Abstract
Objective:
Despite advances in echocardiography and hemodynamic monitoring, limited progress has been made to effectively quantify left ventricular function during cardiac surgery. Traditional measures including left ventricular ejection fraction (LVEF) and cardiac index remain dependent on loading conditions; more complex measures remain impractical in a dynamic surgical setting. However, the Smith-Madigan Inotropy Index (SMII) and potential-to-kinetic energy ratio (PKR) offer promise as measures calculable during cardiac surgery and potentially predictive of outcomes. Using echocardiographic and hemodynamic monitoring data, we aimed to calculate SMII and PKR values after cardiopulmonary bypass, and understand associations with postoperative outcomes, adjusting for previously identified risk factors.
Design:
Observational cohort study.
Setting:
Tertiary care academic hospital.
Patients:
189 elective adult cardiac surgical procedures from 2015 to 2016.
Intervention:
None.
Measurements and Main Results:
The primary outcome was postoperative mortality or organ system complication (stroke, prolonged ventilation, reintubation, cardiac arrest, acute kidney injury, new-onset atrial fibrillation). After adjustment, SMII <0.83 W/m2 independently predicted the primary outcome (adjusted odds ratio 2.19, 95% confidence interval 1.08–4.42) whereas PKR, LVEF, and cardiac index demonstrated no associations. When SMII and PKR were incorporated into a EuroSCORE II risk model, predictive performance improved (net reclassification index improvement 0.457, p = 0.001) whereas a model incorporating LVEF and cardiac index demonstrated no improvement (0.130, p = 0.318).
Conclusion:
We demonstrate that SMII, but not PKR, as a measure of cardiac function is associated with major complications. Our data may guide investigations of more suitable perioperative goal-directed therapies to reduce complications following cardiac surgery.
Within a cardiac surgical context, left ventricular ejection fraction (LVEF) and cardiac index provide simple, rapid means for approximating ventricular function; however, such methods are heavily dependent on loading conditions1–3 and routinely fail to characterize inotropic state.4 Conversely, alternative echocardiographic measures of LV function, which have gained traction in cardiology literature, have failed to penetrate the perioperative setting due to practicality: calculations either require concomitant findings (e.g. mitral regurgitation for dP/dt5), repeated measurements under varying preload conditions potentially harmful to the cardiac surgery patient,6,7 or precise images challenging to obtain in the acute perioperative setting8,9. Furthermore, echocardiography has inherent limitations to precisely modelling indices of myocardial contractility (e.g. end-systolic elastance, preload recruitable stroke work) derived invasively from LV pressure-volume relations.10
Beyond limitations inherent to widely used echocardiographic measures, relationships between LV function, inotropy, and postoperative outcomes remain unresolved. Potentially addressing these shortcomings is the Smith-Madigan inotropy index (SMII), a pragmatic echocardiographic and hemodynamic measure describing the total kinetic and potential cardiac energy expenditure during systole. The SMII has been previously described11 and is summarized in Figures 1 and 2. Additionally, a related measure derived from similar parameters is the potential to kinetic energy ratio (PKR), used to describe myocardial efficiency for yielding perfusion pressure versus blood flow.11 SMII and PKR represent underutilized but potentially useful measurements for describing hemodynamic derangements in a clinically actionable way; however their uptake into clinical practice has been limited, potentially due to a lack of studies demonstrating the utility of such measures for predicting adverse events, including postoperative complications following cardiac surgery. In this study of a cardiac surgical population, we aimed to calculate SMII and PKR as measures of inotropy and myocardial efficiency, respectively, and explore their independent associations with postoperative complications. We hypothesized that cases with favorable states of SMII and PKR are associated with improved outcomes compared to those with unfavorable states, when adjusted for previously identified risk factors. We secondarily explored the utility of SMII and PKR compared to traditional measures of cardiac function - LVEF and cardiac index - for improving existing cardiac surgery risk prediction models.
Figure 1:

Calculation of Smith-Madigan Inotropy Index and Potential:Kinetic Energy Ratio
Figure 2:

Measurement Methods for Parameters Required to Calculate Smith-Madigan Inotropy Index and Potential-to-Kinetic Energy Ratio
METHODS
Institutional Review Board approval was obtained for this retrospective study within our academic tertiary care medical center (Ann Arbor, MI; HUM00052066) and patient consent was waived. The study plan was registered within an internal research forum on November 2, 2016 prior to analysis and is available upon request.12 The Strengthening the Reporting of Observational Studies guidelines were followed throughout the conduct of this study.13
Patient Population
Adult patients (≥18 years) undergoing elective cardiac surgical procedures involving echocardiographic and pulmonary arterial catheter monitoring at a tertiary care medical center were eligible for inclusion. A sample of 189 cases was analyzed over a 14-month study period with intensive data collection from January 2015 to February 2016. Cases were included based on the availability of an experienced cardiac anesthesiology fellow as a supplementary clinical provider, dedicated to acquiring echocardiographic measurements simultaneously with cardiac index measurements. Cardiac surgical procedures included coronary artery bypass, valve replacement, and ascending aortic (excluding arch) procedures. For patients undergoing repeated cardiac surgical procedures during the study period, only the first (index) case was included. Exclusion criteria were American Society of Anesthesiologists physical status classification 5 or 6, emergent procedures, use of postoperative mechanical circulatory support, or cardiac surgery within 365 days (Supplementary Material 1). For all cases, complete post-cardiopulmonary bypass echocardiography examinations were performed and documented using templated electronic procedure notes.
Data Collection
We collected data from three sources: our institution’s local perioperative electronic clinical database as available within the Multicenter Perioperative Outcomes Group, our institution’s electronic health record (Epic Systems Corporation, Verona, WI, USA), and the Society for Thoracic Surgeons Adult Cardiac Surgery Database. Methods for data collection, validation, and extraction of data from each source are described elsewhere14,15 and utilized in multiple prior studies.16–19 Data quality were ensured using pre-specified definitions, validated by nurses with training in data definitions, and via manual review by the study team.
Clinical Care Processes
Anesthetic management was performed at the discretion of the attending anesthesiologist. Intraoperative hemodynamic management was guided by standard monitoring as well as invasive arterial catheter, central venous pressure, and pulmonary artery catheter monitors (as per routine institutional practice); cardiac function was assessed via echocardiography. Hemodynamic targets included a cardiac index >2.2 L/min/m2, mean arterial pressure >65 mmHg, mixed venous oxygen saturation >65%, and an echocardiographic assessment of post-bypass cardiac function compared to pre-bypass function. Echocardiographic assessments were performed using Philips iE-33 probes (Philips Healthcare ©, Andover, MA, USA); cardiac indices were calculated via pulmonary artery catheters (Arrow®, Morrisville, NC, USA) using the Fick thermodilution method. Postoperative management was at the discretion of the intensive care unit team, as based on local protocols and targeting goals discussed during handoff.
Exposure Variables
In addition to collecting data on traditional measures of cardiac function - including LVEF by visual estimation and cardiac index by pulmonary artery catheter measurement - we collected data on novel SMII and PKR values for each case. All values were calculated post-bypass during sternal closure as a reliable index time point indicative of completion of the critical portions of the surgical procedure. To facilitate real-time intraoperative calculation of SMII and PKR as described in Figures 1 and 2, we provide a calculation tool in Supplementary Material 2, with key components of the indices including: i) systolic flow time and mean velocity of aortic valve blood flow (measured via an aortic valve velocity-time integral), ii) cardiac index (measured via a pulmonary artery catheter), iii) heart rate, and iv) mean arterial pressure.
Outcomes
The primary outcome was a composite index of mortality or major organ system-based complication, selected a priori on the basis of availability within the electronic health record or Society of Thoracic Surgeons database, and having a biologically plausible relationship with the study exposure variables, SMII and PKR. Major organ system-based complication components included stroke, cardiac arrest, new-onset atrial fibrillation, prolonged postoperative ventilation >24 hours, reintubation, and acute kidney injury (Appendix 1). Secondary outcomes were postoperative ventilator duration, postoperative inotrope/vasopressor duration, and intensive care unit length of stay.
Covariate Data
For descriptive purposes as well as for risk adjustment, we collected perioperative characteristics as study covariates (Table 1). Using covariate data, we calculated EuroSCORE II values for all patients, as has been previously used for cardiac surgical risk adjustment.20,21 Additional a priori selected covariate data used for risk adjustment but not incorporated into a preoperative EuroSCORE II calculation included body mass index and cardiopulmonary bypass duration. Finally, beyond the preoperative measures of cardiac function including in the EuroSCORE-II calculation (preoperative LVEF and pulmonary artery systolic pressure), intraoperative post-bypass measures of cardiac function (LVEF and cardiac index) were also used for risk adjustment.
Table 1:
Study Cohort Characteristics and Bivariate Analyses
| Mortality or Major Organ System Dysfunction, n / Mean (% / SD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 81) | No (n = 108) | |||||||
| n / mean | % / SD | n / mean | % / SD | n / mean | % / SD | |||
| Age (yr) | 61 | 13 | 66 | 10 | 57 | 14 | <0.001 | |
| Height (cm) | 172 | 10 | 171 | 10 | 173 | 10 | 0.210 | |
| Weight (kg) | 90 | 21 | 89 | 22 | 91 | 20 | 0.598 | |
| Body-Mass Index (kg/m2) | 30.3 | 6.3 | 30.2 | 6.3 | 30.3 | 6.4 | 0.941 | |
| Female sex | 63 | 33 | 25 | 31 | 38 | 35 | 0.640 | |
| Multi-racial/Other | 7 | 3.7 | 6 | 5.6 | 1 | 1.2 | ||
| Patient Medical History | ||||||||
| 4 | 134 | 71 | 62 | 77 | 72 | 67 | ||
| Dialysis (Regardless of CC) | 4 | 2.1 | 2 | 2.5 | 2 | 1.9 | ||
| Extracardiac arteriopathy | 119 | 63 | 55 | 68 | 64 | 59 | 0.287 | |
| Poor mobility | 109 | 58 | 51 | 63 | 58 | 54 | 0.260 | |
| Previous cardiac surgery | 31 | 16 | 14 | 17 | 17 | 16 | 0.932 | |
| Chronic lung disease | 38 | 20 | 21 | 26 | 17 | 16 | 0.122 | |
| Active endocarditis | 5 | 2.6 | 2 | 2.5 | 3 | 2.8 | 0.999 | |
| Critical preoperative state | 2 | 1.1 | 1 | 1.2 | 1 | 0.9 | 0.999 | |
| Diabetes on insulin | 23 | 12 | 14 | 17 | 9 | 8.3 | 0.102 | |
| IV | 9 | 4.8 | 8 | 9.9 | 1 | 0.9 | ||
| CCS class 4 angina | 25 | 13 | 9 | 11 | 16 | 15 | 0.598 | |
| Very Poor (LVEF 20% or less) | 0 | 0.0 | 0 | 0.0 | 0 | 0 | ||
| Recent MI within 90 days | 9 | 4.8 | 2 | 2.5 | 7 | 6.5 | 0.305 | |
| Severe (PA systolic >55 mmHg) | 27 | 14 | 15 | 19 | 12 | 11 | ||
| Intraoperative Data | ||||||||
| 3 Procedures | 16 | 8.5 | 9 | 11 | 7 | 6.5 | ||
| Surgery on thoracic aorta | 60 | 32 | 25 | 31 | 35 | 32 | 0.946 | |
| Urgency Elective Urgent | 184 5 | 97 2.6 | 78 3 |
96 3.7 |
106 2 |
98 1.9 |
0.653 | |
| CPB Duration | 161 | 71 | 171 | 73 | 153 | 69 | 0.092 | |
| Perioperative Risk Summary | EuroSCORE II - Mortality Risk | 5.5 | 6.6 | 7.7 | 9.0 | 3.9 | 3.0 | <0.001 |
| Exposure Variables | Post-CPB SMII (W/m2) | 0.86 | 0.30 | 0.82 | 0.31 | 0.90 | 0.28 | 0.059 |
| Post-CPB PKR | 16.3 | 14.4 | 16.4 | 15.3 | 16.3 | 13.8 | 0.941 | |
| Post-CPB LVEF (%) | 55.2 | 8.7 | 53 | 11 | 57 | 7 | 0.019 | |
| Post-CPB Cardiac Index (L/min/m2) | 2.4 | 0.6 | 2.3 | 0.6 | 2.5 | 0.6 | 0.027 | |
ASA = American Society of Anesthesiologists, CABG = coronary artery bypass graft, CC = creatinine clearance, CCS = Canadian Cardiovascular Society, CPB = cardiopulmonary bypass, LVEF = left ventricular ejection fraction, MI = myocardial infarction, NYHA = New York Heart Association, PA = pulmonary artery, PKR = potential:kinetic energy ratio, SMII = Smith-Madigan Inotropy Index
Statistical Analysis
We performed all analyses in R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and calculated descriptive statistics for all variables. We graphically assessed normality of continuous variables via histograms and Q-Q plots. We next examined variables via univariate analysis, with categorical variable p-values calculated using Fisher’s exact or Pearson chi-square tests, and continuous variable p-values calculated using Student t--tests or Mann-Whitney U tests depending on normality. Using logistic regression and the ‘OptimalCutpoints’ package in R,22 we assessed best thresholds for SMII and PKR by selecting the values that simultaneously maximized the sensitivity and specificity of each covariate for predicting the primary outcome. We performed univariate linear regressions, with post-bypass SMII, PKR, cardiac index, LVEF as independent variables, to assess associations with the continuous outcomes.
We next analyzed independent associations between SMII, PKR (assessed as binary variables based on best thresholds to enable ease of interpretation and target levels for future goal-directed therapy investigations) and outcomes, risk-adjusted for EuroSCORE II components via multivariable logistic regression (primary outcome) and linear regression (continuous secondary outcomes). A threshold of p <0.05 denoted statistical significance. We additionally assessed for improvement in EuroSCORE II multivariable model discrimination with post-bypass SMII and PKR added as binary variables, using net reclassification indices and c-statistics. We compared this to multivariable model discrimination using EuroSCORE II and commonly used thresholds for traditional measures of cardiac function: cardiac index <2.0 L/min/m2 and LVEF <40%.23,24 Finally, we assessed the performance of a multivariable model including EuroSCORE II, exposure variables, and all additional covariates.
Sensitivity Analyses
Given the use of a composite primary outcome, we performed two sensitivity analyses with modified primary outcome definitions and analyzed via similar multivariable regressions. These sensitivity analyses were (i) the acute kidney injury component of the composite outcome restricted to severe stage only (Kidney Disease - Improving Global Outcomes Stage 3 acute kidney injury only) and (ii) new-onset postoperative atrial fibrillation removed from the composite outcome.
Power Analysis
Based upon primary outcome rates of 10% versus 30% in patients with favorable versus unfavorable post-bypass indices respectively, and a sampling ratio of 1, a study with a power of 0.80 to detect a statistically significant difference p <0.05 would require a sample size of 118 patients for analysis. Differences in outcome rates for power analysis were selected a priori based upon prior literature25–27 and clinical judgment of the study team.
RESULTS
Patient Population - Baseline Characteristics
Characteristics of the study cohort are detailed in Table 1. Complete data were available on all patients. The study population had a mean (standard deviation) age of 61 (13) years, 67% were men, and the mean (standard deviation) EuroSCORE II predicted risk of mortality was 5.5 (6.6)%. Common EuroSCORE II risk factors included pulmonary hypertension (72%), extracardiac arteriopathy (63%), and poor mobility (58%). Cardiac surgical procedures included isolated coronary artery bypass grafting (12%), single non-coronary artery bypass grafting (38%), and multiple procedures (50%). Among single non-coronary artery bypass grafting procedures, 50% were aortic valve repairs or replacements, 36% were aortic root and ascending aorta replacements, and 14% were mitral valve repairs or replacements. Among the patients undergoing multiple procedures, 32% were procedures on the aortic valve and ascending aorta, 27% were procedures on multiple valves, 26% were coronary artery bypass and valve procedures, 9% were valve and Maze procedures, 5% were coronary artery bypass and ascending aortic procedures, and 1% were coronary artery bypass, valve, and ascending aorta procedures.
Among traditional measures of cardiac function, the median LVEF observed on post-bypass intraoperative echocardiography was 55% (interquartile range 55–60%; full range 20–80%). The median post-bypass cardiac index was 2.3 L/min/m2 (interquartile range 2.0–2.8; range 1.2–5.0). For the novel measures of cardiac function, median post-bypass SMII and PKR were 0.84 W/m2 (interquartile range 0.63–1.02 W/m2; range 0.30–2.04 W/m2) and 12.3 (interquartile range 8.3–19.3; range 1.1–111) respectively. Each measure demonstrated rightward skew except LVEF (Figure 3).
Figure 3:
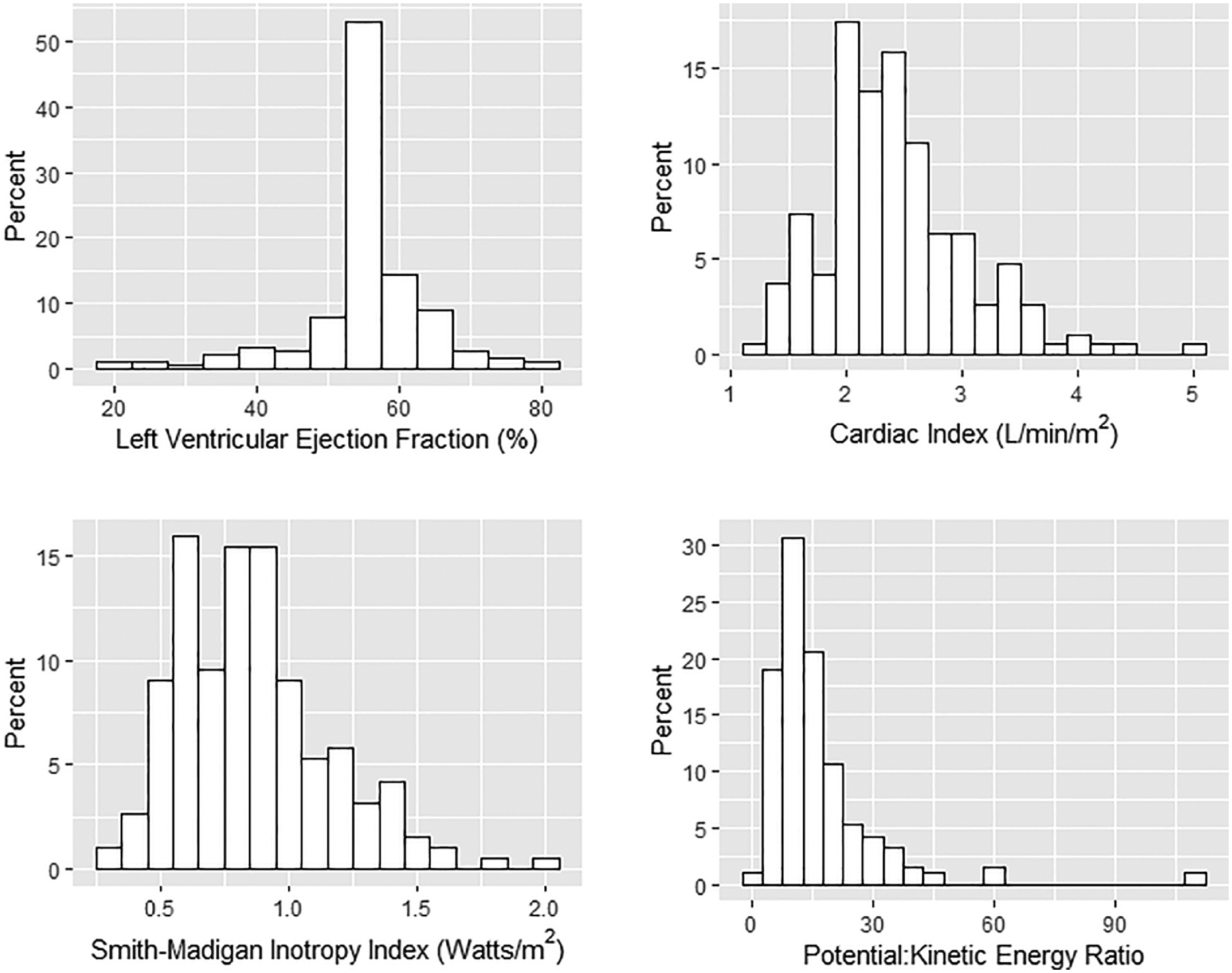
Post-Cardiopulmonary Bypass Measures of Cardiac Function: Left Ventricular Ejection Fraction, Cardiac Output, Smith-Madigan Inotropy Index, Potential:Kinetic Energy Ratio
Postoperative Outcomes
Among the 189 cardiac surgical patients studied, 81 (43%) experienced the primary outcome, including 3 (1.6%) mortalities. Individual components of the primary outcome are described in Table 2. Among secondary outcomes studied, 56% of patients required postoperative inotropic support and 49% of patients required postoperative vasopressor support. All except five patients received postoperative mechanical ventilation; the median postoperative ventilator duration was 6 hours (interquartile range 4–10 hours). The median intensive care unit length of stay was 49 hours (interquartile range 28–78 hours).
Table 2:
Study Outcomes
| n / median | % / IQR | ||
|---|---|---|---|
| Primary Outcome & Components | Composite index, mortality or major organ system dysfunction* | 81 | 43 |
| In-hospital all-cause mortality | 5 | 2.6 | |
| Neurologic - Stroke | 3 | 1.6 | |
| Pulmonary - Prolonged Ventilation >24 hours | 16 | 8.5 | |
| Pulmonary – Reintubation | 2 | 1.1 | |
| Cardiovascular - Cardiac Arrest | 2 | 1.1 | |
| Cardiovascular - New-onset Atrial Fibrillation | 60 | 32 | |
| Renal – Acute Kidney Injury (KDIGO definition)** | 32 | 17 | |
| Secondary Outcomes | Postoperative inotrope support duration (hours) | 6 | [0, 22] |
| Postoperative vasopressor support duration (hours) | 0 | [0, 13] | |
| Postoperative ventilator duration (hours) | 6 | [4, 10] | |
| ICU length of stay (hours) | 49 | [28, 78] |
KDIGO = Kidney Disease - Improving Global Outcomes
Comprised of all individual organ system dysfunction outcome components
Any stage, defined as a serum creatinine ≥1.5 times baseline with 7 days postoperative, or ≥0.3 mg/dL increase within 48 hours postoperative, among patients without pre-existing chronic renal failure (Kidney Disease - Improving Global Outcomes Stage 5). Among patients with acute kidney injury of any stage, 4 patients (2.1% of study cohort) developed KDIGO Stage 3 acute kidney injury, defined as serum creatinine ≥3.0 times baseline or need for renal replacement therapy within 7 days postoperative.
Optimal SMII and PKR Thresholds
Across unadjusted comparisons, patients who developed the primary outcome more commonly were older and had a history of heart failure or renal impairment (Table 1). Such patients more commonly exhibited reduced measures of post-bypass cardiac function, including LVEF, cardiac index, and SMII; however no association between PKR and postoperative mortality or complications was observed. Best thresholds maximizing the sum of univariate sensitivity and specificity were <0.83 W/m2 for SMII (sensitivity 62%, specificity 61%) and <12.6 for PKR (sensitivity 49%, specificity 51%).
Associations with Postoperative Mortality or Major Organ System Complication
Given a total of 81 patients with primary outcomes, all covariates were used in the primary multivariable analyses. Following multivariable adjustment, we observed that a post-bypass SMII <0.83 W/m2, but not PKR, LVEF, or cardiac index, was independently associated with complications (adjusted odds ratio 2.19, 95% confidence interval 1.08–4.42) (Table 3).
Table 3:
Risk-Adjusted Associations of Exposure Variables with Primary Outcome and Overall Model Performance
| Multivariable Model | Variables Included | Adjusted Odds Ratio | 95% CI | Net reclassification index versus EuroSCORE II | 95% CI | C-statistic | 95% CI |
|---|---|---|---|---|---|---|---|
| A | EuroSCORE II | 1.14 | (105, 1.22) | N/A | N/A | 0.641 | (0.56, 0.72) |
| B | EuroSCORE II | 1.13 | (104, 1.22) | 0.457 | (0.18, 0.74) | 0.673 | (0.59, 0.75) |
| SMII <0.83 W/m2 | 2.22 | (120, 4.11) | |||||
| PKR < 12.6 | 1.26 | (0.68, 2.35) | |||||
| C | EuroSCORE II | 1.12 | (104, 1.21) | 0.130 | (−0.13, 0.38) | 0.645 | (0.56, 0.73) |
| LVEF < 40% | 3.21 | (0.61, 16.81) | |||||
| Cardiac Index < 2.0 L/min/m2 | 1.38 | (0.68, 2.82) | |||||
| D | EuroSCORE II | 1.11 | (103, 1.20) | 0.228 | (−0.06, 0.51) | 0.699 | (0.62, 0.78) |
| SMII <0.83 W/m2 | 2.19 | (108, 4.42) | |||||
| PKR < 12.6 | 1.31 | (0.69, 2.48) | |||||
| LVEF < 40% | 3.62 | (0.68, 19.22) | |||||
| Cardiac Index < 2.0 L/min/m2 | 0.92 | (0.41, 2.10) | |||||
| Cardiopulmonary Bypass Duration | 1.00 | (0.96, 1.06) | |||||
| Body Mass Index | 1.01 | (100, 1.01) |
LVEF = left ventricular ejection fraction, SMII = Smith-Madigan Inotropy Index, PKR = Potential:Kinetic Energy Ratio
Exploring Risk Model Performance
In an exploratory multivariate analysis, when novel measures of cardiac function (SMII and PKR) were added to a risk model with EuroSCORE II components, more patients were correctly identified as having complications (net reclassification index improvement of 0.457, p = 0.001) but the overall discrimination of the model yielded a non-significant improvement (c-statistic 0.673 versus 0.641; p = 0.347). Conversely, when traditional measures of cardiac function (intraoperative LVEF and cardiac index) were added to a EuroSCORE II risk model, there was neither an improvement in identifying patients with complications (net reclassification index improvement of 0.130, p = 0.318) nor an improvement in overall model discrimination (c-statistic 0.645 versus 0.641; p = 0.806).
Sensitivity Analyses
Using a primary outcome definition restricting acute kidney injury complications to Stage 3 only, 69 patients developed the composite primary outcome, and SMII <0.83 W/m2 remained independently associated with complications (adjusted odds ratio 2.22, 95% confidence interval 1.20–4.11), whereas PKR, LVEF, and cardiac index continued to lack independent associations (Supplementary Material 3). A multivariable model incorporating SMII and PKR continued to improve model net reclassification index, whereas a model incorporating LVEF and cardiac index continued to lack improvement. When we excluded new-onset postoperative atrial fibrillation from the primary outcome definition, only 43 patients suffered an adverse outcome, and neither SMII nor any other measure of cardiac function was independently associated with complications; however a model including SMII and PKR continued to significantly improve net reclassification index whereas a model including LVEF and cardiac index continued to lack improvement (Supplementary Material 4).
Secondary Outcomes
Among univariate analyses of secondary outcomes, elevated values for both novel measures of cardiac function (SMII and PKR) and traditional measures (LVEF and cardiac index) were associated with shorter durations for all secondary outcomes (Supplementary Material 5).
Following multivariable adjustment, elevated intraoperative LVEF was associated with shorter postoperative inotrope duration as well as shorter intensive care unit length of stay, but not shorter postoperative vasopressor duration or ventilator duration (Supplementary Material 6). Within the same multivariable models, cardiac index was not independently associated with any secondary outcome. Conversely, among multivariable models incorporating SMII and PKR, we observed no independent associations between them and secondary outcomes (Supplementary Material 7).
DISCUSSION
In this observational study, we found that decreased intraoperative post-bypass SMII - but not PKR, LVEF, or cardiac index - was independently associated with postoperative complications. These data reveal potential shortcomings of traditional post-bypass measures of cardiac function, LVEF and cardiac index. These shortcomings may be in part addressed by the consideration of SMII, but not PKR, as a novel measure potentially calculable during cardiac surgery and informative for postoperative risk prediction.
Although the observed mortality rate of 1.6% was substantially less than that predicted by the EuroSCORE-II risk model (5.5%), the mortality rate was comparable to previous studies among similar cardiac surgical populations,28,29 as was the composite complication rate of 43%, primarily driven by new-onset postoperative atrial fibrillation (32%) and acute kidney injury (17%).29,30 In contrast to studies selecting more severe component complications to define a composite primary outcome, we selected component complications for which mechanistic relationships between poor cardiac function and outcomes were biologically plausible - including atrial fibrillation and acute kidney injury of any severity. For a composite outcome excluding new-onset postoperative atrial fibrillation and restricting acute kidney injury to severe stages only - as are commonly excluded from Society of Thoracic Surgeons database-defined major postoperative complications31 - we observed a complication rate of 11%, also comparable to previously reported rates of severe complications.28,29 The performance of prediction models based upon sensitivity analyses excluding these outcome components yielded similar findings to the primary analysis.
In the cardiac surgical cohort studied, the distribution of SMII values observed were similar to those previously reported in a SMII validation study which reliably differentiated healthy patients from patients with acute left ventricular failure presenting to the emergency department or coronary care intensive care unit (SMII mean 0.73 W/m2; range 0.43–0.97 W/m2).11 Conversely, the distribution of PKR values observed were in contrast to the higher values previously reported (PKR mean 124; range 96–174), suggestive of either differences in a cardiac surgical cohort exhibiting greater degrees of vasodilation potentially due to a systemic inflammatory response from the cardiac surgical physiologic insult, or bias in echocardiographic- and pulmonary artery catheter-derived measurements. Other recent studies have demonstrated the value of SMII and PKR for hemodynamic monitoring of neonates,32,33 patients with hypertensive disorders of pregnancy,34 and potentially intensive care unit patients.35 Within the context of cardiac surgery, determinants of SMII may include the use of inotropic and vasoactive medications, cardiovascular depressant anesthetic agents, myocardial stunning and the inflammatory response from exposure to cardiopulmonary bypass, and structural heart changes from the surgical intervention. Further studies are needed to understand the independent contributions of each intraoperative factor influencing SMII, and to validate these findings across similar cardiac surgery and acute care populations.
Among recent high-quality clinical trials investigating goal-directed therapies for hemodynamic management of cardiac surgical patients, parameters used have included central venous pressure, pulmonary artery pressure, and cardiac output,36–39 invasive arterial blood pressure,36–38,40 non-invasive cardiac output monitoring,36–39, central venous oxygen saturation,36–38,41 esophageal doppler,40,42 arterial lactate,38,41 hemoglobin/hematocrit,36,37,39 and urine output36,38. In such trials, inotropes were commonly used, and were specifically initiated and titrated based upon measures of cardiac index,36,37,39,41 central venous oxygen saturation,37 mean arterial pressure,40 stroke volume index,39,40 systemic vascular resistance,36 and pulmonary capillary wedge pressure41. Although such trials commonly used hemodynamic monitors permitting SMII to be calculated, the SMII has not yet been studied as a hemodynamic goal for goal-directed therapies. Goal directed therapies commonly demonstrated benefits during the post-bypass period in such cardiac surgical patient populations39–41 however it remains unclear as to whether the incorporation of SMII into therapeutic goals may lead to further improved outcomes.
Limitations
Our study has multiple limitations which must be considered in the context of generalizing findings to other populations or developing research protocols testing the potential efficacy of SMII as a parameter for goal-directed therapy. First, although the effects of preload and afterload are theoretically independent of SMII,11 this independence between loading conditions and SMII has not been verified using LV pressure-volume loops; further studies are needed to determine how SMII relates to contractility in the setting of manipulations to preload and afterload. Second, although the cohort studied represented a broad range of cardiac surgical procedures, this study is limited by performance at a single center across a limited sample size. Patient populations and practice patterns, such as management of inotropes, vasopressors, and fluids, may not reflect those encountered at other institutions. Third, this study remains observational and was analyzed within the context of clinical care rather than a controlled experimental setting. Intraoperative LVEF, cardiac index, SMII, PKR and were analyzed only during a single time point at sternal closure, and subject to a level of accuracy and precision available from echocardiographic and hemodynamic monitoring used in a clinical setting. Conversely however, our findings represent real-world health care data and reflect what information may be available and clinically actionable for providers at the point of care. Such an approach may be advantageous for informing pragmatic prospective trial design, including investigations of goal-directed therapies using SMII.
Additionally, components of the composite primary outcome may have varying clinical significance, strength of relationship, or effect size, in relation to the modifiable potential risk factors (traditional versus novel measures of cardiac function) studied. Furthermore, patient characteristics such as mitral valve disease and left atrial dilation27 influencing new-onset postoperative atrial fibrillation, the most frequently occurring component complication of the primary outcome, were unaccounted for due to lack of availability or limited sample size precluding a more comprehensive prediction model. Although primary outcome components were selected on the basis of biologically plausible mechanistic relationships with measures of cardiac function and examined via sensitivity analyses using modified component definitions, all potential long-term health consequences were not investigated. Future studies may wish to emphasize the risk of persistent organ dysfunction.43 However, even milder complications such as stage 1 acute kidney injury have been shown to be associated with increased risk of late mortality.44
Finally, whereas LVEF and cardiac index values were available to clinicians at the point of care and were potentially used to inform clinical-decision making, SMII and PKR values were calculated retrospectively. Associations with postoperative inotrope and vasopressor support were limited by LVEF and cardiac indices potentially serving as indications for such therapies rather than markers of true disease severity, and thus such associations must be interpreted with caution. Conversely, this bias did not exist for SMII and PKR values, which were not used to guide clinical care and thus not considered as an indicator for postoperative inotropic or vasopressor support. Across all cases, the indications for inotropic or vasopressor support upon which clinicians acted upon were not documented, and thus existed as a limitation for the secondary outcomes describing postoperative inotropic and vasopressor support.
Conclusions
In summary, we describe the SMII as an innovative measure of cardiac function, independently associated with major complications following cardiac surgery. SMII may offer greater utility for postoperative risk prediction than traditional intraoperative measures of cardiac function including LVEF and cardiac index, and remains practical to obtain intraoperatively by the multi-tasking cardiac anesthesiologist. Given the lack of measures accurately characterizing the inotropic state of patients undergoing cardiac surgery, our results may inform the design of future interventional studies further investigating the potential efficacy of SMII-based goal-directed inotropic therapies for cardiac surgical patients.
Supplementary Material
Acknowledgements:
The authors gratefully acknowledge Michelle Romanowski, M.S. (Department of Anesthesiology, University of Michigan Health System, Ann Arbor, MI, USA) and Jeremy Wolverton, M.S. (Department of Cardiac Surgery, University of Michigan Health System, Ann Arbor, MI, USA) for their contributions in data acquisition and electronic search query programming for this project.
Funding Support:
This work was supported by the US National Institutes of Health - National Heart, Lung, and Blood Institute [grant number K01HL141701]; National Institute of General Medical Sciences [grant number T32GM103730] Bethesda, MD. Additionally this work was supported by the Department of Anesthesiology, University of Michigan Medical School (Ann Arbor, Michigan, USA). The opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of the National Institutes of Health, or any of its employees. Industry contributors have had no role in the study.
Declaration of interests:
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.prf (available on request from the corresponding author) and declare: Dr. Mathis has received a research grant from the US National Institutes of Health - National Heart, Lung, and Blood Institute [grant number K01HL141701]. Dr. Janda is funded by a US National Institutes of Health T32 Grant - National Institute of General Medical Sciences [grant number T32GM103730]. No other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cikes M, Solomon SD: Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur. Heart J 2015; 23:1642–50 [DOI] [PubMed] [Google Scholar]
- 2.Hayley BD, Burwash IG: Heart failure with normal left ventricular ejection fraction: role of echocardiography. Curr. Opin. Cardiol 2012; 27:169–80 [DOI] [PubMed] [Google Scholar]
- 3.Todaro MC, Khandheria BK, Longobardo L, et al. : New diagnostic perspectives on heart failure with preserved ejection fraction: systolic function beyond ejection fraction. J. Cardiovasc. Med 2015; 16:527–37 [DOI] [PubMed] [Google Scholar]
- 4.Carabello BA, Spann JF: The uses and limitations of end-systolic indexes of left ventricular function. Circulation. 1984; 69:1058–64 [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Rodriguez L, Lethor JP, et al. : Continuous wave Doppler echocardiography for noninvasive assessment of left ventricular dP/dt and relaxation time constant from mitral regurgitant spectra in patients. J. Am. Coll. Cardiol 1994; 23:970–6 [DOI] [PubMed] [Google Scholar]
- 6.Gorcsan J 3rd, Romand JA, Mandarino WA, et al. : Assessment of left ventricular performance by on-line pressure-area relations using echocardiographic automated border detection. J. Am. Coll. Cardiol 1994; 23:242–52 [DOI] [PubMed] [Google Scholar]
- 7.Declerck C, Hillel Z, Shih H, et al. : A comparison of left ventricular performance indices measured by transesophageal echocardiography with automated border detection. Anesthesiology. 1998; 89:341–9 [DOI] [PubMed] [Google Scholar]
- 8.Greenberg NL, Firstenberg MS, Castro PL, et al. : Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. 2002; Circulation 105:99–105 [DOI] [PubMed] [Google Scholar]
- 9.Morris DA, Boldt LH, Eichstadt H, et al. : Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ. Heart Fail 2012; 5:610–20 [DOI] [PubMed] [Google Scholar]
- 10.Green P, Kodali S, Leon MB, et al. : Echocardiographic assessment of pressure volume relations in heart failure and valvular heart disease: using imaging to understand physiology. Minerva Cardioangiol. 2011; 59:375–89 [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BE, Madigan VM: Non-invasive method for rapid bedside estimation of inotropy: theory and preliminary clinical validation. Br. J. Anaesth 2013; 111:580–8 [DOI] [PubMed] [Google Scholar]
- 12.University of Michigan Anesthesiology Clinical Research. 2020; Retrieved from: https://anes.med.umich.edu/research/acrc.html
- 13.Von Elm E, Altman DG, Egger M, et al. : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med 2007; 147:573–7 [DOI] [PubMed] [Google Scholar]
- 14.Shroyer AL, Edwards FH, Grover FL: Updates to the Data Quality Review Program: the Society of Thoracic Surgeons Adult Cardiac National Database. Ann. Thorac. Surg 1998; 65:1494–7 [DOI] [PubMed] [Google Scholar]
- 15.Colquhoun DA, Shanks AM, Kapeles SR, et al. : Considerations for Integration of Perioperative Electronic Health Records Across Institutions for Research and Quality Improvement: The Approach Taken by the Multicenter Perioperative Outcomes Group. Anesth. Analg 2020; 130:1133–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender SP, Paganelli WC, Gerety LP, et al. : Intraoperative Lung-Protective Ventilation Trends and Practice Patterns: A Report from the Multicenter Perioperative Outcomes Group. Anesth. Analg 2015; 121:1231–9 [DOI] [PubMed] [Google Scholar]
- 17.Jacobs JP, He X, O’Brien SM, et al. : Variation in ventilation time after coronary artery bypass grafting: an analysis from the society of thoracic surgeons adult cardiac surgery database. Ann. Thorac. Surg 2013; 96:757–62 [DOI] [PubMed] [Google Scholar]
- 18.Shahian DM, O’Brien SM, Filardo G, et al. : The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann. Thorac. Surg 2009; 88:S2–22 [DOI] [PubMed] [Google Scholar]
- 19.Sun E, Mello MM, Rishel CA, et al. : Association of Overlapping Surgery With Perioperative Outcomes. JAMA. 2019; 321:762–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nashef SAM, Roques F, Sharples LD, et al. : EuroSCORE II. European Journal of Cardio-Thoracic Surgery. 2012; 41:734–45 [DOI] [PubMed] [Google Scholar]
- 21.Häkkinen U, Kurki T, Vento A, et al. : Risk adjustment in coronary bypass grafting: How EuroSCORE is related to cost, health-related quality of life, and cost-effectiveness. National Institute for Health and Welfare, 2009. [Google Scholar]
- 22.López-Ratón M, Rodríguez-Álvarez MX, Suárez CC, et al. : OptimalCutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. Journal of Statistical Software. 2014; 61:1–36 [Google Scholar]
- 23.Lomivorotov VV, Efremov SM, Kirov MY, et al. : Low-Cardiac-Output Syndrome After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth 2017; 31:291–308 [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW, Jessup M, Bozkurt B, et al. : 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol 2013; 62:e147–239 [DOI] [PubMed] [Google Scholar]
- 25.Mathis MR, Duggal NM, Likosky DS, et al. : Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology 2019; 131:1046–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vives M, Hernandez A, Parramon F, et al. : Acute kidney injury after cardiac surgery: prevalence, impact and management challenges. Int. J. Nephrol. Renovasc. Dis 2019; 12:153–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran DTT, Perry JJ, Dupuis J-Y, et al. : Predicting New-Onset Postoperative Atrial Fibrillation in Cardiac Surgery Patients. J. Cardiothorac. Vasc. Anesth 2015; 29:1117–26 [DOI] [PubMed] [Google Scholar]
- 28.Shahian DM, He X, Jacobs JP, et al. : The Society of Thoracic Surgeons Composite Measure of Individual Surgeon Performance for Adult Cardiac Surgery: A Report of The Society of Thoracic Surgeons Quality Measurement Task Force. Ann. Thorac. Surg 2015; 100:1315–25 [DOI] [PubMed] [Google Scholar]
- 29.Fernandez FG, Shahian DM, Kormos R, et al. : The Society of Thoracic Surgeons National Database 2019 Annual Report. Ann. Thorac. Surg 2019; 108:1625–32 [DOI] [PubMed] [Google Scholar]
- 30.Huen SC, Parikh CR: Predicting Acute Kidney Injury After Cardiac Surgery: A Systematic Review. Ann. Thorac. Surg 2012; 93:334–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford TC, Magruder JT, Grimm JC, et al. : Complications After Cardiac Operations: All Are Not Created Equal. Ann. Thorac. Surg 103:32–40, 2017 [DOI] [PubMed] [Google Scholar]
- 32.He S-R, Sun X, Zhang C, et al. : Measurement of systemic oxygen delivery and inotropy in healthy term neonates with the Ultrasonic Cardiac Output Monitor (USCOM). Early Hum. Dev 89:289–94, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Zheng M-L, He S-R, Liu Y-M, et al. : Measurement of inotropy and systemic oxygen delivery in term, low- and very-low-birth-weight neonates using the Ultrasonic Cardiac Output Monitor (USCOM). J. Perinat. Med 2020; 48:289–95 [DOI] [PubMed] [Google Scholar]
- 34.Mantovani E, Thilaganathan B, Pagani G, et al. : Inotropy index and ratio of potential to kinetic energy: Two novel parameters derived from continuous-wave Doppler ultrasound. Pregnancy Hypertens. 2013; 42:233 [Google Scholar]
- 35.Smith BE, Madigan VM: Comparison of continuous-wave Doppler ultrasound monitor and echocardiography to assess cardiac output in intensive care patients. Crit. Care Resusc 2018; 20:74. [PubMed] [Google Scholar]
- 36.Kapoor PM, Kakani M, Chowdhury U, et al. : Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Ann. Card. Anaesth 2008; 11:27–34 [DOI] [PubMed] [Google Scholar]
- 37.Smetkin AA, Kirov MY, Kuzkov VV, et al. : Single transpulmonary thermodilution and continuous monitoring of central venous oxygen saturation during off-pump coronary surgery. Acta Anaesthesiol. Scand 2009; 53: 505–14 [DOI] [PubMed] [Google Scholar]
- 38.Johnston LE, Thiele RH, Hawkins RB, et al. : Goal-directed resuscitation following cardiac surgery reduces acute kidney injury: A quality initiative pre–post analysis. J. Thorac. Cardiovasc. Surg 2020; 159:1868–77.e1 [DOI] [PubMed] [Google Scholar]
- 39.Osawa EA, Rhodes A, Landoni G, et al. : Effect of Perioperative Goal-Directed Hemodynamic Resuscitation Therapy on Outcomes Following Cardiac Surgery: A Randomized Clinical Trial and Systematic Review. Crit. Care Med 2016; 44:724–33 [DOI] [PubMed] [Google Scholar]
- 40.McKendry M, McGloin H, Saberi D, et al. : Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ. 2004; 329:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pölönen P, Ruokonen E, Hippeläinen M, et al. : A Prospective, Randomized Study of Goal-Oriented Hemodynamic Therapy in Cardiac Surgical Patients. Anesth Analg. 2000; 90:1052–59 [DOI] [PubMed] [Google Scholar]
- 42.Mythen MG, Webb AR: Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch. Surg 1995; 130:423–9 [DOI] [PubMed] [Google Scholar]
- 43.Stoppe C, McDonald B, Benstoem C, et al. : Evaluation of Persistent Organ Dysfunction Plus Death As a Novel Composite Outcome in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth 2016; 30:30–8 [DOI] [PubMed] [Google Scholar]
- 44.Engoren M, Habib RH, Arslanian-Engoren C, et al. : The effect of acute kidney injury and discharge creatinine level on mortality following cardiac surgery. Crit. Care Med. LWW, 2014; 42:2069app–74 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


