Abstract
Impaired extinction of conditioned fear is associated with anxiety disorders. Common lifestyle factors, like isolation stress and exercise, may alter the ability to extinguish fear. However, the effect of and interplay between these factors on adolescent fear extinction, and the relevant underlying neural mechanisms are unknown. Here we examined the effects of periadolescent social isolation and physical activity on adolescent fear extinction in rats and explored neurogenesis as a potential mechanism. Isolation stress impaired extinction recall in male adolescents, an effect prevented by exercise. Extinction recall in female adolescents was unaffected by isolation stress. However, exercise disrupted extinction recall in isolated females. Extinction recall in isolated females was positively correlated to the number of immature neurons in the ventral hippocampus, suggesting that exercise affected extinction recall via neurogenesis in females. Pharmacologically suppressing cellular proliferation in isolated adolescents using temozolomide blocked the effect of exercise on extinction recall in both sexes. Together, these findings highlight sex-specific outcomes of isolation stress and exercise on adolescent brain and behavior, and highlights neurogenesis as a potential mechanism underlying lifestyle effects on adolescent fear extinction.
Keywords: Stress, Sex differences, Adolescence, Exercise, Neurogenesis
Graphical abstract
Highlights
-
•
Periadolescent isolation stress disrupted extinction recall in male adolescents.
-
•
Running prevented isolation-induced extinction recall deficit in male adolescents.
-
•
Exercise impaired extinction recall in isolated female adolescents.
-
•
Exercise increased hippocampal neurogenesis, except in isolated males.
-
•
Suppression of neurogenesis blocked exercise effects in isolated adolescents.
1. Introduction
Anxiety-related disorders are the most prevalent forms of psychopathology in adolescence, with a world-wide prevalence of 4.7–9.1% (Polanczyk et al., 2015). In fact, adolescents experience anxiety disorders more than any other age group (Merikangas et al., 2009). Development and persistence of these disorders are closely tied to lifestyle factors such as stress and lack of physical activity (Bélair et al., 2018; Kantomaa et al., 2008; Kingery et al., 2010; Scaini et al., 2014; Schiele and Domschke, 2018; Teychenne et al., 2015). Sadly, global trends suggest reduced in-person interactions of children with their peers (George and Odgers, 2015; Griffiths, 1997; Orben et al., 2020; Twenge and Spitzberg, 2020) and increasing childhood inactivity (Brownson et al., 2005; Gray et al., 2014; Guthold et al., 2020; Rosenfeld, 2016; Ziviani et al., 2008). This highlights the critical need to study the contributions of social isolation stress and/or exercise to anxiety disorders in adolescence. Further, how these prevalent factors may differentially affect females and males is poorly understood, despite known sex differences in developmental stress-induced brain changes (Perry et al., 2021) and anxiety-related psychopathology in adolescence (Altemus et al., 2014; Lewinsohn et al., 1998; Merikangas et al., 2009; Roza et al., 2003).
A key feature of anxiety disorders is the failure to appropriately inhibit, or extinguish, fear (Maren et al., 2013; Waters et al., 2009). Typically in fear extinction, a fear-eliciting stimulus is repeatedly presented in the absence of any aversive outcomes, ultimately reducing the fear response (Myers and Davis, 2007). Extinction is evolutionarily conserved between humans and rodents (Sevenster et al., 2018), and extinction studies in humans and rodents have driven our understanding of anxiety disorder treatment (Hofmann and Smits, 2008; Kim and Ganella, 2015; Maren et al., 2013). Relative to adults, adolescent humans and rodents have impaired fear extinction (Ganella et al., 2018a; Kim et al., 2011; McCallum et al., 2010; Pattwell et al., 2012). This is also reflected clinically, with worse treatment outcomes for adolescents compared to other ages (Ollendick and Davis III, 2013). Sex differences in extinction is observed across all stages of development (Baran et al., 2009; Clark et al., 2019; Day and Stevenson, 2019; Park et al., 2017; Perry et al., 2020), supporting the use of fear extinction as a model to understand cognitive-affective processing relevant for anxiety disorders in adolescence.
Sex- and age-specific fear extinction involves interactions between the prefrontal cortex, hippocampus (HPC), and amygdala (Day and Stevenson, 2019; Ganella et al., 2018b; Velasco et al., 2019). Of these regions, the HPC integrates information about our surroundings (Kentner et al., 2019; Rudy, 2009) and adapts to change through neurogenic mechanisms (Kempermann et al., 1997). Notably, periadolescent isolation stress can impair neurogenesis (Ibi et al., 2008) and prevent exercise-induced neurogenesis in adolescence (Kozareva et al., 2019). While neurogenic manipulations in adulthood generally do not alter cue extinction learning (Drew et al., 2010; Kim and Fanselow, 1992; Olsen et al., 2014; Phillips and LeDoux, 1992), their impact on adolescent fear extinction is poorly understood. New neurons are produced and integrated in adolescence at up to four times the rate of adulthood (He and Crews, 2007), which strongly suggests neurogenic manipulations and associated neurogenesis may have profound effects on adolescent fear extinction.
In the present study, we assessed the effects of periadolescent social isolation stress and voluntary running on cued fear extinction in male and female adolescent rats. We also explored whether these factors cause neurogenic changes in the HPC. The dorsal and ventral regions of the HPC (dHPC and vHPC) are neurochemically and functionally distinct (Fanselow and Dong, 2010; Lothmann et al., 2021; Park et al., 2020; Strange et al., 2014), with vHPC proposed to be more stress-responsive than dHPC (Fanselow and Dong, 2010). Therefore, the effects of social isolation and voluntary running on neurogenesis in dHPC and vHPC were examined separately. By pharmacologically inhibiting proliferating cells in isolated adolescent rats, we further examined whether cellular proliferation was a mechanism by which exercise altered cued fear extinction in isolated adolescents. We observed that social isolation attenuated fear extinction recall only in male adolescents. Wheel running rescued extinction recall in isolated males, whereas it disrupted extinction recall only in isolated females. Suppression of proliferating cells during periadolescent isolation attenuated the effects of exercise on extinction recall in both sexes.
2. Materials and methods
2.1. Animals
Sprague-Dawley rats were obtained from the breeding colony at the Florey Institute of Neuroscience and Mental Health (Melbourne, Australia), with breeders from the Animal Resource Centre (Perth, Australia). Each sex was housed in separate rooms on a 12:12hr light:dark cycle (lights on: 07:00). Standard chow and water were provided ad libitum. All procedures were approved by the Animal Ethics Committee at the Florey Institute of Mental Health under the guidelines of the National Health and Medical Research Council Code of Practice for the Care and Use of Animals Experimental Purposes in Australia (National Health and Medical Research Council, 2013).
2.2. Rearing
From post-natal day 21 (P21) until perfusion, rats were randomly allocated to different rearing conditions within an open-top opaque plastic cage (40.64 × 50.8 × 41.91 cm) that contained a 35.56 cm diameter running wheel (Lafayette Instrument Company, Indiana, USA). All conditions were unchanged throughout experimentation (i.e., isolation and running conditions remained throughout fear conditioning, extinction, and testing periods).
2.2.1. Environment
Rats were either same sex grouped (3/cage; ‘grouped’) or isolated (1/cage; ‘isolated’). Cages allowed visual, auditory and olfactory access to other rats.
2.2.2. Exercise
Rats were either unable to use the running wheel (locked wheel; ‘sedentary’) or had continual running wheel access (‘running’). In Experiment 1b only, rats had partial access during their active phase (wheel locked 01:00–07:00, ‘restricted running’). Running was quantified through an infra-red counter using Scurry Activity Monitoring Software (Lafayette Instrument Company).
2.3. Temozolomide (TMZ) treatment
The DNA-alkylating agent TMZ (Temodar®; T2744, Tokyo Chemical Industry Co. Ltd, Japan) was dissolved into 10% dimethyl sulfoxide (DMSO, Sigma Aldrich) in saline. The control vehicle (VEH) solution was prepared similarly with 10% DMSO in saline. The TMZ or VEH solution was intraperitoneally (i.p.) injected at a dose of 25 mg/kg using a standard protocol (i.e., once-daily injection for three consecutive days per week for three weeks (Akers et al., 2014; Garthe et al., 2009)).
2.4. Conditioned fear
From P43, a conditioned fear paradigm was given during the light-phase in previously described apparatus (Park et al., 2017; Zbukvic et al., 2017). Two distinct contexts located in separate rooms were used. In brief, context A had stainless-steel side walls and an opaque Perspex® rear wall with round stickers, with a tray of aspen bedding underneath the flooring, and cleaned with a eucalyptus-scented agent. Context B had a curved white Perspex® wall insert, with a tray lined with two paper towels underneath and cleaned with 80% ethanol. The Med Associates VideoFreeze® System (Med Associates, VT, USA) was used to program the delivery of all auditory and foot-shock stimuli, and to record all freezing behaviors using infrared cameras. The following protocol was used for behavioral testing:
2.4.1. Fear conditioning (P43)
Rats were placed in a novel chamber (context A) and after a 2min baseline period were presented with six 10s tone conditioned stimulus (CS; 5000 Hz, 80 dB) that co-terminated with a 1s foot-shock (1 mA). The mean inter-trial interval (ITI) was 110s.
2.4.2. Extinction 1 (P44) and 2 (P45)
Rats were placed in a new chamber (context B) and after a 2min baseline period 10s CS were presented 60 times (10s ITI) daily for 2 days.
2.4.3. Test (P48)
Rats were placed back into the extinction context to receive a 2min baseline period followed by 15 trials of 10s CS (10s ITI).
2.5. Estrous cycle monitoring
Vaginal lavages on females were performed 2hrs after behavioral testing as previously described (Perry et al., 2020). Males were also handled 2hrs after each behavioral testing to match the females.
2.6. Perfusion and sectioning
Standard perfusion and sectioning protocols were used, as previously described (Charlton et al., 2019). In brief, at P49, rats were transcardially perfused with 50 ml 0.1 M phosphate-buffered saline (PBS) followed by 250 ml 4% paraformaldehyde (PFA, Sigma Aldrich) in PBS. Brains were post-fixed in PFA for 1hr then kept in 20% sucrose PBS overnight at 4 °C. The brains were frozen then coronal sections (40 μm) were taken using a cryostat (Leica Biosystems, NSW, Australia) and stored in sodium azide (0.1% w/v) in PBS at 4 °C.
2.7. Immunohistochemistry
Ki-67 and doublecortin (DCX) were chosen as neurogenesis markers. Ki-67 is a protein present in cells undergoing proliferation (Kee et al., 2002), whilst DCX is expressed in immature neurons (Brown et al., 2003). In brief, the following immunohistochemistry protocol was used. Free-floating sections were pre-treated with 2 M hydrochloric acid (Sigma-Aldrich, CAT 320331) for 30min and directly placed into 0.1 M boric buffer (0.1 M boric acid and 0.02 M sodium tetraborate) for 20min at room temperature (RT). The sections were then blocked for 30 min in a solution containing normal donkey serum (NDS; 1:10; Millipore, USA), Triton X-100 (Tx-100; 1:200; BDH Chemicals, Australia) and 0.1 M phosphate buffer (PB). After this they were incubated in a primary antibody solution for 72hrs at 4 °C which contained: mouse anti-DCX monoclonal antibody (1:500, SC-271390, Santa Cruz Biotechnology), NDS (1:100) and Tx-100 (1:200) in 0.1 M PB. In the last 24hrs of incubation, rabbit anti-Ki-67 monoclonal antibody (1:1000, MA5-14520, Thermofisher Scientific) was added to the solution. Afterwards, sections were blocked for 1hr at RT (1:10 NDS, 1:200 Tx-100, 0.1 M PB). Subsequently they were incubated in a secondary antibody solution for 2hrs at RT that contained: donkey anti-rabbit IgG (1:500, Alexa Fluor® 488, Life Technologies, CA, USA), donkey anti-mouse IgG (1:500, Alexa Fluor® 594) and NDS (1:100) in 0.1 M PB. All sections were washed three times for 10min in 0.1 M PBS between each protocol step unless otherwise stated. Sections were mounted onto gelatin-coated slides and coverslipped with DAKO fluorescent mounting medium (Campbellfield Victoria, Australia). Samples of 3–6 brains per group were assessed with immunohistochemistry for Ki-67 and DCX expression.
2.8. Image acquisition and cell quantification
Fluorescent images were captured using a Zeiss AxioImager M2 (Zeiss, Göttingen, Germany) and the whole dentate gyrus (DG) was examined in 1:8 sections (320 μm apart). An observer blind to experimental group quantified Ki-67+, DCX+ and Ki-67+DCX+ cells in the dorsal (dHPC; AP: −2.8 mm to −4.3 mm) and ventral hippocampus (vHPC; AP: −4.8 mm to −6.3 mm) with the software package ImageJ (Version 1.5, National Institutes of Health, USA) as described in (O'Leary et al., 2019). See Fig. S1 for anatomical definition of dorsal and ventral hippocampus used in immunohistochemical analyses.
2.9. Statistical analysis
Analyses used SPSS IBM (IBM, Armonk NY, USA) and GraphPad Prism (V7, La Jolla, CA), applying analysis of variance (ANOVA), t-tests, and Pearson correlations. Significant interactions were followed up with post hoc tests with Bonferroni corrections. Outliers were defined a priori as previously described (Robinson-Drummer and Stanton, 2014). See Table S1 for the sample sizes and number of exclusions for all experiments. Sample sizes are also reported in figure captions.
Running wheel activity, as measured by mean daily distance per week was analyzed separately for grouped and isolated rats because data were collected per box. The grouped running distance per box is for three rats whereas the isolated running distance per box is for a single rat. Freezing was calculated via automated near-infrared video tracking equipment as previously reported (Ganella et al., 2017; Perry et al., 2020). In extinction sessions, trials were averaged into blocks of five for analysis. For test, the averaged CS-elicited freezing in response to 15 tones was analyzed. Behavioral and neural analyses are reported per Sex due to extensive interactions involving Sex throughout the study (for overall ANOVAs with Sex included, see Supplementary Results 1–4), which led us to hypothesize sex-specific effects of Environment and Exercise. There were no group differences in baseline freezing levels throughout the study. Pearson correlations were performed for each Environment for average % freezing at test against either Ki67+ or DCX+ cell counts.
3. Results
3.1. Sex-specific effects of isolation and voluntary wheel running on adolescent extinction of conditioned fear
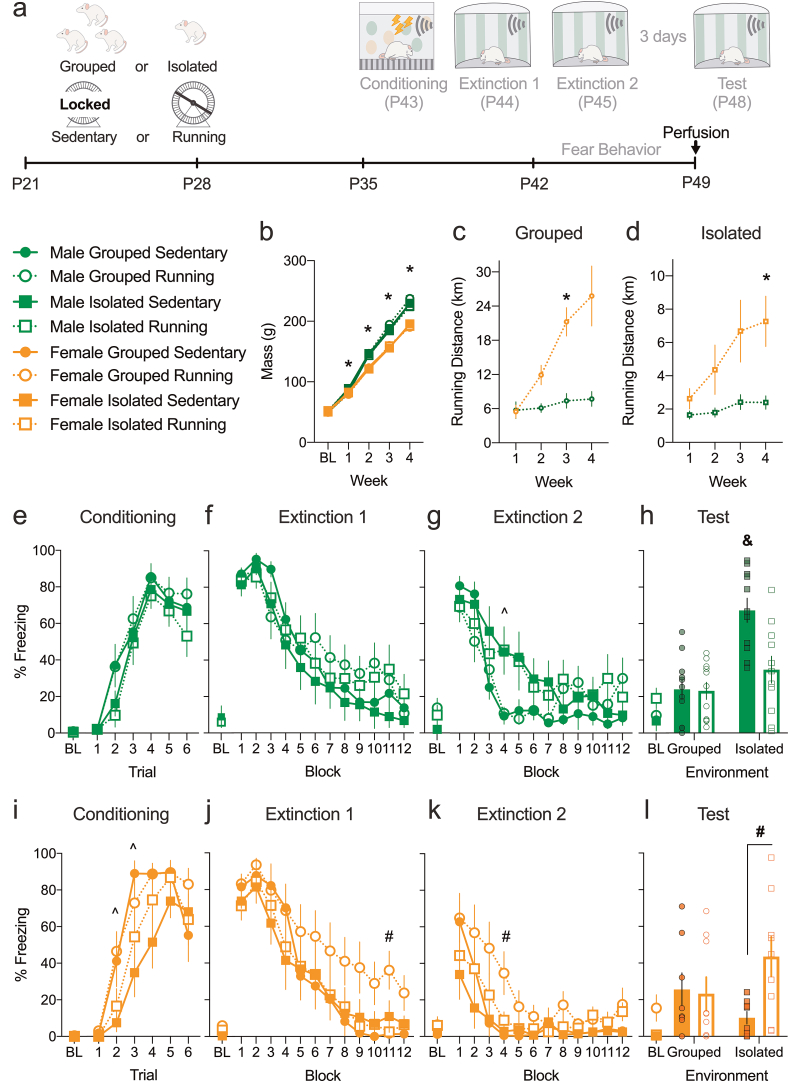
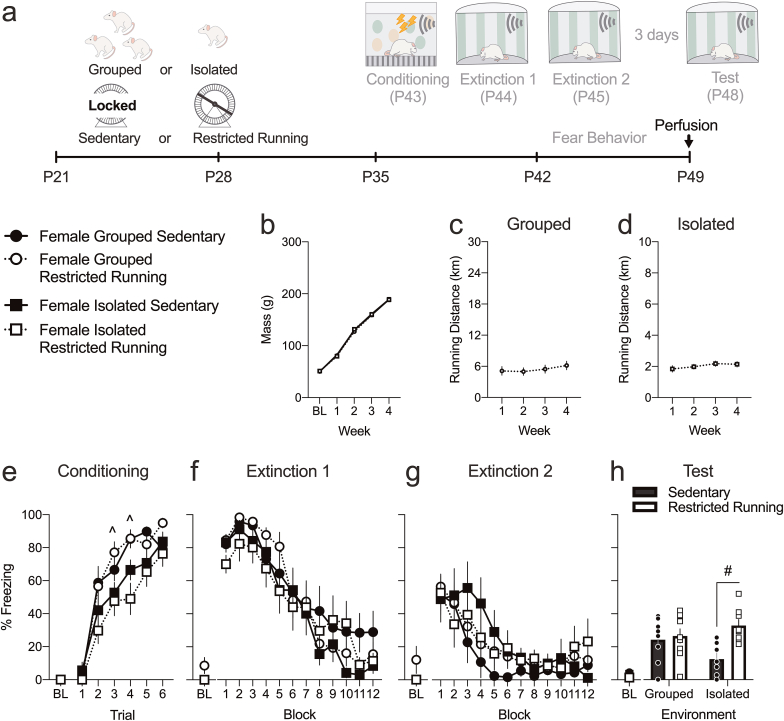
Experiment 1a examined the effect of periadolescent isolation stress and/or voluntary running on cued fear extinction in adolescent rats. Experiment 1a is an Environment (grouped vs isolated) x Exercise (sedentary vs running) design with male and female adolescents (Fig. 1a).
Fig. 1.
Social isolation stress and exercise differentially alter extinction in adolescent males and females. a. Timeline: male and female P21 rats were reared in different conditions and underwent behavioral testing P43–P48. b. Weekly weights were unaffected by rearing conditions. Males were heavier than females at weeks 1–4. Females ran more than males at week 3 for c. grouped and week 4 for d. isolated rats. e & i. Isolated females, but not males, had a transient delay in acquiring CS-elicited freezing during conditioning. f, g, j & k. Exercise transiently delayed acquisition of extinction only in grouped females in extinction session 1 and 2. Extinction 2 was transiently delayed in isolated males. h. Isolated males froze more compared to grouped males at test. Exercise mitigated this effect. l. Exercise increased freezing only in isolated females. Values are means ± SEM. For weight and fear behaviors, n = 7–11 per group per sex. For running, grouped n = 3–4 boxes per sex and individual n = 9–10 boxes per sex. *Post hoc effect of Sex (p < 0.05). ^Post hoc effect of Environment (p < 0.05). #Post hoc effect of Exercise (p < 0.05). &Significant post hoc difference compared to all other groups (ps < 0.05). g, gram; km, kilometers; P, postnatal day; BL, baseline.
3.1.1. Weight
All rats gained weight (Week, F (4,276) = 4486.164, p < 0.001) but females gained less weight compared to males (Sex, F (1,69) = 155.283, p < 0.001; Week × Sex interaction, F (4,276) = 69.967, p < 0.001). Post hoc tests showed significant Sex effects in weeks 1–4 (ps < 0.05). There were no other effects or interactions (Fig. 1b).
3.1.2. Running
In grouped rats, significant effects of Week (F (3,15) = 17.24, p < 0.001), Sex (F (1,5) = 18.593, p = 0.008) and Week × Sex interaction (F (3,15) = 11.757, p < 0.001) indicated that females escalated to higher weekly averaged daily running distance compared to males (Fig. 1c). Post hoc tests showed a significant effect of Sex at week 3 (p = 0.012). Similar results were observed in isolated rats with effects of Week (F (3,48) = 10.776, p < 0.001), Sex (F (1,16) = 5.331, p = 0.035) and Week × Sex interaction (F (3,48) = 4.647, p = 0.006), with post hoc tests indicating females running significantly more at week 4 (p = 0.032, Fig. 1d).
3.1.3. Isolation transiently impaired the acquisition of fear in females
All rats acquired conditioned fear, as indicated by a significant effect of Conditioning Trial for males (F (5,195) = 69.995, p < 0.001) and females (F (5,150) = 62.026, p < 0.001). In males, Environment and Exercise had no effects or interactions on the acquisition of CS-elicited freezing (Fig. 1e). In females, isolation caused a decrease in freezing levels during trials 2 and 3 (Conditioning Trial × Environment interaction, F (5,150) = 3.375, p = 0.006; post hoc tests significant at trials 2 and 3 (p = 0.024 & p = 0.018)), without any Exercise effect or interactions (Fig. 1i). This demonstrates that isolation transiently delays the acquisition of conditioned fear in adolescent females but not males.
3.1.4. Environment and Exercise have sex-specific effects on adolescent extinction learning
All rats extinguished their CS-elicited freezing response in the first extinction session, evident by the significant effects of extinction Block for both males (F (11,429) = 67.315, p < 0.001) and females (F (11,319) = 47.726, p < 0.001). In males, there were no other effects except for Block × Exercise interaction (F (11,429) = 2.244, p = 0.012; Fig. 1f), but post hoc tests were not significant. In females, there was Environment x Exercise × Block interaction (F (11,330) = 2.219, p = 0.013), with post hoc tests significant at block 11 (p = 0.012, Fig. 1j). This suggests that exercise in grouped females delayed extinction acquisition.
Despite no group differences in the first extinction session, isolated male adolescents showed significantly delayed second extinction (Block, F (11,429) = 30.951, p < 0.001; Block × Environment interaction, F (11,429) = 3.167, p < 0.001), with post hoc tests showing an Environment effect at block 4 (p = 0.005) but no others (Fig. 1g). In female adolescents, exercise delayed extinction (Block, F (11,330) = 22.576, p < 0.001; Exercise, F (1,30) = 7.480, p = 0.010; Block × Exercise interaction, F (11,330) = 2.029, p = 0.025). Post hoc tests showed an Exercise effect at block 4 (p = 0.048, Fig. 1k). There were no other effects.
3.1.5. Exercise rescued isolation-induced extinction recall impairment in males, while disrupting it only in isolated females
When extinction recall was tested three days later, males showed effects of Environment (F (1,39) = 13.15, p = 0.001), Exercise (F (1,39) = 4.801, p = 0.034), and Environment × Exercise interaction (F (1,39) = 4.267, p = 0.046). Post hoc tests revealed that isolated-sedentary group was significantly different from all other groups (ps < 0.05), demonstrating that while isolation stress impaired extinction recall in adolescent males, exercise rescued this impairment (Fig. 1h). Females showed effects of Exercise (F (1,30) = 4.783, p = 0.037) and Environment × Exercise interaction (F (1,30) = 6.055, p = 0.020), but no Environment effect (Fig. 1). Post hoc tests revealed that exercise significantly increased freezing only in isolated females (p = 0.034), with no other group differences. Thus, while exercise rescues extinction recall deficits in isolated males, exercise disrupts extinction recall in isolated females.
3.1.6. Isolation attenuated the neurogenic effect of exercise in male but not female adolescents
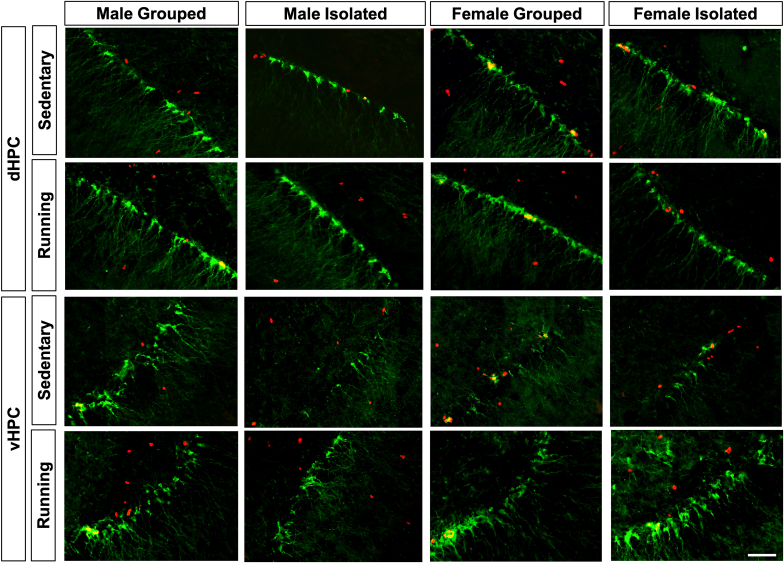
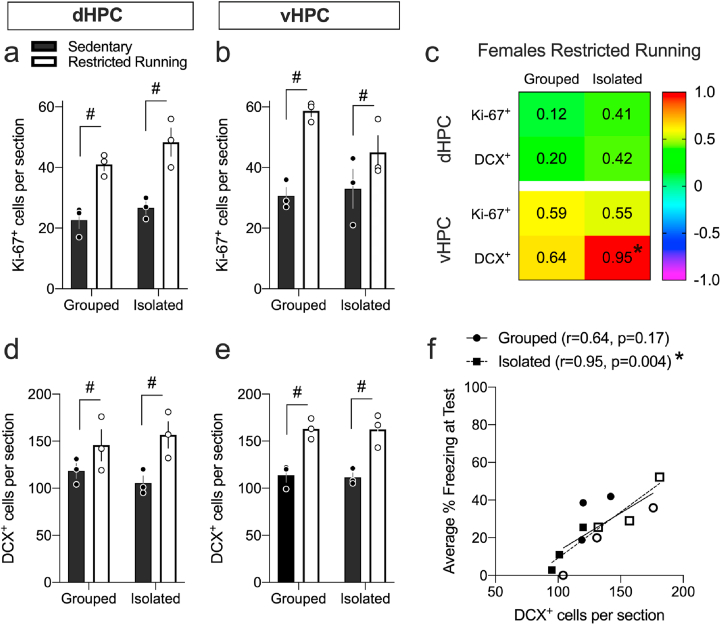
Cells expressing neurogenic markers Ki-67+ and DCX+ were counted (Fig. 2). Dorsal (dHPC) and ventral hippocampus (vHPC) regions counted are defined in Fig. S1.
Fig. 2.
Representative images of the dentate gyrus showing Ki-67+ cells (red), DCX+ cells (green), and double labelled Ki-67+DCX+ cells (yellow). Scale bar = 25 μm dHPC, dorsal hippocampus; vHPC, ventral hippocampus. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
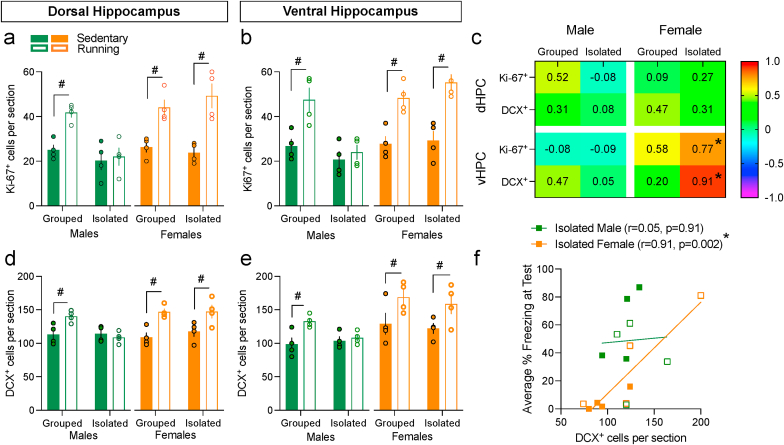
In males, ANOVA revealed significant effects of Environment, Exercise, and Environment × Exercise interaction (ps < 0.05) in the number of Ki-67+ and DCX+ cells in dHPC (Fig. 3a and d) and vHPC (Fig. 3b and e). Post hoc tests showed that running increased Ki-67+ and DCX+ cells only in grouped males in dHPC and vHPC (ps < 0.05). In females, running increased Ki-67+ and DCX+ cells in dHPC and vHPC (ps < 0.05), without any other effects (Fig. 3a, b, d & e). Although there were Ki-67+DCX+ double-labelled cells in dHPC and vHPC (Fig. 2) with ~30% of Ki-67+ cells double-labelled for DCX+ (Table S2), this percentage was not significantly altered by rearing environment. Taken together, isolation alone had no effect on proliferation (Ki-67+) or neuronal differentiation (DCX+) in either sex but the pro-neurogenic effects of running were prevented only in isolated male adolescents.
Fig. 3.
Voluntary wheel running increases hippocampal neurogenesis depending on isolation stress in males but not in females a, b, d & e. Exercise increased Ki-67+ and DCX+ cells in dHPC and vHPC in all groups except in isolated males. c. Pearson's correlation (r) values between average % CS-elicited freezing at Test and Ki-67+ or DCX+ cell counts. A signficant positive association exists in the vHPC of isolated female adolescents only. Color corresponds to the r value. f. As an example, individual data points displayed for the correlations between average % CS-elicited freezing at Test and vHPC DCX+ cell counts in isolated adolescents, showing that there is a signficant correlation in isolated females. #Exercise main or post hoc effect (p < 0.05). *Significant correlation (p < 0.05). Values are means ± SEM. n = 4–5 per group per sex. For correlations, n's were pooled across running condition. dHPC, dorsal hippocampus; vHPC, ventral hippocampus. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Average % CS-elicited freezing at test and Ki-67+ or DCX+ counts in the vHPC of isolated female adolescents significantly and positively correlated (p = 0.026 & p = 0.002, Fig. 3c). There were no other significant correlations. Fig. 3f depicts individual data points plotted for average CS-elicited freezing at Test and vHPC DCX+ cell counts in isolated adolescents as an example. The signficant correlation in isolated females suggest vHPC neurogenesis promotes freezing (i.e., impairs extinction recall) at test.
3.2. Experiment 1b: temporal restriction of running wheel activity in females did not change the effects of running and isolation stress on extinction recall
In Experiment 1a, females ran more than males. To assess whether the changes in behavior and brain observed in Experiment 1a occur in female rats with a level of running comparable to males, Experiment 1b locked the wheels during the last 6 h of dark cycle in a new cohort of female adolescents. Experiment 1b is an Environment (grouped vs isolated) x Exercise (sedentary vs restricted running) design with female adolescents (Fig. 4a).
Fig. 4.
Social isolation stress and restricted voluntary wheel running activity alters extinction of learned fear in adolescent females. a. Timeline: females were reared in different conditions and underwent behavioral testing P43-48. b. Weekly weights of female rats were not affected by Environment and Restricted Exercise factors. Weekly mean daily running wheel activity was stable across weeks for c. grouped and d. isolated. e. During conditioning, isolated females had a transient delay in acquiring CS-elicited freezing compared to grouped females. f & g. All rats simarly decreased their CS-elicited freezing across extinction sessions 1 and 2 regardless of rearing condition. h. Restricted exercise impaired extinction recall in isolated females. Values are means ± SEM. For weight and fear behaviors, n = 7–9 per group per sex. For running data, grouped n = 3 boxes and individual n = 8 boxes per sex. ^Post hoc effect of Environment (p < 0.05). #Post hoc effect of Exercise (p < 0.05). g, gram; km, kilometers; P, postnatal day; BL, baseline.
3.2.1. Weight and running
Rats gained weight over time (Week, (F (4,112) = 2736.273, p < 0.001)). There were no other effects or interactions involving Environment or Restricted Exercise (ps < 0.05, Fig. 4b). Both grouped and isolated female rats ran stable distances, with the effect of Week not significant in either condition (Fig. 4c & d). Running distances recorded in Experiment 1b were similar to those for the males in Experiment 1a (Fig. S2).
3.2.2. Conditioned fear extinction in female adolescents with restricted running
Isolation stress again transiently delayed acquisition of conditioned fear (Fig. 4e). There were significant effects of Conditioning Trial (F (5,140) = 84.967, p < 0.001), Environment (F (1,28) = 14.858, p = 0.001) and Conditioning Trial × Environment interaction (F (5,140) = 2.893, p = 0.016). Post hoc tests showed Environment effect at trials 3 and 4 (p = 0.03 & p = 0.002) but no others. There were no other effects.
In both extinction sessions, only Extinction Block was significant (ps < 0.05, Fig. 4f and g), which suggests the exercise effects on within-session extinction in females in Experiment 1a (Fig. 1j and k) were due to their escalated running.
At test, there was a significant Restricted Exercise × Environment interaction (F (1,28) = 4.626, p=0.0403) and no other effects. Post hoc tests indicated restricted exercise increased freezing only in isolated (p = 0.005) females (Fig. 4h). Together with Experiment 1a, this shows exercise – restricted or free – impairs extinction recall in isolated females.
3.2.3. Restricted voluntary wheel running increased neurogenesis in female adolescents regardless of isolation stress
Analyses of Ki-67+ and DCX+ counts in the dHPC and vHPC revealed significant effects of Restricted Exercise (ps < 0.005, Fig. 5a, b, d & e) but no other effects. The percentage of Ki-67+ cells double-labelled for DCX+ was not significantly altered by rearing conditions (Table S2). There was a significant correlation between average freezing at test and vHPC DCX+ cell counts in isolated females (Fig. 5c and f). There were no other significant correlations.
Fig. 5.
a, b,d & e. Restricted exercise increased Ki-67+ and DCX+ cells in dHPC and vHPC regardless of isolation stress. c. Pearson's correlation (r) values between average % CS-elicited freezing at Test and Ki-67+ or DCX+ cell counts. Color corresponds to the r value. f. A signficant positive correlation was observed between average % CS-elicited freezing at Test and vHPC DCX+ cell counts in isolated, but not grouped, females, showing that there is a signficant correlation in isolated females. #Exercise main effect (p < 0.05). *Significant correlation (p < 0.05). Values are means ± SEM. n = 3 per group per sex. For correlations, n's were pooled across running condition. dHPC, dorsal hippocampus; vHPC, ventral hippocampus. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
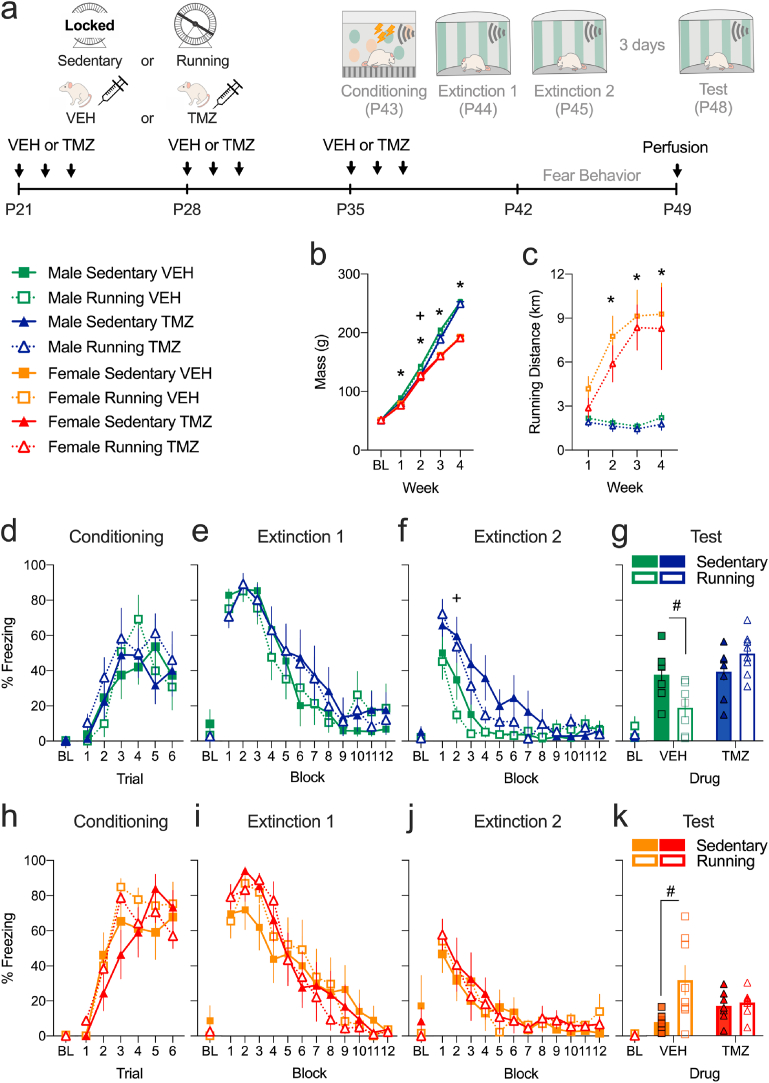
3.3. Experiment 2: pharmacological suppression of neurogenesis in isolated female and male adolescents
In Experiments 1a & b, exercise affected fear extinction recall in isolated stressed adolescent rats. Further, fear extinction recall correlated with Ki-67+ or DCX+ cell counts in vHPC of isolated females only, suggesting a role for neurogenesis in extinction recall in isolated rats. To investigate whether periadolescent exercise mediates its effects in isolated adolescents through a cytogenic mechanism, we pharmacologically suppressed cytogenesis and examined the effect on extinction recall in isolated adolescents. Experiment 2 is Exercise (sedentary vs running) x Drug (TMZ-treated vs VEH-treated) design in male and female adolescents within an isolated environment (Fig. 6a). Considering that the amount of running did not affect exercise-impaired extinction recall in isolated females (Experiments 1a and 1b), all running groups in Experiment 2 had continual access to the running wheel to best match rearing conditions between the sexes.
Fig. 6.
TMZ-treatment blocks the effect of exercise on fear extinction recall in isolated adolescent male and female rats. a. Timeline: isolated adolescents received either TMZ or VEH treatment and underwent behavioral testing P43-8. b. TMZ-treatment reduced weight only at week 2 only in males. c. Females ran more than males at week 2,3 and 4. d & h. Rats similarly acquired conditioned fear. e & i. Extinction 1 was comparable across groups. f & j. TMZ-treatment increased CS-elicited freezing at extinction 2 block 2 only in male adolescents. g. VEH-treated sedentary males froze more than their exercising counterparts when isolation-reared. TMZ-treatment blocked this effect of exercise. k. VEH-treated sedentary females froze less than their exercising counterparts when isolation-reared. TMZ-treatment blocked the effect of exercise. Values are means ± SEM. For running, weight and fear behaviors, n = 7–11 per group per sex. *Post hoc effect of Sex (p < 0.05), +Post hoc effect of Drug (p < 0.05) #Post hoc effect of Exercise (p < 0.05). g, gram; km, kilometers; P, postnatal day; BL, baseline.
3.3.1. Weight and running
All rats gained weight (Week, F (4,212) = 7324.141, p < 0.0001) with males gaining more weight than females (Sex, F (4,212) = 219.774, p < 0.0001; post hoc effects at week 1–4, ps < 0.01). TMZ-treatment transiently delayed weight gain in males (Week x Sex × Drug interaction, F (4,212) = 3.933, p = 0.004; Sex × Drug interaction with Drug effect only in males at week 2, p = 0.005). There was no effect of TMZ-treatment on weight gain by behavioral testing (week 3, Fig. 6b). Consistent with Experiment 1a, females ran more than males (Week (F (3,81) = 7.36, p < 0.001, and Week × Sex interaction (F (3,81) = 9.054, p < 0.001)), with post hoc tests revealing a significant effect of Sex at weeks 2–4 (ps < 0.005). There were no effects/interactions involving Drug on running (Fig. 6c).
3.3.2. Conditioned fear extinction in isolated adolescents following TMZ-treatment
The main effect of Conditioning Trial was significant in both males (F (5,130) = 14.393, p < 0.001) and females (F (5,135) = 49.075, p < 0.001). Neither Drug nor Exercise had effects or interactions on CS-elicited freezing in either sex (Fig. 6d and h).
In the first extinction session, there was a significant effect of Extinction Block in males (F (11,286) = 48.677, p < 0.001) and females (F (11,297) = 50.923, p < 0.001), without any other effects or interactions (Fig. 6e and i). In the second extinction session, CS-elicited freezing was reduced in both male (F (11,286) = 36.96, p < 0.001) and female (F (11,297) = 19.939, p < 0.001) adolescents. In males there was a main effect of Drug (F (1,26) = 8.905, p = 0.006) and Extinction Block × Drug interaction (F (11,286) = 4.541, p < 0.001). Post hoc tests revealed significant effect of Drug at block 2 (p = 0.024, Fig. 6f), indicating a transient delay in second extinction due to TMZ treatment in males. In females there were no significant effects or interactions of either Drug or Exercise (Fig. 6j).
At test, males showed effects of Drug (F (1,26) = 11.611, p = 0.002) and Drug × Exercise interaction (F (1,26) = 9.333, p = 0.005). Post hoc tests revealed that VEH-treated males had impaired extinction recall but exercise rescued this (p = 0.027). However, exercise effects were not observed in TMZ-treated males that showed high levels of freezing (Fig. 6g). In females, there was an effect of Exercise (F (1,27) = 7.207, p = 0.012) and Drug × Exercise interaction (F (1,27) = 5.131, p = 0.032). Post hoc tests revealed that exercise impaired extinction recall in VEH-treated females (p = 0.014) but not in TMZ-treated females that showed low levels of freezing (Fig. 6k). Overall, TMZ-treatment blocked the effects of exercise on extinction recall at test in isolated adolescents.
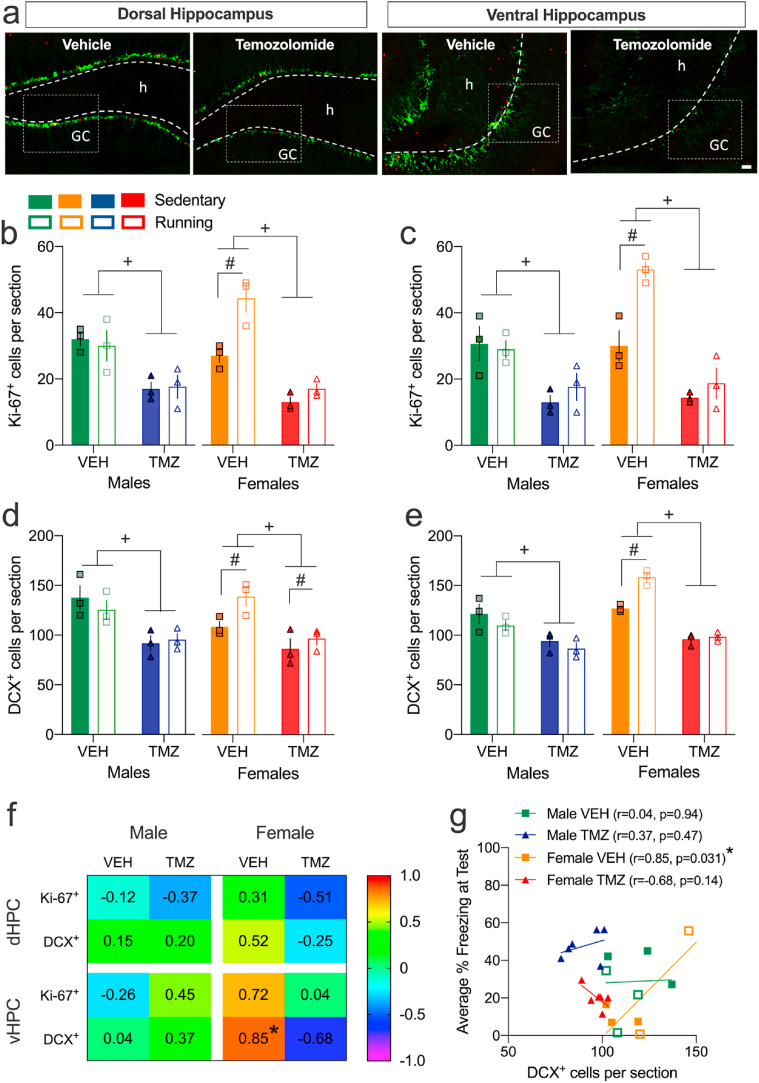
3.3.3. TMZ-treatment suppressed neurogenesis and attenuated the neurogenic effect of voluntary wheel running in female adolescents
TMZ reduced neurogenic markers in dHPC and vHPC (Fig. 7a). In males, analyses of Ki-67+ and DCX+ cells in the dHPC and vHPC revealed significant effects of Drug (ps < 0.05) without any other effects (Fig. 7b–e). In females, there were significant effects of Exercise, Drug and Exercise × Drug interaction for Ki-67+ in each brain region and for DCX+ in vHPC (ps < 0.05). Post hoc tests showed that running increased Ki-67+ or DCX+ cells only in VEH-treated adolescents (ps < 0.05). There were also significant effects of Exercise (F (1,12) = 10.355, p = 0.012) and Drug (F (1,12) = 62.458, p < 0.001) for DCX+ cells in the female dHPC but no interaction (Fig. 7d). Analyses of percentage of Ki-67+ cells double-labelled for DCX+ revealed no effect of Drug, Exercise or any interactions (Table S3). Consistent with Experiments 1a and 1 b, average % CS-elicited freezing at test and vHPC DCX+ cell counts were significantly and positively correlated for VEH-treated females (p = 0.031; Fig. 7f and g). There were no other significant correlations.
Fig. 7.
Pharmacological supression of neurogenesis blocks the effect of exercise on extinction recall in male and female isolated adolescents. a. Representative images of the dHPC and vHPC dentate gyrus after VEH- or TMZ-treatment showing Ki-67+(red), DCX+ (green). Scale bar = 25 μm b-e. TMZ-treatment reduced Ki-67+ and DCX+ cells in the dHPC and vHPC in all groups. In females, TMZ prevented the exercise-induced increase in neurogenesis except for dHPC Ki-67+. f & g. Average % CS-elicited freezing at test and vHPC DCX+ cell counts were significantly and positively correlated for VEH-treated females. There were no other correlations. +Main effect of Drug (p < 0.05). #Main or post hoc effect of Exercise (p < 0.05). *Significant correlation (p < 0.05). Values are means ± SEM. n = 3 per group per sex. For correlations, n's were pooled across running condition. dHPC, dorsal hippocampus; vHPC, ventral hippocampus; h, hilus; GC, granule cell layer. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In the present study, periadolescent isolation stress impaired extinction recall in male adolescents, an effect prevented by exercise. Isolation in female adolescents temporarily delayed conditioned fear acquisition while exercise disrupted extinction recall in isolated females. CS-elicited freezing during extinction recall in isolated females was positively correlated to the number of DCX+ cells in the vHPC. Suppression of cellular proliferation in isolated adolescents blocked the effect of exercise on extinction recall in both sexes.
4.1. Sex-specific interplay between isolation and exercise on extinction of conditioned fear
Periadolescent social isolation delayed the acquisition of CS-elicited freezing during conditioning only in female adolescents. Such sex-specific effects of isolation during conditioning may be unique to adolescence because previous findings demonstrate that isolation rearing had no effects on the acquisition of conditioned fear in adult male or female rats (Weiss et al., 2004). The transient reduction in freezing observed in our study may be due to locomotion, with evidence for hyperlocomotion following isolation rearing in adolescent female rats (Domjan et al., 1977; Jahng et al., 2011; Weiss et al., 2004). Alternatively, there may be sex differences in foot-shock sensitivity or cue inattention in isolated adolescents, although previous investigations in adults have observed no differences in these underlying processes (Fessler and Beatty, 1976; Li et al., 2009). Notably, the acquisition delay was transient and all groups reached similar levels of CS-elicited freezing by the final trial.
Similar to isolation-reared adults (Weiss et al., 2004), isolation stress alone did not affect initial acquisition of extinction. In order to promote sufficient extinction recall, the present study had 2 extinction sessions because extinction recall deficits in group-housed adolescent rats are well-documented (Baker et al., 2016; Kim et al., 2011; Perry et al., 2020; Zbukvic et al., 2017). Isolation delayed extinction reacquisition and impaired extinction recall in males but not females. This is the first observation that periadolescent isolation stress promotes the recovery of extinguished fear in adolescent males. Taken together, recall and reacquisition of extinction appear particularly vulnerable to the effects of isolation stress in adolescent males.
Strikingly, isolated but not grouped adolescents were susceptible to exercise effects on extinction recall, with isolated males and females responding in opposite directions. The lack of exercise effects on grouped adolescents was not the result of one social environment engaging in higher levels of running wheel activity (Fig. S3). It is also not due to handling differences between grouped and isolated conditions, because exercise in the home cage was used to minimize handling (handling in non-home cage running protocols could disrupt isolation-rearing experience (Bouchet et al., 2017)). No effects of exercise on cued fear extinction has been previously observed in group-housed female adult mice (Pietropaolo et al., 2006), which is consistent with our observations in group-housed adolescent rats. In isolated adolescents, exercise enhanced extinction recall (i.e., reduced freezing) in males, but inhibited recall (i.e., increased freezing) in females. These effects were observed in isolated adolescents regardless of whether females ran more than (Fig. 1) or similar to males (Fig. 4). Such effects of exercise may be specific to isolated adolescents. It has been reported that individually-housed adult male mice do not show any effects of chronic running on multiple extinction tests (Dubreucq et al., 2015). Present exercise effects were selective to extinction memory recall, with fear memory recall unaffected as shown in first extinction. Overall, these findings suggest that while periadolescent exercise may not affect cognitive-affective processing of fear cues in an ordinary social environment, it could buffer the effects of social isolation stress in both sexes.
Sex differences in running behavior in maturing rodents have been observed across multiple laboratories (Correa et al., 2014; Eikelboom and Mills, 1988; Gallego et al., 2014; Hancock and Grant, 2009; Rosenfeld, 2016; Smethells et al., 2019) (although see (Ivy et al., 2020; Purohit et al., 2019)). Interestingly, the more running isolated adolescent females engaged in, the more apparent the extinction recall deficit (i.e., increased freezing). Indeed, we observed that unconstrained running in grouped females delayed extinction acquisition (Experiment 1a), an outcome that was eliminated when grouped females ran at comparable levels to males (Experiment 1b). While wheel running activates the hypothalamic-pituitary-adrenal (HPA) axis in both sexes (Stranahan et al., 2008), there is speculation that unconstrained wheel running could be stress-provoking in female rats (James et al., 2014). It is yet unknown whether elevated wheel running in females causes or is caused by the potential sex differences in the HPA axis regulation. Considering that the sex difference in wheel activity emerged over time in the present study, future studies should measure corticosterone levels throughout the whole running period to fully assess the potential relationship between running and HPA axis.
Sex hormones may also play a role in sex differences observed in running (Rosenfeld, 2016). Ovariectomy can reduce heightened wheel running and open-field activity in females (Blizard et al., 1975; Park et al., 2016; Wollnik and Turek, 1988), while testosterone given during the neonatal period in females can induce similar activity patterns to males in late adolescence (Blizard et al., 1975). Sex-specific patterns in activity could also render females more fatigued at the time of behavioral testing (Bono et al., 2006; Hopkins and Bucci, 2010), although this did not appear to affect fear conditioning and extinction in the present study because there were no exercise main effects in females. That is, fatigue would affect both grouped and isolated females, but we only observed exercise effects either in grouped or isolated rats at different days in Experiment 1a.
Voluntary wheel running occurring after conditioning or extinction can augment consolidation processes and then alter fear extinction recall (Bouchet et al., 2017; Siette et al., 2014; Tanner et al., 2018). We thus examined levels of running immediately after conditioning or extinction. However, we found no group differences in the distance ran 2hrs following conditioning or extinction, and the amount ran did not correlate with freezing at test (Fig. S4). We also tested whether acute running for 2hrs after each extinction session affects extinction recall in isolated adolescents with a new cohort of rats, and observed that acute running following extinction sessions had no effect on extinction recall (Fig. S5). Therefore, chronic voluntary running appears to drive the sex-specific effects on extinction recall in isolated adolescents.
4.2. Newborn cells mediate the exercise effects on extinction in isolated adolescents
In response to isolation alone, we found no changes in the number of hippocampal Ki-67+ and DCX+ cells in adolescents, which is consistent with other studies (Bjørnebekk et al., 2007; Kannangara et al., 2009; Spritzer et al., 2011). A pro-neurogenic effect of exercise was observed in all groups, except for isolated males. This prevention of exercise-induced neurogenesis by isolation in males has also been observed in adolescent male mice (Kozareva et al., 2018), and adult males also appear more susceptible than adult females to the suppressive effect of isolation on exercise-induced cytogenesis (Leasure and Decker, 2009; Stranahan et al., 2006). In those studies, proliferating progenitor cells were labelled with bromodeoxyuridine (BrdU+), with Kozareva et al. (2018) and Stranahan et al. (2006) further characterising neuron-specific nuclear protein (NeuN) to specifically assess neurogenesis. Taken together, the present findings on immature new neurons labelled with DCX appear to be consistent with the reports on new neurons labelled with mature neuronal marker NeuN.
To examine the relationship between hippocampal neurogenesis and extinction recall, we correlated the number of hippocampal Ki-67+ or DCX+ cells with CS-elicited freezing at test. We consistently observed a significant positive association between vHPC DCX+ cells and elevated freezing in isolated females. Interestingly, increased DCX+ expression in the hippocampus has been previously shown to interfere with remembering (Akers et al., 2014), which suggests that exercise may interfere also with extinction recall in isolated females via increasing vHPC neurogenesis in the present study. When we chronically suppressed cellular proliferation in isolated rats using an established TMZ-treatment protocol (Nokia et al., 2012), the effects of exercise on extinction recall were blocked in both male and female isolated adolescents. This suggests that at least in isolated females, the number of DCX+ cells within the vHPC directly influences extinction memory recall, adding to the increasing evidence for vHPC in supporting the maintenance and retrieval of extinction memory (Hugues and Garcia, 2007; Park et al., 2020; Sierra-Mercado et al., 2010; Sotres-Bayon et al., 2012).
In contrast, exercise did not increase the number of new cells in the dHPC or vHPC of isolated male adolescents. However, TMZ-treatment still blocked the exercise-induced enhancement of extinction recall in males. These results suggest that while the number of newborn cells in the hippocampus does not directly influence extinction recall, neurogenesis may still play a role in adolescent males via other mechanisms such as: (1) modification to the functional properties of hippocampal newborn (DCX+) cells (Alvarez et al., 2016; Liu et al, 1996, 2000; Sah et al., 2017); (2) altered proliferation leading to different hippocampal cellular populations (e.g., neural stem cells (Dranovsky et al., 2011)); and/or (3) modification number or functional properties of newborn cells in non-hippocampal neurogenic niches within the fear extinction circuit such as the amygdala (Ehninger et al., 2011; Fowler et al., 2008; Jhaveri et al., 2017; Okuda et al., 2009; Sorrells et al., 2019; Yang et al., 2013). These mechanisms may also contribute towards extinction recall in female adolescents.
Our findings suggest that while periadolescent exercise may not affect processing of fear cues in an ordinary social environment, it may buffer the effects isolation stress in both sexes (Fallon et al., 2019; Greenwood and Fleshner, 2011; Mul et al., 2018; Pan-Vazquez et al., 2015; Robinson et al., 2019; Schoenfeld et al., 2013; Tanner et al., 2019). We hypothesize that this is mediated by the actions of proliferating cells within the developing vHPC. In adulthood, neurogenesis in this region confers stress resilience (Anacker et al., 2018). Furthermore, neurogenesis in the dentate gyrus also alters the function of mature granule cells (Lacefield et al., 2012), which could affect the ability of vCA1 afferents to mediate changes in fear extinction recall behavior (Pattwell et al., 2016). Neurogenesis in the maturing brain may sculpt the development of fear extinction circuits to respond to environmental signals. For instance, by modulating the development of vCA1 afferents that compete with afferents from the amygdala to innervate the mPFC during adolescence (Guirado et al., 2016). This likely occurs in a sex-specific manner, due to the known sex differences in brain regions involved in fear extinction (e.g., Cullity et al., 2019; Drzewiecki et al., 2020; Perry et al., 2021; Velasco et al., 2019). Future studies could examine if neurogenesis within the vHPC mediates the maturation of structural connectivity between vHPC, prefrontal cortex and the amygdala, to result in sex-specific effects of periadolescent isolation stress and exercise.
4.3. Limitations and future directions
Our study is underpowered to comprehensively assess the impact of estrous cycling in females, which requires 3–4 times more females than males, as previously reported (Perry et al., 2020). Given this caveat, we found no differences between groups or behavioral testing days in estrous cycling (Fig. S6), but others have reported that social environment can alter estrous cycling (McClintock, 1981). Estrous cycling has been shown to alter freezing behavior in adolescent rats (Perry et al., 2020), although this has not been consistently observed in adult rodents (Chen et al., 2009; Gruene et al., 2015; Zhao et al., 2018).
In our study, individual running was not tracked in a grouped environment. While it may be informative to evaluate how individual variability in running in a grouped environment contributes towards extinction, it is unlikely to have affected overall outcomes in the present study, with a previous investigations reporting no differences in individual running in grouped housing (Stranahan et al., 2006).
Our findings on the effect of TMZ may be extended in future by examining affective and innate anxiety-like behaviors in male and female adolescent rats. In adult male mice, TMZ-treatment can evoke depression-like behavior, as quantified by an increase in novelty suppressed feeding (Egeland et al., 2017). However, along with another antimitotic agent, methylazomethanol acetate, its effects are minimal on adult innate anxiety-like behavior (Egeland et al., 2017; Shors et al., 2002). It will also be critical to further explore whether TMZ effects were mediated by the depletion of neurogenesis in other neurogenic zones (e.g. amygdala and subventricular zone). For instance, olfactory bulb neurogenesis has been implicated in olfactory processing in reversal learning (Alonso et al., 2012; Gheusi et al., 2000; Sakamoto et al., 2014) and thus TMZ-treatment could have influenced olfactory memory associated with the extinction context.
It would also be valuable to assess in future the mechanisms underlying running-induced neurogenesis in adolescence. Putative mediators of exercise-induced neurogenesis include insulin growth factor-1 (Carro et al., 2000), vascular-endothelial growth factor (Fabel et al., 2003), adiponectin (Yau et al., 2014) and lactate (Lev-Vachnish et al., 2019). For instance, adiponectin (a hormone secreted by adipocytes) may modulate the effectiveness of exercise in increasing HPC neurogenesis by adiponectin receptor 1-mediated activation of AMP-activated protein kinase signalling pathway (Yau et al., 2014; Zhang et al., 2016). Interestingly, periadolescent social isolation has been reported to increase adiponectin in female but not in male rats (Krolow et al., 2013). Given the role of adiponectin in fear extinction (Zhang et al., 2017), its role as a potential mediator of exercise effects on extinction warrants further investigation.
Periadolescent isolation stress increases innate anxiety-like behavior in adolescent male rats (Molina-Hernandez et al., 2001; Morinan et al., 1992; Parker and Morinan, 1986; Stanford et al., 1988; Wright et al., 1991) but has inconsistent effects in adolescent female rats (Jahng et al., 2011; Molina-Hernandez et al., 2001) and mice (Abramov et al., 2004; Guo et al., 2004). Notably, changes in measures of innate anxiety (e.g., elevated plus maze, light-dark box) often do not correlate with changes in extinction of conditioned freezing (Short et al., 2016, Short et al., 2017). Therefore, our study focussing on the fear regulation processes of extinction provide novel evidence towards environmental effects on anxiety-relevant behaviours.
Effects of social isolation on adolescent brain and behavior observed in the present study could also be long-lasting. Previous studies demonstrate that post-weaning isolation exerts effects on anxiety-like behaviors in adulthood (Walker et al., 2019), with effects again more consistently observed in males (Hellemans et al., 2004; McCool and Chappell, 2009; Parker and Morinan, 1986; Pritchard et al., 2013; Skelly et al., 2015; Weintraub et al., 2010; Weiss et al., 2004; Wright et al., 1991) than in females (Bourke and Neigh, 2011; Butler et al., 2014; Guo et al., 2004; Jahng et al., 2011; Weintraub et al., 2010; Weiss et al., 2004).
4.4. Conclusions
Our results have implications for understanding emotional regulation in adolescence. We provide evidence suggesting that periadolescent isolation impairs the recall of an extinction memory in adolescent males but not females. Increasing physical activity improved isolation-induced recall deficits in males; however, it impaired recall in isolated females. Moreover, we showed that cellular proliferation mediates these sex-specific effects. Taken together, the present study highlights the need for personalized approaches to exposure-based treatment of fear disorders in adolescence, taking into account lifestyle factors that may have profound effects on fear regulation.
Funding and disclosure
This work was supported by the Research Training Program Scholarship from the Australian Government awarded to KDD, CSL Centenary Fellowship to GJF, Bellberry-Viertel Senior Medical Research fellowship to MEB, National Health and Medical Research Council (NHMRC) and Australian Research Council Dementia Research Development Fellowship (1107144) awarded to CJP; NHMRC Career Development Fellowship (1083309) awarded to JHK, and the Victorian State Government Operational Infrastructure Scheme.
CRediT authorship contribution statement
Katherine D. Drummond: Conceptualization, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Michelle L. Waring: Investigation. Geoffrey J. Faulkner: Supervision, Writing – review & editing. Marnie E. Blewitt: Supervision, Writing – review & editing. Christina J. Perry: Resources, Writing – review & editing. Jee Hyun Kim: Conceptualization, Resources, Formal analysis, Supervision, Writing – review & editing.
Declaration of competing interest
None.
Acknowledgements
We would like to specially thank Georgia Gahtidi and Youenn Travert for their assistance in behavioral testing and tissue processing. We also thank the Florey Core Animal Services. Graphical abstract was created with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100367.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abramov U., Raud S., Kõks S., Innos J., Kurrikoff K., Matsui T., Vasar E. Targeted mutation of CCK2 receptor gene antagonises behavioural changes induced by social isolation in female, but not in male mice. Behav. Brain Res. 2004;155:1–11. doi: 10.1016/j.bbr.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Akers K.G., Martinez-Canabal A., Restivo L., Yiu A.P., Cristofaro A.D., Hsiang H.L.L., Wheeler A.L., Guskjolen A., Niibori Y., Shoji H., Ohira K., Richards B.A., Miyakawa T., Josselyn S.A., Frankland P.W. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Alonso M., Lepousez G., Wagner S., Bardy C., Gabellec M.M., Torquet N., Lledo P.M. Activation of adult-born neurons facilitates learning and memory. Nat. Neurosci. 2012;15:897–904. doi: 10.1038/nn.3108. [DOI] [PubMed] [Google Scholar]
- Altemus M., Sarvaiya N., Epperson C.N. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014;35:320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez D.D., Giacomini D., Yang S.M., Trinchero M.F., Temprana S.G., Buttner K.A., Beltramone N., Schinder A.F. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science. 2016;354:459–465. doi: 10.1126/science.aaf2156. [DOI] [PubMed] [Google Scholar]
- Anacker C., Luna V.M., Stevens G.S., Millette A., Shores R., Jimenez J.C., Chen B., Hen R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559:98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K.D., Bisby M.A., Richardson R. Impaired fear extinction in adolescent rodents: behavioural and neural analyses. Neurosci. Biobehav. Rev. 2016;70:59–73. doi: 10.1016/j.neubiorev.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Baran S.E., Armstrong C.E., Niren D.C., Hanna J.J., Conrad C.D. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol. Learn. Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélair M.A., Kohen D.E., Kingsbury M., Colman I. Relationship between leisure time physical activity, sedentary behaviour and symptoms of depression and anxiety: evidence from a population-based sample of Canadian adolescents. Bmj Open. 2018;8 doi: 10.1136/bmjopen-2017-021119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnebekk A., Mathé A.A., Gruber S.H.M., Brené S. Social isolation increases number of newly proliferated cells in hippocampus in female flinders sensitive line rats. Hippocampus. 2007;17:1193–1200. doi: 10.1002/hipo.20352. [DOI] [PubMed] [Google Scholar]
- Blizard D.A., Lippman H.R., Chen J.J. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol. Behav. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- Bono J.P.D., Adlam D., Paterson D.J., Channon K.M. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiology-regulatory Integr Comp Physiology. 2006;290:R926–R934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- Bouchet C.A., Lloyd B.A., Loetz E.C., Farmer C.E., Ostrovskyy M., Haddad N., Foright R.M., Greenwood B.N. Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex-dependent manner. Learn. Mem. 2017;24:358–368. doi: 10.1101/lm.045195.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke C.H., Neigh G.N. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.P., Després S.C., Kuhn C.M.C., Winkler J., Aigner L., Kuhn H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Brownson R.C., Boehmer T.K., Luke D.A. Declining rates of physical activity in the United States: what are the contributors? Annu. Rev. Publ. Health. 2005;26:421–443. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- Butler T.R., Carter E., Weiner J.L. Adolescent social isolation does not lead to persistent increases in anxiety- like behavior or ethanol intake in female long-evans rats. Alcohol Clin. Exp. Res. 2014;38:2199–2207. doi: 10.1111/acer.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E., Nuñez A., Busiguina S., Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000;20:2926–2933. doi: 10.1523/jneurosci.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton A.J., May C., Luikinga S.J., Burrows E.L., Kim J.H., Lawrence A.J., Perry C.J. Chronic voluntary alcohol consumption causes persistent cognitive deficits and cortical cell loss in a rodent model. Sci. Rep. 2019;9:1–19. doi: 10.1038/s41598-019-55095-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Shields J., Huang W., King J.A. Female fear: influence of estrus cycle on behavioral response and neuronal activation. Behav. Brain Res. 2009;201:8–13. doi: 10.1016/j.bbr.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J.W., Drummond S.P.A., Hoyer D., Jacobson L.H. Sex differences in mouse models of fear inhibition: fear extinction, safety learning, and fear-safety discrimination. Br. J. Pharmacol. 2019;176:4149–4158. doi: 10.1111/bph.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa S.M., Newstrom D.W., Warne J.P., Flandin P., Cheung C.C., Lin-Moore A.T., Pierce A.A., Xu A.W., Rubenstein J.L., Ingraham H.A. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2014;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullity E.R., Madsen H.B., Perry C.J., Kim J.H. Postnatal developmental trajectory of dopamine receptor 1 and 2 expression in cortical and striatal brain regions. J. Comp. Neurol. 2019;527(6):1039–1055. doi: 10.1002/cne.24574. [DOI] [PubMed] [Google Scholar]
- Day H.L.L., Stevenson C.W. The neurobiological basis of sex differences in learned fear and its inhibition. Eur. J. Neurosci. 2019;107:109. doi: 10.1111/ejn.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M., Schorr R., Best M. Early environmental influences on conditioned and unconditioned ingestional and locomotor behaviors. Dev. Psychobiol. 1977;10:499–506. doi: 10.1002/dev.420100603. [DOI] [PubMed] [Google Scholar]
- Dranovsky A., Picchini A.M., Moadel T., Sisti A.C., Yamada A., Kimura S., Leonardo E.D., Hen R. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew M.R., Denny C.A., Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav. Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki C.M., Willing J., Juraska J.M. Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex. Brain Struct. Funct. 2020;225(8):2495–2507. doi: 10.1007/s00429-020-02137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S., Marsicano G., Chaouloff F. Duration- and environment-dependent effects of repeated voluntary exercise on anxiety and cued fear in mice. Behav. Brain Res. 2015;282:1–5. doi: 10.1016/j.bbr.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Egeland M., Guinaudie C., Preez A.D., Musaelyan K., Zunszain P.A., Fernandes C., Pariante C.M., Thuret S. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.68. e1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D., Wang L.P., Klempin F., Römer B., Kettenmann H., Kempermann G. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelboom R., Mills R. A microanalysis of wheel running in male and female rats. Physiol. Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Fabel Klaus, Fabel Konstanze, Tam B., Kaufer D., Baiker A., Simmons N., Kuo C.J., Palmer T.D. VEGF is necessary for exercise‐induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fallon I.P., Tanner M.K., Greenwood B.N., Baratta M.V. Sex differences in resilience: experiential factors and their mechanisms. Eur. J. Neurosci. 2019;52:2530–2547. doi: 10.1111/ejn.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler R.G., Beatty W.W. Variations in postweaning environment and sensitivity to electric shock in male and female rats. Behav. Biol. 1976;16:535–538. doi: 10.1016/s0091-6773(76)91747-8. [DOI] [PubMed] [Google Scholar]
- Fowler C.D., Liu Y., Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res. Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego X., Cox R.J., Funk E., Foster R.A., Ehringer M.A. Voluntary exercise decreases ethanol preference and consumption in C57BL/6 adolescent mice: sex differences and hippocampal BDNF expression. Physiol. Behav. 2014;138:28–36. doi: 10.1016/j.physbeh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella D.E., Drummond K.D., Ganella E.P., Whittle S., Kim J.H. Extinction of conditioned fear in adolescents and adults: a human fMRI study. Front. Hum. Neurosci. 2018;11:2638. doi: 10.3389/fnhum.2017.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella D.E., Lee-Kardashyan L., Luikinga S.J., Nguyen D.L.D., Madsen H.B., Zbukvic I.C., Coulthard R., Lawrence A.J., Kim J.H. Aripiprazole facilitates extinction of conditioned fear in adolescent rats. Front. Behav. Neurosci. 2017;11:65. doi: 10.3389/fnbeh.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella D.E., Nguyen L.D., Lee-Kardashyan L., Kim L.E., Paolini A.G., Kim J.H. Neurocircuitry of fear extinction in adult and juvenile rats. Behav. Brain Res. 2018;351:161–167. doi: 10.1016/j.bbr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Garthe A., Behr J., Kempermann G. Adult-Generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PloS One. 2009;4 doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M.J., Odgers C.L. Seven fears and the science of how mobile Technologies may Be influencing adolescents in the digital age. Perspect. Psychol. Sci. 2015;10:832–851. doi: 10.1177/1745691615596788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G., Cremer H., McLean H., Chazal G., Vincent J.D., Lledo P.M. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C.E., Larouche R., Barnes J.D., Colley R.C., Bonne J.C., Arthur M., Cameron C., Chaput J.P., Faulkner G., Janssen I., Kolen A.M., Manske S.R., Salmon A., Spence J.C., Timmons B.W., Tremblay M.S. Are we driving our kids to unhealthy habits? Results of the active healthy kids Canada 2013 report card on physical activity for children and youth. Int J Environ Res Pu. 2014;11:6009–6020. doi: 10.3390/ijerph110606009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B.N., Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc. Sport Sci. Rev. 2011;39:140–149. doi: 10.1097/jes.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. Friendship and social development in children and adolescents: the impact of electronic technology. Educ. Child Psychol. 1997:25–37. [Google Scholar]
- Gruene T.M., Flick K., Stefano A., Shea S.D., Shansky R.M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi: 10.7554/elife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirado R., Umemori J., Sipilä P., Castrén E. Evidence for competition for target innervation in the medial prefrontal cortex. Cerebr. Cortex. 2016;26:1287–1294. doi: 10.1093/cercor/bhv280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Wu C.F., Liu W., Yang J.Y., Chen D. Sex difference in psychological behavior changes induced by long-term social isolation in mice. Prog Neuro-psychopharmacology Biological Psychiatry. 2004;28:115–121. doi: 10.1016/j.pnpbp.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Guthold R., Stevens G.A., Riley L.M., Bull F.C. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. The Lancet. Child & adolescent health. 2020;4:23–35. doi: 10.1016/s2352-4642(19)30323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock S.D., Grant V.L. Sexually dimorphic effects of postnatal treatment on the development of activity-based anorexia in adolescent and adult rats. Dev. Psychobiol. 2009;51:679–695. doi: 10.1002/dev.20403. [DOI] [PubMed] [Google Scholar]
- He J., Crews F.T. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol., Biochem. Behav. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Hellemans K.G.C., Benge L.C., Olmstead M.C. Adolescent enrichment partially reverses the social isolation syndrome. Dev. Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Smits J.A.J. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J. Clin. Psychiatr. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins M.E., Bucci D.J. Interpreting the effects of exercise on fear conditioning: the influence of time of day. Behav. Neurosci. 2010;124:868–872. doi: 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S., Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Amp Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D., Takuma K., Koike H., Mizoguchi H., Tsuritani K., Kuwahara Y., Kamei H., Nagai T., Yoneda Y., Nabeshima T., Yamada K. Social isolation rearing‐induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion‐related behaviors in juvenile mice. J. Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Ivy A.S., Yu T., Kramár E., Parievsky S., Sohn F., Vu T. A unique mouse model of early life exercise enables hippocampal memory and synaptic plasticity. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-66116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng J.W., Yoo S.B., Ryu V., Lee J.H. Hyperphagia and depression-like behavior by adolescence social isolation in female rats. Int J Dev Neurosci Official J Int Soc Dev Neurosci. 2011;30:47–53. doi: 10.1016/j.ijdevneu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- James M.H., Campbell E.J., Walker F.R., Smith D.W., Richardson H.N., Hodgson D.M., Dayas C.V. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front. Behav. Neurosci. 2014;8:864. doi: 10.3389/fnbeh.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri D.J., Tedoldi A., Hunt S., Sullivan R., Watts N.R., Power J.M., Bartlett P.F., Sah P. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol. Psychiatr. 2017;23:521–532. doi: 10.1038/mp.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara T.S., Webber A., Gil-Mohapel J., Christie B.R. Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice. Hippocampus. 2009;19:889–897. doi: 10.1002/hipo.20514. [DOI] [PubMed] [Google Scholar]
- Kantomaa M.T., Tammelin T.H., Ebeling H.E., Taanila A.M. Emotional and behavioral problems in relation to physical activity in youth. Med. Sci. Sports Exerc. 2008;40:1749–1756. doi: 10.1249/mss.0b013e31817b8e82. [DOI] [PubMed] [Google Scholar]
- Kee N., Sivalingam S., Boonstra R., Wojtowicz J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H.G., Gage F.H. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner A.C., Cryan J.F., Brummelte S. Resilience priming: translational models for understanding resiliency and adaptation to early life adversity. Dev. Psychobiol. 2019;61:350–375. doi: 10.1002/dev.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Ganella D.E. A review of preclinical studies to understand fear during adolescence. Aust. Psychol. 2015;50:25–31. doi: 10.1111/ap.12066. [DOI] [Google Scholar]
- Kim J.H., Li S., Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cerebr. Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kingery J.N., Erdley C.A., Marshall K.C., Whitaker K.G., Reuter T.R. Peer experiences of anxious and socially withdrawn youth: an integrative review of the developmental and clinical literature. Clin Child Fam Psych. 2010;13:91–128. doi: 10.1007/s10567-009-0063-2. [DOI] [PubMed] [Google Scholar]
- Kozareva D.A., Cryan J.F., Nolan Y.M. Born this way: hippocampal neurogenesis across the lifespan. Aging Cell. 2019;18:232. doi: 10.1111/acel.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozareva D.A., O'Leary O.F., Cryan J.F., Nolan Y.M. Deletion of TLX and social isolation impairs exercise-induced neurogenesis in the adolescent hippocampus. Hippocampus. 2018;28:3–11. doi: 10.1002/hipo.22805. [DOI] [PubMed] [Google Scholar]
- Krolow R., Noschang C., Arcego D.M., Huffell A.P., Marcolin M.L., Benitz A.N., Lampert C., Fitarelli R.D., Dalmaz C. Sex-specific effects of isolation stress and consumption of palatable diet during the prepubertal period on metabolic parameters. Metabolism. 2013;62:1268–1278. doi: 10.1016/j.metabol.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Lacefield C.O., Itskov V., Reardon T., Hen R., Gordon J.A. Effects of adult‐generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure J.L., Decker L. Social isolation prevents exercise‐induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Lev-Vachnish Y., Cadury S., Rotter-Maskowitz A., Feldman N., Roichman A., Illouz T., Varvak A., Nicola R., Madar R., Okun E. L-lactate promotes adult hippocampal neurogenesis. Front Neurosci-switz. 2019;13:403. doi: 10.3389/fnins.2019.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P.M., Gotlib I.H., Lewinsohn M., Seeley J.R., Allen N.B. Gender differences in anxiety disorders and anxiety symptoms in adolescents. J. Abnorm. Psychol. 1998;107:109–117. doi: 10.1037/0021-843x.107.1.109. [DOI] [PubMed] [Google Scholar]
- Li C., Zhang B., Li Z., Huang F., Tian S., Hu X. Effects of abnormal early rearing environments on fear memory in adult rats: effects of abnormal early rearing environments on fear memory in adult rats. Zool. Res. 2009;30:31–37. doi: 10.3724/sp.j.1141.2009.01031. [DOI] [Google Scholar]
- Liu X.S., Tilwalli S., Ye G., Lio P.A., Pasternak J.F., Trommer B.L. Morphologic and electrophysiologic maturation in developing dentate gyrus granule cells. Brain Res. 2000;856:202–212. doi: 10.1016/s0006-8993(99)02421-x. [DOI] [PubMed] [Google Scholar]
- Liu Y.B., Lio P.A., Pasternak J.F., Trommer B.L. Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J. Neurophysiol. 1996;76:1074–1088. doi: 10.1152/jn.1996.76.2.1074. [DOI] [PubMed] [Google Scholar]
- Lothmann K., Deitersen J., Zilles K., Amunts K., Herold C. New boundaries and dissociation of the mouse hippocampus along the dorsal‐ventral axis based on glutamatergic, GABAergic and catecholaminergic receptor densities. Hippocampus. 2021;31:56–78. doi: 10.1002/hipo.23262. [DOI] [PubMed] [Google Scholar]
- Maren S., Phan K.L., Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum J., Kim J.H., Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock M.K. Social control of the ovarian cycle. Bioscience. 1981;31:138–139. doi: 10.2307/1308257. [DOI] [Google Scholar]
- McCool B.A., Chappell A.M. Early social isolation in male long‐evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self‐administration. Alcohol Clin. Exp. Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K.R., Nakamura E.F., Kessler R.C. Epidemiology of mental disorders in children and adolescents. Dialogues Clin. Neurosci. 2009;11:7–20. doi: 10.31887/DCNS.2009.11.1/krmerikangas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Hernandez M., Tellez-Alcantara P., Perez-Garcia J. Isolation rearing induced fear-like behavior without affecting learning abilities of Wistar rats. Prog Neuro-psychopharmacology Biological Psychiatry. 2001;25:1111–1123. doi: 10.1016/s0278-5846(01)00168-3. [DOI] [PubMed] [Google Scholar]
- Morinan A., Parker V., Rich D.A., Cariuk P., Horton R.W. Social isolation does not alter brain regional benzodiazepine binding site numbers, affinity and coupling in the rat. Psychopharmacology. 1992;106:565–569. doi: 10.1007/bf02244831. [DOI] [PubMed] [Google Scholar]
- Mul J.D., Soto M., Cahill M.E., Ryan R.E., Takahashi H., So K., Zheng J., Croote D.E., Hirshman M.F., Fleur S.E. la, Nestler E.J., Goodyear L.J. Voluntary wheel running promotes resilience to chronic social defeat stress in mice: a role for nucleus accumbens ΔFosB. Neuropsychopharmacol Official Publ Am Coll Neuropsychopharmacol. 2018;43 doi: 10.1038/s41386-018-0103-z. 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K.M., Davis M. Mechanisms of fear extinction. Mol. Psychiatr. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council . Australian code for the care and use of animals for scientific purposes. 8th. National Health and Medical Research Council; Canberra: 2013. [Google Scholar]
- Nokia M.S., Anderson M.L., Shors T.J. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur. J. Neurosci. 2012;36:3521–3530. doi: 10.1111/ejn.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda H., Tatsumi K., Makinodan M., Yamauchi T., Kishimoto T., Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- O'Leary J.D., Hoban A.E., Murphy A., O'Leary O.F., Cryan J.F., Nolan Y.M. Differential effects of adolescent and adult‐initiated exercise on cognition and hippocampal neurogenesis. Hippocampus. 2019;29:352–365. doi: 10.1002/hipo.23032. [DOI] [PubMed] [Google Scholar]
- Ollendick T.H., Davis III T.E. One-session treatment for specific phobias: a review of öst’s single-session exposure with children and adolescents. Cognit. Behav. Ther. 2013;42:275–283. doi: 10.1080/16506073.2013.773062. [DOI] [PubMed] [Google Scholar]
- Olsen R.H.J., Marzulla T., Raber J. Impairment in extinction of contextual and cued fear following post-training whole-body irradiation. Front. Behav. Neurosci. 2014;8:214. doi: 10.3389/fnbeh.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orben A., Tomova L., Blakemore S.J. The effects of social deprivation on adolescent development and mental health. Lancet Child Adolesc Heal. 2020;4:634–640. doi: 10.1016/s2352-4642(20)30186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Vazquez A., Rye N., Ameri M., McSparron B., Smallwood G., Bickerdyke J., Rathbone A., Dajas-Bailador F., Toledo-Rodriguez M. Impact of voluntary exercise and housing conditions on hippocampal glucocorticoid receptor, miR-124 and anxiety. Mol. Brain. 2015;8:40. doi: 10.1186/s13041-015-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H.J., Ganella D.E., Kim J.H. A dissociation between renewal and contextual fear conditioning in juvenile rats. Dev. Psychobiol. 2017;59:515–522. doi: 10.1002/dev.21516. [DOI] [PubMed] [Google Scholar]
- Park C.H.J., Ganella D.E., Perry C.J., Kim J.H. Dissociated roles of dorsal and ventral hippocampus in recall and extinction of conditioned fear in male and female juvenile rats. Exp. Neurol. 2020;329:113306. doi: 10.1016/j.expneurol.2020.113306. [DOI] [PubMed] [Google Scholar]
- Park Y.M., Kanaley J.A., Padilla J., Zidon T., Welly R.J., Will M.J., Britton S.L., Koch L.G., Ruegsegger G.N., Booth F.W., Thyfault J.P., Vieira-Potter V.J. Effects of intrinsic aerobic capacity and ovariectomy on voluntary wheel running and nucleus accumbens dopamine receptor gene expression. Physiol. Behav. 2016;164:383–389. doi: 10.1016/j.physbeh.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker V., Morinan A. The socially-isolated rat as a model for anxiety. Neuropharmacology. 1986;25:663–664. doi: 10.1016/0028-3908(86)90224-8. [DOI] [Google Scholar]
- Pattwell S.S., Duhoux S., Hartley C.A., Johnson D.C., Jing D., Elliott M.D., Ruberry E.J., Powers A., Mehta N., Yang R.R., Soliman F., Glatt C.E., Casey B.J., Ninan I., Lee F.S. Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S.S., Liston C., Jing D., Ninan I., Yang R.R., Witztum J., Murdock M.H., Dincheva I., Bath K.G., Casey B.J., Deisseroth K., Lee F.S. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat. Commun. 2016;7:11475. doi: 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C.J., Campbell E.J., Drummond K.D., Lum J.S, Kim J.H. Sex differences in the neurochemistry of frontal cortex: Impact of early life stress. J. Neurochem. 2021;157(4):963–981. doi: 10.1111/jnc.15208. [DOI] [PubMed] [Google Scholar]
- Perry C.J., Ganella D.E., Nguyen L.D., Du X., Drummond K.D., Whittle S., Pang T.Y., Kim J.H. Assessment of conditioned fear extinction in male and female adolescent rats. Psychoneuroendocrinology. 2020;116:104670. doi: 10.1016/j.psyneuen.2020.104670. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S., Feldon J., Alleva E., Cirulli F., Yee B.K. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behav. Neurosci. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- Polanczyk G.V., Salum G.A., Sugaya L.S., Caye A., Rohde L.A. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry Allied Discip. 2015;56:345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- Pritchard L.M., Kempen T.A.V., Zimmerberg B. Behavioral effects of repeated handling differ in rats reared in social isolation and environmental enrichment. Neurosci. Lett. 2013;536:47–51. doi: 10.1016/j.neulet.2012.12.048. [DOI] [PubMed] [Google Scholar]
- Purohit D.C., Mandyam A.D., Terranova M.J., Mandyam C.D. Voluntary wheel running during adolescence distinctly alters running output in adulthood in male and female rats. Behav. Brain Res. 2019;377:112235. doi: 10.1016/j.bbr.2019.112235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S., Christ C.C., Cahill M.M., Aldrich S.J., Taylor-Yeremeeva E. Voluntary exercise or systemic propranolol ameliorates stress-related maladaptive behaviors in female rats. Physiol. Behav. 2019;198:120–133. doi: 10.1016/j.physbeh.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Robinson-Drummer P.A., Stanton M.E. Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol. Behav. 2014;148:22–28. doi: 10.1016/j.physbeh.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld C.S. Sex-dependent differences in voluntary physical activity: physical activity and sex differences. J. Neurosci. Res. 2016;95:279–290. doi: 10.1002/jnr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza S.J., Hofstra M.B., van der Ende J., Verhulst F.C. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. Am. J. Psychiatr. 2003;160:2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- Rudy J.W. Context representations, context functions, and the parahippocampal-hippocampal system. Learn. Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah N., Peterson B.D., Lubejko S.T., Vivar C., van Praag H. Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Scientific reports. 2017;7:10903. doi: 10.1038/s41598-017-11268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M., Ieki N., Miyoshi G., Mochimaru D., Miyachi H., Imura T., Yamaguchi M., Fishell G., Mori K., Kageyama R., Imayoshi I. Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. J. Neurosci. 2014;34:5788–5799. doi: 10.1523/jneurosci.0674-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaini S., Belotti R., Ogliari A. Genetic and environmental contributions to social anxiety across different ages: a meta-analytic approach to twin data. J. Anxiety Disord. 2014;28:650–656. doi: 10.1016/j.janxdis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Schiele M.A., Domschke K. Epigenetics at the crossroads between genes, environment and resilience in anxiety disorders. Gene Brain Behav. 2018;17 doi: 10.1111/gbb.12423. [DOI] [PubMed] [Google Scholar]
- Schoenfeld T.J., Rada P., Pieruzzini P.R., Hsueh B., Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J Neurosci Official J Soc Neurosci. 2013;33:7770–7777. doi: 10.1523/jneurosci.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D., Visser R.M., D'Hooge R. A translational perspective on neural circuits of fear extinction: current promises and challenges. Neurobiol. Learn. Mem. 2018;155:113–126. doi: 10.1016/j.nlm.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T.J., Townsend D.A., Zhao M., Kozorovitskiy Y., Gould E. Neurogenesis may relate to some but not all types of hippocampal‐dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short A.K., Fennell K.A., Perreau V.M., Fox A., O’Bryan M.K., Kim J.H., Bredy T.W., Pang T.Y., Hannan A.J. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry. 2016;6(6) doi: 10.1038/tp.2016.109. e837-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short A.K., Yeshurun S., Powell R., Perreau V.M., Fox A., Kim J.H., Pang T.Y., Hannan A.J. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl. Psychiatry. 2017;7(5):e1114. doi: 10.1038/tp.2017.82. 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D., Padilla-Coreano N., Quirk G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacol Official Publ Am Coll Neuropsychopharmacol. 2010;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J., Reichelt A.C., Westbrook R.F. A bout of voluntary running enhances context conditioned fear, its extinction, and its reconsolidation. Learn. Mem. 2014;21:73–81. doi: 10.1101/lm.032557.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly M.J., Chappell A.E., Carter E., Weiner J.L. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: possible role of disrupted noradrenergic signaling. Neuropharmacology. 2015;97:149–159. doi: 10.1016/j.neuropharm.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethells J.R., Greer A., Dougen B., Carroll M.E. Effects of voluntary exercise and sex on multiply-triggered heroin reinstatement in male and female rats. Psychopharmacology. 2019;237:453–463. doi: 10.1007/s00213-019-05381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S.F., Paredes M.F., Velmeshev D., Herranz-Pérez V., Sandoval K., Mayer S., Chang E.F., Insausti R., Kriegstein A.R., Rubenstein J.L., Garcia-Verdugo J.M., Huang E.J., Alvarez-Buylla A. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019;10:2748. doi: 10.1038/s41467-019-10765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F., Sierra-Mercado D., Pardilla-Delgado E., Quirk G.J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer M.D., Ibler E., Inglis W., Curtis M.G. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. doi: 10.1016/j.neuroscience.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford S.C., Parker V., Morinan A. Deficits in exploratory behaviour in socially isolated rats are not accompanied by changes in cerebral cortical adrenoceptor binding. J. Affect. Disord. 1988;15:175–180. doi: 10.1016/0165-0327(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Stranahan A.M., Khalil D., Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan A.M., Lee K., Mattson M.P. Central mechanisms of HPA Axis regulation by voluntary exercise. Neuromol Med. 2008;10:118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B.A., Witter M.P., Lein E.S., Moser E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Tanner M.K., Fallon I.P., Baratta M.V., Greenwood B.N. Voluntary exercise enables stress resistance in females. Behav. Brain Res. 2019;369:111923. doi: 10.1016/j.bbr.2019.111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M.K., Hake H.S., Bouchet C.A., Greenwood B.N. Running from fear: exercise modulation of fear extinction. Neurobiol. Learn. Mem. 2018;151:28–34. doi: 10.1016/j.nlm.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teychenne M., Costigan S.A., Parker K. The association between sedentary behaviour and risk of anxiety: a systematic review. BMC Publ. Health. 2015;15:513. doi: 10.1186/s12889-015-1843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge J.M., Spitzberg B.H. Declines in non‐digital social interaction among Americans, 2003–2017. J. Appl. Soc. Psychol. 2020;50:363–367. doi: 10.1111/jasp.12665. [DOI] [Google Scholar]
- Velasco E.R., Florido A., Milad M.R., Andero R. Sex differences in fear extinction. Neurosci. Biobehav. Rev. 2019;103:81–108. doi: 10.1016/j.neubiorev.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.M., Cunningham A.M., Gregory J.K., Nestler E.J. Long-term behavioral effects of post-weaning social isolation in males and females. Front. Behav. Neurosci. 2019;13:1929. doi: 10.3389/fnbeh.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.M., Henry J., Neumann D.L. Aversive Pavlovian conditioning in childhood anxiety disorders: impaired response inhibition and resistance to extinction. J. Abnorm. Psychol. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- Weintraub A., Singaravelu J., Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Weiss I.C., Pryce C.R., Jongen-Rêlo A.L., Nanz-Bahr N.I., Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Wollnik F., Turek F.W. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol. Behav. 1988;43:389–396. doi: 10.1016/0031-9384(88)90204-1. [DOI] [PubMed] [Google Scholar]
- Wright I.K., Upton N., Marsden C.A. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol. Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Yang J.M., Zhang J., Chen X.J., Geng H.-Y., Ye M., Spitzer N.C., Luo J.H., Duan S.M., Li X.M. Development of GABA circuitry of fast-spiking basket interneurons in the medial prefrontal cortex of erbb4-mutant mice. J. Neurosci. 2013;33:19724–19733. doi: 10.1523/jneurosci.1584-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau S.Y., Li A., Hoo R.L.C., Ching Y.P., Christie B.R., Lee T.M.C., Xu A., So K.F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:15810–15815. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbukvic I.C., Park C.H.J., Ganella D.E., Lawrence A.J., Kim J.H. Prefrontal dopaminergic mechanisms of extinction in adolescence compared to adulthood in rats. Front. Behav. Neurosci. 2017;11:65. doi: 10.3389/fnbeh.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Wang X., Lu X.Y. Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology. 2016;157:2853–2869. doi: 10.1210/en.2015-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Wang X., Wang B., Garza J.C., Fang X., Wang J., Scherer P.E., Brenner R., Zhang W., Lu X.Y. Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Mol. Psychiatr. 2017;22:1044–1055. doi: 10.1038/mp.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Bijlsma E.Y., Verdouw M.P., Groenink L. No effect of sex and estrous cycle on the fear potentiated startle response in rats. Behav. Brain Res. 2018;351:24–33. doi: 10.1016/j.bbr.2018.05.022. [DOI] [PubMed] [Google Scholar]
- Ziviani J., Wadley D., Ward H., Macdonald D., Jenkins D., Rodger S. A place to play: socioeconomic and spatial factors in children's physical activity. Aust. Occup. Ther. J. 2008;55:2–11. doi: 10.1111/j.1440-1630.2006.00646.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.