Abstract
Inhibitory control is the ability to suppress inappropriate movements and unwanted actions, allowing to regulate impulses and responses. This ability can be measured via the Stop Signal Task, which provides a temporal index of response inhibition, namely the stop signal reaction time (SSRT). At the neural level, Transcranial Magnetic Stimulation (TMS) allows to investigate motor inhibition within the primary motor cortex (M1), such as the cortical silent period (CSP) which is an index of GABAB-mediated intracortical inhibition within M1. Although there is strong evidence that intracortical inhibition varies during action stopping, it is still not clear whether differences in the neurophysiological markers of intracortical inhibition contribute to behavioral differences in actual inhibitory capacities. Hence, here we explored the relationship between intracortical inhibition within M1 and behavioral response inhibition. GABABergic-mediated inhibition in M1 was determined by the duration of CSP, while behavioral inhibition was assessed by the SSRT. We found a significant positive correlation between CSP’s duration and SSRT, namely that individuals with greater levels of GABABergic-mediated inhibition seem to perform overall worse in inhibiting behavioral responses. These results support the assumption that individual differences in intracortical inhibition are mirrored by individual differences in action stopping abilities.
Subject terms: Neuroscience, Motor control
Introduction
Inhibitory control is a central executive function that allows to temporarily withhold or completely suppress inappropriate or unintended responses, even after these are already initiated. This ability plays a pivotal role in everyday life because behaving in a goal-directed manner constantly requires a quick and efficient regulation of our impulses and responses1. Lacking an efficient inhibitory control may result in a number of different dysfunctional behaviors, as evidenced in several medical and psychiatric conditions2 such as attention-deficit/hyperactivity disorder3, eating disorders4 substance abuse disorders5 and obsessive–compulsive disorder6.
At the behavioral level, one of the most reliable paradigms employed for measuring response inhibition is the Stop Signal Task7–10 (SST). This task allows estimating individuals’ ability to suppress a response already initiated, as it measures the temporal dynamics underlying successful response inhibition8,9,11–13. Performance in this task is highly variable across the normal population and reaches abnormally longer values in clinical conditions2 including attention-deficit hyperactivity disorder3 (ADHD) and obsessive compulsive disorder6 (OCD), as well as Gambling Disorder14. Hence finding biomarkers of response inhibition is desirable.
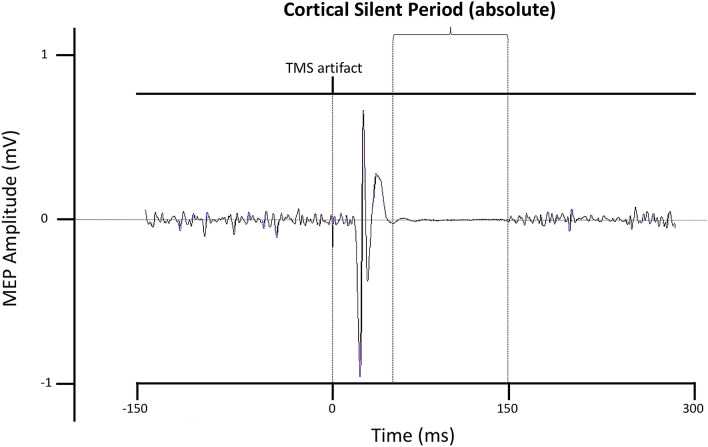
At the neural level, Transcranial Magnetic Stimulation (TMS) has been widely employed to investigate the electrophysiological markers of motor inhibition in the brain1,15–23. Different TMS-EMG protocols can be used to measure levels of intracortical inhibition within the primary motor cortex (M1). Specifically, intracortical inhibition can be quantified either from the intensity of Short-interval intracortical inhibition (SICI, GABAA-mediated inhibition) and Long-interval intracortical inhibition (LICI, GABAB-mediated inhibition), obtained with two different paired-pulses procedures, or from the duration of the cortical silent period (CSP, GABAB-mediated inhibition), measured following specific single pulse procedures24–26. The CSP is a cessation in the background voluntary muscle activity induced by a single suprathreshold TMS pulse delivered on M1 during tonic contraction of the target muscle24,25,27 (Fig. 1). In particular, absolute CSP is obtained by measuring the time interval between the offset of the MEP and the restoration of the muscle activity, and it is expressed in milliseconds (ms). The first part of the CSP (50–75 ms) is thought to be partially influenced by spinal cord inhibition contributions, while its latter part is entirely mediated by motor cortical postsynaptic inhibition24,25. Overall, the duration of the CSP is considered an index of the levels of slower inhibitory postsynaptic potentials GABAB inhibition within M125,28,. Crucially, while SICI and LICI provide an amplitude measure of intracortical inhibition, the CSP provides a temporal measure of this process. Hence, even though both LICI and CSP could be treated as markers of GABAB-mediated inhibition, these two measures do not overlap, as they reflect different aspects29,30. Specifically, LICI is an electric potential difference expressed in millivolts which represents the magnitude of inhibition, while CSP represents the duration of intracortical inhibition.
Figure 1.
Representative traces of the FDI’s cortical silent period at 30% maximal voluntary contraction (MVC).
Given the complementary nature of these different measures, several works have already tried to employ TMS during the SST to probe the fluctuations in the levels of corticospinal and intracortical inhibition within M1 associated with concurrent action preparation and action stopping31. Interestingly, studies in which TMS was delivered online on participants during the SST revealed that behavioral motor inhibition is deeply influenced by the ongoing electrophysiological modulation of corticospinal motor excitability and inhibition within M131,32. For example, in go trials, action preparation induces a significant progressive increase in the levels of corticospinal excitability in the contralateral M118,32–34 (≈130–175 ms after the onset of the go signal), while during stop trials action inhibition induces both a widespread decrease in corticospinal excitability33 (≈140 ms after the onset of the stop signal) and, concurrently, a significant increase in SICI.
Overall, the ongoing modulation of corticospinal excitability and intracortical inhibition within M1 appears to be critical to the successful restraint and cancellation of actions. Nevertheless, it is still unclear whether individual differences in these neurophysiological markers of intracortical inhibition might be related to actual behavioral individual differences in inhibitory control efficiency14,35. Recently, quite a few studies14,35–42 have investigated whether and to what extent individual levels of resting-state SICI and LICI measured offline might reflect individual differences in the efficiency of the inhibitory process, indexed by the length of the Stop Signal Reaction Time (SSRT). Taken together, these studies support the hypothesis that trait-like individual differences in the neurophysiological markers of intracortical inhibition (and SICI in particular) can predict an individual’s actual behavioral motor inhibition capacities14. Hence, TMS-derived measures of intracortical inhibition might be effectively employed as biomarkers of motor inhibition performance. However, to date, no studies have investigated whether the CSP’s duration, measured offline, might also be considered a viable biomarker of motor inhibition, notwithstanding this being the only TMS-based parameter measured as a time interval. This is the aim and the novelty of the current study.
Results
Behavioral data
Raw data were processed via a customized R software (version 3.6.2) for Windows, using the code for the analysis provided by Verbruggen and Colleagues9. The average SSRT was 215 ms (SD = 20.6 ms). The table below (Table 1) shows the average and SD of the main measures obtained from the Stop Signal Task: Stop Signal Reaction Time (SSRT), Stop Signal Delay (SSD), RT on go trials (goRT), probability of responding on a stop trial [p(respond|signal)], RT on unsuccessful stop trials (sRT), probability of go omissions (miss), and accuracy rate on go trials (acc).
Table 1.
Descriptive statistics of the Stop Signal Task. Values in the table represent means and standard deviations of the descriptive statistics of the Stop Signal Task. Legend: SSRT = Stop Signal Reaction Time; SSD = Stop Signal Delay; goRT = RT on go trials; p(respond|signal) = probability of responding on a stop trial; sRT = RT of go responses on unsuccessful stop trials; miss = probability of go omissions; acc = accuracy rate on go trials.
| Stop Signal Task | Mean ± SD |
|---|---|
| SSRT | 215 ms ± 21 |
| SSD | 318 ms ± 117 |
| goRT | 546 ms ± 105 |
| p(respond|signal) | 49% ± 1 |
| sRT | 480 ms ± 89 |
| miss | 2% ± 3 |
| acc | 99.6% ± 0.4 |
Neurophysiological data
The CSP duration was defined as the time elapsed between the offset of the MEP and the time at which the post-stimulus EMG activity reverted to the pre-stimulus level (absolute CSP). The analysis of CSPs was carried out using Signal 6.04 software (Cambridge Electronic Design, Cambridge, UK). Overall, the mean CSP duration was 106 ms (SD = 26 ms). Average MEP amplitude was 0.49 mV (SD = 0.29 mV), while MEP duration was 35 ms (SD = 2 ms). The average rMT was 55% of the maximum stimulator output (ranging from 48 to 61%, SD = 4%).
Correlation analysis
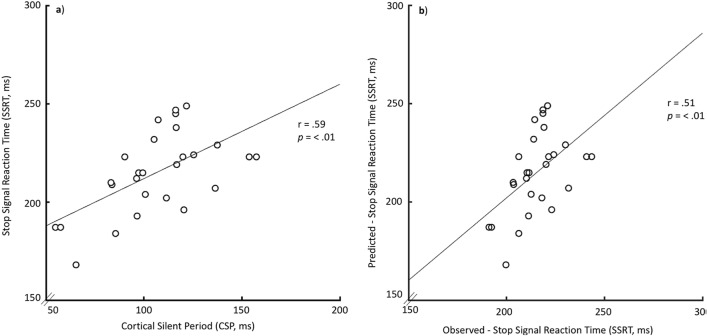
Four Pearson correlations were carried out to look for the relationships between SSRT and the relevant TMS-derived neurophysiological variables (CSP, rMT, MEP amplitude, MEP duration). These correlations were tested against a Bonferroni-adjusted alpha level of 0.012 (0.05/4). The linear correlation between the CSP and the SSRT was significant (Pearson r (25) = 0.59; p = 0.001; CI = [0.25 0.92], Fig. 2a) and survived robust correlation (skipped Pearson r (25) = 0.59; p = 0.001; CI = [0.34 0.77]). Most importantly, the results of the leave-one-out cross-validation analysis showed a significant correlation between the model-predicted and observed SSRT values (r(25) = 0.51; p = 0.007, Fig. 2b). There was no significant correlation between SSRT and any of the other relevant TMS-derived neurophysiological variables (Table 2).
Figure 2.
(a) Linear association between cortical silent period (CSP) and Stop Signal Reaction Time (SSRT). (b) Leave one out cross-validation analysis showing a significant correlation between the model-predicted and observed SSRT values.
Table 2.
Correlations between the TMS-derived neurophysiological parameters and SSRT. Values in the table represent Pearson correlation coefficient and significance of the Pearson correlations between each significant TMS-derived Neurophysiological parameter and the SSRT. Legend: rMT = Resting Motor Threshold; MEP (mV) = Motor Evoked Potential amplitude; MEP (ms) Motor Evoked Potential duration; CSP = Cortical Silent Period (absolute); SSRT = see Table 1; ** = correlation is significant at the 0.01 level (2-tailed).
| rMT | MEP (mV) | MEP (ms) | CSP | |
|---|---|---|---|---|
| SSRT | r(25) = .07; p = .716 | r(25) = .24; p = .239 | r(25) = .269; p = .175 | r(25) = .587; p = .001** |
Discussion
The present study aimed at investigating whether individual differences in the temporal aspect of intracortical inhibition might act as a neurophysiological trait marker reflecting individual response inhibition capacities. Our results revealed a clear relationship between the duration of the cortical silent period (CSP) and the stop signal reaction time (SSRT), obtained from the Stop Signal Task (SST). In particular, individuals with longer CSP performed worse at the Stop Signal Task, as indexed by longer SSRT, compared to individuals with shorter CSP. The duration of CSP is a neurophysiological marker of the levels of intracortical inhibition within M124,25. Lengthening of the CSP is observed after disruption of motor attention by sedative drugs such as ethanol or benzodiazepines. Indirect pharmacological evidence supports a largely GABAB-mediated origin of the CSP43–46. On the other hand, SSRT is a precise index of the duration of the whole chain of processes underlying response inhibition, and so a longer SSRT indicates lower levels of inhibitory control, while a shorter SSRT denotes a better response inhibition. Therefore, our results suggest that CSP might provide a valid trait bio-marker of the quality of action restraint and response inhibition, namely individual inhibitory control capacities.
In general, the relationship between ongoing corticospinal brain activity and behavioral motor functioning has been extensively investigated31. Recently, quite a few studies14,35–42 have investigated the relationship between offline TMS-derived GABA-ergic inhibitory biomarkers (resting-state SICI, LICI) and behavioral motor-inhibitory efficiency. In particular, in their study Chowdhury and colleagues14 showed a negative correlation between individual GABAA-ergic intracortical motor inhibition (measured via SICI’s amplitude) and SSRT’s length, indicating that subjects with stronger resting state SICI tend to be faster at inhibiting their responses, and so better at action stopping.
Hence, our results complemented those reported by Chowdhury and colleagues14. Indeed, intracortical inhibition is not modulated through a single process, but it is the synergic result of the interaction between two functionally distinct neural populations mediating either short-lasting ionotropic GABAA postsynaptic inhibition or long-lasting metabotropic GABAB postsynaptic inhibition. Such two mechanisms are indexed by SICI and LICI/CSP respectively47,48.
According to the resulting model of interaction between these different cortical inhibitory systems, LICI and CSP do not only inhibit cortical outputs via postsynaptic GABAB receptors, but they even selectively suppress SICI via presynaptic GABAB receptors, causing an overall reduction of GABA release26,49–51. This GABAB mediated suppression of GABAA effects has been corroborated by converging evidence from in vitro studies47,52, as well as pharmacological and TMS studies both at rest and during voluntary movement26,30,51,53. Hence, not only SICI and LICI/CSP are mediated by different neural circuits, but the latter can inhibit the first, and so SICI inhibition might result not only from the actual activity of SICI circuits but also as a consequence of increasing GABAB-mediated inhibition51. Given that CSP is regulated by a GABAB-mediated inhibitory network and that longer CSPs are considered an index of upregulated intracortical inhibition54, we hypothesize that stronger GABAB-ergic circuits, indexed by longer CSP, might unbalance intracortical inhibition during action stopping, resulting in a global reduction of SICI-mediated reactive inhibition and so in a longer SSRT. Interestingly, a similar detrimental effect of CSP’s upregulation has been recently associated with eating disorders, and specifically with Binge Eating Disorder55. Keeping this in mind, the negative correlation between SICI’s strength and SSRT found by Chowdhury and Colleagues14 and the positive correlation between CSP and SSRT reported here might originate from the same overall inhibitory mechanism. Indeed, it is entirely plausible that these two correlations reflect the opposite effects of these two distinct neural populations mediating different inhibitory sub-processes during action stopping, and so that stronger levels of GABAB inhibition might unbalance intracortical inhibition diminishing the GABAA-mediated inhibition during action stopping, resulting in worse performance and consequently in a longer SSRT.
Overall, our results suggest that the duration of CSP measured off-task should be considered as a neurophysiological inhibitory biomarker reflecting individual response inhibition capacities, and specifically that individuals with longer CSP performed worse at action stopping (longer SSRT), compared to individuals with shorter CSP (shorter SSRT). Our results also support the idea that TMS-derived biomarkers might provide a reliable methodology to investigate behavioral individual differences in motor inhibition.
Methods
Participants
Twenty-seven 27 (11 males, mean age = 27.84 years; SD = 3.8; range = 23–38) right-handed (self-reported) naïve participants with normal or corrected-to-normal vision took part in the present study. The sample size of 27 was calculated by using G Power software56,57 (v3.1.9.6) analysis assuming an effect size (r) of 0.62 (based on Chowdhury and Colleagues14), an acceptable minimum level of significance (α) of 0.05, and an expected power (1-β) of 0.80.
During the recruitment stage, participants were preliminarily screened for history of neurological disorders and current mental health problems, as well as for hearing and visual difficulties, and completed a questionnaire to check whether they were eligible for a TMS-based study. None of the participants here included reported neither having TMS contraindicators nor having been diagnosed with any psychiatric or neurological disorder, as self-reported. Participants provided written informed consent before taking part in the study. None of the participants reported any negative side effects during or after the TMS procedure. The whole study took place at ITAB (Institute for Advanced Biomedical Technologies) in Chieti and lasted 1 h and 15 min on average. The study was approved by the Ethics Committee of the “G. d’Annunzio” University of Chieti-Pescara and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Measures
EMG recording preparation
The surface EMG signal was recorded from the right First Dorsal Interosseous (FDI) hand muscle using three self-adhesive EMG electrodes connected to a CED Micro 1401 (Cambridge Electronic Design, Cambridge, UK). Before electrode placement, recording areas were wiped using an alcohol swab and a pad with abrasive skin prep. Three electrically conductive adhesive hydrogels surface electrodes were then placed along with the target areas of the right hand. Specifically, the positive electrode was placed over the FDI muscle, the negative electrode was placed on the outer side of the thumb knuckle, and the ground electrode was placed on the ulnar styloid process. EMG raw signals were amplified (by a factor of 1000), digitized at a sampling rate of 8 kHz, and filtered using an analogical online band-pass (20 Hz to 250 Hz) and a 50 Hz notch filter. EMG recordings were then stored on a computer for offline analysis with Signal 6.04 software (Cambridge Electronic Design, Cambridge, UK).
Transcranial magnetic stimulation
Before TMS administration, participants wore a hypoallergenic cotton helmet which was used to mark the exact location of the FDI hotspot over the left primary motor cortex (M1). Single-pulse monophasic TMS was delivered over the left primary motor cortex using a double 70 mm Alpha coil connected to BiStim4 Magstim stimulators (Magstim, Whitland, UK) to induce a posterior-anterior current flow in the brain. The coil was positioned tangentially to the scalp following the orthodox method27, with the handle pointed backward and angled 45 degrees from the midline, perpendicular to the central sulcus. The FDI optimal scalp position for stimulation was identified by maneuvering the coil around the left M1 hand area in steps of 1 cm until eliciting the maximum amplitude motor-evoked potentials (MEPs) in the contralateral FDI muscle using slightly suprathreshold stimuli. Once identified and marked the hotspot on the helmet, coil position was fastened through mechanical support, and its position was constantly monitored by the experimenter. Participants were asked to avoid any head movement throughout the whole TMS session and were also firmly cushioned using ergonomic pads. Afterward, individuals’ resting motor threshold (rMT) was estimated by consistently adjusting the stimulator to find the lowest percentage of the maximum stimulator output necessary to elicit MEPs with a peak-to-peak amplitude of more than 50 μV during muscle relaxation in 5 out of 10 trials27. For each participant, rMT was used to determine the specific intensity of TMS suprathreshold stimulation, which was set at 120% of this individual value. This level of stimulation intensity is considered appropriate for studying CSP57,58.
Intracortical inhibition
Individuals’ CSP was assessed delivering 20 suprathreshold pulses at 120% of rMT while participants were performing an opposition pinch grip at 30% of their FDI’s maximal voluntary isometric contraction (MVC) and maintaining both a static hand posture and a constant level of muscle activity. Individuals’ MVC was used as the reference contraction to normalize the muscular activity between subjects59,60 and it was determined by averaging the mean peak-to-peak amplitude of the EMG signal (μV) recorded across three trials lasting 3 s each. Before measuring MVC, the experimenter emphasized to the participants the importance of performing at their best and of trying to keep the contraction stable during EMG recording. Once determined participants’ MVC, the level of muscular activation was constantly monitored by the experimenter via online data inspection throughout the whole TMS session. Before the TMS session, each participant took part in a short preliminary training session (never longer than 5 min) to learn how to constantly perform and maintain the appropriate level of FDI contraction (30% MVC) while receiving constant EMG visual feedback displayed on the computer monitor. The TMS session (hotspot mapping procedure, resting motor threshold estimation, and actual experimental session) started only after participants became able to reproduce the adequate level of EMG activity requested without the support of the EMG visual feedback. Each single-pulse TMS stimulation was delivered with an inter-stimulus interval jittered between 8 and 15 s to avoid any habituation effect. Coherently with the short duration of the CSP recording session and with the relatively low level of FDI contraction requested (30% MVC), no participant reported experiencing muscle fatigue at any stage of the recording procedure. Trials were rejected if the participant displayed any pronounced head movement before or during the stimulation. For each trial, CSP duration was first quantified as the time between the offset of the MEP and the return of EMG activity to the pre-stimulus level (± 2 SD) and then double-checked following a standard procedure58,61. CSPs preliminary inspection, analysis, and offline extraction were all carried out using Signal 6.04 software (Cambridge Electronic Design, Cambridge, UK). CSPs were inspected before processing the Stop Signal Task data, and so the inspector was blinded to the relative behavioral results.
Behavioral level—motor Inhibition
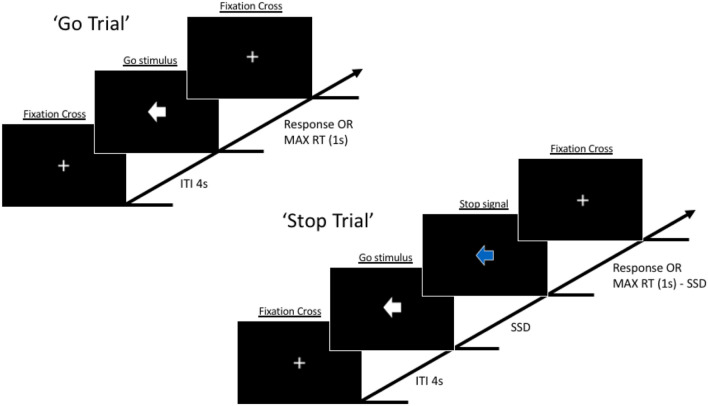
To measure motor inhibition at the behavioral level we employed “STOP-IT”62, a Matlab and Psychtoolbox63–65 version of the Stop Signal Task. In the beginning, participants were instructed to place their right hand on a specific site of the computer keyboard (the right index finger over the left arrow key and the right ring finger over the right arrow key) and to maintain this position throughout the whole experiment. The task required participants to perform a speeded response go task while discriminating between two different go stimuli, a left-pointing white arrow, and a right-pointing white arrow, responding to both of them as quickly as possible pressing the left arrow key of the computer keyboard with the right index finger and the right arrow key with the right ring finger, respectively (go trials). However, in 25% of the trials (stop trials), the white go arrow would turn blue after a variable delay (stop signal delay (SSD)), indicating to the participants to withhold their response, whether possible (Fig. 3). Crucially, the instructions provided with the task explicitly emphasized that in half of the stop trials the stop signal would appear soon after the go stimulus, making response inhibition easier, while on the other half of these trials the stop signal would be displayed late making response inhibition difficult or even impossible for the participant. Thus, participants were instructed that the task was difficult and that failing in half of the trial was an inherent characteristic of the task itself. They were also instructed to always try to respond to the go signal as fast and accurately as possible, without withholding their response to wait for a possible stop signal occurrence.
Figure 3.
Visual representation of the sequence of events in the Stop Signal Task employed in the present study.
In the go trials, go stimuli lasted 1 s (maximum RT), while in stop trials the combination of the go, the SSD, and the stop signal lasted 1 s in total, with SSD being initially set at 250 ms. Inter-trial interval lasted 4 s. Crucially, after each trial the SSD automatically varied in steps of 50 ms as a function of participants previous stop performance, decreasing after each unsuccessful stop trial and increasing after each successful stop trial, ensuring an overall successful inhibition rate near 50%, and so a p(respond|signal) ≅ 0.50. The task included an initial practice block providing feedback after each trial. Relevant descriptive statistics obtained from the task included: the probability of go omissions, probability of choice errors on go trials, RT on go trials, probability of responding on a stop trial, Stop Signal Delay, Stop Signal Reaction Time, RT of go responses on unsuccessful stop trials. The Stop Signal data collected from each participant were screened according to the following outlier rejection criteria66: (1) probability of responding on stop trials < 40% or > 60%; (2) probability of go omissions > 25%; (3) probability of choice errors on go trial > 10%; (4) violation of the independent race model (RT of go responses on unsuccessful stop trials > RT on go trials); (5) either negative SSRT or SSRT < 50 ms. These exclusion criteria were adopted because they help to ensure that participants followed the instruction and got engaged throughout the task66. One participant was excluded for matching exclusion criteria (4) and replaced with another one. In the present study, we used a non-parametric approach for SSRT’s estimation using the integration method with the replacement of go omissions with the maximum goRT9. Overall, the whole task comprised 1 practice block of 32 trials (8 stop trials) and 5 experimental blocks of 96 trials (24 stop trials) each. Between blocks, participants were reminded about the instructions and provided with block-based feedback on their performance.
Procedure
Participants took part in either the TMS session or the behavioral task, administered in random order on the same day with a 10 min break between them. The TMS session took place in the TMS/EMG laboratory of ITAB for about 35 min, following the same procedure already described above for each participant. The behavioral task took place in one of the Data Collecting Booths of the TEAMLab of ITAB for about 40 min. During the behavioral task, participants sat on a comfortable chair in front of a computer monitor with a resolution of 1024 horizontal pixels by 768 vertical pixels, at a distance of approximately 56–57 cm. The tasks were administered on Windows 7 using MATLAB R2016b. The computer monitor refresh rate was set to 60 Hz. Once finished the two parts of the experiment, participants were debriefed.
Data analysis
To investigate the relationship between behavioral and cortical inhibition we look for a possible correlation between SSRT and CSP.
To control for the specificity of the effect, we run a regression analysis between each of the individual Stop Signal Task significant parameters (SSRT, SSD, goRT, sRT, p(respond|signal), acc. ns, miss. ns) and each significant TMS-derived neurophysiological parameter (rMT, amplitude of the accompanying MEP, and duration of CSP). CSPs inspection and extraction were performed before processing the Stop Signal Task data, and so the inspector was blinded to behavioral results. Moreover, to test the robustness of the relationship we computed skipped parametric (Pearson) correlations67 using the Robust Correlation toolbox68 and conducted null hypothesis statistical significance testing using the nonparametric percentile bootstrap test (2000 resamples; 95% confidence interval, corresponding to an alpha level of 0.05), which is more robust against heteroscedasticity compared with the traditional t-test68. Then, we employed a leave-one-out cross-validation analysis69 (i.e., internal validation) to test whether participants’ CSP could reliably predict the SSRT. Specifically, at each round of cross-validation, a linear regression model was trained on n-1 subjects' values and tested on the left-out participant. Pearson correlations between observed and predicted SSRT values were used to assess predictive power. All statistical tests were two-tailed. To account for the non-independence of the leave-one-out folds, we conducted a permutation test by randomly shuffling the SSRT scores 5000 times and rerunning the prediction pipeline, to create a null distribution of r values. The p values of the empirical correlation values, based on their corresponding null distribution, were then computed.
Author contributions
M.P., F.F., and M.C. developed the study concept and design. M.P. and M.G.P. developed the experimental setup. M.P. and G.D.C. performed the behavioral data collection. M.P. performed the TMS data acquisition. M.G.P. provided technical support during data acquisition. M.P. and G.D.C. analyzed the data. M.P. and M.C. interpreted the data. M.P. wrote the manuscript. M.C. contributed to the drafting of the manuscript. All authors contributed to and approved the final manuscript.
Data availability
The data analyzed during this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Francesca Ferri and Marcello Costantini.
References
- 1.Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb. Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipszyc, J. & Schachar, R. Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. J. Int. Neuropsychol. Soc.16, 1064–1076 (2010). [DOI] [PubMed]
- 3.Oosterlaan J, Sergeant JA. Inhibition in ADHD, aggressive, and anxious children: A biologically based model of child psychopathology. J. Abnorm. Child Psychol. 1996;24:19–36. doi: 10.1007/BF01448371. [DOI] [PubMed] [Google Scholar]
- 4.Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci. Biobehav. Rev. 2016;64:35–62. doi: 10.1016/j.neubiorev.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug Alcohol Depend. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Menzies L, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 7.Lappin JS, Eriksen CW. Use of a delayed signal to stop a visual reaction-time response. J. Exp. Psychol. 1966;72:805–811. doi: 10.1037/h0021266. [DOI] [Google Scholar]
- 8.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984;91:295–327. doi: 10.1037/0033-295X.91.3.295. [DOI] [PubMed] [Google Scholar]
- 9.Verbruggen, F. et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife8, e46323 (2019). [DOI] [PMC free article] [PubMed]
- 10.Vince MA. The intermittency of control movements and the psychological refractory period1. Br. J. Psychol. Gen. Sect. 1948;38:149–157. doi: 10.1111/j.2044-8295.1948.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 11.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol. Sci. 1997;8:60–64. doi: 10.1111/j.1467-9280.1997.tb00545.x. [DOI] [Google Scholar]
- 12.Logan GD, Van Zandt T, Verbruggen F, Wagenmakers E-J. On the ability to inhibit thought and action: General and special theories of an act of control. Psychol. Rev. 2014;121:66–95. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 13.Verbruggen F, Chambers CD, Logan GD. Fictitious inhibitory differences. Psychol. Sci. 2013;24:352–362. doi: 10.1177/0956797612457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury NS, Livesey EJ, Blaszczynski A, Harris JA. Variations in response control within at-risk gamblers and non-gambling controls explained by GABAergic inhibition in the motor cortex. Cortex. 2018;103:153–163. doi: 10.1016/j.cortex.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J. Neurosci. 2010;30:3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenhouse I, King M, Noah S, Maddock RJ, Ivry RB. Individual differences in resting corticospinal excitability are correlated with reaction time and GABA content in motor cortex. J. Neurosci. 2017;37:2686–2696. doi: 10.1523/JNEUROSCI.3129-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has global or nonglobal effects on the motor system depending on preparation. J. Neurophysiol. 2012;107:384–392. doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenhouse I, Saks D, Hoang T, Ivry RB. Inhibition during response preparation is sensitive to response complexity. J. Neurophysiol. 2015;113:2792–2800. doi: 10.1152/jn.00999.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenhouse I, Sias A, Labruna L, Ivry RB. Nonspecific inhibition of the motor system during response preparation. J. Neurosci. 2015;35:10675–10684. doi: 10.1523/JNEUROSCI.1436-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasbroucq T, et al. Cortico-spinal inhibition reflects time but not event preparation: Neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Physiol. (Oxf.) 1999;101:243–266. doi: 10.1016/s0001-6918(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 21.Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Leone ALVARO, et al. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115:1045–1059. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- 23.Starr A, Caramia M, Zarola F, Rossini PM. Enhancement of motor cortical excitability in humans by non-invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr. Clin. Neurophysiol. 1988;70:26–32. doi: 10.1016/0013-4694(88)90191-5. [DOI] [PubMed] [Google Scholar]
- 24.Hallett M. Transcranial magnetic stimulation: A primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Paulus W, et al. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardellicchio P, Dolfini E, Hilt PM, Fadiga L, D’Ausilio A. Motor cortical inhibition during concurrent action execution and action observation. Neuroimage. 2020;208:116445. doi: 10.1016/j.neuroimage.2019.116445. [DOI] [PubMed] [Google Scholar]
- 29.Inghilleri, M., Berardelli, A., Marchetti, P. & Manfredi, M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp. Brain Res.109, 467-472 (1996). [DOI] [PubMed]
- 30.McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 31.Duque J, Greenhouse I, Labruna L, Ivry RB. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 2017;40:219–236. doi: 10.1016/j.tins.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Wildenberg WP, et al. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: A TMS study. J. Cogn. Neurosci. 2010;22:225–239. doi: 10.1162/jocn.2009.21248. [DOI] [PubMed] [Google Scholar]
- 33.Coxon JP, Stinear CM, Byblow WD. Intracortical Inhibition During Volitional Inhibition of Prepared Action. J. Neurophysiol. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald JA, Beauchamp MH, Crigan JA, Anderson PJ. Age-related differences in inhibitory control in the early school years. Child Neuropsychol. 2013;20:509–526. doi: 10.1080/09297049.2013.822060. [DOI] [PubMed] [Google Scholar]
- 35.He JL, et al. Individual differences in intracortical inhibition predict motor-inhibitory performance. Exp. Brain Res. 2019;237:2715–2727. doi: 10.1007/s00221-019-05622-y. [DOI] [PubMed] [Google Scholar]
- 36.Chowdhury NS, Livesey EJ, Harris JA. Contralateral and ipsilateral relationships between intracortical inhibition and stopping efficiency. Neuroscience. 2019;415:10–17. doi: 10.1016/j.neuroscience.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Chowdhury NS, Livesey EJ, Harris JA. Individual differences in intracortical inhibition during behavioural inhibition. Neuropsychologia. 2019;124:55–65. doi: 10.1016/j.neuropsychologia.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury NS, Livesey EJ, Harris JA. Stop signal task training strengthens GABA-mediated neurotransmission within the primary motor cortex. J. Cogn. Neurosci. 2020;32:1984–2000. doi: 10.1162/jocn_a_01597. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury, N. S., Livesey, E. J., Blaszczynski, A. & Harris, J. A. Motor cortex dysfunction in problem gamblers. Addict. Biol.26, e12871 (2020). [DOI] [PubMed]
- 40.Chowdhury NS, Livesey EJ, Blaszczynski A, Harris JA. Pathological gambling and motor impulsivity: A systematic review with meta-analysis. J. Gambl. Stud. 2017;33:1213–1239. doi: 10.1007/s10899-017-9683-5. [DOI] [PubMed] [Google Scholar]
- 41.Hermans L, et al. Age-related alterations in the modulation of intracortical inhibition during stopping of actions. Aging. 2019;11:371–385. doi: 10.18632/aging.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermans L, et al. Brain GABA levels are associated with inhibitory control deficits in older adults. J. Neurosci. 2018;38:7844–7851. doi: 10.1523/JNEUROSCI.0760-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziemann U, et al. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Ziemann, U. Pharmaco-transcranial magnetic stimulation studies of motor excitability. In Handbook of Clinical Neurology 116, 387–397 (2013). [DOI] [PubMed]
- 45.Ziemann U, Lönnecker S, Paulus W. Inhibition of human motor cortex by ethanol A transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 46.Ziemann, U., Lnnecker, S., Steinhoff, B. J. & Paulus, W. The effect of lorazepam on the motor cortical excitability in man. Exp. Brain Res.109, 127-135 (1996). [DOI] [PubMed]
- 47.Deisz R. GABAB receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. doi: 10.1016/S0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 48.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J. Clin. Neurophysiol. 1992;9:212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- 49.Chu J, Gunraj C, Chen R. Possible differences between the time courses of presynaptic and postsynaptic GABAB mediated inhibition in the human motor cortex. Exp. Brain Res. 2007;184:571–577. doi: 10.1007/s00221-007-1125-7. [DOI] [PubMed] [Google Scholar]
- 50.Craig MT, McBain CJ. The emerging role of GABAB receptors as regulators of network dynamics: Fast actions from a ‘slow’ receptor? Curr. Opin. Neurobiol. 2014;26:15–21. doi: 10.1016/j.conb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J. Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J. Physiol. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J. Physiol. 2007;583:971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaves AR, Kelly LP, Moore CS, Stefanelli M, Ploughman M. Prolonged cortical silent period is related to poor fitness and fatigue, but not tumor necrosis factor, in Multiple Sclerosis. Clin Neurophysiol. 2019;130:474–483. doi: 10.1016/j.clinph.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 55.Antunes, L. C. et al. Longer cortical silent period length is associated to binge eating disorder: An exploratory study. Front. Psychiatry11, (2020). [DOI] [PMC free article] [PubMed]
- 56.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 57.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 58.Säisänen L, et al. Factors influencing cortical silent period: Optimized stimulus location, intensity and muscle contraction. J. Neurosci. Methods. 2008;169:231–238. doi: 10.1016/j.jneumeth.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Burden A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25years of research. J. Electromyogr. Kinesiol. 2010;20:1023–1035. doi: 10.1016/j.jelekin.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Mathiassen SE, Winkel J, Hägg GM. Normalization of surface EMG amplitude from the upper trapezius muscle in ergonomic studies—A review. J. Electromyogr. Kinesiol. 1995;5:197–226. doi: 10.1016/1050-6411(94)00014-X. [DOI] [PubMed] [Google Scholar]
- 61.Farzan F, et al. The EEG correlates of the TMS-induced EMG silent period in humans. Neuroimage. 2013;83:120–134. doi: 10.1016/j.neuroimage.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verbruggen F, Logan GD, Stevens MA. STOP-IT: Windows executable software for the stop-signal paradigm. Behav. Res. Methods. 2008;40:479–483. doi: 10.3758/BRM.40.2.479. [DOI] [PubMed] [Google Scholar]
- 63.Brainard DH. The psychophysics toolbox. Spat. Vis. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- 64.Kleiner, M., Brainard, D., Pelli, D., Ingling, A., Murray, R. & Broussard, C. What's New in Psychtoolbox-3. Perception36, 1–16 (2007).
- 65.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997;10:437–442. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- 66.Congdon, E., Mumford, J. A., Cohen, J. R., Galvan, A., Canli, T. & Poldrack, R. A. Measurement and Reliability of Response Inhibition. Front. Psychol.3, 1-10 (2012). [DOI] [PMC free article] [PubMed]
- 67.Wilcox R. Inferences based on a skipped correlation coefficient. J. Appl. Stat. 2004;31:131–143. doi: 10.1080/0266476032000148821. [DOI] [Google Scholar]
- 68.Pernet, C. R., Wilcox, R. & Rousselet, G. A. Robust correlation analyses: False positive and power validation using a new open source matlab toolbox. Front. Psychol.3, 606 (2013). [DOI] [PMC free article] [PubMed]
- 69.Koul, A., Becchio, C. & Cavallo, A. Cross-validation approaches for replicability in psychology. Front. Psychol.9, 1117 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed during this study are available from the corresponding author upon request.