Abstract
Patients with rheumatologic conditions can have complex dermatologic manifestations. In addition, immunosuppressing treatment for autoimmune disorders can also increase incidence of infectious complications. Skin conditions in rheumatologic patients present particular challenges and this case highlights a rare infectious complication.
Keywords: fungal infection, skin rash, Sporothrix, sporotrichosis
CASE REPORT
A 44-year-old woman was transferred to the University of Washington Medical Center for disfiguring generalized rash, and cellulitis with tenosynovitis of her right hand (Figure 1). She had a history of cutaneous sarcoidosis and was taking chronic oral prednisone 20 mg daily; she had also recently received methotrexate. The first lesion appeared on her hand and was noted 2 months prior; it began as a nodule approximately 1–2 weeks after she was scratched by her cat. The nodule progressively became large and ulcerated. Other lesions appeared in her extremities and trunk, and they became painful and pruritic. For 2–3 weeks prior to admission, she also reported subjective fevers, chills, and night sweats. Two weeks prior to transfer, she was seen by her primary care provider, and her prednisone dose was increased from 20 mg to 60 mg for presumed cutaneous sarcoid lesions; after that, her lesions rapidly worsened, which prompted admission to an outside hospital. She received broad-spectrum antibiotics (vancomycin later switched to linezolid and ceftriaxone) without improvement of her lesions.
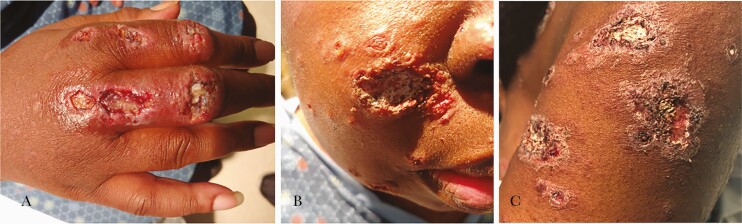
Figure 1.
Initial disseminated lesions. A, Right hand lesions. B, Facial lesions. C, Left arm lesions. Photos show deep ulcerative lesions with irregular borders (A and B) and flatter lesions with scaling and irregular borders (C).
Additional pertinent medical and social history included extrapulmonary/cutaneous sarcoidosis diagnosed in June 2018 with noncaseating granuloma seen on skin biopsy at an outside hospital, ductal carcinoma in situ of breast status post bilateral mastectomy with left-sided lymph node removal and subsequent reconstruction × 2 in 2017, chronic nonalcoholic liver disease, hypertension, and gastroesophageal reflux. She is originally from California but had lived in Washington for 8 years with no recent travel and no sick contacts, and had never visited the Midwest. The patient owned a cat and dog and lived on a large property; she reported seeing bats many times before but never inside the house. The patient had not had prior tuberculosis exposure or testing, and worked as home caregiver for her son and at her mother’s daycare. She reported occasional cannabis use for pain but denied any other illicit habits or heavy alcohol intake.
Tissue and blood cultures were performed with findings below (Figure 2.)
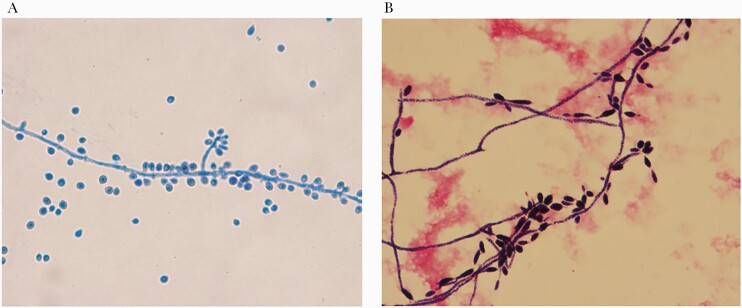
Figure 2.
Microbiology slides. A, Findings from tissue culture lactophenol cotton blue prep for mold showing “rosette-like” clusters of sessile conidia [18, 19]. B, Peripheral blood culture showing microconidia on gram stain with original magnification ×100. Photo credit: Ryan Morse, MD, University of Washington, Department of Pathology and Laboratory Medicine.
WHAT IS YOUR DIAGNOSIS?
Diagnosis: Disseminated Sporotrichosis
The initial differential diagnosis included sporotrichosis, histoplasmosis, and cryptococcosis, for which treatment was started empirically with liposomal amphotericin B infusion (5 mg/kg daily). Immunosuppressive therapy was also tapered. There was evidence of superimposed bacterial cellulitis in some of the lesions, as well as tenosynovitis of the patient’s right third finger, for which she received ceftriaxone and vancomycin for a 7-day course. Tissue cultures were performed (Inhibitory Mold Agar and Sabouraud Dextrose with BHI, Remel), with evidence of yeast on direct fungal stain from both a right arm wound and a deep tissue culture at room temperature of hand wound. These cultures grew Sporothrix schenckii after 3 days, and blood cultures (VersaTREK, ThermoFisher Scientific) also grew S schenckii after 3 days of incubation (Figure 2). The organism identification of S schenckii was confirmed by polymerase chain reaction (PCR) amplification of the internal tandem spacer regions 1 and 2. Sanger sequencing and bioinformatics analysis identified the organism as S schenckii with 99.76% nucleotide identity to type strain CBS 359.36 from the National Center for Bioinformatics Reference Sequence Database (accession KX590842) and 99.5%–100% nucleotide identity to 95 published sequences of S schenckii [1, 2]. Previous tissue biopsy from an outside hospital was obtained, which revealed histiocytic cells containing numerous intracellular organisms, round to oval in shape, with positive fungal gramstain stain showing clusters of yeast forms and short hyphal forms. Additional testing included cryptococcal serum antigen (IMMY), Histoplasma urine antigen (Mayo Clinic Laboratories, IMMY), and human immunodeficiency virus test (Abbott Architect), all of which were negative. Given fungemia, the patient underwent additional workup, which included a transthoracic echo negative for cardiac valvular vegetations and a maxillofacial and head computed tomographic scan with no evidence of sinusitis or invasive fungal disease. She was evaluated by otolaryngology and had no evidence of oropharyngeal, nasal, or sinus mucosal involvement. Ophthalmic evaluation revealed a low-grade anterior uveitis initially managed with topical steroids. Her course was complicated by acute kidney injury for which amphotericin B was switched to itraconazole orally (200 mg orally twice daily). The patient received 14 months of itraconazole with near resolution of all her skin lesions (Figure 3). Unfortunately, her eye disease progressed with development of bilateral endogenous endophthalmitis (vitreous fluid culture and PCR positive for S schenckii) requiring numerous intraocular amphotericin B injections. She ultimately required pars plana vitrectomy and cataract surgery of the left eye. She developed a few additional skin nodules while on treatment that had fungal elements on pathologic examination and positive PCR (as above) for S schenckii, but no growth on cultures.
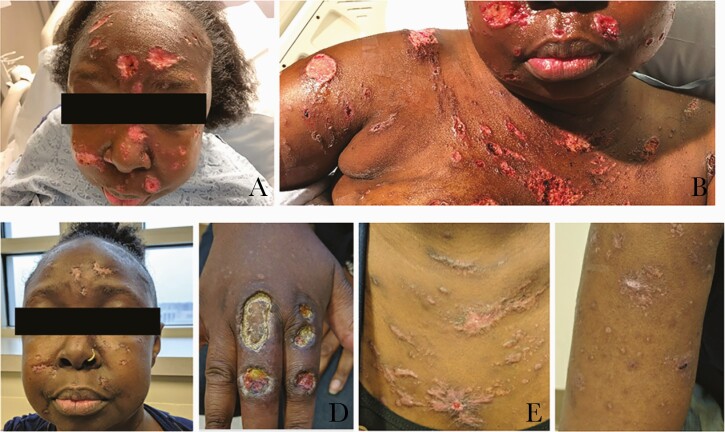
Figure 3.
Disseminated lesions at 7 days and 2 months after treatment. A and B, Skin lesions at 7 days after starting treatment. C–F, Skin lesions after 2 months of treatment.
After approximately 1 year on itraconazole treatment, she re-presented with new fever and skin lesions. Terbinafine was added in addition to her itraconazole. She had several areas of abscess over her prior breast implants and required removal of both implants. The removed implants were both positive by culture for S schenckii with the same sensitivities as the prior isolate. Additional sensitivities were obtained including an amphotericin B minimum inhibitory concentration (MIC) of 1 µg/mL and posaconazole MIC of 0.5 µg/mL. She was subsequently transitioned to posaconazole and terbinafine due to concerns about itraconazole absorption. Her skin lesions stabilized on this regimen. She underwent cataract removal in her right eye with amphotericin B injection approximately 2 months later with no detection of fungal DNA on vitreous fluid PCR.
DISCUSSION
Sporotrichosis is a subcutaneous mycosis that infects both humans and animals. It has a worldwide distribution, particularly in tropical and temperate regions, and is commonly found in soil and on decaying vegetation [3–5]. Infection is caused by a dimorphic fungus and was first isolated in 1896 by Benjamin Schenk. Two forms can be found, both mold and yeast, depending on temperature; this is classic for thermally dimorphic fungi [6].
Infection occurs by inoculation of soil, plants, and organic matter that is contaminated with Sporothrix fungal elements. The incubation period depends on the size of the inocula; for cutaneous cases, an average incubation time is 3 weeks [6]. Sporotrichosis is associated with risk factors including gardening and farming; it is also associated with cat scratches or bites as the organism can be part of their oral flora [3]. Sporotrichosis usually starts as a local subcutaneous infection and may cause nodular lymphangitic spread in up to 90% of cases [3–7].
Four clinical variants have been described: lymphocutaneous, fixed cutaneous, disseminated cutaneous, and extracutaneous. Disseminated cutaneous disease is defined by 3 or more lesions in at least 2 different sites [7]. The main risk factor for disseminated disease is immunocompromised state; however, alcoholism and diabetes have also been described as risk factors for disseminated disease as cellular immunity is impaired [3–10]. Variation in genotype and phenotype cause virulence of the strain and that, combined with the immune response of host, may contribute to its different clinical manifestations [6].
The gold standard for diagnosis of disseminated sporotrichosis is positive culture from a tissue biopsy. The organism generally takes at least 3–5 days for growth in culture and can take up to 2 weeks. Histopathology of biopsied tissue often shows granulomas, most commonly suppurative granulomas [11]. The fungus may not be visible in tissue specimens, but when present, demonstrates yeast forms that are oval or cigar shaped and 3–5 μm in length. Blood cultures are helpful when positive, as seen in this case. There are multiple noncommercial laboratory PCR tests developed for fungal identification. Where available, these assays can be run on tissue and body fluid for more rapid diagnosis [12].
Although S schenckii is generally identified as a single species in most laboratories, genetic analysis has found that this group is made up of at least 6 genetically distinct species. These different species within the S schenckii complex tend to have distinct geographical distributions: The isolates from Europe have the most distinct differences from isolates from the Americas, while isolates from South Africa are more closely related to the American than the European isolates [11]. In general, the different species have similar sensitivities and are treated the same.
The backbone of therapy for disseminated sporotrichosis is liposomal amphotericin B 3–5 mg/kg daily. Although no specific clinical trials exist for this form of disease, patients typically respond to amphotericin B, followed by itraconazole as continuation therapy. The timing of continuation therapy is dependent on clinical improvement. Treatment should be continued for a minimum of 12 months, but in patients for whom immunosuppression cannot be fully reversed, lifelong suppression with itraconazole may be required [13]. Terbinafine has also been used in occasional case reports [14–16] and is active in vitro against Sporothrix species, but clinical experience is limited. Endogenous endophthalmitis, as seen in this patient, can often be treated with systemic therapy alone, but in severe cases, intravitreal amphotericin B and vitrectomy are sometimes required [17].
In summary, sporotrichosis should be considered in persons with nodular skin lesions and/or tenosynovitis, especially for those with gardening and cat exposure and/or immunosuppression. Identifying exposure history and determining underlying risk factors, particularly immunosuppression, may be key to identifying this disease in lower-endemicity environments. In particular, disseminated skin disease not responding to therapy or worsening in the setting of increased immunosuppression should raise concern for fungal disease including sporotrichosis. Delay of diagnosis, such as in our case, can lead to worsened clinical outcomes, as the disease can disseminate to protected spaces resulting in meningeal or ocular disease.
Notes
Acknowledgments. The authors express gratitude to our patient for allowing us to publish this case and to the medical resident on our team, Katherine Smolinski, DO, for her compassionate care. We also thank Ryan Morse, MD, University of Washington Department of Pathology and Laboratory Medicine, who provided the microbiology images, and the University of Washington Department of Dermatology for performing the patient’s biopsy.
Author contributions. All authors contributed equally to this work.
Patient consent statement. The patient’s written consent was obtained prior to the preparation and publication of the case report. This work conforms to standards currently applied in the United States regarding patient information published in the literature and did not require University of Washington institutional review board approval.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, NIH (grant number T32AI007044).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rakeman JL, Bui U, Lafe K, et al. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J Clin Microbiol 2005; 43:3324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Irinyi L, Serena C, Garcia-Hermoso D, et al. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database—the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med Mycol 2015; 53:313–37. [DOI] [PubMed] [Google Scholar]
- 3. Kaadan MI, Dennis M, Desai N, et al. One health education for future physicians: a case report of cat-transmitted sporotrichosis. Open Forum Infect Dis 2020;7:ofaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia 2012; 173:265–73. [DOI] [PubMed] [Google Scholar]
- 5. White M, Adams L, Phan C, et al. Disseminated sporotrichosis following iatrogenic immunosuppression for suspected pyoderma gangrenosum. Lancet Infect Dis 2019; 19:e385–91. [DOI] [PubMed] [Google Scholar]
- 6. Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi 2017; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rueda M, Torres N, Bravo F. Disseminated cutaneous sporotrichosis: an unusual case. Dermatol Online J 2018; 24:6. [PubMed] [Google Scholar]
- 8. Bonifaz A, Tirado-Sanchez A, Paredes-Solis V, et al. Cutaneous disseminated sporotrichosis: clinical experience of 24 cases. Eur Acad Dermatol Venereol 2018; 32:e77–9. [DOI] [PubMed] [Google Scholar]
- 9. Morgan MA, Cockerill FR III, Cortese DA, Roberts GD. Disseminated sporotrichosis with Sporothrix schenckii fungemia. Diagn Microbiol Infect Dis 1984; 2:151–5. [DOI] [PubMed] [Google Scholar]
- 10. Sharon VR, Kim J, Sudhakar S, et al. Disseminated cutaneous sporotrichosis. Lancet Infect Dis 2013; 13:95. [DOI] [PubMed] [Google Scholar]
- 11. Quintella LP, Lambert Passos SR, Francesconi do Vale AC, et al. Histopathology of cutaneous sporotrichosis in Rio de Janeiro: a series of 119 consecutive cases. J Cutan Pathol 2011; 38:25–32. [DOI] [PubMed] [Google Scholar]
- 12. Rodrigues AM, Sybren de Hoog G, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis 2015; 9:e0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marimon R, Gené J, Cano J, et al. Molecular phylogeny of Sporothrix schenckii. J Clin Microbiol 2006; 44:3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kauffman CA, Bustamante B, Chapman SW, Pappas PG. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:1255–65. [DOI] [PubMed] [Google Scholar]
- 15. Guo K, Wang S, Wang Z, Zhang L. Effective treatment using itraconazole with terbinafine in the treatment of nasal sporotrichosis. Medicine 2019; 98:e17155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahajan VK. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract 2014; 2014:272376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coskun B, Saral Y, Akpolat N, et al. Sporotrichosis successfully treated with terbinafine and potassium iodide: case report and review of the literature. Mycopathologia 2004; 158:53–6. [DOI] [PubMed] [Google Scholar]
- 18. Soto MCR. Differences in clinical ocular outcomes between exogenous and endogenous endophthalmitis caused by Sporothrix: a systematic review of published literature. Br J Ophthalmol 2018; 102:977–82. [DOI] [PubMed] [Google Scholar]
- 19. Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol 2007; 45:3198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]