Abstract
Pediatric gastroenterologists took on a variety of challenges during the coronavirus disease 2019 pandemic, including learning about a new disease and how to recognize and manage it, prevent its spread among their patients and health professions colleagues, and make decisions about managing patients with chronic gastrointestinal and liver problems in light of the threat. They adapted their practice to accommodate drastically decreased numbers of in-person visits, adopting telehealth technologies, and instituting new protocols to perform endoscopies safely. The workforce pipeline was also affected by the impact of the pandemic on trainee education, clinical experience, research, and job searches.
Keywords: COVID-19, Pediatric gastroenterology, Telehealth, Endoscopy
Key points
-
•
Coronavirus disease 2019 and multisystem inflammatory syndrome in children and adolescents can present with gastrointestinal symptoms and liver injury.
-
•
Special populations of children and adolescents with chronic liver disease or immune suppression may be at greater risk of coronavirus disease 2019 or have attenuated responses to vaccination.
-
•
Pediatric gastroenterologists have faced challenges of fewer patient care visits, delays in presentation of gastrointestinal illnesses, curtailed endoscopy, the adoption of telehealth, and fewer opportunities for clinical and research training.
Introduction
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for the coronavirus disease 2019 (COVID-19), was first recognized in Wuhan, China, in December 2019 and rapidly spread. Globally, there have been more than 127 million cases with 2.8 million deaths; the United States has contributed more than 30 million cases and 500,000 deaths as of April 2021. The impact of this disease cannot be understated, with far-reaching changes in the world and medicine. Our goal is to provide a review tailored to the pediatric gastroenterologist that focuses on caring for patients with COVID-19, preventing the disease in patients with chronic gastrointestinal (GI) and liver disorders, and adapting to the associated widespread changes to clinical practice and training.
Epidemiology
In children and adolescents, COVID-19 generally presents similar to a viral upper respiratory infection with the most common presenting symptoms being cough (48.5%), pharyngeal erythema (46.2%), fever (41.5%), diarrhea (8.8%), fatigue (7.6%), rhinorrhea (7.6%), and vomiting (6.4%).1 When symptomatic, pediatric patients are more likely to have mild disease (53%) that can be managed in the outpatient setting.2 A meta-analysis by Viner and colleagues3 showed that children have a significantly lower susceptibility to COVID-19 infection with an odds ratio of 0.56 compared with adults. Saleh and colleagues4 specifically investigated hospitalized children with COVID-19 and found that the most common presentations were fever (95%), headache (60.3%), fatigue (57.8%), and shock (21.8%). Acute pancreatitis (1.5%) was the most common atypical presentation of COVID-19 in this study.4 Common laboratory abnormalities in hospitalized patients with COVID-19 include leukopenia, lymphopenia, elevated inflammatory markers, and abnormal liver tests.4, 5, 6 Mortality rates remain low in children with 0.17 per 100,000 or 0.48% of the estimated total mortality from all causes in a normal year.7 Older children have a higher mortality rate than young children; however, the rate is still low compared with adults.7
Pathophysiology
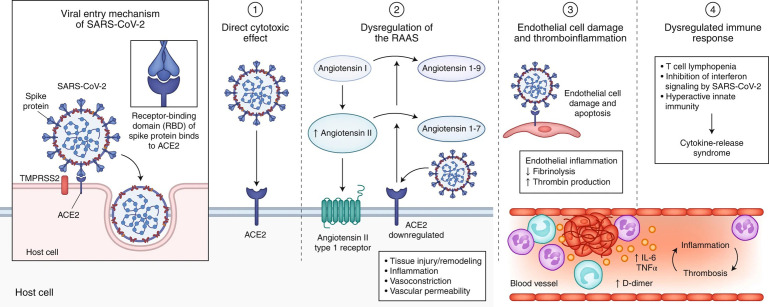
SARS-CoV-2 is a positive-sense single-stranded RNA virus.8 It enters target cells via interaction between the viral spike protein and angiotensin-converting enzyme 2 (ACE-2) receptors. Injury is likely due to direct cytotoxic effect of the virus, dysregulation of the renin–angiotensin–aldosterone system, cell endothelial damage, thromboinflammation, and a dysregulated immune response (Fig. 1 ).9
Fig. 1.
SARS-CoV-2 enters host cells through interaction of its spike protein with the entry receptor ACE2 in the presence of TMPRSS2 (far left). Proposed mechanisms for COVID-19 caused by infection with SARS-CoV-2 include (1) direct virus-mediated cell damage; (2) dysregulation of the renin–angiotensin–aldosterone system (RAAS) as a consequence of downregulation of ACE2 related to viral entry, which leads to decreased cleavage of angiotensin I and angiotensin II; (3) endothelial cell damage and thromboinflammation; and (4) dysregulation of the immune response and hyperinflammation caused by inhibition of interferon signaling by the virus, T-cell lymphodepletion, and the production of proinflammatory cytokines, particularly IL-6 and tumor necrosis factor (TNF)-α.
(From Gupta A, Madhavan MV, et al. Extrapulmonary Manifestations of COVID-19. Nat Med. 2020; 26: 1017-2032, with permission.)
GI injury is multifactorial. There is a high prevalence of ACE-2 receptors in enterocytes with known viral replication in the GI tract given the presence of live virus in patient stool.9 , 10 There is also diffuse microvascular small bowel injury and inflammation-mediated tissue damage of the stomach, duodenum, and rectum.9 Hepatobiliary injury is also multifactorial and may be due to ACE-2–mediated entry of SARS-CoV-2 directly into cholangiocytes damaging the biliary ducts.9 , 11 Although some series reported that up to 37% of patients had abnormal liver tests,12 a pooled data meta-analysis indicated no greater risk of abnormal transaminases or total bilirubin.13 Drug-induced hepatic injury related to COVID-19 treatment is also possible. Some early reports of remdesivir treatment for COVID-19 included increased transaminases and bilirubin,14 but a meta-analysis of multiple reports15 and studies in the pediatric16 and transplant populations17 , 18 found no increased risk of liver injury. Pancreatic injury is also multifactorial and hypothetically due to cytotoxicity of the virus via ACE-2 receptors in the pancreas, drug-induced injury, and damage secondary to the cytokine storm caused by immune dysregulation in severe infections.19 Of note, pancreas injury secondary to SARS-CoV-2 is controversial, although the pathophysiology is plausible.19, 20, 21
Complications
Although the risk of serious illness is highest in the elderly, children are not exempt from serious illness. A later consequence of COVID-19 infection is multisystem inflammatory syndrome in children (MIS-C). The Centers for Disease Control and Prevention diagnostic criteria for MIS-C are fever greater than 38 °C for at least 24 hours or subjective fever of at least 24 hours, laboratory evidence of inflammation (elevated C-reactive protein, erythrocyte sedimentation rate, ferritin, etc), a minimum of 2 organ systems involved, and recent or current SARS-CoV-2 infection or exposure.22 This syndrome typically occurs 2 to 4 weeks after COVID-19 infection and rapid deterioration is common.23 Treatment is currently focused on decreasing this dysregulated inflammatory response and cytokine storm with intravenous immune globulin, and steroids, as well as other biologic agents if needed.24
Of particular interest to the pediatric gastroenterologist is the high prevalence of GI manifestations, which is the most common organ system involved.23 The most common GI manifestations are abdominal pain, diarrhea, and nausea and vomiting.25 These symptoms are secondary to inflammation along the GI tract, with the ileum and colon most commonly effected.25 In severe cases there can be bowel wall thickening, causing luminal narrowing and obstruction. Fortunately, most children will have resolution of their manifestations with appropriate medical management; however, rarely patients have required surgical resection.25 Interestingly, when patients with severe abdominal pain underwent computed tomography imaging, approximately 85% showed inflammatory bowel changes including marked terminal ileitis, inflammation of the cecum, and mesenteric fat stranding.25 Mucosal hyperenhancement, fibrostenosis, and penetrating lesions were not seen. On histopathologic assessment of the surgically removed tissue, there was noted to be marked transmural lymphocytic inflammation, venous microthrombi, arteritis, and necrotizing lymphadenitis, which was distinct from chronic inflammatory bowel disease (IBD).25
Acute hepatitis and pancreatitis have also been linked to MIS-C. Previous studies in adults have shown that as many as 43% of patients with MIS-C have hepatitis during the course of their illness.26 Patients with hepatitis were noted to have more severe disease with higher inflammatory cytokine levels, longer hospitalizations, and increased respiratory support.26 Although long-term data are not available currently, more than 50% of patients had persistent aspartate aminotransferase and alanine aminotransferase elevation at 1-month follow-up visits.26 There is less literature on pancreatitis during MIS-C; however, adult studies have reported a 3% prevalence.23
Discussion
Special Populations
Inflammatory bowel disease
Patients with IBD who are on immunosuppressive medication are at greater risk for infections. Comorbidities are a risk factor for severe COVID-19 infection, so there is particular concern for worse outcomes in pediatric patients with IBD. Fortunately, current pediatric data are encouraging, showing that COVID-19 is well-tolerated in this population.27 Large tertiary care centers in the United States, China and, Italy have reported low rates of COVID-19 infection in pediatric patients with IBD and when symptomatic the vast majority of cases have been mild.27 , 28 There is also no difference in severity of COVID-19 infection between patients with Crohn’s disease, ulcerative colitis, or unspecified IBD.27 , 28
Mesalamine and chronic steroids confer an increased risk of COVID-19 infection, with steroids conferring the highest risk.29, 30, 31 Other risk factors associated with COVID-19 incidence and severity in a mixed population of adults and children were older age, male sex, and comorbidities such as cardiovascular disease and diabetes.31 Immunomodulators and biologic medications including infliximab and vedolizumab for IBD do not confer an increased risk of COVID-19 infection or increased severity when contracted.31 , 32 The most common complication recognized in patients with IBD was not related to COVID-19 specifically, but rather delays in therapy or follow-up owing to changes in hospital policies or parental concerns.28 Patients should receive their regularly scheduled infusions during the pandemic, because a delay increases the risk for exacerbation of the underlying disease.28 Interestingly, biologics may be protective against severe COVID-19 infection and many immunomodulators are currently under investigation as possible treatments for the aberrant inflammatory response caused by COVID-19.27
One of the most pressing issues for pediatric gastroenterologist is whether they should be recommending the COVID-19 vaccine to their patients with IBD. Multiple vaccines have been approved and have shown excellent efficacy and safety in the general population.33 , 34 A recent study by Kennedy and colleagues35 investigated the COVID-19 vaccine in patients with IBD treated with infliximab and found that, similar to other vaccines, infliximab is associated with an attenuated immune response; however, most patients will still have seroconversion after a second dose of the vaccine. The International Organization for the Study of Inflammatory Bowel Disease has recommended providers give the COVID-19 vaccination to patients with IBD because the benefits of COVID-19 prevention outweigh the risks of the vaccine.36
Chronic liver disease
Overall, chronic liver disease from any cause is likely a risk factor for severe COVID-19 infection, although the literature in pediatrics is mixed. The adult data support chronic liver disease as a risk factor; Singh and colleagues37 report an increased relative risk of mortality as compared with propensity-matched patients. Kehar and colleagues38 examined pediatric chronic liver disease in comparison with liver transplant recipients. Their reported data are similar to adults, with higher admission rates, pediatric intensive care management, mechanical ventilation, and death in patients with chronic liver disease as compared with liver transplant recipients. An observational cohort study by Di Giorgio and colleagues39 contradicted this result. Their data showed no difference in the severity of COVID-19 in patients with liver disease; however, they did show a higher observed incidence of infection than the estimated incidence in the population. Of note, their study reported 12% of their population as symptomatic for COVID-19 based on exposures; however, only 0.5% of patients had a confirmed case via testing, causing these data to be difficult to interpret. Regardless of the potential increased risk, treating patients with preexisting liver disease is further complicated by the hepatotoxicity of many of the medications used to treat COVID-19.9 , 11 The adult data support cirrhosis as a risk factor for severe disease; however, again, the data in pediatrics are unclear.38,39 When investigating autoimmune hepatitis as compared with other etiologies of liver disease, there was no difference in rate of severe disease.40 Similar to IBD, immunosuppression with biologic agents was not a risk factor for severe disease secondary to COVID-19 for patients with autoimmune hepatitis.40
Metabolic-associated fatty liver disease
Similar to other chronic liver diseases, metabolic-associated fatty liver disease (MAFLD), formerly known as nonalcoholic fatty liver disease, has been shown to have higher risk of disease progression and longer viral shedding as compared with patients without MAFLD, although specific pediatric data are lacking.41 Obesity, diabetes, and hypertension are all prominent risk factors for severe COVID-19 infection and are common comorbidities in patients with MAFLD.11 Patients with MAFLD may have more severe COVID-19 infections owing to inflammation-suppressing M2 macrophages activation rather than M1 macrophages; however, this correlation is hypothetical.11 Pediatric gastroenterologists should be prepared to see a to higher prevalence and worsening severity of MAFLD because the COVID-19 pandemic has caused a significant decrease in activity for many children that could worsen patients’ obesity.42 It is vital for pediatric gastroenterologist to help families identify safe effective ways to increase physical activity to help delay and potentially reverse the progression of MAFLD.
Liver transplantation
The risk of infection with SARS-CoV-2 in liver transplant recipients is unclear.11 , 38 The literature is limited, and results are mixed. Large adult registry data around the world have shown no increased incidence of infection, but higher mortality rates.11 Colmenero and colleagues43 reported the opposite of this finding in their prospective cohort study with a higher incidence of COVID-19 infections, but a lower mortality rate as compared with matched members of the general population. Two case reports of COVID-19 and MIS-C in pediatric liver transplant recipients had poor outcomes44 , 45; however, the observational cohort study by Kehar and colleagues38 specifically assessing pediatric liver transplant recipients showed improved outcomes as compared with patients with chronic liver disease. Consistent with data in other diseases, immunosuppression has not been shown to be a risk factor for increased severity of COVID-19 infection.11 , 43 There has been a decrease in all solid organ transplants since the start of the COVID-19 pandemic.46
Early research into the COVID-19 vaccine for solid organ transplant recipients is encouraging. Multiple studies have shown that the vaccine is safe and effective, although the immune response is attenuated as compared with the general population.47, 48, 49 The COVID-19 vaccine is recommended for solid organ transplant recipients, pretransplant patients, and all close contacts of solid organ transplant recipients by numerous societies, including the International Liver Transplantation society.50, 51, 52
Impact on clinical practice
Fewer Patient Visits
When the pandemic was identified and advice to decrease in-person visits to health care facilities and postpone elective surgical procedures, it had a large impact on pediatric gastroenterology practices. Outpatient visits decreased,8 , 53 and most endoscopic procedures came to a halt.54 Pediatric gastroenterologists considered the risk of transmission of SARS-CoV-2 to patients, staff, and themselves; the risk of delayed diagnosis for new patients; the jeopardized health of children with chronic disease owing to delays in follow-up care; medication or dietary adherence; fewer clinical opportunities for trainees; and the financial impact of fewer visits and endoscopies. Concerns about delays in diagnosis were realized in reports of severe illness and deaths owing to diabetic ketoacidosis, pyloric stenosis, sepsis, and cancer in children presenting to the hospital after delays attributed to fear of COVID-19 and decreased access to care.55 , 56 Delays in presentation were suspected to contribute to an increase the rate of complicated appendicitis in more than 1 series.56, 57, 58, 59 A study of a large sample of emergency departments in the United States revealed a decrease in pediatric visits by up to 72%, compared with the same week 1 year prior.60 Comparing the same 3-month period, March to June, there was a 22% decrease in visits for serious conditions, including appendicitis and intussusception, and a 62% decrease in visits for abdominal pain.60 Patients were not visiting the gastroenterology clinic either; the largest pediatric GI practice in Iowa saw 20% and 90% decreases in face-to-face encounters in March and April 2020, respectively, compared with 2019.53 That report and others highlighted the pivot to telehealth to see patients.53 , 61 , 62
Telehealth
Telehealth use increased in response to the pandemic,63 facilitated by the relaxation of some regulatory requirements and affirmations of reimbursement by public and private payers.64 Pediatric gastroenterology practices used existing and new technology via electronic health records and mobile and computer teleconferencing applications. A timely publication offered guidance specifically to pediatric gastroenterologists adopting telehealth.62 A few centers have reported successful experiences using telehealth, including adapting multidisciplinary subspecialized disease based clinics,65 and an approach to triaging referrals as e-consults, telehealth, or in-person visits.66 Several studies have demonstrated the usefulness of telehealth for pediatric primary and subspecialty care,67 , 68 but inequities in use and technology have been seen and merit addressing.69 , 70
The future of telehealth beyond the pandemic is bright, but some uncertainty remains. According to a survey of large employers, 53% of employers plan to implement more virtual care solutions for their health plans.71 The increased access to coverage for telehealth for Medicare beneficiaries has depended on a temporary waiver during the public health emergency and will continue through the end of the calendar year of the end of the public health emergency. Medicaid rules can vary by state, but some have continued coverage beyond the initial emergency period.72 Some expanded telehealth services for rural communities were made permanent in the Centers for Medicare and Medicaid Services Physician Fee Schedule final rule released in December 2020.73 Although the pandemic helped to demonstrate the usefulness and feasibility of telehealth, the permanent and widespread adoption of expanded coverage for telehealth services is complex and faces several hurdles.74
Endoscopy
Several considerations related to the risk of transmission or patient complications owing to SARS-CoV-2 infection led to massive disruption of pediatric GI endoscopy. With the stay-at-home orders that were enacted in the spring of 2020, all but urgent and emergent endoscopies came to a halt. Recognizing that endoscopy can aerosolize patients’ mucosal secretions, and because SARS-CoV-2 is frequently present in the stool of infected patients, upper endoscopies and colonoscopies carried a possibly higher risk of transmission of virus. Early guidance for weighing risks and benefits of performing a procedure, the implementation of screening and testing patients for SARS-CoV-2 infection, and the effective use of personal protective equipment came from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition.54 This initial guidance aligned with American Gastroenterological Association recommendations in recommending N95 masks rather than standard surgical masks and using negative pressure rooms, in part based on concerns that children with SARS-CoV-2 infection may not have symptoms, testing may not be available or perfectly sensitive to detect infection, and the virus can be shed in stool beyond the period it is found in the nasopharynx. Subsequently, Hsu and colleagues,75 proposed an alternative perspective reporting institutional practice of universal preprocedure polymerase chain reaction–based testing, to allow standard personal protective equipment, including surgical masks, for patients who test negative. A report from an endoscopy unit in Wuhan, China, described experience performing 159 endoscopies, including 17 in patients previously infected or thought to be carriers of the virus, and no cases of transmission were identified.76 A more recent set of recommendations from the American Gastroenterological Association suggests using a preprocedure testing based on estimated local prevalence of asymptomatic infection.77 To date, we found no reports of transmission of virus to health care workers attributed to an endoscopic procedure.
With the decrease in endoscopic procedures, outpatient visits, and hospital admissions, the opportunities for the typical clinical training of fellows were diminished during the first several months of the pandemic.61 , 78 A survey of program directors revealed that endoscopy and typical outpatient clinical experiences were drastically decreased, but fellows were included with the rapid adoption of telehealth for outpatient visits.61 Fellows’ research activities were curtailed owing to a lack of access to research subjects and to the laboratory. Indeed, many fellows altered their research methodology or refocused on an alternative project. When fellows were surveyed, large proportions reported subjective negative impact on clinical (52%), research (46%), and procedural confidence (41%).78 A small but important proportion of fellows graduating in the summer of 2020 reported that their postfellowship employment contracts were altered or rescinded owing to hiring freezes attributed to the pandemic. Fellows in their first and second years of 3-year fellowships reported high levels of concern in finding a job after graduation.
Summary
Pediatric gastroenterologists took on a variety of challenges during the COVID-19 pandemic, including learning about a new disease and how to recognize and manage it, prevent its spread among their patients and health professions colleagues, and make decisions about managing patients with chronic GI and liver problems considering the threat (Table 1 ). They adapted their practice to accommodate drastically reduced in-person visits, adopting telehealth, and instituting new protocols to perform endoscopies safely. The workforce pipeline was also affected by the pandemic because of its impact on trainee education, clinical experience, research, and job searches.
Table 1.
Special population considerations and recommendations
| IBD | There is no apparent increased risk of COVID-19 infection owing to IBD, but oral corticosteroids do increase the risk of severe COVID-19. |
| There is no difference in disease severity between ulcerative colitis, Crohn’s disease, or unspecified IBD. | |
| Immunosuppression is safe and regimens should not be changed prophylactically, except to minimize steroid use. | |
| COVID-19 vaccine is strongly recommended for patients with IBD. | |
| Chronic liver disease | There is significantly increased risk of COVID-19 infection, particularly in patients with MAFLD. |
| The treatment of COVID-19 infection is complicated by the hepatotoxic effects of medications. | |
| The incidence of MAFLD likely increased during the pandemic owing to decreased exercise. | |
| Liver transplant | There is an increased risk of COVID-19 infection, however it is less than patients with chronic liver disease. |
| Immunosuppression is safe and regimens should not be changed prophylactically, except to minimize steroid use. | |
| Vaccination is recommended for recipients, candidates, and close contacts. |
Future directions
Driven by vaccinations against COVID-19, the rate of infections in the United States is decreasing,79 and public health measures such as mask mandates and restrictions on public gatherings are being lifted. As patients have returned to clinics, hospitals have filled again and procedures have resumed, pediatric gastroenterologists will consider ways to integrate telehealth into future practice, address questions about vaccinating and possibly revaccinating patients and continue to consider COVID-19 as a potential cause of GI illness.
Clinics care points
-
•
COVID-19 infection and MIS-C often have GI manifestations that can be severe and the initial presenting symptoms.
-
•
Patients requiring immunosuppression should not have their regimens altered prophylactically, except to minimize chronic steroid use, because the risk of underlying disease exacerbation greatly outweighs COVID-19 infection risk.
-
•
The COVID-19 vaccination should be given to all patients; however, those with chronic disease and immunosuppression are likely to have an attenuated immune response.
-
•
Office visits and procedures significantly decreased during the pandemic and are slowly returning to normal volumes.
-
•
Telehealth greatly expanded over the last year and is likely to continue to be a popular visit option for patients.
-
•
Typical training experiences were decreased for fellows with lower patient volumes, procedures, and research opportunities during the start of the pandemic.
Acknowledgments
Disclosure
The authors have nothing to disclose.
References
- 1.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Viner R.M., Mytton O.T., Bonell C., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleh N.Y., Aboelghar H.M., Salem S.S., et al. The severity and atypical presentations of COVID-19 infection in pediatrics. BMC Pediatr. 2021;21(1):144. doi: 10.1186/s12887-021-02614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Q., Huang D., Yu H., et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi X., Liu C., Jiang Z., et al. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol. 2020;73(2):455–458. doi: 10.1016/j.jhep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhopal S.S., Bagaria J., Olabi B., et al. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health. 2021;5(5):e12–e13. doi: 10.1016/S2352-4642(21)00066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray K.F., Gold B.D., Shamir R., et al. Coronavirus disease 2019 and the pediatric gastroenterologist. J Pediatr Gastroenterol Nutr. 2020;70(6):720–726. doi: 10.1097/MPG.0000000000002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos V.S., Gurgel R.Q., Cuevas L.E., et al. Prolonged fecal shedding of SARS-CoV-2 in pediatric patients: a quantitative evidence synthesis. J Pediatr Gastroenterol Nutr. 2020;71(2):150–152. doi: 10.1097/MPG.0000000000002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammed A., Paranji N., Chen P.H., et al. COVID-19 in chronic liver disease and liver transplantation: a clinical review. J Clin Gastroenterol. 2021;55(3):187–194. doi: 10.1097/MCG.0000000000001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Z., Chen L., Li J., et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bzeizi K., Abdulla M., Mohammed N., et al. Effect of COVID-19 on liver abnormalities: a systematic review and meta-analysis. Sci Rep. 2021;11(1):10599. doi: 10.1038/s41598-021-89513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinner C.D., Gottlieb R.L., Criner G.J., et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasavon Gholamhoseini M., Yazdi-Feyzabadi V., Goudarzi R., et al. Safety and efficacy of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. J Pharm Pharm Sci. 2021;24:237–245. doi: 10.18433/jpps31870. [DOI] [PubMed] [Google Scholar]
- 16.Méndez-Echevarría A., Pérez-Martínez A., Gonzalez Del Valle L., et al. Compassionate use of remdesivir in children with COVID-19. Eur J Pediatr. 2021;180(4):1317–1322. doi: 10.1007/s00431-020-03876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale M., Sogawa H., Seyedsaadat S.M., et al. Successful management of COVID-19 infection in 2 early post-liver transplant recipients. Transplant Proc. 2021;53(4):1175–1179. doi: 10.1016/j.transproceed.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meshram H.S., Kute V.B., Patel H., et al. Feasibility and safety of remdesivir in SARS-CoV2 infected renal transplant recipients: a retrospective cohort from a developing nation. Transpl Infect Dis. 2021:e13629. doi: 10.1111/tid.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samanta J., Gupta R., Singh M.P., et al. Coronavirus disease 2019 and the pancreas. Pancreatology. 2020;20(8):1567–1575. doi: 10.1016/j.pan.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inamdar S., Benias P.C., Liu Y., et al. Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology. 2020;159(6):2226–2228.e2. doi: 10.1053/j.gastro.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de-Madaria E., Capurso G. COVID-19 and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol. 2021;18(1):3–4. doi: 10.1038/s41575-020-00389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://emergency.cdc.gov/han/2020/han00432.asp Available at: Accessed May 8, 2021.
- 23.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics Multisystem inflammatory syndrome in children (MIS-C) interim guidance. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/#:∼:text=Patients%20with%20MIS%2DC%20are,the%20infusion%20of%20IVIG%20therapy Available at: Accessed May 8, 2021.
- 25.Sahn B., Eze O.P., Edelman M.C., et al. Features of intestinal disease associated with COVID-related multisystem inflammatory syndrome in children. J Pediatr Gastroenterol Nutr. 2021;72(3):384–387. doi: 10.1097/MPG.0000000000002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantor A., Miller J., Zachariah P., et al. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single-center report. Hepatology. 2020;72(5):1522–1527. doi: 10.1002/hep.31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sultan K., Mone A., Durbin L., et al. Review of inflammatory bowel disease and COVID-19. World J Gastroenterol. 2020;26(37):5534–5542. doi: 10.3748/wjg.v26.i37.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner D., Huang Y., Martín-de-Carpi J., et al. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2020;70(6):727–733. doi: 10.1097/MPG.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner E.J., Pigneur B., Focht G., et al. Benign evolution of SARS-Cov2 infections in children with inflammatory bowel disease: results from two international databases. Clin Gastroenterol Hepatol. 2021;19(2):394–396.e5. doi: 10.1016/j.cgh.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan N., Mahmud N., Trivedi C., et al. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut. 2021 doi: 10.1136/gutjnl-2021-324356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperger J., Shah K.S., Lu M., et al. Development and validation of multivariable prediction models for adverse COVID-19 outcomes in IBD patients. medRxiv. 2021 doi: 10.1101/2021.01.15.21249889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Arcangelo G., Distante M., Raso T., et al. Safety of biological therapy in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2021;72(5):736–741. doi: 10.1097/MPG.0000000000003044. [DOI] [PubMed] [Google Scholar]
- 33.D'Amico F., Rabaud C., Peyrin-Biroulet L., et al. SARS-CoV-2 vaccination in IBD: more pros than cons. Nat Rev Gastroenterol Hepatol. 2021;18(4):211–213. doi: 10.1038/s41575-021-00420-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olliaro P., Torreele E., Vaillant M. COVID-19 vaccine efficacy and effectiveness—the elephant (not) in the room. Lancet Microbe. 2021;2(7):e279–e280. doi: 10.1016/S2666-5247(21)00069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy N.A., Lin S., Goodhand J.R., et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021 doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 36.Siegel C.A., Melmed G.Y., McGovern D.P., et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70(4):635–640. doi: 10.1136/gutjnl-2020-324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh S., Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(2):768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kehar M., Ebel N.H., Ng V.L., et al. SARS-CoV2 infection in children with liver transplant and native liver disease: an international observational registry study. J Pediatr Gastroenterol Nutr. 2021;72(6):807–814. doi: 10.1097/MPG.0000000000003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Giorgio A., Nicastro E., Arnaboldi S., et al. Health status of children with chronic liver disease during the SARS-CoV-2 outbreak: results from a multicentre study. Clin Res Hepatol Gastroenterol. 2021;45(2):101610. doi: 10.1016/j.clinre.2020.101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marjot T., Buescher G., Sebode M., et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74(6):1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y.H., Rios R.S., Zheng K.I., et al. Recommendations and clinical guidance for children with metabolic-associated fatty liver disease during the COVID-19 pandemic. J Clin Transl Hepatol. 2021;9(1):1–2. doi: 10.14218/JCTH.2020.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colmenero J., Rodríguez-Perálvarez M., Salcedo M., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikoupour H., Kazemi K., Arasteh P., et al. Pediatric liver transplantation and COVID-19: a case report. BMC Surg. 2020;20(1):224. doi: 10.1186/s12893-020-00878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petters L.M., Vogel T.P., Munoz F.M., et al. Multisystem inflammatory syndrome in children associated with SARS-CoV-2 in a solid organ transplant recipient. Am J Transplant. 2021;21(7):2596–2599. doi: 10.1111/ajt.16572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doná D., Torres Canizales J., Benetti E., et al. Pediatric transplantation in Europe during the COVID-19 pandemic: early impact on activity and healthcare. Clin Transpl. 2020;34(10):e14063. doi: 10.1111/ctr.14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabinowich L., Grupper A., Baruch R., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peled Y., Ram E., Lavee J., et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American Society of Transplantation Statement on COVID-19 vaccination in solid organ transplant recipients. 2021. https://www.myast.org/statement-covid-19-vaccination-solid-organ-transplant-recipients# Available at: Accessed May 29, 2021.
- 51.Cornberg M., Buti M., Eberhardt C.S., et al. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74(4):944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fix O.K., Blumberg E.A., Chang K.M., et al. AASLD Expert Panel Consensus Statement: vaccines to prevent COVID-19 infection in patients with liver disease. Hepatology. 2021 doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kriem J., Rahhal R. COVID-19 pandemic and challenges in pediatric gastroenterology practice. World J Gastroenterol. 2020;26(36):5387–5394. doi: 10.3748/wjg.v26.i36.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh C.M., Fishman D.S., Lerner D.G. Pediatric endoscopy in the era of coronavirus disease 2019: a North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition position paper. J Pediatr Gastroenterol Nutr. 2020;70(6):741–750. doi: 10.1097/MPG.0000000000002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazzerini M., Barbi E., Apicella A., et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolescent Health. 2020;4(5):e10–e11. doi: 10.1016/S2352-4642(20)30108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding Y.Y., Ramakrishna S., Long A.H., et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr Blood Cancer. 2020;67(9):e28427. doi: 10.1002/pbc.28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonilla L., Gálvez C., Medrano L., et al. [Impact of COVID-19 on the presentation and course of acute appendicitis in paediatrics] An Pediatr (Engl Ed) 2021;94(4):245–251. doi: 10.1016/j.anpedi.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delgado-Miguel C., Muñoz-Serrano A.J., Miguel-Ferrero M., et al. Complicated acute appendicitis during COVID-19 pandemic: the hidden epidemic in children. Eur J Pediatr Surg. 2021 doi: 10.1055/s-0041-1723992. [DOI] [PubMed] [Google Scholar]
- 59.Snapiri O., Rosenberg Danziger C., Krause I., et al. Delayed diagnosis of paediatric appendicitis during the COVID-19 pandemic. Acta Paediatr. 2020;109(8):1672–1676. doi: 10.1111/apa.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pines J.M., Zocchi M.S., Black B.S., et al. Characterizing pediatric emergency department visits during the COVID-19 pandemic. Am J Emerg Med. 2021;41:201–204. doi: 10.1016/j.ajem.2020.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallon D., Pohl J.F., Phatak U.P., et al. Impact of COVID-19 on pediatric gastroenterology fellow training in North America. J Pediatr Gastroenterol Nutr. 2020;71(1):6–11. doi: 10.1097/MPG.0000000000002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berg E.A., Picoraro J.A., Miller S.D., et al. COVID-19 - a guide to rapid implementation of telehealth services: a playbook for the pediatric gastroenterologist. J Pediatr Gastroenterol Nutr. 2020;70(6):734–740. doi: 10.1097/MPG.0000000000002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wosik J., Fudim M., Cameron B., et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957–962. doi: 10.1093/jamia/ocaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Medicare and Medicaid Services Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf Available at: Accessed April 19, 2020.
- 65.Verstraete S.G., Sola A.M., Ali S.A. Telemedicine for pediatric inflammatory bowel disease in the era of COVID-19. J Pediatr Gastroenterol Nutr. 2020;70(6):e140. doi: 10.1097/MPG.0000000000002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leinwand K., Blodgett N., Ramraj R. Telehealth in pediatric gastroenterology can be a sustainable long-term option: a single-center experience. Permanente J. 2021;25:1. doi: 10.7812/TPP/20.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah A.C., Badawy S.M. Telemedicine in pediatrics: systematic review of randomized controlled trials. JMIR Pediatr parenting. 2021;4(1):e22696. doi: 10.2196/22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haynes S.C., Marcin J.P., Dayal P., et al. Impact of telemedicine on visit attendance for paediatric patients receiving endocrinology specialty care. J Telemed Telecare. 2020 doi: 10.1177/1357633X20972911. 1357633x20972911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eberly L.A., Kallan M.J., Julien H.M., et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID-19 pandemic. JAMA Netw Open. 2020;3(12):e2031640. doi: 10.1001/jamanetworkopen.2020.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ray K.N., Mehrotra A., Yabes J.G., et al. Telemedicine and outpatient subspecialty visits among pediatric Medicaid beneficiaries. Acad Pediatr. 2020;20(5):642–651. doi: 10.1016/j.acap.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Business Group on Health. 2021 large employers’ health care strategy and plan design survey. August 2020. https://www.businessgrouphealth.org/resources/2021-large-employers-health-care-strategy-and-plan-design-survey Available at: Accessed May 8, 2021.
- 72.Ohio Department of Medicaid . 2020. Telehealth billing guidelines.https://medicaid.ohio.gov/Portals/0/Providers/COVID19/Telehealth-Billing-Guidelines-on-or-after-11-15-2020.pdf Available at: [Google Scholar]
- 73.Centers for Medicare and Medicaid Services (CMS): Trump Administration Finalizes Permanent Expansion of Medicare Telehealth Services and Improved Payment for Time Doctors Spend with Patients [press release]. December 2020. Available at: https://www.cms.gov/newsroom/press-releases/trump-administration-finalizes-permanent-expansion-medicare-telehealth-services-and-improved-payment. Accessed May 8, 2021.
- 74.Turner Lee N, Karsten J, Roberts J. Removing regulatory barriers to telehealth before and after COVID-19. May 2020. Available at: https://www.brookings.edu/research/removing-regulatory-barriers-to-telehealth-before-and-after-covid-19. Accessed May 8, 2020.
- 75.Hsu E.K., Ambartsumyan L., Wahbeh G.T., et al. Pediatric endoscopy during the COVID-19 pandemic: addressing the implications of universal preprocedural testing for PPE utilization. J Pediatr Gastroenterol Nutr. 2021;72(1):e25–e26. doi: 10.1097/MPG.0000000000002962. [DOI] [PubMed] [Google Scholar]
- 76.Yu Q., Xu P., Gan H., et al. Comprehensive gastroenterology endoscopy unit workflow and infection prevention during the COVID-19 pandemic: experience with 159 cases in Wuhan, China. Dig Endosc. 2021;33(1):195–202. doi: 10.1111/den.13832. [DOI] [PubMed] [Google Scholar]
- 77.Sultan S., Siddique S.M., Altayar O., et al. AGA Institute Rapid Review and Recommendations on the role of pre-procedure SARS-CoV-2 testing and endoscopy. Gastroenterology. 2020;159(5):1935–1948.e5. doi: 10.1053/j.gastro.2020.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irastorza L.E., Hopson P., Ta A., et al. The impact of COVID-19 on job prospects and educational training for pediatric gastroenterology fellows. J Pediatr Gastroenterol Nutr. 2021;72(4):514–519. doi: 10.1097/MPG.0000000000003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.CDC. COVID data tracker. 2021. 2021. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases Available at: Accessed May 26, 2021.