Abstract
Late-life depression is common and often inadequately managed using existing therapies. Depression is also associated with increased markers of inflammation, suggesting a potential role for anti-inflammatory agents. ASPREE-D is a sub-study of ASPREE, a large multi-centre, population-based, double-blind, placebo-controlled trial of aspirin vs placebo in older Australian and American adults (median follow-up: 4.7 years) of whom 1,879 were depressed at baseline. Participants were given 100mg daily dose of aspirin or placebo. Depressive symptoms were assessed annually using the validated, self-rated short version of the Center for Epidemiological Studies Depression (CES-D-10) scale. There was a significant increase in depressive scores (0.6; 95% CI 0.2 to 0.9; Chi-square (1) = 10.37; p = 0.001) and a decreased score in the mental health component of a quality of life scale (−0.7; 95% CI −1.4 to −0.1; Chi-square (1) =4.74; p = 0.029) in the aspirin group compared to the placebo group. These effects were greater in the first year of follow-up and persisted throughout the study, albeit with small to very small effect sizes. This study failed to demonstrate any benefit of aspirin in the long-term course of depression in this community-dwelling sample of older adults over a 5-year period, and identified an adverse effect of aspirin in the course of depression in those with pre-existing depressive symptoms.
Keywords: Immunology, Antidepressants, Biomarkers, Aspirin, Inflammation, Risk, Prevention, Depression, Psychiatry, Mental Health
One Sentence Summary:
Contrary to hypotheses, this study demonstrates for the first time that aspirin might have an adverse effect on the mood of older adults with pre-existing depression.
Introduction
Finding effective strategies to address the rising burden of depression in later life might significantly improve outcomes and Health Related Quality of Life (HRQoL) in a progressively ageing global population. Compared to depression in other age groups, late-life depression has a more chronic course, poorer prognosis, more refractory symptoms and an increased chance of relapse (1). Furthermore, older individuals are more prone to develop adverse effects of commonly prescribed antidepressants and manifest suboptimal responses to available treatments (2). Recently, the American Geriatrics Society reclassified some classes of antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, as potentially inappropriate medications for older adults because of associated risks, regardless if used as antidepressants. This highlights the pressing need to find new therapeutic strategies for depression in this population (3).
Strong evidence exists for a bidirectional association between inflammation and depression in later life (4). Therefore, suppression of inflammation might be a useful strategy to treat depression in this age group (5). While a recent meta-analysis found evidence for a beneficial effect of several anti-inflammatory drugs as adjunct or monotherapy options for depression (6), few studies addressed the role of these drugs for depression in later life. Preclinical, pharmacoepidemiologic and pilot clinical trial data suggest that aspirin, a drug with anti-inflammatory properties, may have therapeutic potential for depression (7,8), negative data notwithstanding (9). Aspirin is a non-steroidal anti-inflammatory agent that acts as an irreversible inhibitor of both cyclooxygenase (COX)-1 and COX-2. Through a wide range of mechanisms, aspirin may lower levels of inflammatory biomarkers previously associated with depression (8). Aspirin has shown positive signals in animal models of depression (10) and as an adjunctive agent for the treatment of major depressive episodes in unipolar and bipolar depression (11,12).
Aspirin for the Prevention of Depression in the Elderly (ASPREE-D) is a sub-study of the Aspirin in Reducing Events in the Elderly (ASPREE) randomised controlled trial (ClinicalTrials.gov Identifier: NCT01038583) (13). ASPREE-D aimed to explore the effects of low dose aspirin for the prevention and treatment of depression in later life (5). We previously published data suggesting that low-dose aspirin was not effective in preventing depression in the elderly (14) and now report a pre-specified outcome, the long-term effects of aspirin among older individuals presenting with depressive symptoms at baseline. The primary hypothesis was that low dose aspirin in those with pre-existing depression, as measured by a validated depression scale, will reduce the severity of depressive symptoms over time when compared to placebo.
Material and Methods
Study design and participants
This sub-study forms part of a large multi-centre, population-based, double-blind, placebo-controlled trial investigating the effects of aspirin on several endpoints in older adults living in Australia and in the US. The ASPREE trial recruited 19,114 participants between 2010 and 2014 from primary care services in Australia and through clinic-based mailing lists, electronic records and advertisements in the United States, as detailed elsewhere (13). ASPREE-D is a pre-specified sub-study of the ASPREE main trial, whose design has been published in detail previously (5).
The study included community-dwelling men and women aged 70 years and older (65 years of age and older for US minorities) who were willing and able to provide informed consent. Participants were excluded if they had a current indication for, or contraindication to the use of aspirin, or had any component of the composite primary outcomes. The exclusion criteria were a previous cardiovascular event or established cardiovascular disease or atrial fibrillation; dementia or a score of <78 on the Modified Mini-Mental State examination; the presence of significant disability (defined by severe difficulty or inability to perform any one of the Katz activities of daily living); a condition with a high current or recurrent risk of bleeding; anaemia; a condition likely to cause death within 5 years; current use of other antiplatelet or antithrombotic medication, current use of aspirin for secondary prevention, or severe uncontrolled hypertension (i.e. systolic blood pressure (SBP) of ≥ 180mmHg or a diastolic blood pressure (DBP) of ≥ 105mmHg). To investigate the possible role of low dose aspirin on the treatment and long term management of depression, this study only included participants who met the threshold for the definition of depression (CES-D ≥8) at baseline (n = 1,879; 9.8%) using the Center for Epidemiological Studies Depression (CES-D-10) scale.
The trial was conducted according to the Australian National Statement on Ethical Conduct in Human Research, the Australian Code for the Responsible Conduct of Research, the 2008 Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice E6 and was approved by institutional review boards at all sites. The protocol was published (5), developed in accordance with Standard Protocol Items Recommendations for Intervention Trials (SPIRIT) 2013 guidelines, reported using the Consolidated Standards of Reporting Trials (CONSORT) guidelines and according to the ICH E9 Statistical Principles for Clinical Trials and was registered on ClinicalTrials.gov (Identifier: NCT01038583).
Randomisation and masking
Eligible participants for the ASPREE main trial underwent a one-month placebo run-in phase and those with an adherence of 80% or greater were randomised to receive either 100 mg of enteric coated aspirin (n = 9,525) or an enteric coated placebo (n = 9,589), of identical appearance. Randomisation of trial medications followed a block randomisation procedure with a ratio of 1:1 and was stratified by site and age (65–79 and >80 years). For this sub-study, participants already randomised according to the ASPREE main trial were selected based on their depressive status prior to randomisation (CES-D-10 ≥ 8). Study participants, investigators and general practitioner co-investigators were blind to group allocation.
Procedures
Depressive symptoms were assessed using the short version of the Centre for Epidemiological Studies Depression (CES-D-10) scale. The CES-D-10 is a self-reported questionnaire that scores the severity of depressive symptoms “during the past week”. Self-report questionnaires avoid the possibility of observer bias associated with clinical outcomes. This instrument has shown comparable performance to the full version of the CES-D (k = 0.97) in classifying participants with depressive symptoms (15). Construct validity of the CES-D-10 showed that a single score was a reliable and valid measure of depression in an older population (16). When compared to a formal psychiatric diagnosis of depression in old age, the scale demonstrated a sensitivity of 97% and a specificity of 84% (17). In line with previous research, a cut-off of ≥ 8 was operationalised as positive screen for depression for ASPREE (18). The CES-D-10 was initially administered at baseline, and at years one, three, five and seven. Upon receipt of further funding (NHMRC GNT1081901), CES-D-10 annual assessments were added to years two, four and six. Eligible participants were classified as depressed at baseline (i.e. CES-D-10 ≥8) and were assessed for depression annually until the end of their participation in the study.
The Quality of Life Short Form 12 (SF-12) questionnaire was used to rate HRQoL and was administered annually. Baseline HRQoL of ASPREE participants was slightly higher than that reported in population-based studies of older individuals (19). The mental component summary score (MCS) of this scale was a secondary outcome measure of ASPREE-D.
Outcomes
The primary outcome of this study was between-group difference in the CES-D-10 score across annual follow-ups.
Secondary outcomes included between-group difference in the MCS scores of the SF-12 scale over time, persistence/recurrence rates in both groups (defined as a CES-D ≥ 8 in any follow-up visit), as well as rates of hospitalisation due to depression during the study period. The use of dichotomised depressive symptoms using higher thresholds (i.e. CES-D-10 ≥ 10; ≥ 12) was also a pre-defined secondary outcome.
Statistical Analysis
The primary analysis compared the aspirin and placebo groups regarding between-group difference in CES-D-10 score at annual follow-up visits. Treatment effects for the repeated CES-D-10 outcomes were determined via a population average model. The model was calculated using the generalised estimating equation (GEE) approach with Gaussian distribution and identity link function. Following a modified intention to treat approach, all participants with at least one valid follow-up measure were included in the study. The GEE model accounted for within-individual repeated measures using an exchangeable working correlation matrix, and a robust sandwich variance estimator was used. The model contained the fixed effect of intervention allocation and nominal values of measurement time points (annual visits) as main effects and baseline CES-D-10 scores as a covariate. Similar analytical approaches were used for the SF-12 mental component outcome, sub-group analyses and mediator and moderator analyses. Cohen’s d effect size for differential change between groups was reported with effects being interpreted as small (0.20 – 0.49), medium (0.50 – 0.79), or large (≥ 0.80) (20). Treatment effects for the repeated binary CES-D-10 outcomes were determined via population average models using the GEE approach with logit link function. The two-way interaction between intervention allocation and annual visit was tested in additional models. The intervention allocation estimates the average effect of aspirin across all annual visits in the first model and the two-way interaction estimates the effect of aspirin at each annual visit in the later model.
Cox proportional-hazards models were used to compare persistence/recurrence rates (defined as another CES-D-10 ≥ 8) at annual follow-ups between the aspirin group and the placebo group. Hazard ratios and 95% CI were reported.
Logistic regression was used to compare the overall proportion of hospitalisation due to depression during the study period in both treatment groups.
Mediators and moderators of treatment effects was evaluated by testing the two-way interactions between group allocation and potential modifying/ moderating factor GEE models. The GEE models contained the fixed effect of intervention allocation and nominal values of measurement time points (annual visits) as main effects as well as baseline CES-D-10 scores and potential modifying/moderating variables in order to evaluate interactions between treatment allocation or time points with potential modifying/moderating variable.
All tests of treatment effects were two-sided and conducted using an alpha level of 0.05. Confidence intervals and p values of secondary outcomes were not adjusted for multiple comparisons due to exploratory nature of these comparisons. Cohen’s d effect sizes were calculated for the primary and all secondary comparisons and were taken into account in interpretation in order to mitigate false discovery rate from multiple comparisons. All analyses were performed using Stata software, version 15.0. (StataCorp. 2017. College Station, TX: StataCorp LLC).
Results
Study Sample
A total of 19,114 participants were recruited into the ASPREE main trial. The trial was terminated after a median follow-up of 4.7 years after determination of futility regarding the primary endpoints and a significant increased risk of bleeding in the aspirin group (21,22). Adherence to treatment was similar between groups (22). From the entire ASPREE cohort, 1,879 (9.8%) participants presented with a CES-D-10 score ≥ 8 at baseline and were therefore included in this sub-study. Of these 1,760 had at least one valid CES-D-10 score at annual follow-up and were included in the study following a modified intention to treat approach. Eligible participants were allocated within the ASPREE main trial to receive either aspirin (n = 925; 49.2%) or placebo (n = 954; 50.8%). The CONSORT flowchart is available in Supplement Figure 1.
Details of participants’ demographic and baseline characteristics are presented in Table 1. The mean (±SD) age was 75.3 (4.8) in the aspirin and 75.1 (4.6) in the placebo groups respectively. There were 595 (47.7) females in the aspirin group and 653 (52.3) in the placebo group. Most participants were community dwelling, white/Caucasian, English-speaking, and non-smoking (Table 1). Baseline characteristics of these participants have been described in detail elsewhere (18). Although the distribution of participants characteristics were similar across study groups in the ASPREE trial, participants in this sub-group with high depressive symptom scores at baseline were more likely to be women, living at home alone, ethnic minorities, current smokers, and had increased rates of self-reported history of depression.
Table 1.
Demographic Characteristics of Participants with a CES-D ≥ 8 at baseline.
| Demographic categories | Aspirin, n = 925 n (%)1 |
Placebo, n = 954 n (%)1 |
|---|---|---|
| Age, M (SD) | 75.3 (4.8) | 75.1 (4.6) |
| Weight, M (SD) | 77.6 (16.6) | 76.6 (15.8) |
| BMI, M (SD) | 28.8 (5.4) | 28.6 (5.3) |
| Gender: Female | 595 (47.7) | 653 (52.3) |
| Country | ||
| Australia | 795 (49.8) | 800 (50.2) |
| United States | 130 (45.8) | 154 (54.2) |
| Living status | ||
| At home alone | 387 (48.3) | 414 (51.7) |
| At home with family, friends or a spouse | 529 (49.8) | 534 (50.24) |
| In a residential home2 | 9 (60.0) | 6 (40.0) |
| Ethnicity | ||
| Hispanic or Latino | 25 (39.1) | 39 (60.9) |
| Race | ||
| White/ Caucasian | 832 (49.3) | 855 (50.7) |
| Black/ African American | 66 (50.4) | 65 (49.6) |
| Other | 27 (44.3) | 34 (55.7) |
| Language | ||
| English | 885 (49.3) | 912 (50.7) |
| Born overseas | ||
| Yes | 215 (52.4) | 195 (47.6) |
| Education | ||
| > 12 years | 339 (46.6) | 388 (53.4) |
| Smoking status | ||
| Current | 54 (49.1) | 56 (50.9) |
| Former | 389 (51.5) | 367 (48.5) |
| Never | 482 (47.6) | 531 (52.4) |
| Alcohol drinking | ||
| Current | 664 (48.6) | 701 (51.4) |
| Former | 78 (50.3) | 77 (49.7) |
| Never | 183 (51.0) | 176 (49.0) |
| Antidepressant use at baseline | ||
| 233 (25.2) | 223 (23.4) | |
| Depression history 3 | ||
| Unsure | 8 (40.0) | 12 (60.0) |
| No | 238 (52.4) | 216 (47.6) |
| Yes | 202 (49.5) | 206 (50.5) |
M: Mean, SD: Standard deviation;
supervised care or assisted living;
the question of a history of depression was only asked after June 2013 (n=882).
The mean CES-D-10 score at baseline was 10.6 (± 3.2) in the aspirin and 10.6 (± 3.1) in the placebo group. Rates of previous depressive episodes were similar between groups. A total of 456 participants were taking an antidepressant medication at baseline (25.2% in the aspirin and 23.4% in the placebo arms) and 662 participants had an antidepressant prescription at any of the follow-up annual visits (36.7% in the aspirin arm and 33.9% in the placebo arm).
Primary outcome: continuous CES-D-10 scores
In total, 7,483 annual CES-D-10 measurements were conducted across the study period, with a mean of 4.0 measurements per participant. The overall effect of aspirin across all follow-up years on the continuous CES-D-10 scores was 0.6 (95% CI 0.2 to 0.9; Chi-square (1) =10.37; p = 0.001), illustrating a significant increase in depression scores in the aspirin group. The mean CES-D-10 scores in the aspirin group were higher at all annual follow-ups compared to the placebo group (Table 2). The between-group differential changes from baseline (aspirin vs placebo) ranged from 0.4 to 0.8 (Cohen’s d effect size 0.07 to 0.15) illustrating small differences in favour of the placebo group. Interestingly, the largest difference emerged in the first year and there was no group by time interaction effect, meaning that the effect persisted and did not increase throughout annual follow-ups.
Table 2.
CES-D-10 and Mental Health Component score comparison in Aspirin and Placebo groups: a) all participants (primary comparison)
| n | Aspirin Mean (SD) | Placebo Mean (SD) | Differential change# (95%CI) | Effect size (Cohen’s D) | |
|---|---|---|---|---|---|
| a) CES-D-10 | |||||
| Baseline | 1,879 | 10.6 (3.2) | 10.6 (3.1) | ||
| Year 1 | 1,734 | 9.0 (5.0) | 8.2 (4.8) | 0.8 (0.3, 1.3) | 0.15 |
| Year 2 | 729 | 8.3 (5.2) | 7.9 (4.8) | 0.3 (−0.3, 1.0) | 0.09 |
| Year 3 | 1,404 | 8.6 (5.2) | 8.2 (4.8) | 0.4 (−0.1, 0.9) | 0.06 |
| Year 4 | 932 | 8.8 (5.3) | 8.1 (5.0) | 0.6 (0.0, 1.2) | 0.08 |
| Year 5 | 611 | 8.8 (5.1) | 8.3 (4.7) | 0.5 (−0.2, 1.2) | 0.07 |
| Year 6 | 194 | 8.8 (4.6) | 8.0 (4.4) | 0.8 (−0.3, 1.9) | 0.20 |
| b) Mental Health Component SF-12 | |||||
| Baseline | 1,879 | 48.1 (9.5) | 47.9 (9.3) | ||
| Year 1 | 1,732 | 48.6 (10.0) | 49.3 (8.9) | −0.9 (−1.8, 0.0) | −0.03 |
| Year 2 | 1,641 | 49.6 (9.7) | 49.5 (9.2) | 0.0 (−1.0, 0.9) | −0.00 |
| Year 3 | 1,398 | 48.7 (10.2) | 50.2 (9.0) | −1.5 (−2.6, −0.5) | −0.01 |
| Year 4 | 1,002 | 49.1 (9.9) | 49.9 (9.2) | −0.6 (−1.8, 0.6) | −0.2 |
| Year 5 | 607 | 47.8 (9.7) | 49.7 (8.8) | −1.3 (−2.7, 0.0) | −0.04 |
| Year 6 | 607 | 47.8 (9.7) | 49.7 (8.8) | −0.2 (−2.4, 2.0) | −0.07 |
Note:
Differential change from baseline at follow-ups (aspirin vs placebo) was estimated from two-way interaction of intervention allocation and follow-up annual visit from a GEE model that includes fixed effect of group allocation and follow-up time point and their two-way interactions; reference groups: baseline measurement and placebo group.
Secondary outcomes
SF-12 Mental Component Summary (MCS)
The mean SF-12 MCS score at baseline was 48.1 (± 9.5) in the aspirin, and 47.9 (± 9.3) in the placebo group. The overall effect of aspirin across all follow-up years was −0·7 (95% CI −1.4 to −0.1; Chi-square (1) = 4.74; p = 0.029), showing a significant decline. The between-group differential changes from baseline showed a uniform greater decline in the aspirin group as compared to placebo (Supplement Table 1), although Cohen’s d effect sizes illustrated small differences (Cohen’s d effect size −0.00 to −0.20).
Dichotomised CES-D-10 scores
Beyond inclusion criteria (i.e. CES-D-10 ≥8), two other a priori cut-off points were considered for dichotomising depressive symptoms (i.e. CES-D-10 ≥ 10; and ≥ 12). A total of 954 individuals had a CES-D-10 ≥10 at baseline, with 470 (49.3%) in the aspirin and 484 (50.7%) in the placebo group, and 524 participants had a CES-D-10 ≥ 12, with 263 in the aspirin and (50.2%) and 261 (49.8%) in the placebo group. The overall effect of aspirin (compared to placebo) on CES-D-10 ≥ 8 outcome across all follow-ups was OR (odds ratio) = 1.15 (95% CI 1.00 to 1.32; Chi-square (1) = 3.78; p = 0.05). For the CES-D-10 ≥10 outcome, the OR was 1.31 (95% CI 1.07 to 1.61; Chi-square (1) = 6.84; p < 0.01), and for CES-D-10 ≥12 outcome the OR was 1.51 (95% CI 1.15 to 1.99; Chi-square (1) = 8.71; p < 0.01). The results for the overall effect of aspirin on CES-D-10 across all follow-ups from above models showed a moderately large positive relationship between aspirin intake and higher CES-D-10 rates. Model-based marginal prevalence differences between CES-D-10 dichotomised outcomes (aspirin vs placebo adjusted for baseline CES-D-10) at follow-up years were notably higher in the aspirin group (Table 3). The prevalence differences ranged from 1.0% to 6.5% for the CES-D-10 ≥ 8 outcome, −1.3% to 18.0% for CES-D-10 ≥ 10; and −4.5% to 12.7% for CES-D-10 ≥ 12 illustrating a relatively large absolute elevation in the prevalence of depressive symptoms in those taking aspirin. Again, the largest differences in prevalence emerged in the first year of follow-up and were subsequently maintained for all defined outcomes (Table 3).
Table 3.
Comparison of depressive syndromes (CES-D-10) prevalence between aspirin and placebo groups each year of follow-up.
| n | Aspirin n (%) | Placebo n (%) | Intervention effect# (95%CI) | |
|---|---|---|---|---|
| CES-D-10 ≥ 8 1 | ||||
| Baseline | 1,879 | 925 (49.2) | 954 (50.8) | |
| Year 1 | 1,734 | 483 (56.4) | 439 (50.0) | 6.5 (1.8, 11.2) |
| Year 2 | 729 | 198 (51.7) | 171 (49.4) | 1.7 (−5.1, 8.5) |
| Year 3 | 1,404 | 360 (52.2) | 369 (51.6) | 1.0 (−4.2, 6.1) |
| Year 4 | 932 | 227 (52.1) | 241 (48.6) | 3.8 (−2.4, 10.0) |
| Year 5 | 611 | 154 (53.9) | 171 (52.6) | 1.1 (−6.4, 8.5) |
| Year 6 | 194 | 51 (58.6) | 53 (49.5) | 5.1 (−7.6, 17.8) |
| CES-D-10 ≥ 10 2 | ||||
| Baseline | 954 | 470 (49.3) | 484 (50.7) | |
| Year 1 | 868 | 226 (52.3) | 185 (42.4) | 10.1 (3.5, 16.7) |
| Year 2 | 369 | 86 (44.6) | 79 (44.9) | −1.3 (−10.8, 8.1) |
| Year 3 | 687 | 164 (47.0) | 141 (41.7) | 4.9 (−2.3, 12.2) |
| Year 4 | 458 | 109 (48.9) | 100 (42.6) | 6.2 (−2.5, 14.8) |
| Year 5 | 290 | 72 (50.7) | 64 (43.2) | 9.6 (−1.4, 20.6) |
| Year 6 | 93 | 22 (55.0) | 23 (43.4) | 18.0 (−0.8, 36.9) |
| CES-D-10 ≥ 12 3 | ||||
| Baseline | 524 | 263 (50.2) | 261 (49.8) | |
| Year 1 | 474 | 113 (46.9) | 87 (37.3) | 12.7 (3.8, 21.6) |
| Year 2 | 200 | 43 (40.2) | 36 (38.7) | −4.5 (−17.4, 8.4) |
| Year 3 | 366 | 82 (42.7) | 52 (29.9) | 11.6 (15.9, 21.5) |
| Year 4 | 243 | 63 (50.4) | 40 (33.9) | 9.8 (−2.1, 21.7) |
| Year 5 | 146 | 27 (39.1) | 28 (36.4) | 11.2 (−4.5, 27.0) |
| Year 6 | 44 | 7 (43.8) | 11 (39.3) | 5.4 (24.4, 35.1) |
P-value and (test statistic) for joint test of intervention effect across visit years: Chi-square 8·74, df 6, P=0·189.
P-value and (test statistic) for joint test of intervention effect across visit years: Chi-square 14·23, df 6, P=0·027.
P-value and (test statistic) for joint test of intervention effect across visit years: Chi-square 13·84, df 6, P=0·032.
Aspirin effect: Model-based marginal prevalence difference (aspirin vs placebo) at follow-up years; estimated form a GEE with binary outcome and logistic link including treatment allocation and follow-up visit as factor and two-way interaction between treatment allocation and follow-up visit.
Depressive symptoms recurrence/persistence rate (CES-D-10 ≥ 8)
A total of 681 participants in the aspirin group (337 events per 1000 person-years) and 657 in the placebo group (291 events per 1000 person-years) had another CES-D-10 ≥ 8 score at an annual follow-up, suggesting recurrence or persistence of depressive symptoms. The between-group difference was significant (hazard ratio, 1.15; 95% CI, 1.03 to 1.27; p = 0.012) (Figure 1).
Figure 1:
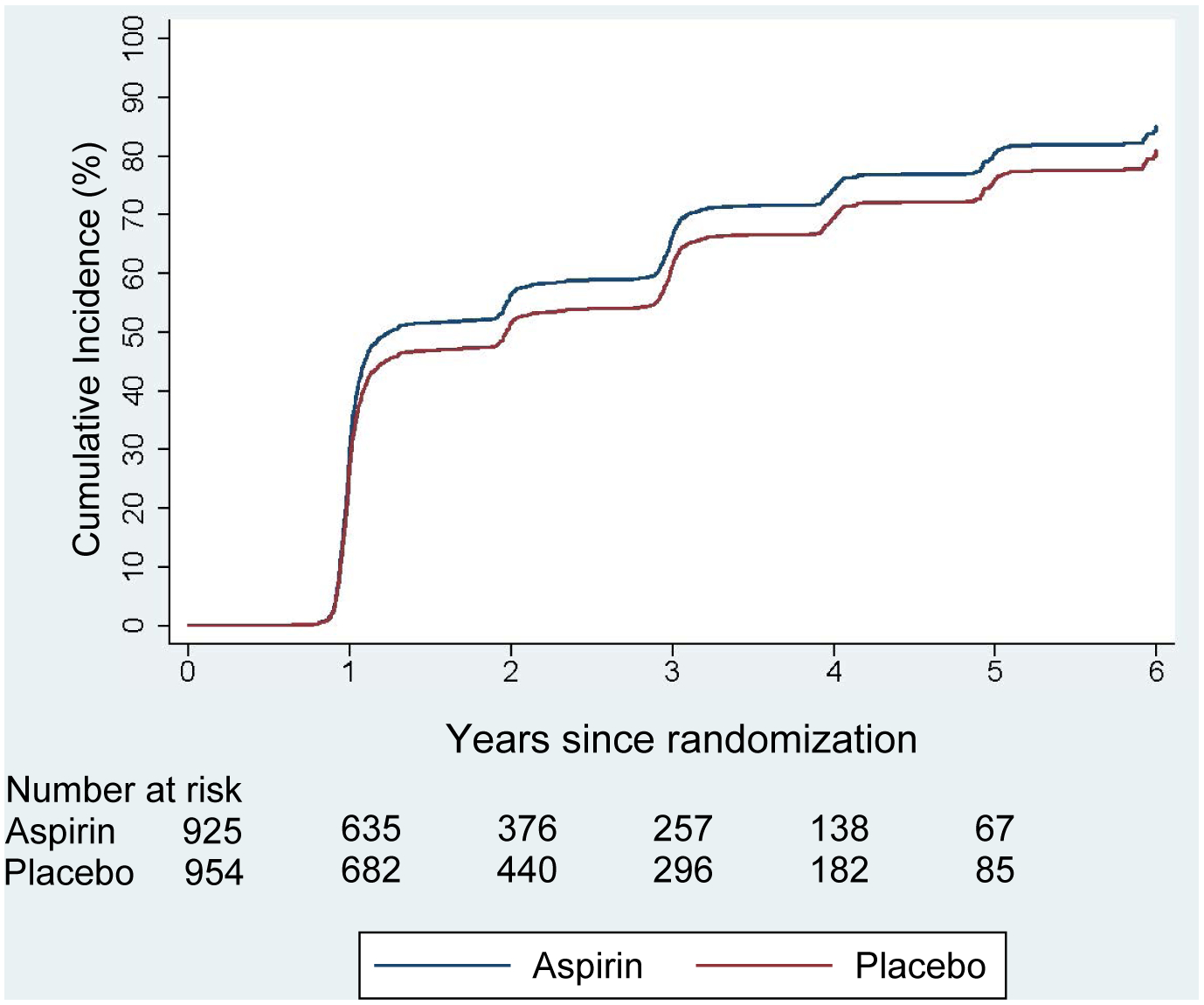
Cumulative rates of Persistence/ Recurrence of depression according to another CES-D-10 ≥ 8 score at yearly follow-ups
Hospitalisation due to depression
Twelve participants were hospitalised for depression during the study period, of whom 6 (0.65%) were on aspirin and 6 (0.63%) on placebo (OR = 1.03, 95% CI, 0.27 to 3.87).
Sub-group analyses
The impact of gender, age (≤75, 75–85, and ≥85 years), smoking (current, former, and never), alcohol consumption (current, former, and never), abdominal circumference (dichotomised factor), and BMI (≤25, 25–35, ≥35 kg/m²) were examined through separate GEE models. There was no significant effect modification for any of the above-mentioned factors. Figure 2 illustrates no evidence that any of these factors modified the link between aspirin intake and CES-D-10 at follow-ups.
Figure 2:

Sub-group analyses - Main effect of investigated effect modifications on continuous CES-D-10 outcome
Antidepressant use at baseline
Effect of antidepressant use as an effect modifier was investigated through further sub-group analyses. In those individuals taking an antidepressant at baseline, aspirin intake was associated with a significant increase in mean CES-D-10 scores. The overall effect of aspirin across all follow-up years was 1.1 units. (95% CI 0.3 to 1.8; Chi-square (1) = 7.61; p < 0.01). The differential change from baseline was higher across all annual visits (except for year 4) in the aspirin group with effect sizes ranging from 0.16 to 0.59, illustrating small to moderate effect sizes (Supplement Table 1). Similarly, in people with no history of antidepressant use, aspirin intake was associated with higher mean CES-D-10 scores across all annual visits (Table 2), although the difference was not statistically significant. Overall, the effect of aspirin was 0.4 (95% CI 0.0 to 0.8; Chi-square (1) = 3.82; p = 0.05) with effect sizes ranging from 0.01 to 0.14 illustrating very small to small effect sizes.
A possible causal pathway for the joint effect of aspirin and antidepressants on CES-D-10 scores was investigated by testing the two-way interaction between time-updating antidepressant intake (i.e. potential mediator) and aspirin use. The two-way interaction showed that antidepressant use was independent from the effect of aspirin on CES-D-10; mediation effect: −0.03 (95% CI −0.75 to 0.71; Chi-square (1) = 0.00; p = 0.945). Effect of antidepressant use at baseline (i.e. time invariant) as an effect modifier was investigated by testing the two-way interaction between antidepressant intake at baseline and aspirin use. The two-way interaction was not statistically significant; effect modification: 0.5 (95% CI −0.3 to 1.4; Chi-square (1) = 1.51; p = 0.220).
Discussion
Contrary to the primary hypothesis of this study, not only did aspirin not improve depressive symptoms in this population, this data suggests it might have an adverse effect on the long-term course of depression in this population. This primary finding was supported by the secondary pre-specified outcomes, showing a negative effect of aspirin on the mental component of a validated HRQoL score, as well as in those with more severe depressive symptoms. These findings suggest that low dose aspirin is unlikely to be effective in the treatment of late-life depression and should raise awareness about aspirin’s potential adverse mood effects among older adults with pre-existing depressive symptoms. Interestingly, the largest effect of aspirin on mood was seen in the first year of follow-up and in individuals with more severe depressive scores.
The growing appreciation of a potential central role for inflammation in the pathogenesis and treatment of depression provided a new therapeutic avenue for repurposed drugs that target different components of this intricate system. Antidepressants have immunomodulatory properties and a growing body of evidence suggests that anti-inflammatory strategies might have a role in the treatment of depression in other age groups (6,23). However, few studies focused on geriatric populations and the only trial (to our knowledge) that investigated the mood effects of anti-inflammatory drugs in an older population was the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) (24). In this population of more than 2,000 older adults, with sociodemographic characteristics very similar to those in ASPREE (median age 74.5 years), the use of celecoxib (200mg, 2x/day) or naproxen (220mg, 2x/day) showed no benefit over placebo on depressive symptoms, concordant with a recent trial of vitamin D for depression prevention (VITAL-DEP) (25) and our own null findings of aspirin and vitamin D for the prevention of depression in the elderly (14,26). Use of an otherwise healthy sample makes it difficult to extrapolate results for the general older population, a limitation shared by the ASPREE study. However, the relatively small sample, short follow-up period (2 years) and the multiple groups design (decreasing statistical power) employed in the ADAPT trial might have hindered their ability to detect small differences. In secondary analyses, individuals classified as depressed at baseline (using a Geriatric Depression Scale score > 5) (n = 449) showed no significant improvement in the course of depressive symptoms in either treatment group. This result from the ADAPT trial contrasts with our findings of worsening depressive scores in the aspirin group, which were more pronounced in the first year of follow-up and were maintained across the study.
There are several potential explanations as to why aspirin may worsen depression in this specific older population with pre-existing depressive symptoms. In other age groups, the effects of anti-inflammatory strategies may be dependent on levels of inflammatory biomarkers. In a proof of concept study of omega-3 fatty acids in major depression, higher levels of inflammatory markers seemed to predict response to treatment when compared to placebo, where low levels of inflammation were associated with worsening of depressive symptoms (27). Another study of infliximab for treatment-resistant depression found that the benefits of treatment were only seen in those with elevated levels of inflammatory markers at baseline, while individuals with low levels of inflammation did worse than those treated with placebo (28). When the same drug was used in a sample of clinically depressed bipolar patients, selected on the basis of a proxy clinical “inflammatory phenotype”, infliximab had no statistically significant effect on depressive symptoms when compared to placebo (29). Taken together, these studies provide mixed evidence that anti-inflammatory strategies might have an iatrogenic effect on the mood of individuals without baseline inflammation (30). Because ours is a relatively healthier sample than the general population, due to stringent exclusion criteria, and due to the fact that not all depression in later life is necessarily connected to inflammation, we might speculate that this is a heterogeneous group in terms of levels of inflammatory biomarkers. This might also explain the small, although significant, effect sizes we found in this study.
In sub-group analyses, aspirin’s effect on mood was greater in those taking an antidepressant medication at baseline. This might reflect antidepressants as a marker of a longer duration and/or severity of disease (prompting recognition and treatment), but it may also imply pharmacological interactions between aspirin and antidepressant drugs. Recently available capsule endoscopic evidence suggests a high and previously underestimated frequency of small bowel lesions associated with aspirin, with numbers estimated around 80% among chronic users. Antidepressants have synergistic antiplatelet effects with aspirin and are associated with increased risk of bleeding in aspirin users (31). Therefore, it is reasonable to believe that their combination might increase the frequency of small-bowel lesions, with the potential to alter intestinal permeability, increase oxidative stress and alter the gut microenvironment – pathological changes previously associated with depression (32,33).
Another possible mechanism for the depressogenic effect of aspirin is through its effects on the arachidonic acid pathway. Arachidonic acid is implicated in mood disorders, and elevated levels of this fatty-acid (relative to other fatty-acids) have been linked to depression (34). A recent neuroimaging study suggests a direct effect of arachidonic acid on brain serotonin transporters, which in turn correlated with depression severity (35). Therefore, by inhibiting arachidonic acid metabolism, aspirin might interfere with serotonin systems involved in mood regulation. A recent study in younger adults with depression corroborates this hypothesis, with numerical (albeit not significant) worsening of depressive symptoms in the aspirin treated group compared to placebo (36).
Several methodological characteristics of this study must be considered when interpreting these results. It is strengthened by its size and representativeness: ASPREE is one of the largest and best-characterised sample of community-dwelling older adults ever involved in a randomised controlled trial, which allowed us to investigate pre-specified outcomes implementing well-powered statistical models. The use of a well-validated instrument for depression assessment and a comprehensive evaluation of participants is another strength. The consistency of results from the different scales and outcomes employed provide further support for a real effect. Recruiting mainly from primary care (where most cases of late-life depression are treated) also provided a more naturalistic setting. The extended follow-up period is also one of the longest seen in trials conducted in an older population.
There are also some limitations. First, notwithstanding the fact that the CES-D-10 is a validated tool for depression screening, it is not akin to a formal diagnosis of depression. Nevertheless, the recognised impact of subthreshold depressive symptoms in old age makes this a valuable and reliable instrument for the purpose of this study (16,37) The use of higher thresholds in CES-D-10 as sensitivity analyses also increases specificity of the diagnosis (at the expense of sensitivity and statistical power). The use of annual follow-ups is another limitation, since it misses mood changes that might happen between those periods. For this reason, we refer to the cumulative incidence seen as persistence/recurrence, since they are indistinguishable with this design. The dose and duration of aspirin is another issue, since it is not clear whether low dose aspirin can uniformly decrease peripheral levels of inflammation. That we do not yet have biomarkers to test this and other biological hypotheses is another limitation. Aspirin differs mechanistically from other anti-inflammatory agents, and this data does not preclude efficacy of mechanistically diverse agents. The generalisability of our findings must also be considered. Population cohorts, especially in terms of depression, may differ meaningfully from clinical cohorts. Since the ASPREE study excluded subjects with severe life-limiting diseases, disability, dementia, uncontrolled hypertension, and individuals with a history of heart disease, these sub-groups were not addressed in this study. These are groups where the benefits of aspirin might surpass its potential risks. Because our sample is composed mainly of white older adults who may be generally healthier than their counterparts, generalisability of these results are limited to this specific population. Lastly, although older individuals tend to have higher levels of inflammation, and one might expect that anti-inflammatory strategies might be more useful in this population, one cannot generalise these findings to younger cohorts.
In conclusion, our finding that aspirin may have an adverse effect in older individuals with depressive symptoms has potential implications for therapeutic and preventive strategies in aged care. Mental health outcomes need to be considered when prescribing for an older population and the mood effects of individual drugs must be weighed against potential benefits in other health domains. Not only does this study not support the use of aspirin in otherwise healthy older adults with depression, the results of this trial point to an adverse effect of aspirin in this population, although the mechanisms driving this effect remain uncertain.
Supplementary Material
Acknowledgments
Funding
This paper was supported by a grant (U01AG029824) from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants (1081901, 334047 and 1127060) from the National Health and Medical Research Council of Australia. MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072).
Role of Funding Source
The study sponsors had no role in study design, the collection, analysis, interpretation of data, the writing of the report or the decision to submit the paper for publication.
Footnotes
Trial registration: ClinicalTrials.gov Identifier: NCT01038583.
Competing Interests:
MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. PBF has received equipment for research from MagVenture A/S, Medtronic Ltd, Neuronetics and Brainsway Ltd and funding for research from Neuronetics. He is on scientific advisory boards for Bionomics Ltd and LivaNova and is a founder of TMS Clinics Australia: all unrelated to this work. MRN received travel and advisory board support from Bayer AG who provided product for the ASPREE study.
Data and materials availability
The individual participant data that underlie the results reported in this article will be made available to others, after de-identification. Requests for data access will be via the ASPREE Principal Investigators with details for applications provided through the web site, www.ASPREE.org, and in accord with the NIH policy on data sharing, details available at https://grants.nih.gov/grants/policy/data_sharing. Data availability will commence on publication of this article. The supporting Protocol and Statistical Analysis Plan is already available as an independently published article. The supporting documents will be available at NEJM.org. This data will be available to investigators whose proposed use of the data has been approved by a review committee in order to achieve the aims in the approved proposal. This data will be available through a web-based data portal safe haven, based at Monash University, Australia.
References
- 1.Kok RM, Reynolds CF. Management of depression in older adults: A review. JAMA - J Am Med Assoc. 2017;317(20):2114–22. [DOI] [PubMed] [Google Scholar]
- 2.Sobieraj DM, Martinez BK, Hernandez AV, Coleman CI, Ross JS, Berg KM, et al. Adverse Effects of Pharmacologic Treatments of Major Depression in Older Adults. 2019;1–11. [PubMed] [Google Scholar]
- 3.Fick DM, Semla TP, Steinman M, Beizer J, Brandt N, Dombrowski R, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria®for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–94. [DOI] [PubMed] [Google Scholar]
- 4.Smith KJ. The association between C-reactive protein , Interleukin-6 and depression among older adults in the community : A systematic review and meta- analysis. Exp Gerontol. 2018;102(December 2017):109–32. [DOI] [PubMed] [Google Scholar]
- 5.Berk M, Woods RL, Nelson MR, Shah RC, Reid CM, Storey E, et al. ASPREE-D: Aspirin for the prevention of depression in the elderly. Int Psychogeriatrics. 2016;28(10):1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köhler-Forsberg O N. Lydholm C, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404–19. [DOI] [PubMed] [Google Scholar]
- 7.Kessing LV, Rytgaard HC, Gerds TA, Berk M, Ekstrøm CT, Andersen PK. New drug candidates for depression – a nationwide population-based study. Acta Psychiatr Scand. 2019;139(1):68–77. [DOI] [PubMed] [Google Scholar]
- 8.Berk M, Dean O, Drexhage H, McNeil JJ, Moylan S, O’Neil A, et al. Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med. 2013;11(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molero P, Ruiz-Estigarribia L, Lahortiga-Ramos F, Sánchez-Villegas A, Bes-Rastrollo M, Escobar-González M, et al. Use of non-steroidal anti-inflammatory drugs, aspirin and the risk of depression: The “Seguimiento Universidad de Navarra (SUN)” cohort. J Affect Disord. 2019;247(January):161–7. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Yang F, Liu YF, Gao F, Jiang W. Acetylsalicylic acid as an augmentation agent in fluoxetine treatment resistant depressive rats. Neurosci Lett. 2011;499(2):74–9. [DOI] [PubMed] [Google Scholar]
- 11.Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, et al. Treatment of bipolar depression with minocycline and/or aspirin: An adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sepehrmanesh Z, Fahimi H, Akasheh G, Davoudi M, Gilasi H, Ghaderi A. The effects of combined sertraline and aspirin therapy on depression severity among patients with major depressive disorder: A randomized clinical trial. Electron Physician. 2017;9(11):5770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): A randomized, controlled trial. Contemp Clin Trials. 2013;36(2):555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berk M, Berk M, Berk M, Berk M, Woods RL, Nelson MR, et al. Effect of Aspirin vs Placebo on the Prevention of Depression in Older People: A Randomized Clinical Trial. JAMA Psychiatry. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang K-F, Weng L-J. Screening for depressive symptoms among older adults in Taiwan: Cutoff of a short form of the Center for Epidemiologic Studies Depression Scale. Health (Irvine Calif). 2013;05(03):588–94. [Google Scholar]
- 16.Mohebbi M, Nguyen V, McNeil JJ, Woods RL, Nelson MR, Shah RC, et al. Psychometric properties of a short form of the Center for Epidemiologic Studies Depression (CES-D-10) scale for screening depressive symptoms in healthy community dwelling older adults. Gen Hosp Psychiatry. 2018;51:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin M, Artin KH, Oxman MN. Screening for Depression in the Older Adult. Arch Intern Med. 1999;159(15):1701. [DOI] [PubMed] [Google Scholar]
- 18.Mohebbi M, Agustini B, Woods RL, McNeil JJ, Nelson MR, Shah RC, et al. Prevalence of depressive symptoms and its associated factors among healthy community-dwelling older adults living in Australia and the United States. Int J Geriatr Psychiatry. 2019;34(8):1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stocks NP, González-Chica DA, Woods RL, Lockery JE, Wolfe RSJ, Murray AM, et al. Quality of Life for 19,114 participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study and their association with sociodemographic and modifiable lifestyle risk factors. Qual Life Res. 2019;28(4):935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J Statistical power analysis for the behavioral sciences 2nd edn. Hillsdale, NJ: Erlbaum Associates, Hillsdale; 1988. [Google Scholar]
- 21.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379(16):1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain Behav Immun. 2019;79(February):24–38. [DOI] [PubMed] [Google Scholar]
- 24.Fields C, Drye L, Vaidya V, Lyketsos C. Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: Findings from a randomized controlled trial. Am J Geriatr Psychiatry. 2012;20(6):505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okereke OI, Reynolds CF, Mischoulon D, Chang G, Vyas CM, Cook NR, et al. Effect of Long-term Vitamin D3Supplementation vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA - J Am Med Assoc. 2020;324(5):471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, et al. Annual high-dose vitamin D3 and mental well-being: Randomised controlled trial. Br J Psychiatry. 2011;198(5):357–64. [DOI] [PubMed] [Google Scholar]
- 27.Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: A proof-of-concept study. Mol Psychiatry. 2016;21(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. Arch Gen Psychiatry. 2013;70(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, et al. Efficacy of Adjunctive Infliximab vs Placebo in the Treatment of Adults With Bipolar I/II Depression: A Randomized Clinical Trial. JAMA psychiatry. 2019. May;76(8):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berk M, Walker AJ, Nierenberg AA. Biomarker-Guided Anti-inflammatory Therapies: From Promise to Reality Check. JAMA Psychiatry. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, Van Zyl LT, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: The Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) platelet substudy. Circulation. 2003;108(8):939–44. [DOI] [PubMed] [Google Scholar]
- 32.Handa O, Majima A, Onozawa Y, Horie H, Uehara Y, Fukui A, et al. The role of mitochondria-derived reactive oxygen species in the pathogenesis of non-steroidal anti-inflammatory drug-induced small intestinal injury. In: Free Radical Research. 2014. p. 1095–9. [DOI] [PubMed] [Google Scholar]
- 33.Burgueño JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol [Internet]. 2020;1–16. Available from: http://www.nature.com/articles/s41575-019-0261-4 [DOI] [PubMed] [Google Scholar]
- 34.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68(2):140–7. [DOI] [PubMed] [Google Scholar]
- 35.Gopaldas M, Zanderigo F, Zhan S, Ogden RT, Miller JM, Rubin-Falcone H, et al. Brain serotonin transporter binding, plasma arachidonic acid and depression severity: A positron emission tomography study of major depression. J Affect Disord. 2019;257(December 2018):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berk M, Mohebbi M, Dean OM, Cotton SM, Chanen AM, Dodd S, et al. Youth Depression Alleviation with Anti-inflammatory Agents (YoDA-A): a randomised clinical trial of rosuvastatin and aspirin. BMC Med. 2020;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


