Abstract
Aim
The present study evaluated the clinical and radiological stability of hard and soft tissues following alveolar socket preservation (ASP) procedure with a follow-up of 5 year from implant insertion.
Materials and methods
The initial sample consisted of seven patients who underwent single tooth extraction and ASP procedure by means of demineralized bovine bone mineral particles covered with a porcine-derived non-cross-linked collagen matrix (CM). Each patient received a submerged single implant in the healed site. Mesial and distal peri-implant marginal bone resorption (MBR) rates were assessed radiographically at 1 year (T1) and 5 years (T2) after implant placement (baseline value).
Results and Statistics
No dropouts occurred up to 5 years. At T1, the MBR was 0.08 ± 0.16 mm at the mesial aspect and 0.1 ± 0.12 mm at the distal aspect. This difference was not statistically significant (P = 0.867). At T2, the mesial MBR was 0.15 ± 0.17 mm and the distal MBR was 0.11 ± 0.14 mm, with a non-statistically significant difference (P = 0.532). Therefore, no statistically significant differences were detected comparing mesial and distal MBR at any time point. With respect to the intra-group comparisons, no differences were observed comparing the different study periods within each variable. Indeed, the comparison between T0, T1 and T2 was non-statistically significant at both mesial (P = 0.06) and distal (P = 0.06) aspects. After 5 years, the volume of the soft tissues appeared clinically well maintained with a natural aspect around dental implants and adjacent teeth.
Conclusion
ASP using demineralized bovine bone mineral in combination with CM proved to be an effective technique to maintain stable dimensional volumes of both hard and soft tissues.
Keywords: Bone regeneration, Bone substitutes, Socket preservation, Tooth loss, Mucograft-seal
Introduction
Following tooth loss, a physiological remodeling process of hard and soft tissues might occur. This results in a dimensional shrinkage of the alveolar ridge in both height and width, depending on multiple aspects [1]. The significant number of factors involved in the healing of empty sockets leads to a substantial variability of clinical scenarios. Accordingly, the shrinkage of the alveolar process may prevent implant placement in a prosthetically driven position, jeopardizing the functional and aesthetic outcomes of the prosthetic rehabilitation. Thus, ridge preservation treatment protocols have been advocated to minimize the inevitable alveolar bone resorption and to ensure the support of an adequate ridge profile. Alveolar socket preservation (ASP) procedure is generally characterized by the placement of a grafting material in the socket of the extracted tooth, with or without the use of barrier membranes or autogenous soft tissue grafts to protect the graft. The said technique aims to preserve or improve the original ridge dimensions and to allow an ideal implant placement.
The use of deproteinized bovine bone mineral (DBBM) as grafting material during ASP procedures has been widely investigated in clinical and preclinical studies [1, 2]. L histological study performed on humans proved that, after six months from its placement in fresh extraction sockets, the DBBM delayed healing, but on the other hand, facilitated the preservation of the edentulous ridge [3]. This was corroborated by the comparison between grafted and non-grafted sockets. A significant reduction in dimensional bone loss has been observed when post-extractive sockets were filled with bone substitutes [4]. The key factor of the technique must be identified in the slow resorption rate of DBBM granules. These particles can still be observed in the grafted site after 7 months from the ridge preservation [5]. Carmagnola et al. observed an adequate quality and quantity of alveolar bone required for a correct implant placement after a healing period of 9 months following ASP procedures [6]. It can therefore be stated that DBBM plays a pivotal role in the preservation of hard tissues. The same importance must be addressed on the soft tissue coverage of post-extractive sockets. Soft tissue management should aim at maintaining an optimal gingival contour of the implant-supported restoration, along with biological and functional demands. The use of autogenous soft tissue grafts to cover the socket is associated with several disadvantages, such as the need for a secondary site, risk of graft necrosis, aesthetical discrepancies with the neighboring tissues at the recipient site, increased operative time and patient morbidity [7]. Conversely, the use of xenogeneic collagen matrices to seal the grafted socket and preserve soft tissue volumes showed more than the promising results [8]. A new xenogeneic, porcine-derived non-cross-linked bilayered resorbable collagen matrix (CM) consisting of pure type I and III collagen has been recently introduced in the field of soft tissue regeneration [9]. A safe integration of the CM with no sign of inflammation has been observed clinically and histologically. The placement of the CM induced a greater width and thickness of the keratinized mucosa together with a better color match compared to the spontaneous healing [10, 11]. The use of the CM for ASP procedures in association with a biomaterial provided the encouraging results in terms of soft tissue preservation and ridge resorption in all directions [12, 13].
While most of the studies focused on the early healing of ASP procedures, medium- and long-term evaluations of MBR around implants placed in preserved sockets remain still lacking. A recent meta-analysis found better outcomes in terms of MBR in grafted compared to not grafted sites [14]. However, it has also been stressed how the included studies showed wide heterogeneity with respect to study design, statistical analysis and population, together with a limited follow-up.
Moreover, different techniques and biomaterials have been investigated, sometimes leading to the contrasting results [15]. In a recent retrospective study, preserved and non-preserved sites showed similar success rates of 51% and 58%, respectively; however, the patient population included also smokers, diabetic subjects and patients diagnosed with peri-implantitis [16]. Furthermore, the soft tissues evaluation and management have not always been considered. Cosyn et al., together with the need of ridge preservation, evaluated the concomitant possibility to use a connective tissue graft harvested from the palate, showing better outcomes in terms of MBR and aesthetics [17]. However, not many other studies investigated this option, or the use of soft tissue substitutes such as collagen matrices.
In view of the above, the aim of the present study was to evaluate the marginal bone resorption (MBR) around dental implants placed in sites preserved with DBBM covered with CM over a 5-year follow-up.
Methods
Study Design
The present study was designed as a clinical and radiological prospective evaluation conducted in a cohort of patients previously treated with ASP procedures between January 2014 and January 2015 in a university setting as described by Maiorana et al. [18]. The study was approved by the local institutional review board, and it was conducted according to the principles articulated in the Helsinki Declaration of 1975 for biomedical research involving human subjects, as revised in 2000. All patients were informed about the nature of the study and gave their written consent.
Patient Population
All subjects initially included were non-smoking patients with ≥ 18 years of age, with no local or systemic contraindications to implant surgery. Clinically, patients had to present with a hopeless tooth in the upper jaw between the second premolars that required extraction and immediate replacement with an implant. All extraction sites had adjacent teeth present. Subjects were excluded in case of concomitant acute infection or presence of pus in or close to the site intended for extraction. Patients were also excluded if the cortical walls of the alveolus were not intact upon probing following tooth extraction.
Alveolar Socket Preservation Procedures
All preoperative and surgical phases were explained in detail in a previous publication [18]. In brief, professional oral hygiene procedures were performed one week before the scheduled surgical procedure. Tooth extraction was carried out with a flapless minimally invasive approach taking care to preserve the adjacent soft tissues and the bone walls. De-epithelialization of the inner layer of the gingival walls at the socket orifice was conducted with a diamond bur under copious irrigation with sterile saline to increase the blood supply by exposing the vascularized lamina propria. At this point, DBBM particles (Bio-Oss®, Geistlich Pharma AG, Wolhusen, Switzerland) were grafted in the empty socket up to the coronal portion of the alveolar bone. The bone substitute was finally covered with a porcine-derived non-cross-linked resorbable CM sutured with non-resorbable 6–0 simple interrupted stitches to seal the grafted socket.
Implant Placement Procedures
After a healing period of 6 months, a full-thickness flap was raised and implant bed preparation was performed in accordance with the protocol provided by the manufacturer’s instructions. The implant was placed at the bone level, the cover screw was connected to the fixture, and a first-intention healing was accomplished with 4–0 simple interrupted suturing (PROLENE®, Ethicon Inc., Somerville, NJ, USA).
Radiological Evaluation
Before tooth extraction, a film holder (Rinn® XCP, Dentsply, York, PA, USA) customized directly in the patient's mouth using auto-polymerizing acrylic resin was made to reproduce the same position of the film each time relative to the teeth adjacent to the surgical site, thereby obtaining superimposable dental radiographs at different intervals.
For the radiographic assessment of the mesial and distal MBR, the intraoral peri-apical radiographs taken immediately after implant insertion were used as the baseline radiological reference (T0). To evaluate the MBR over time, additional intraoral peri-apical radiographs were taken after 1 year (T1) and 5 years (T2) from implant placement with the same customized film holder.
The peri-apical radiographs were taken perpendicularly to the long axis of the alveolus with a long-cone parallel technique using the patient-specific customized film holder in order to increase the degree of reproducibility and to standardize the projection geometry between the pair of serially acquired images. Thereafter, the radiographs were scanned to obtain standardized digital images with a resolution of 1200 dpi. These images were imported and superimposed using specialized computer software (ImageJ 1.49v, Research Services Branch, National Institute of Health, Bethesda, MD, USA). The calibration of the pixel/millimeter ratio was performed on the basis of a known distance (i.e., the length of the film), which was 41 mm in each radiograph.
The MBR was calculated as the distance between the most apical bone-to-implant contact visible in the scanned images and the implant-abutment connection level.
Statistical Analyses
Statistical analyses were performed using IBM SPSS Statistics 24.0 (IBM Corp, Armonk, NY), adopting the implant as the statistical unit. Descriptive statistics of the measured continuous values were used to explore the marginal bone resorption (MBR) at the mesial and distal aspects, including mean ± standard deviation and 95% confidence interval (CI). The Shapiro–Wilk test was used to assess the normality of data distribution. Because the distribution of the data met the requirements for normality and homogeneity of variance assumptions (P > 0.05), quantitative data were analyzed using parametric tests. An independent-sample t test was used to compare mesial and distal marginal bone resorption at each time point independently. One-way analysis of variance was used to compare the mean marginal bone resorption at different study periods within each group. The level of significance was set at 0.05.
Results
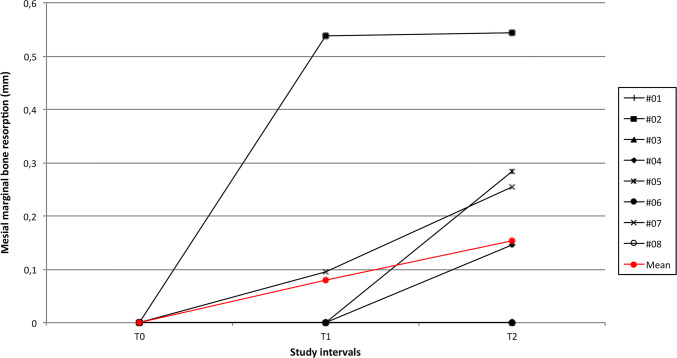
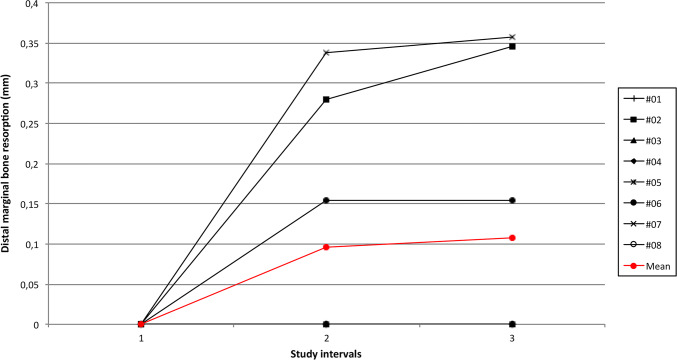
A total of seven patients were originally enrolled in the present prospective study. An additional patient fulfilled the inclusion criteria and was therefore included. Overall, eight patients completed the study. No surgical complications occurred and the healing proceeded uneventfully. During the entire study period from implant insertion up to the last follow-up visit, no biological or prosthetic complications occurred. All the patients were evaluated radiologically at T0 (Fig. 1), T1 (Fig. 2) and T2 (Fig. 3). The modification of marginal bone levels is reported in Table 1 and illustrated in Figs. 4 and 5.
Fig. 1.
Radiological evaluation at baseline
Fig. 2.
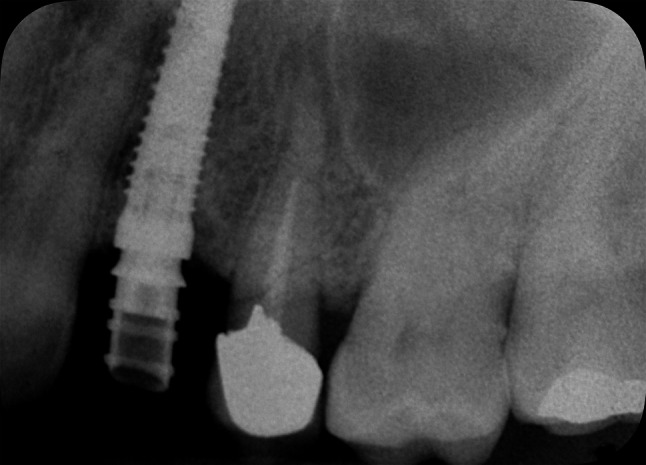
Radiological evaluation at 12 months from the ASP procedure
Fig. 3.
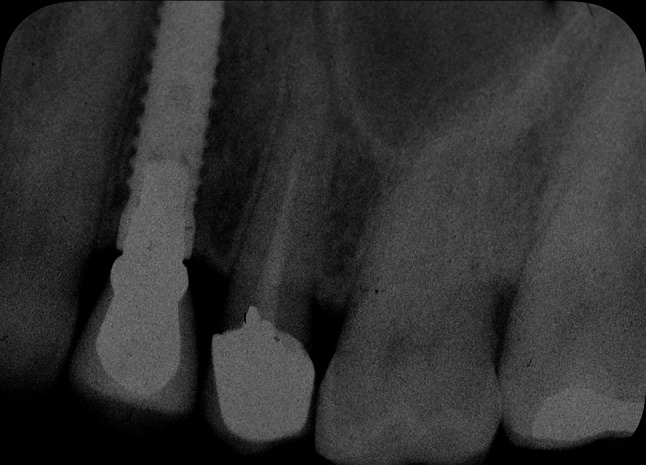
Radiological evaluation at 5 year from the ASP procedure
Table 1.
Marginal bone resorption recorded in mm at the mesial and distal aspect of each implant at T0, T1, and T2
| Patient ID | T0 | T1 | T2 | |||
|---|---|---|---|---|---|---|
| Mesial | Distal | Mesial | Distal | Mesial | Distal | |
| ASP#1 | 0 | 0 | 0 | 0 | 0 | 0 |
| ASP#2 | 0 | 0 | 0.538 | 0.28 | 0.543 | 0.346 |
| ASP#3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ASP#4 | 0 | 0 | 0 | 0 | 0.146 | 0 |
| ASP#5 | 0 | 0 | 0 | 0 | 0.284 | 0 |
| ASP#6 | 0 | 0 | 0 | 0.154 | 0 | 0.154 |
| ASP#7 | 0 | 0 | 0.095 | 0.338 | 0.255 | 0.357 |
| ASP#8 | 0 | 0 | 0 | 0 | 0 | 0 |
Fig. 4.
Graph illustrating the trend of MBR at the mesial aspect of each implant
Fig. 5.
Graph illustrating the trend of MBR at the distal aspect of each implant
At T1, the MBR was 0.08 ± 0.16 mm at the mesial aspect and 0.1 ± 0.12 mm at the distal aspect. This difference was not statistically significant (P = 0.867). At T2, the mesial MBR was 0.15 ± 0.17 mm and the distal MBR was 0.11 ± 0.14 mm, with a non-statistically significant difference (P = 0.532). Therefore, no statistically significant differences were detected comparing mesial and distal MBR at any time point.
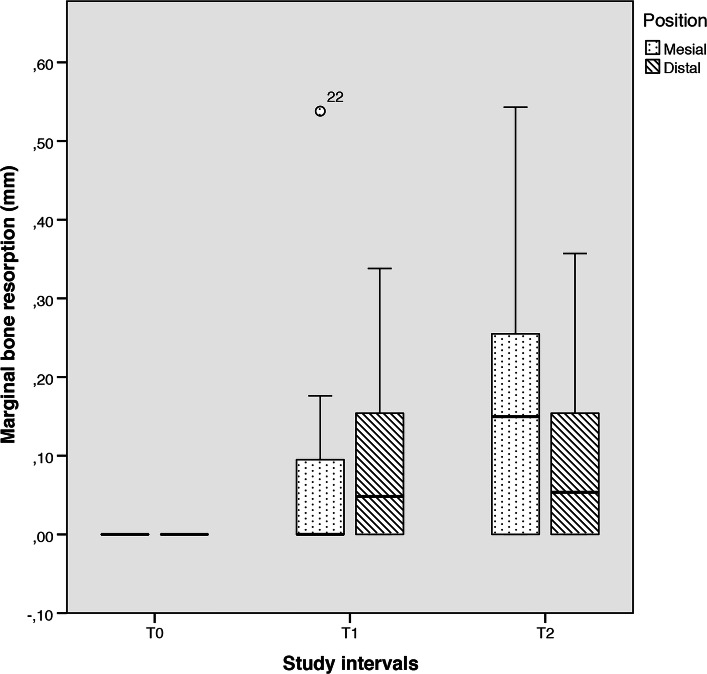
With respect to the intra-group comparisons, no differences were observed comparing the different study periods within each variable. Indeed, the comparison between T0, T1 and T2 was non-statistically significant at both mesial (F = 3.137; P = 0.06) and distal (F = 3.136; P = 0.06) aspects (Fig. 6).
Fig. 6.
Box plot illustrating the overall mesial and distal MBR according to each study period
After 5 years, the peri-implant soft tissues appeared clinically healthy and well-integrated within the surrounding tissues. Soft tissue volumes and colors were more than adequate resulting in a good aesthetic as shown in Figs. 7 and 8.
Fig. 7.
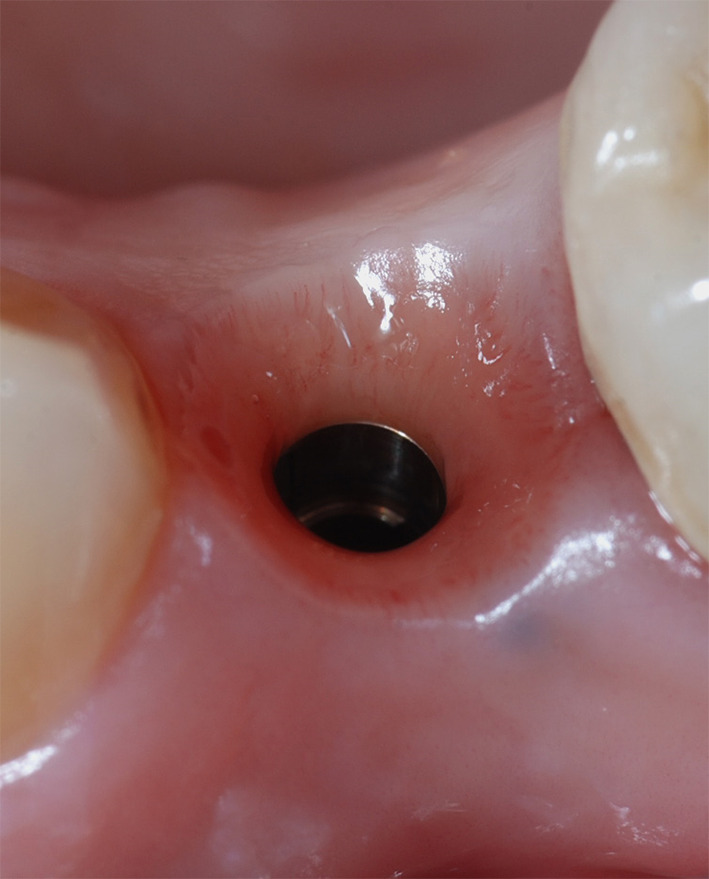
Transmucosal portion of the soft tissues after 5 years of maturation following implant insertion
Fig. 8.
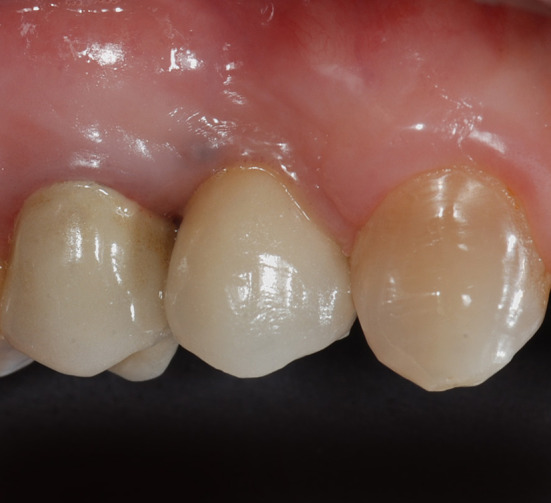
Buccal aspect of the final restoration after 5 years following implant insertion
Discussion
Preservation of alveolar sockets is a surgical technique developed to preserve hard and soft tissue volumes after dental extraction for implant placement purposes. Even if socket intervention therapies might reduce the dimensional remodeling of the alveolus, resorption cannot be prevented or stopped in any case [19]. This has been clearly pointed out in a previous study that investigated histologically the bone healing in sites treated with ASP procedures at 6 months after tooth extraction [18]. The sockets were grafted with DBBM and covered with a bioabsorbable porcine-derived CM. Radiographically, the dimensions of the grafted sockets remained nearly stable, as highlighted by a mean resorption in height and width of 0.46 mm and 1.21 mm, respectively [18]. This was consistent with a similar trial where ASP was obtained with DBBM and bioabsorbable collagen membranes. The authors observed how the original ridge dimensions were maintained up to 92.74% after a healing period of 4 months [20]. Similarly, a recent retrospective analysis recorded a volume loss of 9.9% when the ASP was performed with DBBM covered by a resorbable collagen barrier and left to heal for 6 months [1]. Several reviews already clarified how the horizontal bone loss is, in general, more pronounced compared to the vertical remodeling [21, 22]. It can be thus concluded that ASP procedures may reduce horizontal and vertical ridge alterations compared to non-grafted sockets [23]. This finding was confirmed by a recent meta-analysis showing how ASP could preserve approximately 1.31 mm to 1.54 mm of bone width and 0.95 mm to 1.12 mm of bone height at 6 months [8]. In particular, if compared to spontaneous healing, ASP by means of DBBM and CM showed significantly less reduction in ridge width (4.48 ± 0.65 mm versus 1.04 ± 1.08 mm, respectively) and height (1.54 ± 0.33 mm versus 0.46 ± 0.46 mm, respectively) [20]. Not only the bucco-lingual/palatal dimension, but even the mean vertical ridge resorption is generally more pronounced in the control sockets compared to preserved sites [24]. Therefore, it is safe to assume that fresh extraction sockets filled with DBBM fared better in terms of buccal plate resorption than non-grafted sockets [25].
The management of the buccal plate is of paramount importance in consideration of the aesthetic outcome of the implant therapy. A facial bone thickness of less than 1 mm has been related to a greater vertical bone loss [26]. According to this, grafting the socket with an osteoconductive biomaterial would reduce the loss of the buccal plate and the resulting drawbacks in implant treatment. Several clinical results have been validated by histological and histomorphometric analyses [1, 3, 6, 27].
According to Cardaropoli et al. [20], ASP enabled the maintenance of most of the original ridge dimensions and allowed implant placement without the need for bone augmentation. This might be related to the use of a low resorption rate bone substitute that enables the volumetric preservation of the grafted site and promotes hard tissue formation by acting as a scaffold with osteoconductive characteristics.
The preservation of the hard tissue must be accompanied by the correct management of the overlying soft tissues to obtain optimal aesthetic outcomes. To reduce patient morbidity at donor site in case of soft tissue autografts, collagen matrices of porcine origin have been introduced as soft tissue substitutes. Such resorbable matrices aim to increase the width of keratinized gingiva with the results comparable to those obtained with connective tissue grafts, but with a significantly lower patient morbidity [10, 28].
Furthermore, collagen matrices showed accelerated wound healing compared to spontaneous healing, providing at the same time better color matching and less wound sensitivity [11].
All of these considerations concerning preservation of hard and soft tissue volumes were validated in the present cohort of patient with positive outcomes [18]. Healing of the sockets following ridge preservation occurred without complications, and the exposed portions of the membranes were slowly replaced by mature keratinized tissue in two postoperative months. The re-epithelialization of the graft was also improved by de-epithelializing the soft tissue walls of the socket to favor the nourishment and revascularization of the CM.
Hence, the present study aimed to evaluate the stability of mesial and distal peri-implant marginal bone levels up to 5 years from implant placement in preserved sockets. The results coming from the comparison between T0 and T2 showed an average bone loss of 0.15 ± 0.17 mm and 0.11 ± 0.14 mm at the mesial and distal aspects, respectively. An increased bone loss was observed between implant placement and 12 months, stabilizing thereafter up to 5 years, with no statistically significant differences between the different study periods. It must be noted at this point, the evaluation of ASP outcomes with follow-up longer than 12 months is extremely lacking in the literature. Marconcini and coworkers in a 4-year evaluation observed survival and success rates of 100%, defining the success as radiographic bone loss lower than 1.5 mm during both the first year of function and the following follow-up periods [29]. Interestingly, a recent meta-analysis concluded that survival and success rates and marginal bone changes around dental implants placed in preserved sites were similar to that of implants placed in untreated sockets [30]. However, only trials with a follow-up equal or shorter than 12 months were examined. Wey et al. evaluated three patients who underwent implant placement following ASP in molar sockets with advanced periodontitis [31]. In the radiological evaluation, they observed an average marginal bone loss of 0.43 mm (range 0.25–0.6 mm) at 1-year post-loading and up to 0.51 mm (range 0.33–0.75 mm) after a follow-up period of 30 months. These values, even if still comparable, are slightly higher than those obtained in the present study. The reduced sample and the advanced periodontitis status of the patients at the recruitment might explain the variability of these outcomes.
Conclusions
From the results obtained in the present study, it can be concluded that ASP with DBBM and CM is able to maintain stable peri-implant marginal bone levels up to 5 years from implant insertion.
Author Contributions
CM and MB conceived the ideas. MM collected the data. PPP and MM analyzed the data. MB and FS led the writing. PPP and RV led the review. All authors gave final approval of the manuscript and agree to be accountable for all aspects of the work.
Funding
None reported.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are exposed in Table 1. Pictures are, however, available from the corresponding author upon reasonable request.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
The study was approved by the local institutional review board, and it was conducted according to the principles articulated in the Helsinki Declaration of 1975 for biomedical research involving human subjects, as revised in 2000. All patients were informed about the nature of the study and gave their written consent.
Consent for Publication
Consent to publish was obtained from the participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Araujo MG, Liljenberg B, Lindhe J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: an experimental study in the dog. Clin Oral Implants Res. 2010;21:55–64. doi: 10.1111/j.1600-0501.2009.01854.x. [DOI] [PubMed] [Google Scholar]
- 2.Sbordone C, Toti P, Martuscelli R, Guidetti F, Ramaglia L, Sbordone L. Retrospective volume analysis of bone remodeling after tooth extraction with and without deproteinized bovine bone mineral insertion. Clin Oral Implants Res. 2015;27:1152–1159. doi: 10.1111/clr.12712. [DOI] [PubMed] [Google Scholar]
- 3.Lindhe J, Cecchinato D, Donati M, Tomasi C, Liljenberg B. Ridge preservation with the use of deproteinized bovine bone mineral. Clin Oral Implants Res. 2014;25:786–790. doi: 10.1111/clr.12170. [DOI] [PubMed] [Google Scholar]
- 4.Cardaropoli D, Tamagnone L, Roffredo A, Gaveglio L. Relationship between the buccal bone plate thickness and the healing of postextraction sockets with/without ridge preservation. Int J Periodontics Restor Dent. 2014;34:211–217. doi: 10.11607/prd.1885. [DOI] [PubMed] [Google Scholar]
- 5.Becker W, Clokie C, Sennerby L, Urist MR, Becker BE. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol. 1998;69:414–421. doi: 10.1902/jop.1998.69.4.414. [DOI] [PubMed] [Google Scholar]
- 6.Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003;14:137–143. doi: 10.1034/j.1600-0501.2003.140201.x. [DOI] [PubMed] [Google Scholar]
- 7.Sisti A, Canullo L, Mottola MP, Covani U, Barone A, Botticelli D. Clinical evaluation of a ridge augmentation procedure for the severely resorbed alveolar socket: multicenter randomized controlled trial, preliminary results. Clin Oral Implants Res. 2012;23:526–535. doi: 10.1111/j.1600-0501.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- 8.Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res. 2014;93:950–958. doi: 10.1177/0022034514541127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghanaati S, Schlee M, Webber MJ, Willershausen I, Barbeck M, Balic E, et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater. 2011;6:015010. doi: 10.1088/1748-6041/6/1/015010. [DOI] [PubMed] [Google Scholar]
- 10.Sanz M, Lorenzo R, Aranda JJ, Martin C, Orsini M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: a randomized prospective clinical trial. J Clin Periodontol. 2009;36:868–876. doi: 10.1111/j.1600-051X.2009.01460.x. [DOI] [PubMed] [Google Scholar]
- 11.Thoma DS, Sancho-Puchades M, Ettlin DA, Hammerle CH, Jung RE. Impact of a collagen matrix on early healing, aesthetics and patient morbidity in oral mucosal wounds—a randomized study in humans. J Clin Periodontol. 2012;39:157–165. doi: 10.1111/j.1600-051X.2011.01823.x. [DOI] [PubMed] [Google Scholar]
- 12.Jung RE, Hurzeler MB, Thoma DS, Khraisat A, Hammerle CH. Local tolerance and efficiency of two prototype collagen matrices to increase the width of keratinized tissue. J Clin Periodontol. 2011;38:173–179. doi: 10.1111/j.1600-051X.2010.01640.x. [DOI] [PubMed] [Google Scholar]
- 13.Parashis AO, Hawley CE, Stark PC, Ganguly R, Hanley JB, Steffensen B. Prospective clinical and radiographic study of alveolar ridge preservation combining freeze-dried bone allograft with two xenogeneic collagen matrices. J Periodontol. 2016;87:416–425. doi: 10.1902/jop.2016.150500. [DOI] [PubMed] [Google Scholar]
- 14.Iocca O, Farcomeni A, Pardinas Lopez S, Talib HS. Alveolar ridge preservation after tooth extraction: a Bayesian Network meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. J Clin Periodontol. 2017;44:104–114. doi: 10.1111/jcpe.12633. [DOI] [PubMed] [Google Scholar]
- 15.Willenbacher M, Al-Nawas B, Berres M, Kammerer PW, Schiegnitz E. The effects of alveolar ridge preservation: a meta-analysis. Clin Implant Dent Relat Res. 2016;18:1248–1268. doi: 10.1111/cid.12364. [DOI] [PubMed] [Google Scholar]
- 16.Apostolopoulos P, Darby I. Retrospective success and survival rates of dental implants placed after a ridge preservation procedure. Clin Oral Implant Res. 2017;28:461–468. doi: 10.1111/clr.12820. [DOI] [PubMed] [Google Scholar]
- 17.Cosyn J, Pollaris L, Van der Linden F, De Bruyn H. Minimally Invasive Single Implant Treatment (MISIT) based on ridge preservation and contour augmentation in patients with a high aesthetic risk profile: 1-year results. J Clin Periodontol. 2015;42:398–405. doi: 10.1111/jcpe.12384. [DOI] [PubMed] [Google Scholar]
- 18.Maiorana C, Poli PP, Deflorian M, Testori T, Mandelli F, Nagursky H, et al. Alveolar socket preservation with demineralised bovine bone mineral and a collagen matrix. J Periodontal Implant Sci. 2017;47:194–210. doi: 10.5051/jpis.2017.47.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morjaria KR, Wilson R, Palmer RM. Bone healing after tooth extraction with or without an intervention: a systematic review of randomized controlled trials. Clin Implant Dent Relat Res. 2014;16:1–20. doi: 10.1111/j.1708-8208.2012.00450.x. [DOI] [PubMed] [Google Scholar]
- 20.Cardaropoli D, Tamagnone L, Roffredo A, Gaveglio L, Cardaropoli G. Socket preservation using bovine bone mineral and collagen membrane: a randomized controlled clinical trial with histologic analysis. Int J Periodontics Restorat Dent. 2012;32:421–430. [PubMed] [Google Scholar]
- 21.Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23(Suppl 5):1–21. doi: 10.1111/j.1600-0501.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- 22.Darby I, Chen ST, Buser D. Ridge preservation techniques for implant therapy. Int J Oral Maxillofac Implants. 2009;24(Suppl):260–271. [PubMed] [Google Scholar]
- 23.Troiano G, Zhurakivska K, Lo Muzio L, Laino L, Cicciu M, Lo RL. Combination of bone graft and resorbable membrane for alveolar ridge preservation: a systematic review, meta-analysis, and trial sequential analysis. J Periodontol. 2018;89:46–57. doi: 10.1002/JPER.17-0576. [DOI] [PubMed] [Google Scholar]
- 24.Festa VM, Addabbo F, Laino L, Femiano F, Rullo R. Porcine-derived xenograft combined with a soft cortical membrane versus extraction alone for implant site development: a clinical study in humans. Clin Implant Dent Relat Res. 2013;15:707–713. doi: 10.1111/j.1708-8208.2011.00398.x. [DOI] [PubMed] [Google Scholar]
- 25.Nevins M, Camelo M, De Paoli S, Friedland B, Schenk RK, Parma-Benfenati S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorat Dent. 2006;26:19–29. [PubMed] [Google Scholar]
- 26.Chappuis V, Engel O, Reyes M, Shahim K, Nolte LP, Buser D. Ridge alterations post-extraction in the esthetic zone: a 3D analysis with CBCT. J Dent Res. 2013;92:195s–201s. doi: 10.1177/0022034513506713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallman M, Lundgren S, Sennerby L. Histologic analysis of clinical biopsies taken 6 months and 3 years after maxillary sinus floor augmentation with 80% bovine hydroxyapatite and 20% autogenous bone mixed with fibrin glue. Clin Implant Dent Relat Res. 2001;3:87–96. doi: 10.1111/j.1708-8208.2001.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 28.Meloni SM, Tallarico M, Lolli FM, Deledda A, Pisano M, Jovanovic SA. Postextraction socket preservation using epithelial connective tissue graft vs porcine collagen matrix. 1-year results of a randomised controlled trial. Eur J Oral Implantol. 2015;8:39–48. [PubMed] [Google Scholar]
- 29.Marconcini S, Giammarinaro E, Derchi G, Alfonsi F, Covani U, Barone A. Clinical outcomes of implants placed in ridge-preserved versus nonpreserved sites: a 4-year randomized clinical trial. Clin Implant Dent Relat Res. 2018;20:906–914. doi: 10.1111/cid.12682. [DOI] [PubMed] [Google Scholar]
- 30.Mardas N, Chadha V, Donos N. Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: a randomized, controlled clinical trial. Clin Oral Implants Res. 2010;21:688–698. doi: 10.1111/j.1600-0501.2010.01918.x. [DOI] [PubMed] [Google Scholar]
- 31.Yiping Wei XB, Hu WJ, Yalin Z, Yunsong L, Kwok-Hung C. Evaluation of dental implants following ridge preservation in molar extraction sockets affected by advanced periodontitis: a 30-month postloading case series. Dentistry. 2018;8:8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are exposed in Table 1. Pictures are, however, available from the corresponding author upon reasonable request.