarming not only because of the greater risk of mortality and hospitalization but the recent outbreak in India with B.1.617 and its mutated variants (B.1.617.1, B.1.617.2, B.1.617.3, B.1.617.x) has become more serious and also reported in failure with conventional detection. Experts suspect that this new variant B.1.617 has more capacity for a human to human transmission than the others [17]. Though present vaccine provider companies (Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca) states that their vaccines still active in B.1.1.7 variant but show reduced efficacy on B.1.351 phenotype [18].
4. Recent treatment protocol
SARS-CoV-2 spike protein configuration is different from its ancestor SARS-CoV and MERS-CoV (Middle East respiratory syndrome coronavirus) which makes COVID-19 management complicated. Due to the fast infection rate, mutation, and complex mechanism of action, conventional anti-viral therapy leads to poor management [19]. Many clinical trials are going on for the search on suitable COVID-19 treatment plans.
On February 9, 2021, FDA had issued Emergency use authorization for monoclonal antibody (bamlanivimab plus etesevimab) treatment for the outpatient with mild to moderate symptoms but not for the hospitalized patients or those who required oxygen therapy [20]. Remdesivir or Remdesivir plus Dexamethasone or Dexamethasone treatment is recommended for those who are hospitalized and require supplemental oxygen. Only Dexamethasone for the hospitalized patient with invasive mechanical ventilation [21].
5. An alternative approach to COVID-19 management
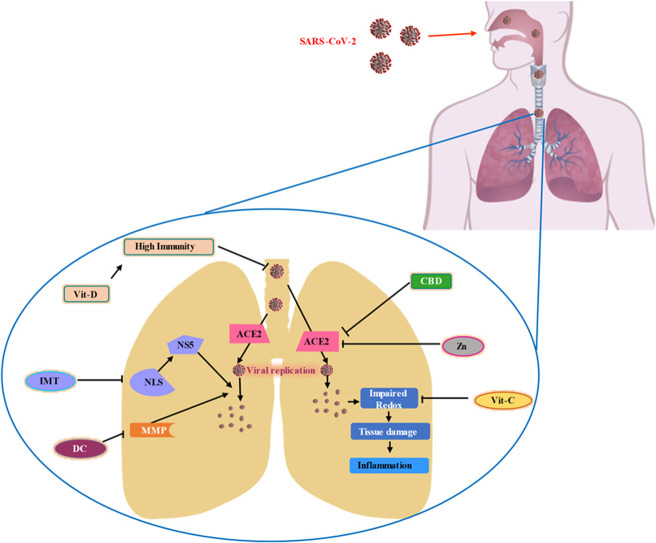
On September 24th of 2020, my cousin and her whole family (5 members) got infected with COVID-19 and they followed the prescription which follows Ivermectin (12 mg) for 5 days, Doxycycline (200 mg) for 7 days, Vitamin-D (2000IU) 15 days, Vitamin C (chewable table) 1 month. They were quarantined and had these medications according to the prescription. In the following 21 days, they all were recovered. This real event intrigued me to investigate more about these drugs against the COVID-19. Another most important reason which interests me that these drugs are available in every country and their safety profile is outstanding. Side by side, countries with lower economic growth have little reachability to the vaccine which made them even more vulnerable to COVID-19. Scientists and clinical practitioners are more driven in the search for alternative management because of poor outcomes from the recent treatment protocol. We have discussed below six potential candidates ( Fig. 3) for better management based on several clinical trials ( Table 2). These drugs might not only approachable for COVID-19 treatment but also might protect if taken in a prophylactic manner.
Fig. 3.
Potential candidate for COVID-19 management; this diagram displays six promising conventional molecules (2D structure) that might present a life savior option to treat COVID-19.
Table 2.
List of clinical trials and case studies. The clinical studies related to the IMT, DC, Zn, Vit-C, Vit-D, and CBD are listed in this table with their clinical trial identification number and actual or estimated participants amount. The studies which don’t have identification numbers are referred to a case studies.
| Title | Number of patients enrolled | Identification number |
|---|---|---|
| A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. | 72 | none |
| Clinical Trial of Ivermectin Plus Doxycycline for the Treatment of Confirmed Covid-19 Infection. | 400 | NCT04523831 |
| Effectiveness of Ivermectin and Doxycycline on COVID-19 Patients. | 140 | NCT04591600 |
| Clinical Outcomes of Early Treatment with Doxycycline for 89 High-Risk COVID-19 Patients in Long-Term Care Facilities in New York. | 89 | none |
| Coronavirus 2019 (COVID-19)- Using Ascorbic Acid and Zinc Supplementation (COVIDAtoZ). | 520 | NCT04342728 |
| Vitamin D Supplementation in Patients With COVID-19. | 240 | NCT04449718 |
| Short Term, High Dose Vitamin D Supplementation for COVID-19 (SHADE). | 30 | NCT04459247 |
| Prophylactic Ivermectin in COVID-19 Contacts. | 340 | NCT04422561 |
| Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study. | 117 | none |
| Zinc Versus Multivitamin Micronutrient Supplementation in the Setting of COVID-19 (ZnCOVID-19). | 4500 (estimated) | NCT04551339 |
| A Preventive Treatment for Migrant Workers at High-risk of COVID-19. | 4257 | NCT04446104 |
| A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection (HELPCOVID-19). | 600 (estimated) | NCT04335084 |
| Safety and Efficacy of Hydroxychloroquine for the Treatment & Prevention of Coronavirus Disease 2019 (COVID-19) Caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) | 5000 (estimated) | NCT04590274 |
| Povidone-Iodine Vs Essential Oil Vs Tap Water Gargling For COVID-19 Patients (GARGLES). | 20 | NCT04410159 |
| Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment. | 596 | NCT04292730 |
| Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) | 397 | NCT04292899 |
| Outcomes Mandate National Integration with Cannabis as Medicine for Prevention and Treatment of COVID-19 (OMNI-Can) | 200,000 (estimated) | NCT03944447 |
| Cannabidiol for CoviD-19 patients With Mild to Moderate Symptoms (CANDIDATE) | 104 (estimated) | NCT04467918 |
6. Ivermectin
Ivermectin (IMT) is a macrocyclic lactone derived from the Nobel prize-winning compound avermectin [22]. IMT is a broad-spectrum anti-parasitic drug (FDA approved) [23] and also proven as an antiviral drug in a wide range [24], [25], [26], anti-cancer [27], topical anti-inflammatory and wound healing [28], [29], antibacterial [30], anti-mitotic activity [31].
Nonstructural protein 5 (NS5) is a crucial component to replicate RNA viruses like ZIKA, Dengue, West Nile, and HIV-1 [32], [33]. NS5 complies with the importin β1 nuclear localization signal (NLS) and α/β NLS [34]. Various investigations proved that IMT could inhibit NLS by disassociating importin α/β1 heterodimer and thus blind NS5 to recognize importin mediated NLS [32]. Coronavirus is also a positive-sense single-stranded RNA virus, making it a suitable target for IMT treatment [19]. Clay et al. was the first to investigate the antiviral effect of IMT on Covid-19 in vitro and reported results elucidated an efficient remedy with ~ 5000-fold reduction of viral RNA in 48 h [35]. IMT also can downregulate the inflammation through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and MAPK pathway [36]. Recently five days randomized clinical trial was conducted on 72 patients infected with mild Covid-19. Patients were divided into three groups (IMT, IMT + doxycycline, and placebo) and received 12 mg of IMT or IMT 12 mg and doxycycline (DC) 200 mg once a day for five days except for the control group. Interestingly the viral load was found negative in 9.7 days and fully recovered within 14 days. No adverse effect or drug-drug interaction was noticed during this trial [37]. Additionally, another phase-3 clinical trial (NCT04523831) was conducted on 400 patients with mild symptoms by combining IMT and DC In this study, patients have received IMT 6 mg twice on the first day of the treatment and discontinued for the rest of the course. DC 100 mg twice a day (1 + 0 + 1) was continued for five days. Within seven days, Body temperature and oxygen saturation become normal (37.5 °C and SpO2, > 93%) for 60.7% of the treatment group, 12 days for 23%, 14 days for 7.7%, and one month for 8.7%. Interestingly the mortality rate was 0.0%, which was very encouraging. Another small trial (NCT04591600) on IMT also suggested the same indication, but they also included that early administration of IMT can lead to better management [38].
7. Doxycycline
Doxycycline (DC) is an FDA-approved antibiotic that comes from the tetracycline family. DC possesses good intra-cellular penetration and broad-spectrum antibacterial effect [39]. DC has been reported for various infectious disease control including pasteurellosis, brucellosis, borreliosis, trepanomatosis, rickettsioses, leptospirosis, cholera, Q fever, urinary and pulmonary infections by Chlamydia and Mycoplasma, anthrax, and gonococci. It is also useful for malaria prophylaxis because of its anti-Plasmodium capabilities [40]. In dermatology, DC is indicated for rosacea and acne [41]. DC can bind with bacterial 30S ribosomal subunit and inhibit bacterial protein synthesis [42]. Besides its core effect, DC is also studied for its antiviral activity against the chikungunya virus, Japanese encephalitis virus, Respiratory Syncytial Virus, and Dengue virus [43], [44], [45], [46]. Rothan et al. showed that DC is very potent to inhibit RNA viruses, e.g., chikungunya [44]. DC can inhibit matrix metalloproteinases and bind with E2 envelope glycoprotein, which is important for viral replication and survival [47]. Investigation results elucidated that DC can have a potential effect on SARS-CoV-2 entry and its post replication. Side by side, it can act as an anti-inflammatory agent by reducing various pro-inflammatory cytokines expressions [48]. In May 2020, a small study was conducted on 89 COVID-19 positive patients with DC (100 mg) for seven days. Initially, they were treated with hydroxychloroquine and azithromycin, but afterward, medication was replaced with DC because of both of their cardiotoxic behavior. This study reported a recovery rate of 85%, and only 3% of patients were rebounded for hospitalization due to their clinical deuteriation after ten days [49]. As previously described, the IMT + DC combination was remarkable on COVID-19 recovery (NCT04523831, NCT04591600).
8. Zinc
Zinc (Zn) is an essential macronutrient that involves growth development, neurosensory functions, DNA synthesis, gene expression, and immune system regulation. Nowadays, 17.3% of the global population is Zn deficient, and Zn deficiency can lead to growth retardation, diarrhea, hair loss (alopecia), inflammation of the tongue (glossitis), nail dystrophy, and premature birth [50]. As Zn's source, Oysters comprise the most, but the major Zn comes from red meat and poultry. Refined rice only contains 20% Zn of daily requirement although, the countries where rice is the staple food has more susceptible to Zn deficiency [51], [52]. Insufficient Zn can downregulate antibody production and, consequently, reduce cell-mediated immunity, leading to more infection vulnerability [53]. Admiration of Zn can reduce the duration of common cold symptoms [54] and upper respiratory tract infection morbidity [55]. It also can inhibit the hepatitis C virus through IFN-λ3 signaling [56] and ease people living with HIV infections [57]. In vitro study showed that Zn cations could inhibit SARS-CoV-2 RNA polymerase activity by decreasing replication [58]. Zn with or without chloroquine can increase Zn abundance inside the cells, which can intensify cell-mediated immunity [59]. SARS-CoV-2 penetrates the cells through angiotensin-converting enzyme 2 (ACE2), and Zn was studied on the modulation of ACE2. Exposure of Zn reduced recombinant human ACE-2 activity in rat lungs, which might be a potential approach against COVID-19 [60]. Five hundred twenty patients with COVID-19 were recruited for a clinical trial (NCT04342728) with ascorbic acid (8000 mg/day) and Zn (50 mg/day) for 28 days. This group was divided into four subgroups (Ascorbic Acid or Zinc Gluconate or Ascorbic Acid and Zinc Gluconate Dietary Supplement and Standard of Care). This study was expected to reduce symptoms in 28 days, though the results are yet to develop. A case study of four patients who received a high dose of Zn improved significantly after one day of treatment, and the researcher suggested that Zn might play a crucial part in the recovery phase from COVID-19 [61].
9. Vitamin D
Vitamin D (Vit-D) is one of the essential vitamins that can be involved in calcium homeostasis and involves the immune system modulation of our body. Lack of Vit-D can lead to autoimmune diseases and increase the vulnerability to infections [62]. Vit-D deficiency (serum 25-hydroxyvitamin D < 20 ng/ml or 50 nmol/L) is very common worldwide. Currently, 40% in Europe, 37% in Canada, 24% in the U.S., > 20% population in India, Tunisia, Pakistan, and Afghanistan are reported as Vit-D deficient [63]. Generally, adults [64], darker skin [65], and obese [66] people are more susceptible to Vit-D lacking. Elizabeth et al. recorded 10,926 death from 17,278,392 COVID-19 patients and found that more elderly, obese, and darker skin people died [67], which might have a co-relation with Vit-D deficiency. Another study showed that COVID-19 mortality and infection are inversely proportional to the population's Vit-D level [68]. A blood clot in the lung tissue and ventilation is more prevalent to lower Vit-D leveled people and has a greater mortality rate [69], [70].
Vit-D can be obtained by sun exposure, diet, and dietary supplement; however, direct sunlight to the skin is the primary source of Vit-D [71]. A small portion can be obtained from fish, egg yolk, animal liver, and milk [72]. The daily necessary intake of Vit-D for an adult is 15 μg (600 IU) to 100 μg (4000 IU) [73], but due to lifestyle and lack of sunlight, 50% of the world population is at risk of Vit-D deficiency [74]. A clinical trial (NCT04449718) of 240 patients displayed that one single bolus dose of Vit-D did not improve the outcome. Another small study showed that a high dose (60,000 IU/day) for seven days might help against COVID-19 and a significant decrease in fibrinogen [75].
10. Vitamin C
Vitamin C (Vit-C) or ascorbic acid is a powerful antioxidant that helps decrease inflammation and improve immune function. Vit-C deficiency or low dietary intake can lead to low immunity and greater susceptibility to viral infection [76]. Vit-C has well documented against the common cold including various studies against herpes simplex virus-1, influenza type A virus, AIDS, poliovirus type 1, viral upper respiratory tract infections, acute respiratory infections, and rhinovirus have proved this [77]. In a viral infection, redox homeostasis is imbalanced and causes tissue damage, leading to inflammation [78]. Studies showed that Vit-C could shorten the length of ICU (intensive care unit) with respiratory diseases [79] and I.V. (intravenous) dose helps patients to recover from septic shock [80]. In ICU, Vit-C can improve blood pressure, bronchoconstriction, infections, atrial fibrillation, and acute renal injury [81]. So, a mega-dose of Vit-C might rescue severely ill patients from COVID-19 [82]. During the pandemic in China, 10 g and 20 g Vit-C I.V. was been prescribed from moderate to severe COVID-19 patients for 5–7 days and that improved the disease resolution period [77]. Though regular 250 mg (twice daily) Vit-C supplement might present a preventive measure to the general population[83].
Besides Vit-C, quercetin (QCT) is a natural plant-derived flavonoid, can act as a viral protease inhibitor thus block RNA production and viral replication of different RNA viruses like HIV, Hepatitis-C, and SARS-CoV-1. Concurrent administration of Vit-C, QCT, and Vit-B3 can reduce the vulnerability and mortality from influenza H1N1. QCT is also believed to bind with the 3CL-like-protease of SARS-CoV-2 and inhibit its proteolytic activity. Ascorbate (conjugate base form of Vit-C) able to recycle QCT and increase its bioavailability [84], [85].
11. Cannabidiol
Cannabidiol (CBD) is a non-psychoactive cannabinoid derived from the cannabis plant. CBD exerts a broad range of therapeutic effects like anti‐inflammatory, antioxidant, sedative, anticonvulsive, neuroprotective, hypnotic, antipsychotic, and anti‐cancer activities. Recently CBD has been hypothesized against COVID-19 [86]. SARS-CoV-2 binds with ACE2 receptor followed by the consequent activation of NF-κB and stormy release of interleukin-6 (IL-6), tumor necrosis factor-α, IL-1α/β, chemokine ligand-2, and C-X-C motif chemokine ligand-10. This event leads to activation of T-cells and reduction of total T-cells amount leads to disrupt immunity system [87]. Wang et al. illustrated that CBD might able to manifest a possible hindrance at the infiltration gateway by reducing the expression of ACE2 and transmembrane serine protease-2 (TMPRSS2) which helps the spike protein to bind with the ACE2 receptor [88]. Another study suggested that CBD can modulate apelin (a secondary catalytic substrate for ACE2) and might improve COVID-19 related acute respiratory distress syndrome (ARDS) [89]. A phase 2 clinical study (NCT03944447) is undergoing to evaluate the application of CBD as a preventive and diseased management tactic. Another small randomized, double-blinded phase 2 study (NCT04467918) is going on with CBD (300 mg/day) for 14 days. This study aims to observe changes in proinflammatory cytokine concentration, parenchymal lung damage, and adverse event with CBD treatment.
12. Prophylactic use of the proposed drugs
A randomized clinical trial (NCT04422561) was done with 340 participants in Egypt to evaluate the prophylactic use of IMT. In this study, they reported only 15 participants out of 203 developed symptoms who receive prophylactic IMT after 14 days. Similarly, a group of health professionals in India received IMT at a dose of 300 μg/kg for 72 h. These healthcare workers were directly in contact with COVID-19 patients, and 73% of these people were uninfected for the following month [90].
DC showed promising results in preventing viral infection, replication, and associated inflammation. This drug has also been used in several clinical trials against COVID-19, suggesting that a low dose of DC can be given (40 mg/day) to take preventive measures [91].
At the time of writing this article, a clinical study (NCT04551339) of 4500 patients is being conducted where the researcher proposed 11 mg of Zn tablet per day for the next three months. This study will be conducted to observe the prophylaxis of Zn. Similar kinds of clinical trials (NCT04446104, NCT04335084, NCT04590274) are also ongoing, but they also see how Zn is standing out hydroxychloroquine, DC, IMT, Vit-C, Vit-D, Azithromycin, and Quercetin.
A study based on the Israeli population was done on 7807 individuals with serum Vit-D test reports and found that only 10.1% were COVID-19 positive, and the rest were not infected. The Vit-D level in the infected group was lower than the non-infected group, which suggests that the Vit-D can boost the immune system to fight against COVID-19 and prevent from getting infected [92].
Different studies against different viral respiratory tract infections showed that Vit-C could decrease the severity of illness and mortality rate [93], [94]. Several randomized clinical trials suggested that early intake of Vit-C can reduce the 50% chance of getting caught with the common cold [95].
ACE2 and TMPRSS2 inhibitors can restrict SARS-CoV-2 invasion [96] so, high‐cannabidiol containing mouth wash can be a preventive strategy due to the ability of ACE2 downregulation in EpiIntestinal and EpiAirway tissues [86], [88].
13. Safety profile
Though IMT is a very potent anti-parasites, its antiviral capability becomes more prominent in COVID-19 treatment. IMT has a good safety profile and has a minimum adverse effect when taken orally. The safe therapeutic doses of IMT range from 20 to 80 ng/ml in the blood [97].
Like other tetracyclines, DC is also contraindicated in pregnant women, especially after the second trimester, breastfeeding mothers and children. The overuse of DC can also lead to antimicrobial resistance, leading to higher medical costs, prolonged hospital stays, and increased mortality [98]. However, DC is a well-tolerated drug with some side effects like stomach upset and some cutaneous abnormalities [39].
Daily recommended Zn intake for men and women is 7.5–12.7 mg/day and 9.4–16.3 mg/day, respectively. The upper limit is set at 40 mg/day, but higher and prolonged-term intake might cause Zn toxicity, leading to nausea, vomiting, fatigue, epigastric pain, lethargy, and permanent loss of smell [99].
Vit-D has been considered a very safe medication, even in a very high dose. If the serum 25-hydroxyvitamin D has to exceed 150 ng/ml (375 nM/L) with higher calcium intake, Vit-D toxicity might occur, which is extremely rare [100]. According to the European food safety authority (EFSA), 250 µg/day was established as no observed adverse effect level (NOAEL) [101].
Though the daily recommended dose of Vit-C for adults is 90 mg/day during acute infection, this can be raised to 2 g. Does higher than 2 g per day might cause diarrhea, nausea, and abdominal pain [102]. A larger dose (10 mg/day) might cause oxalate kidney stones, but nothing was observed in sepsis in a clinical trial [103].
CBD is non-psychoactive but the smoking of cannabis is not recommended. CBD might lead to hepatic abnormalities, vomiting, diarrhea, fatigue, and CBD-induced drug-drug interactions [104]. So, medical CBD has to be applied under strict monitoring.
14. Comparison between Remdesivir and proposed drug combination
At present, Remdesivir (RDV) is the only drug that has been approved by the FDA for COVID-19 management. The antiviral activity of this drug acts by inhibiting viral replication. The active metabolite of RDV (Remdesivir triphosphate or, GS-443902) is a synthetic analog and competes with the natural nucleosides which inhibit viral RNA replication [105]. RDV nucleoside analog inhibits or delays transportation of RNA-dependent RNA polymerase of SARS-CoV-2 and suppresses its viral replication [106]. Two clinical trials (NCT04292730 and NCT04292899) were conducted with RDV for moderate to severe COVID-19 patients where they reported that RDV is more suitable for moderate patients when followed five days regime [107], [108]. Similar results were also shown by IMT and DC clinical studies. Eweas et al. exhibited that both IMT and RVD were very potent candidates against SARS-CoV-2 [109].
Our proposed drugs act on SARS-CoV-2 in multiple sites ( Table 1) and side by side the most advantageous point is the ability of prophylactic uses which might not be attained with RDV. Other advantages of these drugs are not only cost-effective but also accessible for most of these drugs.
Table 1.
Alternative option of COVID-19 management; the list of drugs and supplements with their dose and regime to use either as preventive measure or treatment approach against COVID-19.
| Generic name | Dose | Dose regimen (prophylaxis) | Dose regimen (treatment) | Availability | Role on COVID-19 |
|---|---|---|---|---|---|
| Ivermectin | 12 mg/day | Once | Physician’s decision | On prescription | Inhibit viral replication, Immunomodulation. |
| Doxycycline | 40–200 mg/day | 5 days | Physician’s decision | On prescription | Inhibit viral replication, anti-inflammation. |
| Zinc | 15–50 mg/day | 20–30 days | Physician’s decision | OTC | Inhibit viral activity. |
| Vitamin-D | 1000–4000 IU/day | 20–30 days | Physician’s decision | OTC | Increase immunity. |
| Vitamin-C | 250–1000 mg/day | 20–30 days | Physician’s decision | OTC | Reduce viral infection chance, redox balance. |
| Cannabidiol | Physician’s decision | Physician’s decision | Physician’s decision | On prescription | Incorporate viral invasion, reduce ARDS |
15. Expert opinion
The most fundamental way to stay away from COVID-19 infection is to wear a mask, wash hands frequently, avoid touching the nose and eyes with unwashed hands, maintain social distance, and stay healthy as much as possible [110]. Apart from these, taking essential vitamins and minerals can give a fighting chance to stay uninfected.
As previously discussed, the IMT and DC have been used for the preventive approach on COVID-19. However, the main concern is the dose regimen because frequent intake as a preventive measure can make this drug-resistant, especially DC IMT and DC are not available as OTC (over the counter) drugs so, before starting to take these drugs, one has to discuss with the physician or licensed pharmacist to get a prescription. During these meditations, each individual should be under daily monitoring regarding any side effects.
Zn level in the body is inversely proportional to the interleukin-6, and in the case of COVID-19, Zn was found low. For the patients who died in COVID-19, their Zn levels were much lower than those who survived [111]. Another study also suggested that insufficient Zn level results in poor outcomes in COVID-19 [112].
Vit-D is a very safe supplement, but the dose must be adjusted according to the patient body mass index (BMI) to obtain the optimum level. Vit-D is fat-soluble, so overweight individuals (BMI = 26–30) are suggested to take a 1.5 times greater dose than healthy weighed (BMI ≤ 25) individuals. For obese people (BMI > 30), a high dose (even up to 3-fold) is recommended to obtain the average Vit-D level [113]. EFSA suggested 100 µg/day of Vit-D as an upper limit for adults, including pregnant and lactating women. For 1–10 years old, the upper limit was set at 50 µg/day [101].
Vit-C is widely available in many foods and fruits naturally, but people might need to take supplements to maintain daily requirements. We also need to keep in mind the safe upper limit (2000 mg/day) of Vit-C [102]. The last thing we do not want is to get sick before getting infected. The last thing we do not want is to get sick before getting infected.
An interesting study (NCT04410159) was conducted on Povidone-Iodine solution (PIs) for COVID-19. They used 10 ml of PIs to gargle 30 s (three times a day) for seven days and found promising results. A similar study (NCT04549376) is also currently going on, suggesting a prophylactic use of PIs against COVID-19 [114].
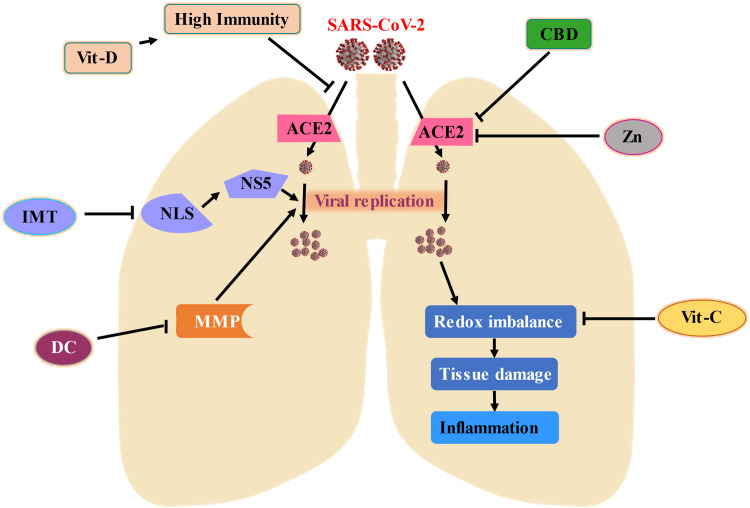
At the present state, the whole world cannot be covered with vaccines which might take a very long time but the spread and the mutation of SARS-CoV-2 will continue. Besides the mainstay therapeutic approach, these compounds might be helpful to contain this pandemic under control. The suggested mechanism ( Fig. 4) indicates that IMT, DC, Zn, Vit-C, Vit-D, and CBD can be useful to be a preventive and therapeutic candidate against COVID-19.
Fig. 4.
Proposed mechanism of action against SARS-CoV-2 infection; this illustration reflects a probable mechanism of action with the combination of the Ivermectin (IMT), Doxycycline (DC), Zinc (Zn), Vitamin-D (Vit-D), Vitamin-C (Vit-C), and Cannabidiol (CBD) might present preventive measure against COVID-19. SARS-CoV-2 invades the host body through Angiotensin-Converting Enzyme 2 (ACE2) and consumes host machinery to replicate itself which leads to extensive tissue damage, immune reaction, redox imbalance, and inflammation. IMT induces conformational changes in nuclear localization signal (NLS) and inhibits the contribution of nonstructural protein-5 (NS5) to replicate SARS-CoV-2 along with the immunomodulation to the host. Matrix metalloproteinase (MMP) is also a vital component for viral replication which can be repressed by DC. Vit-C is a well-known antioxidant that can help to counterbalance redox stress. Zn and CBD might modulate ACE2 which encumber SARS-CoV-2 to penetrate the host body. Vit-D can boost the immunity system and might give an extra layer of protection.
16. Conclusion
COVID-19 presented a significant challenge in front of humankind, but we are about the edge of a breakthrough. Many scientists worldwide are working day and night to find a suitable solution to prevent and cure this pandemic. Different pharmaceutical companies already completed some promising vaccines, which is the shortest time to invent a vaccine in human history. Nevertheless, the long-term safety and efficacy of these vaccines are still under investigation. Meanwhile, old drugs like IMT, DC, some essential vitamins like (Vit-D, Vit-C), minerals (Zn), and natural plant extract (CBD) can be considered an excellent shield to improve the fighting chance as prophylaxis treatment COVID-19 at the same time.
CRediT authorship contribution statement
Apu Chowdhury: Conceptualization, Methodology, Data curation, Writing – original draft. Nabila Jahan: Conceptualization. Muhammad Sajid: Writing – review & editing, Software. Temitope Isaac Adelusi: Writing – review & editing. Pulak Maitra: Validation. Sicen Wang: Supervision, Revision. Yuan Gao: Supervision, Revision. Guolian Yin: Supervision, Revision. Xudong Wu: Supervision, Revision.
Author contributions
A. Chowdhury and N. Jahan design the study. A. Chowdhury collected data and wrote the manuscript. M. Sajid, T. Isaac Adelusi, P. Maitra, W. Sicen, Y. Gao, G. Yin, and X. Wu revised the manuscript.
Ethics approval
Ethics approval is not applicable.
Conflict of interest statement
All authors declared that there is no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2021.111956.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Burrell C.J., Howard C.R., Murphy F.A. In: Fenner and White’s Medical Virology. Burrell C.J., Howard C.R., Murphy F.A., editors. Academic Press; London: 2017. Chapter 31 – Coronaviruses; pp. 437–446. [Google Scholar]
- 2.Chan J.F., To K.K., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21(10):544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., Laredo L., Laosa O., Centeno-Soto G.A., Ángeles Gálvez M., Puerro M., González-Rojano E., Pedraza L., de Pablo I., Abad-Santos F., Rodríguez-Mañas L., Gil M., Tobías A., Rodríguez-Miguel A., Rodríguez-Puyol D. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395(10238):1705–1714. doi: 10.1016/S0140-6736(20)31030-8. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D’Angelo A., De Cobelli F., Rovere-Querini P., Tresoldi M., Dagna L., Zangrillo A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO, Coronavirus Disease (COVID-19), 2020. 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19#:~:text=symptoms〉. (Accessed 11 January 2021).
- 7.CDC, People with Certain Medical Conditions, 2020. 〈https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html〉. (Accessed 11 January 2021).
- 8.J. Zhao, Y. Yang, H. Huang, D. Li, D. Gu, X. Lu, Z. Zhang, L. Liu, T. Liu, Y. Liu, Y. He, B. Sun, M. Wei, G. Yang, X. Wang, L. Zhang, X. Zhou, M. Xing, P.G. Wang, Relationship Between the ABO Blood Group and the COVID-19 Susceptibility, 2020. 〈 10.1101/2020.03.11.20031096〉. [DOI] [PMC free article] [PubMed]
- 9.Rubin D., Chan-Tack K., Farley J., Sherwat A. FDA approval of Remdesivir – a step in the right direction. N. Engl. J. Med. 2020;383(27):2598–2600. doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y., Wang X., Goff S.P., Gao G. Translational repression precedes and is required for ZAP-mediated mRNA decay. EMBO J. 2012;31(21):4236–4246. doi: 10.1038/emboj.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali A., Allen E.R., Allen L., Almeida T.C.D.S.C., Alves M.P.S., Amorim F., Andritsou F., Anslow R., Appleby M., Arbe-Barnes E.H., Ariaans M.P., Arns B., Arruda L., Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O’Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., Carvalho Yd.M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.-S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis J., Davis J., De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., van Eck S., Edwards M., Edwards N.J., El Muhanna O.M., Elias S.C., Elmore M., English M., Esmail A., Essack Y.M., Farmer E., Farooq M., Farrar M., Farrugia L., Faulkner B., Fedosyuk S., Felle S., Feng S., Ferreira Da Silva C., Field S., Fisher R., Flaxman A., Fletcher J., Fofie H., Fok H., Ford K.J., Fowler J., Fraiman P.H.A., Francis E., Franco M.M., Frater J., Freire M.S.M., Fry S.H., Fudge S., Furze J., Fuskova M., Galian-Rubio P., Galiza E., Garlant H., Gavrila M., Geddes A., Gibbons K.A., Gilbride C., Gill H., Glynn S., Godwin K., Gokani K., Goldoni U.C., Goncalves M., Gonzalez I.G.S., Goodwin J., Goondiwala A., Gordon-Quayle K., Gorini G., Grab J., Gracie L., Greenland M., Greenwood N., Greffrath J., Groenewald M.M., Grossi L., Gupta G., Hackett M., Hallis B., Hamaluba M., Hamilton E., Hamlyn J., Hammersley D., Hanrath A.T., Hanumunthadu B., Harris S.A., Harris C., Harris T., Harrison T.D., Harrison D., Hart T.C., Hartnell B., Hassan S., Haughney J., Hawkins S., Hay J., Head I., Henry J., Hermosin Herrera M., Hettle D.B., Hill J., Hodges G., Horne E., Hou M.M., Houlihan C., Howe E., Howell N., Humphreys J., Humphries H.E., Hurley K., Huson C., Hyder-Wright A., Hyams C., Ikram S., Ishwarbhai A., Ivan M., Iveson P., Iyer V., Jackson F., De Jager J., Jaumdally S., Jeffers H., Jesudason N., Jones B., Jones K., Jones E., Jones C., Jorge M.R., Jose A., Joshi A., Júnior E.A.M.S., Kadziola J., Kailath R., Kana F., Karampatsas K., Kasanyinga M., Keen J., Kelly E.J., Kelly D.M., Kelly D., Kelly S., Kerr D., Kfouri Rd.Á., Khan L., Khozoee B., Kidd S., Killen A., Kinch J., Kinch P., King L.D.W., King T.B., Kingham L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Lang M., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lazarus E.M., Leach A., Lees E.A., Lemm N.-M., Lessa A., Leung S., Li Y., Lias A.M., Liatsikos K., Linder A., Lipworth S., Liu S., Liu X., Lloyd A., Lloyd S., Loew L., Lopez Ramon R., Lora L., Lowthorpe V., Luz K., MacDonald J.C., MacGregor G., Madhavan M., Mainwaring D.O., Makambwa E., Makinson R., Malahleha M., Malamatsho R., Mallett G., Mansatta K., Maoko T., Mapetla K., Marchevsky N.G., Marinou S., Marlow E., Marques G.N., Marriott P., Marshall R.P., Marshall J.L., Martins F.J., Masenya M., Masilela M., Masters S.K., Mathew M., Matlebjane H., Matshidiso K., Mazur O., Mazzella A., McCaughan H., McEwan J., McGlashan J., McInroy L., McIntyre Z., McLenaghan D., McRobert N., McSwiggan S., Megson C., Mehdipour S., Meijs W., Mendonça R.N.Á., Mentzer A.J., Mirtorabi N., Mitton C., Mnyakeni S., Moghaddas F., Molapo K., Moloi M., Moore M., Moraes-Pinto M.I., Moran M., Morey E., Morgans R., Morris S., Morris S., Morris H.C., Morselli F., Morshead G., Morter R., Mottal L., Moultrie A., Moya N., Mpelembue M., Msomi S., Mugodi Y., Mukhopadhyay E., Muller J., Munro A., Munro C., Murphy S., Mweu P., Myasaki C.H., Naik G., Naker K., Nastouli E., Nazir A., Ndlovu B., Neffa F., Njenga C., Noal H., Noé A., Novaes G., Nugent F.L., Nunes G., O’Brien K., O’Connor D., Odam M., Oelofse S., Oguti B., Olchawski V., Oldfield N.J., Oliveira M.G., Oliveira C., Oosthuizen A., O’Reilly P., Osborne P., Owen D.R.J., Owen L., Owens D., Owino N., Pacurar M., Paiva B.V.B., Palhares E.M.F., Palmer S., Parkinson S., Parracho H.M.R.T., Parsons K., Patel D., Patel B., Patel F., Patel K., Patrick-Smith M., Payne R.O., Peng Y., Penn E.J., Pennington A., Peralta Alvarez M.P., Perring J., Perry N., Perumal R., Petkar S., Philip T., Phillips D.J., Phillips J., Phohu M.K., Pickup L., Pieterse S., Piper J., Pipini D., Plank M., Du Plessis J., Pollard S., Pooley J., Pooran A., Poulton I., Powers C., Presa F.B., Price D.A., Price V., Primeira M., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Quaid S., Rabara R., Radford A., Radia K., Rajapaska D., Rajeswaran T., Ramos A.S.F., Ramos Lopez F., Rampling T., Rand J., Ratcliffe H., Rawlinson T., Rea D., Rees B., Reiné J., Resuello-Dauti M., Reyes Pabon E., Ribiero C.M., Ricamara M., Richter A., Ritchie N., Ritchie A.J., Robbins A.J., Roberts H., Robinson R.E., Robinson H., Rocchetti T.T., Rocha B.P., Roche S., Rollier C., Rose L., Ross Russell A.L., Rossouw L., Royal S., Rudiansyah I., Ruiz S., Saich S., Sala C., Sale J., Salman A.M., Salvador N., Salvador S., Sampaio M., Samson A.D., Sanchez-Gonzalez A., Sanders H., Sanders K., Santos E., Santos Guerra M.F.S., Satti I., Saunders J.E., Saunders C., Sayed A., Schim van der Loeff I., Schmid A.B., Schofield E., Screaton G., Seddiqi S., Segireddy R.R., Senger R., Serrano S., Shah R., Shaik I., Sharpe H.E., Sharrocks K., Shaw R., Shea A., Shepherd A., Shepherd J.G., Shiham F., Sidhom E., Silk S.E., da Silva Moraes A.C., Silva-Junior G., Silva-Reyes L., Silveira A.D., Silveira M.B.V., Sinha J., Skelly D.T., Smith D.C., Smith N., Smith H.E., Smith D.J., Smith C.C., Soares A., Soares T., Solórzano C., Sorio G.L., Sorley K., Sosa-Rodriguez T., Souza C.M.C.D.L., Souza B.S.D.F., Souza A.R., Spencer A.J., Spina F., Spoors L., Stafford L., Stamford I., Starinskij I., Stein R., Steven J., Stockdale L., Stockwell L.V., Strickland L.H., Stuart A.C., Sturdy A., Sutton N., Szigeti A., Tahiri-Alaoui A., Tanner R., Taoushanis C., Tarr A.W., Taylor K., Taylor U., Taylor I.J., Taylor J., te Water Naude R., Themistocleous Y., Themistocleous A., Thomas M., Thomas K., Thomas T.M., Thombrayil A., Thompson F., Thompson A., Thompson K., Thompson A., Thomson J., Thornton-Jones V., Tighe P.J., Tinoco L.A., Tiongson G., Tladinyane B., Tomasicchio M., Tomic A., Tonks S., Towner J., Tran N., Tree J., Trillana G., Trinham C., Trivett R., Truby A., Tsheko B.L., Turabi A., Turner R., Turner C., Ulaszewska M., Underwood B.R., Varughese R., Verbart D., Verheul M., Vichos I., Vieira T., Waddington C.S., Walker L., Wallis E., Wand M., Warbick D., Wardell T., Warimwe G., Warren S.C., Watkins B., Watson E., Webb S., Webb-Bridges A., Webster A., Welch J., Wells J., West A., White C., White R., Williams P., Williams R.L., Winslow R., Woodyer M., Worth A.T., Wright D., Wroblewska M., Yao A., Zimmer R., Zizi D., Zuidewind P. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO, Weekly Epidemiological Update, 2 March 2021. 〈https://www.who.int/publications/m/item/weekly-epidemiological-update---2-march-2021〉. (Accessed 6 March 2021).
- 13.J.H. University, COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), 2021. 〈https://coronavirus.jhu.edu/map.html〉. (Accessed 6 March 2021).
- 14.WHO, Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update, 2021. 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports〉. (Accessed 20 May 2021).
- 15.Atangana A. Modeling and forecasting the spread of COVID-19 with stochastic and deterministic approaches: Africa and Europe. Adv. Differ. Equ. 2021;2021(2021):57. doi: 10.1186/s13662-021-03213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burki T. Understanding variants of SARS-CoV-2. Lancet. 2021;397(10273):462. doi: 10.1016/S0140-6736(21)00298-1. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam D. What scientists know about new, fast-spreading coronavirus variants. Nature. 2021;594:19–20. doi: 10.1038/d41586-021-01390-4. [DOI] [PubMed] [Google Scholar]
- 18.L.J. Abu-Raddad, H. Chemaitelly, A.A. Butt, Effectiveness of the BNT162b2 Covid-19 Vaccine Against the B.1.1.7 and B.1.351 Variants, 2021. [DOI] [PMC free article] [PubMed]
- 19.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020 doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA, Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab, 2021. 〈https://www.fda.gov/media/145802/download〉. (Accessed 15 March 2021).
- 21.N.I.o. Health, Therapeutic Management of Adults With COVID-19, 2021. 〈https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/〉. (Accessed 15 March 2021).
- 22.Tambo E., Khater E.I.M., Chen J.-H., Bergquist R., Zhou X.-N. Nobel prize for the artemisinin and ivermectin discoveries: a great boost towards elimination of the global infectious diseases of poverty. Infect. Dis. Poverty. 2015;4:58. doi: 10.1186/s40249-015-0091-8. 58-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canga A.G., Prieto A.M.S., Liébana M.J.D., Martínez N.F., Vega M.S., Vieitez J.J.G. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croci R., Bottaro E., Chan K.W., Watanabe S., Pezzullo M., Mastrangelo E., Nastruzzi C. Liposomal systems as nanocarriers for the antiviral agent Ivermectin. Int. J. Biomater. 2016;2016 doi: 10.1155/2016/8043983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese F.S., Kaukinen P., Gläsker S., Bespalov M., Hanski L., Wennerberg K., Kümmerer B.M., Ahola T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016;126:117–124. doi: 10.1016/j.antiviral.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Xu T.L., Han Y., Liu W., Pang X.Y., Zheng B., Zhang Y., Zhou X.N. Antivirus effectiveness of ivermectin on dengue virus type 2 in Aedes albopictus. PLOS Negl. Trop. Dis. 2018;12(11) doi: 10.1371/journal.pntd.0006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang L., Wang P., Sun Y.J., Wu Y.J. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway. J. Exp. Clin. Cancer Res. CR. 2019;38(1):265. doi: 10.1186/s13046-019-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sia D.K., Mensah K.B., Opoku-Agyemang T., Folitse R.D., Darko D.O. Mechanisms of ivermectin-induced wound healing. BMC Vet. Res. 2020;16(1):397. doi: 10.1186/s12917-020-02612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventre E., Rozières A., Lenief V., Albert F., Rossio P., Laoubi L., Dombrowicz D., Staels B., Ulmann L., Julia V., Vial E., Jomard A., Hacini-Rachinel F., Nicolas J.F., Vocanson M. Topical ivermectin improves allergic skin inflammation. Allergy. 2017;72(8):1212–1221. doi: 10.1111/all.13118. [DOI] [PubMed] [Google Scholar]
- 30.Ashraf S., Chaudhry U., Raza A., Ghosh D., Zhao X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob. Resist. Infect. Control. 2018;7:27. doi: 10.1186/s13756-018-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashraf S., Prichard R. Ivermectin exhibits potent anti-mitotic activity. Vet. Parasitol. 2016;226:1–4. doi: 10.1016/j.vetpar.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Yang S.N.Y., Atkinson S.C., Wang C., Lee A., Bogoyevitch M.A., Borg N.A., Jans D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020;177 doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- 33.Wagstaff K.M., Rawlinson S.M., Hearps A.C., Jans D.A. An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen. 2011;16(2):192–200. doi: 10.1177/1087057110390360. [DOI] [PubMed] [Google Scholar]
- 34.Ng I.H.W., Chan K.W.-K., Tan M.J.A., Gwee C.P., Smith K.M., Jeffress S.J., Saw W.-G., Swarbrick C.M.D., Watanabe S., Jans D.A., Grüber G., Forwood J.K., Vasudevan S.G. Zika virus NS5 forms supramolecular nuclear bodies that sequester importin-α and modulate the host immune and pro-inflammatory response in neuronal cells. ACS Infect. Dis. 2019;5(6):932–948. doi: 10.1021/acsinfecdis.8b00373. [DOI] [PubMed] [Google Scholar]
- 35.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ci X., Li H., Yu Q., Zhang X., Yu L., Chen N., Song Y., Deng X. Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam. Clin. Pharmacol. 2009;23(4):449–455. doi: 10.1111/j.1472-8206.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., Phru C.S., Rahman M., Zaman K., Somani J., Yasmin R., Hasnat M.A., Kabir A., Aziz A.B., Khan W.A. A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.H.A. Hashim, M.F. Maulood, A.M. Rasheed, D.F. Fatak, K.K. Kabah, A.S. Abdulamir, Controlled Randomized Clinical Trial on Using Ivermectin with Doxycycline for Treating COVID-19 Patients in Baghdad, Iraq, medRxiv, 2020. 〈 10.1101/2020.10.26.20219345〉. [DOI]
- 39.Bonnetblanc J.M. Doxycycline. Ann. Dermatol. Venereol. 2002;129:874–882. [PubMed] [Google Scholar]
- 40.Tan K.R., Magill A.J., Parise M.E., Arguin P.M., Centers for Disease Control and Prevention Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 2011;84(4):517–531. doi: 10.4269/ajtmh.2011.10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aupee O., Almeras D., Le Garlantezec P., Bohand X. Doxycycline. Med. Trop. Rev. Corps Sante Colonia. 2009;69(6):556–558. [PubMed] [Google Scholar]
- 42.Cunha B.A., Sibley C.M., Ristuccia A.M. Doxycycline. Ther. Drug Monit. 1982;4(2):115–135. doi: 10.1097/00007691-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Fredeking T.M., Zavala-Castro J.E., González-Martínez P., Moguel-Rodríguez W., Sanchez E.C., Foster M.J., Diaz-Quijano F.A. Dengue patients treated with doxycycline showed lower mortality associated to a reduction in IL-6 and TNF levels. Recent Pat. Anti-infect. Drug Discov. 2015;10(1):51–58. doi: 10.2174/1574891x10666150410153839. [DOI] [PubMed] [Google Scholar]
- 44.Rothan H.A., Bahrani H., Mohamed Z., Teoh T.C., Shankar E.M., Rahman N.A., Yusof R. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLOS One. 2015;10(5) doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topno R., Khan S.A., Chowdhury P., Mahanta J. Pharmacodynamics of aminoglycosides and tetracycline derivatives against Japanese encephalitis virus. Asian Pac. J. Trop. Med. 2016;9(3):241–246. doi: 10.1016/j.apjtm.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 46.Charles P.G., Whitby M., Fuller A.J., Stirling R., Wright A.A., Korman T.M., Holmes P.W., Christiansen K.J., Waterer G.W., Pierce R.J., Mayall B.C., Armstrong J.G., Catton M.G., Nimmo G.R., Johnson B., Hooy M., Grayson M.L. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008;46(10):1513–1521. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 47.Ng H.H., Narasaraju T., Phoon M.C., Sim M.K., Seet J.E., Chow V.T. Doxycycline treatment attenuates acute lung injury in mice infected with virulent influenza H3N2 virus: involvement of matrix metalloproteinases. Exp. Mol. Pathol. 2012;92(3):287–295. doi: 10.1016/j.yexmp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Gendrot M., Andreani J., Jardot P., Hutter S., Delandre O., Boxberger M., Mosnier J., Le Bideau M., Duflot I., Fonta I., Rolland C., Bogreau H., La Scola B., Pradines B. In vitro antiviral activity of doxycycline against SARS-CoV-2. Molecules. 2020;25(21):5064. doi: 10.3390/molecules25215064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alam M.M., Mahmud S., Rahman M.M., Simpson J., Aggarwal S., Ahmed Z. Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Cureus. 2020;12(8):9658. doi: 10.7759/cureus.9658. e9658-e9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLOS One. 2012;7(11):50568. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medicine Io. The National Academies Press; Washington, DC: 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. [PubMed] [Google Scholar]
- 52.Sharma A., Patni B., Shankhdhar D., Shankhdhar S.C. Zinc – an indispensable micronutrient. Physiol. Mol. Biol. Plants. 2013;19(1):11–20. doi: 10.1007/s12298-012-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraker P.J., King L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 54.Singh M., Das R.R. WITHDRAWN: zinc for the common cold. Cochrane Database Syst. Rev. 2015;4(2015) doi: 10.1002/14651858.CD001364.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kartasurya M.I., Ahmed F., Subagio H.W., Rahfiludin M.Z., Marks G.C. Zinc combined with vitamin A reduces upper respiratory tract infection morbidity in a randomised trial in preschool children in Indonesia. Br. J. Nutr. 2012;108(12):2251–2260. doi: 10.1017/S0007114512000499. [DOI] [PubMed] [Google Scholar]
- 56.Read S.A., O’Connor K.S., Suppiah V., Ahlenstiel C.L.E., Obeid S., Cook K.M., Cunningham A., Douglas M.W., Hogg P.J., Booth D., George J., Ahlenstiel G. Zinc is a potent and specific inhibitor of IFN-λ3 signalling. Nat. Commun. 2017;8:15245. doi: 10.1038/ncomms15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dirajlal-Fargo S., Yu J., Kulkarni M., Sattar A., Funderburg N., Barkoukis H., McComsey G.A. Brief report: zinc supplementation and inflammation in treated HIV. J. Acquir. Immune Defic. Syndr. 2019;82(3):275–280. doi: 10.1097/QAI.0000000000002129. (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guastalegname M., Vallone A. Could chloroquine/hydroxychloroquine be harmful in coronavirus disease 2019 (COVID-19) treatment? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71(15):888–889. doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chilvers M.A., McKean M., Rutman A., Myint B.S., Silverman M., O’Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur. Respir. J. 2001;18(6):965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 61.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int. J. Infect. Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., Martucci G., Pilz S., Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74(11):1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 65.Harris S.S. Vitamin D and African Americans. J. Nutr. 2006;136(4):1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 66.Vranić L., Mikolašević I., Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina. 2019;55(9):541. doi: 10.3390/medicina55090541. (Kaunas) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S.J.W., Smeeth L., Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32(7):1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baktash V., Hosack T., Patel N., Shah S., Kandiah P., Van Den Abbeele K., Mandal A.K.J., Missouris C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021;97:442–447. doi: 10.1136/postgradmedj-2020-138712. postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radujkovic A., Hippchen T., Tiwari-Heckler S., Dreher S., Boxberger M., Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12(9) doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saraff V., Shaw N. Sunshine and vitamin D. Arch. Dis. Child. 2016;101(2):190–192. doi: 10.1136/archdischild-2014-307214. [DOI] [PubMed] [Google Scholar]
- 72.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006;92(1):33–38. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Žmitek K., Hribar M., Hristov H., Pravst I.J.N. Efficiency of vitamin D supplementation in healthy adults is associated with body mass index and baseline serum 25-hydroxyvitamin D level. Nutrients. 2020;12(5):1268. doi: 10.3390/nu12051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nair R., Maseeh A. Vitamin D: the “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012;3(2):118–126. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., Puri G.D., Malhotra P. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-139065. postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 76.Hemilä H. Vitamin C and infections. Nutrients. 2017;9(4) doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abobaker A., Alzwi A., Alraied A.H.A. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol. Rep. 2020;72(6):1517–1528. doi: 10.1007/s43440-020-00176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoang B.X., Shaw G., Fang W., Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J. Glob. Antimicrob. Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hemilä H., Chalker E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J. Intensive Care. 2020;8(1):15. doi: 10.1186/s40560-020-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bharara A., Grossman C., Grinnan D., Syed A., Fisher B., DeWilde C., Natarajan R., Fowler A.A. Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome. Case Rep. Crit. Care. 2016;2016 doi: 10.1155/2016/8560871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hemilä H., Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11(4):708. doi: 10.3390/nu11040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davey Smith G., Blastland M., Munafò M. Covid-19’s known unknowns. BMJ (Clin. Res. Ed.) 2020;371:3979. doi: 10.1136/bmj.m3979. [DOI] [PubMed] [Google Scholar]
- 83.Simonson W. Vitamin C and coronavirus. Geriatr. Nurs. 2020;41(3):331–332. doi: 10.1016/j.gerinurse.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Russo M., Moccia S., Spagnuolo C., Tedesco I., Russo G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020;328 doi: 10.1016/j.cbi.2020.109211. 109211-109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front. Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. 1451-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Esposito G., Pesce M., Seguella L., Sanseverino W., Lu J., Corpetti C., Sarnelli G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020;177(21):4967–4970. doi: 10.1111/bph.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. 827-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang B., Kovalchuk A., Li D., Rodriguez-Juarez R., Ilnytskyy Y., Kovalchuk I., Kovalchuk O. In search of preventive strategies: novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging. 2020;12(22):22425–22444. doi: 10.18632/aging.202225. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salles É.L., Khodadadi H., Jarrahi A., Ahluwalia M., Paffaro V.A., Jr., Costigliola V., Yu J.C., Hess D.C., Dhandapani K.M., Baban B. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J. Cell. Mol. Med. 2020;24(21):12869–12872. doi: 10.1111/jcmm.15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.P. Behera, B.K. Patro, A.K. Singh, P.D. Chandanshive, R.K. S.R, S.K. Pradhan, S.S.K. Pentapati, G. Batmanabane, B.M. Padhy, S. Bal, S.R. Singh, R.R. Mohanty, Role of Ivermectin in the Prevention of COVID-19 Infection among Healthcare Workers in India: A Matched Case-Control Study, 2020. 〈 10.1101/2020.10.29.20222661〉. [DOI] [PMC free article] [PubMed]
- 91.Yates P.A., Newman S.A., Oshry L.J., Glassman R.H., Leone A.M., Reichel E. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620951053. 1753466620951053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., Frenkel-Morgenstern M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jungeblut C.W. Further observations on vitamin C therapy in experimental poliomyelitis. J. Exp. Med. 1937;66(4):459–477. doi: 10.1084/jem.66.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hunt C., Chakravorty N.K., Annan G., Habibzadeh N., Schorah C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam. Nutr. Res. Int. Z. Vitam. Ernahr. J. Int. Vitaminol. Nutr. 1994;64(3):212–219. [PubMed] [Google Scholar]
- 95.Anderson T.W., Reid D.B., Beaton G.H. Vitamin C and the common cold: a double-blind trial. Can. Med. Assoc. J. 1972;107(6):503–508. [PMC free article] [PubMed] [Google Scholar]
- 96.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heidary F., Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020;73(9):593–602. doi: 10.1038/s41429-020-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Narendrakumar L., Joseph I., Thomas S. Potential effectiveness and adverse implications of repurposing doxycycline in COVID-19 treatment. Expert Rev. Anti-infect. Ther. 2020:1–8. doi: 10.1080/14787210.2021.1865803. [DOI] [PubMed] [Google Scholar]
- 99.Fosmire G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990;51(2):225–227. doi: 10.1093/ajcn/51.2.225. [DOI] [PubMed] [Google Scholar]
- 100.Marcinowska-Suchowierska E., Kupisz-Urbańska M., Łukaszkiewicz J., Płudowski P., Jones G. Vitamin D toxicity-a clinical. Front. Endocrinol. 2018;9:550. doi: 10.3389/fendo.2018.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.N. Products, Scientific opinion on the tolerable upper intake level of vitamin D, EFSA J., 10, 2012.
- 102.Hemilä H., Louhiala P. Vitamin C may affect lung infections. J. R. Soc. Med. 2007;100(11):495–498. doi: 10.1258/jrsm.100.11.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kashiouris M.G., L’Heureux M., Cable C.A., Fisher B.J., Leichtle S.W., Fowler A.A. The emerging role of vitamin C as a treatment for sepsis. Nutrients. 2020;12(2) doi: 10.3390/nu12020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huestis M.A., Solimini R., Pichini S., Pacifici R., Carlier J., Busardò F.P. Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 2019;17(10):974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malin J.J., Suárez I., Priesner V., Fätkenheuer G., Rybniker J. Remdesivir against COVID-19 and other viral diseases. Clin. Microbiol. Rev. 2020;34(1) doi: 10.1128/CMR.00162-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Farnung L., Siewert A., Höbartner C., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12(1):279. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., Chai L.Y.A., Roestenberg M., Tsang O.T.Y., Bernasconi E., Le Turnier P., Chang S.C., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wang H., Gaggar A., Brainard D.M., McPhail M.J., Bhagani S., Ahn M.Y., Sanyal A.J., Huhn G., Marty F.M. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.Y., Nahass R.G., Chen Y.S., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wei X., Gaggar A., Brainard D.M., Towner W.J., Muñoz J., Mullane K.M., Marty F.M., Tashima K.T., Diaz G., Subramanian A. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eweas A.F., Alhossary A.A., Abdel-Moneim A.S. Molecular docking reveals Ivermectin and Remdesivir as potential repurposed drugs against SARS-CoV-2. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.592908. 592908-592908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chowdhury A., Jahan N., Wang S. One month of the novel coronavirus 2019 outbreak: is it still a threat? VirusDisease. 2020;31(2):174–178. doi: 10.1007/s13337-020-00579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.M. Vogel-González, M. Talló-Parra, V. Herrera-Fernández, G. Pérez-Vilaró, M. Chillón, X. Nogués, S. Gómez-Zorrilla, I. López-Montesinos, J. Villar, M.L. Sorli-Redó, J.P. Horcajada, N. García-Giralt, J. Pascual, J. Díez, R. Vicente, R. Güerri-Fernández, Low Zinc Levels at Clinical Admission Associates with Poor Outcomes in COVID-19, 2020. 〈 10.1101/2020.10.07.20208645〉. [DOI]
- 112.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., Manoharan S., Ramani V., Narasimhan G., Kaliamoorthy I., Rela M. COVID-19: poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ekwaru J.P., Zwicker J.D., Holick M.F., Giovannucci E., Veugelers P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLOS One. 2014;9(11) doi: 10.1371/journal.pone.0111265. e111265-e111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naqvi S.H.S., Citardi M.J., Cattano D., Ostrosky-Zeichner L., Knackstedt M.I., Karni R.J. Povidone-iodine solution as SARS-CoV-2 prophylaxis for procedures of the upper aerodigestive tract a theoretical framework. J. Otolaryngol. Head Neck Surg. J. d’oto-rhino-laryngol. chir. cervico-fac. 2020;49(1):77. doi: 10.1186/s40463-020-00474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material