Abstract
Chest radiography (CXR) is most likely to be the utilized modality for diagnosing COVID-19 and following up on any lung-associated abnormalities. This review provides a meta-analysis of the current literature on CXR imaging findings to determine the most common appearances of lung abnormalities in COVID-19 patients in order to equip medical researchers and healthcare professionals in their efforts to combat this pandemic. Twelve studies met the inclusion criteria and were analyzed. The inclusion criteria consisted of: (1) published in English literature; (2) original research study; (3) sample size of at least 5 patients; (4) reporting clinical characteristics of COVID-19 patients as well as CXR imaging features; and (5) noting the number of patients with each corresponding imaging feature. A total of 1948 patients were included in this study. To perform the meta-analysis, a random-effects model calculated the pooled prevalence and 95% confidence intervals of abnormal CXR imaging findings. Seventy-four percent (74%) (95% CI: 51–92%) of patients with COVID-19 had an abnormal CXR at the initial time of diagnosis or sometime during the disease course. While there was no single feature on CXR that was diagnostic of COVID-19 viral pneumonia, a characteristic set of findings were obvious. The most common abnormalities were consolidation (28%, 95% CI: 8–54%) and ground-glass opacities (29%, 95% CI: 10–53%). The distribution was most frequently bilateral (43%, 95% CI: 27–60%), peripheral (51%, 95% CI: 36–66%), and basal zone (56%, 95% CI: 37–74%) predominant. Contrary to parenchymal abnormalities, pneumothorax (1%, 95% CI: 0–3%) and pleural effusions (6%, 95% CI: 1–16%) were rare.
Abbreviations: ES, Effect size; CI, Confidence interval; COVID-19, Coronavirus disease of 2019; CT, Computed tomography; CXR, Chest X-ray; GGO, Ground-glass opacity; ICU, Intensive care unit; RT-PCR, Reverse transcription polymerase chain reaction
Keywords: COVID-19, Imaging, Chest radiograph, CXR, Coronavirus, SARS-CoV-2, Meta-analysis
1. Introduction
The first case of coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China in December 2019 and it was declared a pandemic by the World Health Organization (WHO) in March 20201. Healthcare workers need diagnostic tools to study cases of potential COVID-19 that are both sensitive and specific7. The gold standard for diagnosing COVID-19 patients is a viral nucleic acid test conducted by reverse transcription-polymerase chain reaction (RT-PCR) with a sensitivity of 79% and specificity of 100%2. Chest CT has been found to have comparable diagnostic performance with a sensitivity of 77% and specificity of 96%3.
The pulmonary system is primarily affected by COVID-19, as such, it is common practice to request a chest radiograph (CXR) in suspicious cases as the initial imaging exam. The diagnostic performance of CXR in the early stages of COVID-19 is, however, limited and pathological findings may not be detected on radiography that is identifiable on chest CT8. CXR obtained from confirmed and symptomatic COVID-19 patients have had a lower sensitivity at 69%4. Although CXR is less sensitive, it is available in urgent care centers, clinics and hospitals and may help with COVID-19 diagnosis7.
Available information on CXR features of COVID-19 is dispersed among various published papers in the scientific literature, and a consolidated systematic review has yet to be assembled. This review intends to analyze the characteristics of the rapidly progressive COVID-19 viral pneumonia on CXR. The value of radiographic imaging is in generating results that are clinically actionable either for establishing a diagnosis or for guiding patient management. We aim to determine the most common appearances of lung abnormalities in COVID-19 patients to provide insight into the initial CXR as well as follow-up CXR characteristics of this infectious disease. The characteristic imaging findings during the different stages of the disease and the variation in the prevalence of the different patterns are also studied. A review detailing the most common patterns of lung abnormalities on CXR will help equip medical researchers and healthcare professionals in their efforts to combat this pandemic.
2. Methods
2.1. Literature search
This meta-analysis was conducted using “Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines”9. It includes a systematic literature search of the World Health Organization database, PubMed, Elsevier and Google Scholar, using the keywords “COVID-19 OR coronavirus OR SARS-Cov-2” and “chest x-ray OR CXR”.
2.2. Eligibility criteria
The resulting studies that meet the following inclusion criteria were included in this review's analysis: (1) published in English literature; (2) original research study; (3) sample size of at least 5 patients; (4) reporting clinical characteristics of COVID-19 patients as well as CXR imaging features; and (5) noting the number of patients with each corresponding imaging feature. Studies that lacked research data or outcome parameters were excluded.
2.3. Data extraction
An aggregate of 401 non-duplicate citations was identified by using the aforementioned search strategies from which 37 potentially applicable papers were evaluated. Ultimately, 12 studies were eligible with reported cases ranging from 5 to 636 were included in the meta-analysis (Table 1, Table 2, Table 3 ). In total, these studies contained 1948 patients, 643 cases with normal CXR and 1305 cases with abnormal CXR findings (Fig. 1 ). Table 1 contains the basic demographic of the participant population in each study as well as the reported CXR findings.
Table 1.
Basic clinical characteristics of studies in COVID-19 patients.
| Reference | Country of Origin | Date Published (d/m/y) | Number of Participants | Gender Male: Female |
Average Age | Method of Diagnosis | Patient Type | Symptom Severity | Onset of symptoms prior to radiograph taken |
|---|---|---|---|---|---|---|---|---|---|
| Arentz et al. | USA | 28/4/2020 | 21 | 11:10 | 70 | RT-PCR | Inpatient | SOB (76%), fever (52%), cough (48%) | 3.5 days |
| Chen et al. | China | 30/1/2020 | 99 | 67:32 | 55.5 | RT-PCR (by WHO interim guidance) | Inpatient | Cough and fever (82%), SOB (30%) | – |
| Cozzi et al. | Italy | 9/6/2020 | 234 | 153:81 | 66 | RT-PCR | Inpatient | – | – |
| Guan et al. | China | 30/4/2020 | 274 | – | 47.0 | RT-PCR | Inpatient | Fever (88.7%), cough (67.8%) | 4 days |
| Ippolito et al. | Italy | 22/4/2020 | 468 | 328:140 | 64.7 | RT-PCR | Inpatient | Fever (90.6%), cough (57.7%), dyspnea (56.8%) | >5 days for majority of patients (57.7%) |
| Kim et al. | Korea | 6/4/2020 | 28 | – | 42.6 | RT-PCR | Inpatient | Cough (28.6%), sore throat (28.6), fever, myalgia, and headache (25%) | – |
| Ng et al. | China | 2/2/2020 | 5 | – | 56 | RT-PCR | – | Fever (90%), cough (48%), sore throat (10%), diarrhea (10%) | 3 |
| Russel et al. | USA | −/5/2020 | 636 | 363:273 | 50 | RT-PCR | – | – | – |
| Toussie et al. | USA | 14/5/2020 | 338 | 210:128 | 39 | RT-PCR | Inpatient | – | 4 days |
| Vancheri et al. | Italy | 30/5/2020 | 240 | 169:71 | 65 | RT-PCR | Inpatient | – | – |
| Wong et al. | China | 27/5/2020 | 64 | 26:38 | 56 | RT-PCR | Inpatient | Fever (59%), cough (41%), asymptomatic (14%) | 10–12 days |
| Yoon et al. | Korea | 21/4/2020 | 9 | 4:5 | 54 | RT-PCR | Inpatient | – | – |
Table 2.
Chest radiograph findings of studies in COVID-19 patients.
| Reference |
Baseline |
Lung Involvement |
Consolidation (%) |
Ground Glass Opacities (%) |
Distribution |
Zone Predominance |
Other Atypical Features (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal (%) | Abnormal (%) | Normal Turned Abnormal (%) | Right (%) |
Left (%) | Bilateral (%) | Diffuse (%) | Peripheral (%) | Perihilar (%) | None (%) | Superior (%) | Basal (%) |
||||
| Arentz et al. | 1 (4.8) | 20 (95.2) | 0 (0) |
– | – | 11 (52.4) | 4 (19.0) |
10 (47.6) | – | – | – | – | – | – | Pleural Effusion: 6 (28.6) Pulmonary Edema: 2 (9.5) Atelectasis: 1 (4.8) |
| Chen et al. | 0 (0) |
99 (100) | 0 (0) |
– | – | 74 (74.7) |
– | 14 (14.1) |
– | – | – | – | – | – | Pneumothorax: 1 (1) |
| Cozzi et al. | 13 (5.6) |
221 (94.4) | 0 (0) |
29 (12.4) | 21 (9.0) | 162 (69.2) | 135 (57.7) |
147 (62.8) | 37 (16.5) | 135 (60.5) | 51 (22.8) | 55 (24.6) | 31 (13.9) | 137 (61.4) | Cardiomegaly: 70 (29.9) Pleural Effusion: 39 (16.7) Pneumothorax: 5 (2.4) |
| Guan et al. | 112 (40.9) |
162 (59.1) | 0 (0) |
– | – | 100 (36.5) | – | 55 (20.1) | – | – | – | – | – | – | – |
| Ippolito et al. | – | – | – | – | – | 301 (64.5) | 245 (52.5) |
335 (63.1) | – | 292 (62.5) | – | – | – | 335 (71.1) | Pleural Effusion: 57 (12.2) |
| Kim et al. | 15 (53.6) |
13 (46.4) | 0 (0) |
– | – | 6 (21.4) | – | – | – | – | – | – | – | – | – |
| Ng et al. | 2 (40.0) |
3 (60.0) |
0 (0) |
– | – | 2 (40.0) |
3 (60.0) |
– | – | – | – | 2 (40.0) |
0 (0) |
1 (20.0) |
– |
| Russel et al. | 371 (58.3) |
265 (41.7) | 0 (0) |
– | – | 133 (20.9) | 34 (5.3) |
120 (18.9) | 6 (0.9) |
225 (35.4) | 45 (7.1) |
6 (0.9) |
128 (20.1) |
215 (33.8) |
Pleural Effusion: 2 (0.3) |
| Toussie et al. | – | – | – | – | – | – | – | – | – | – | – | – | 23 (6.8) |
270 (79.9) |
Pleural Effusion: 0 (0) Pneumothorax: 0 (0) |
| Vancheri et al. | 60 (25.0) |
180 (75.0) |
0 (0) |
24 (10.0) | 24 (10.0) | 132 (55.0) | 71 (29.6) |
124 (51.7) | 71 (29.6) | 89 (37.1) |
20 (8.3) |
– | 66 (27.5) | 158 (65.8) | Pleural Effusion: 12 (5.0) |
| Wong et al. | 20 (31.3) |
44 (68.8) |
7 (10.9) |
10 (15.6) |
9 (14.1) |
32 (50.0) | 30 (46.8) |
21 (32.8) |
19 (29.7) | 26 (40.6) |
6 (9.4) |
19 (29.7) |
0 (0) |
32 (50.0) |
Pleural Effusion: 2 (3) |
| Yoon et al. | 4 (44.4) |
5 (55.6) |
0 (0) |
5 (55.6) | 5 (55.6) | – | 8 (88.9) |
2 (22.2) |
2 (22.2) | 6 (66.6) |
2 (22.2) |
– | 2 (22.2) |
5 (55.6) |
– |
Table 3.
Compilation of pooled prevalence of chest radiograph imaging findings from each study.
| Meta-Analyses: Pooled Prevalence of CXR Imaging Findings (95% CI) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Baseline |
Lung Involvement |
Consolidation | Ground Glass Opacities | Distribution |
Zone Predominance |
Pleural Effusion | ||||||||
| Normal | Abnormal | Normal Turned Abnormal | Right | Left | Bilateral | Diffuse | Peripheral | Perihilar | None | Superior | Basal | ||||
| Arentz et al. | 0.05 (0.00, 0.24) | 0.95 (0.76, 1.00) |
0.00 (0.00, 0.16) |
– | – | 0.52 (0.30, 0.74) |
0.19 (0.05, 0.42) |
0.48 (0.26, 0.70) |
– | – | – | – | – | – | 0.29 (0.11, 0.52) |
| Chen et al. | 0.00 (0.00, 0.04) | 1.00 (0.96, 1.00) |
0.00 (0.00, 0.04) |
– | – | 0.75 (0.65, 0.83) |
– | 0.14 (0.08, 0.23) |
– | – | – | – | – | – | – |
| Cozzi et al. | 0.06 (0.00, 0.09) | 0.94 (0.91, 0.97) |
0.00 (0.00, 0.02) |
0.12 (0.08, 0.17) |
0.09 (0.06, 0.13) |
0.69 (0.63, 0.75) |
0.58 (0.51, 0.64) |
0.63 (0.56, 0.69) |
0.16 (0.11, 0.21) |
0.58 (0.51, 0.64) |
0.22 (0.17, 0.28) |
0.24 (0.18, 0.29) |
0.13 (0.09, 0.18) |
0.59 (0.52, 0.65) |
0.17 (0.12, 0.22) |
| Guan et al. | 0.41 (0.35, 0.47) | 0.59 (0.53, 0.65) |
0.00 (0.00, 0.01) |
– | – | 0.36 (0.31, 0.43) |
– | 0.20 (0.15, 0.25) |
– | – | – | – | – | – | – |
| Ippolito et al. | – | – | – | – | – | 0.64 (0.60, 0.69) |
0.52 (0.48, 0.57) |
0.72 (0.67, 0.76) |
– | 0.62 (0.59, 0.67) |
– | – | – | 0.72 (0.67, 0.76) |
0.12 (0.09, 0.15) |
| Kim et al. | 0.54 (0.34, 0.72) | 0.46 (0.28, 0.66) |
0.00 (0.00, 0.12) |
– | – | 0.21 (0.08, 0.41) |
– | – | – | – | – | – | – | – | – |
| Ng et al. | 0.40 (0.05, 0.85) | 0.60 (0.15, 0.95) |
0.00 (0.00, 0.52) |
– | – | 0.40 (0.05, 0.85) |
0.60 (0.15, 0.95) |
– | – | – | – | 0.40 (0.05, 0.85) |
0.00 (0.00, 0.52) |
0.20 (0.01, 0.72) |
– |
| Russel et al. | 0.58 (0.62, 0.54) | 0.42 (0.38, 0.46) |
0.00 (0.00, 0.01) |
– | – | 0.21 (0.18, 0.24) |
0.05 (0.04, 0.07) |
0.19 (0.16. 0.22) |
0.01 (0.00, 0.02) |
0.35 (0.32, 0.39) |
0.07 (0.05, 0.09) |
0.01 (0.00, 0.02) |
0.20 (0.17, 0.23) |
0.34 (0.30, 0.38) |
0.00 (0.00. 0.01) |
| Toussie et al. | – | – | – | – | – | – | – | – | – | – | – | – | 0.07 (0.04, 0.10) |
0.80 (0.75, 0.84) |
0.00 (0.00. 0.01) |
| Vancheri et al. | 0.25 (0.20, 0.31) | 0.75 (0.69, 0.80) |
0.00 (0.00, 0.02) |
0.10 (0.07, 0.15) |
0.10 (0.07, 0.15) |
0.55 (0.49, 0.61) |
0.30 (0.24, 0.36) |
0.52 (0.45, 0.58) |
0.30 (0.24, 0.36) |
0.37 (0.31, 0.44) |
0.08 (0.05, 0.13) |
– | 0.28 (0.22, 0.34) |
0.66 (0.59, 0.72) |
0.05 (0.02, 0.09 |
| Wong et al. | 0.31 (0.20, 0.44) | 0.69 (0.56, 0.80) |
0.11 (0.05, 0.21) |
0.16 (0.08, 0.27) |
0.14 (0.07, 0.25) |
0.50 (0.37, 0.63) |
0.47 (0.34, 0.60) |
0.33 (0.22, 0.46) |
0.30 (0.19, 0.42) |
0.41 (0.29, 0.54) |
0.09 (0.04, 0.19) |
30 (0.19, 0.42) |
0.00 (0.00, 0.06) |
0.50 (0.37, 0.63) |
0.03 (0.00, 0.11) |
| Yoon et al. | 0.44 (0.14, 0.79) | 0.56 (0.21, 0.86) |
0.00 (0.00, 0.34) |
0.56 (0.21, 0.86) |
0.56 (0.21, 0.86) |
– | 0.89 (0.52, 1.00) |
0.22 (0.03, 0.60) |
0.22 (0.03, 0.60) |
0.67 (0.30, 0.93) |
0.22 (0.03, 0.60) |
– | 0.22 (0.03, 0.60) |
0.56 (0.21, 0.86) |
– |
| Overall I2 | 98.18% | 98.18% | 70.54% | 76.22% | 91.48% | 97.54% | 99.03% | 99.14% | 97.19% | 95.67% | 91.07% | 98.01% | 92.14% | 97.79% | 97.41% |
| Overall p | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
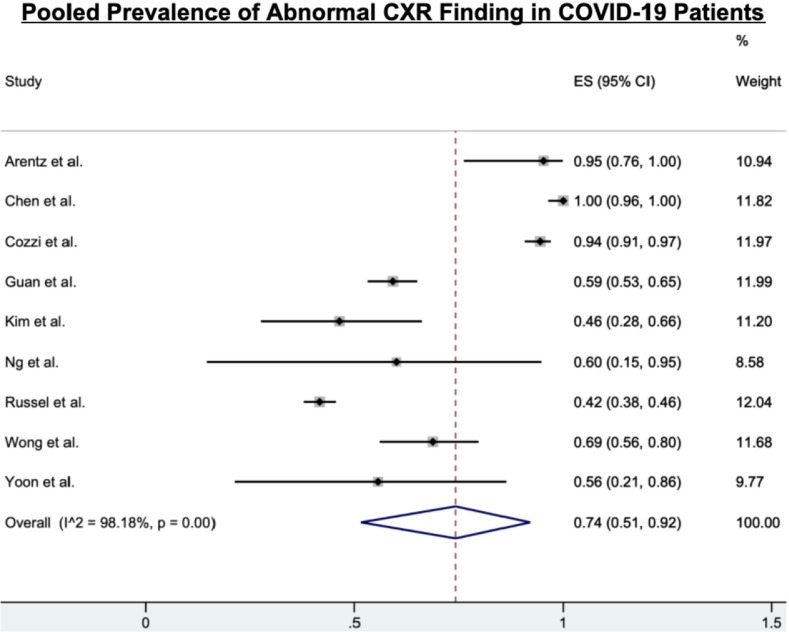
Fig. 1.
A forest plot illustrating results of the meta-analysis on pooled prevalence values for abnormal CXR in COVID-19 patients. Each study is represented by one line in this figure, depicting the 95% CIs, and each box in the center describes the proportion for that study comparison. The effect size of each box to the hollow blue diamond represents the strength or the weight that particular study gives to the overall meta-analysis (% Weight). The hollow blue diamond denotes the prevalence value from the pooled-effects model; the center of the diamond gives the overall proportion while the extremities illustrate the 95% CI. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the following sections, we present an analysis and review of CXR findings in COVID-19 patients as gathered from the eligible studies. The ensuing info was collected from each published paper for the analysis of CXR findings: total number of patients in the study; gender of participants and average age; lung lesion distribution (diffuse, peripheral or perihilar); lung involvement (right, left, or bilateral); typical abnormalities such as consolidation and ground-glass opacities; frequency of zone predominance (superior or basal); and less common findings such as pleural effusion, pulmonary edema, atelectasis and pneumothorax. Stata version 15.0 was utilized to perform the statistical analysis. Table 1, Table 2 summarize the collected data. The figures displaying the CXR findings were obtained with permission via email from Radiopedia.org.
2.4. Statistical analysis
A meta-analysis was conducted using a variance components model to determine the pooled proportion values and the analogous 95% confidence intervals (CI) of distinctive imaging features linked to patients with COVID-19. The pooled-effects meta-analysis model involved an assumption that the effect size (ES) being approximated in the different studies are not alike; rather, they adhere to some distribution (Fig. 1). The center of the symmetric distribution characterizes the average of the effects, whilst the width describes the degree of heterogeneity10. Statistical heterogeneity was calculated using the I2 test (p < 0.05 represented significant statistical heterogeneity). Data was entered into Stata version 15.0 to perform statistical analyses.
The pooled-effects model was calculated for each CXR finding. All the graphs for the meta-analyses and forest plots can be found in the supplementary document. Statistically significant heterogeneity was found among all the studies in regards to the data extracted from their findings (p < 0.05). The results from the graphs were summarized in Table 3.
3. Results
CXR was utilized in 12 studies diagnosing COVID-19. Both CXR and CT were used in 4 studies, with chest CT proving to be superior to CXR in the assessment and detection of each type of lung abnormality, demonstrating the limitations of CXR11., 12., 13., 14.. There is some heterogeneity between studies in regards to the average age of the patient cohort. In addition, the diverse array of findings on CXR sometimes made it hard to make sense of because of vague and nonstandard terminology such as “patchy opacities,” “nodules,” “infiltrates,” “airspace disease,” and “airspace opacities”, which may contribute to the limitations in CXR analysis.
Of the 12 studies, 9 (75.0%) found normal CXR findings in COVID-19 patients. The proportion of patients with normal CXR findings varied from 5% at the low end of the spectrum to 58% at the higher end; this range was found to be dependent on how COVID-19 presented in patients, with high normal CXR percentages being reported in asymptomatic patients or those with mild symptoms. The overall pooled prevalence was 26% (95% CI: 8–49%). Furthermore, ≥25% of normal CXR findings was reported in 70.0% (7/10) studies. A total of 11 studies (91.7%) noted information in regards to bilateral, right, or left lung involvement (Table 1); from which 9 studies noted a greater proportion of bilateral lung involvement (43%, 95% CI: 27–60%) when compared to right lung involvement (19%, 95% CI: 7–33%) or left lung involvement (1%, 95% CI: 0–4%) (Table 3, Table 4 ). All 11 studies noted that bilateral lung involvement was more commonly seen in urgent or severe cases of COVID-19 and that bilateral lung involvement was much greater than the involvement of the right or left lung in COVID-19 patients.
Table 4.
Overall summary of meta-analyses of chest radiograph imaging findings.
| Summary: Pooled Prevalence of CXR Imaging Findings | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Baseline |
Lung Involvement |
Consolidation | Ground Glass Opacities | Distribution |
Zone Predominance |
Pleural Effusion | ||||||||
| Normal | Abnormal | Normal Turned Abnormal | Right | Left | Bilateral | Diffuse | Peripheral | Perihilar | None | Superior | Basal | ||||
| ES | 0.26 | 0.74 | 0.00 | 0.19 | 0.01 | 0.43 | 0.28 | 0.29 | 0.13 | 0.51 | 0.13 | 0.17 | 0.08 | 0.56 | 0.06 |
| 95% CI | (0.08, 0.49) | (0.51, 0.92) | (0.00, 0.01) | (0.07, 0.33) | (0.00, 0.04) | (0.27, 0.60) | (0.08, 0.54) | (0.10, 0.53) | (0.01, 0.35) | (0.36, 0.66) | (0.04, 0.24) | (0.00, 0.46) | (0.02, 0.16) | (0.37. 0.74) | (0.01, 0.16) |
Six studies (50.0%) noted the proportions on the distribution of lesions or abnormalities in the perihilar or peripheral lung regions or diffuse lung distribution on CXR, with peripheral distribution (51%, 95% CI: 36–66%) showing significant dominance compared to perihilar distribution (13%, 95% CI: 4–24%) and diffuse distribution (13%, 95% CI: 1–35%). Eight studies (66.7%) reported the details of zone predominance of abnormalities or lesions in the basal or superior or neither lung regions, with basal predominance (56%, 95% CI: 37–74%) being a more common finding compared to superior predominance (8%, 95% CI: 2–16%) or no predominance (17%, 95% CI: 0–46%). Ippolito et al. noted information only on the involvement of peripheral lung distribution and basal predominance15.
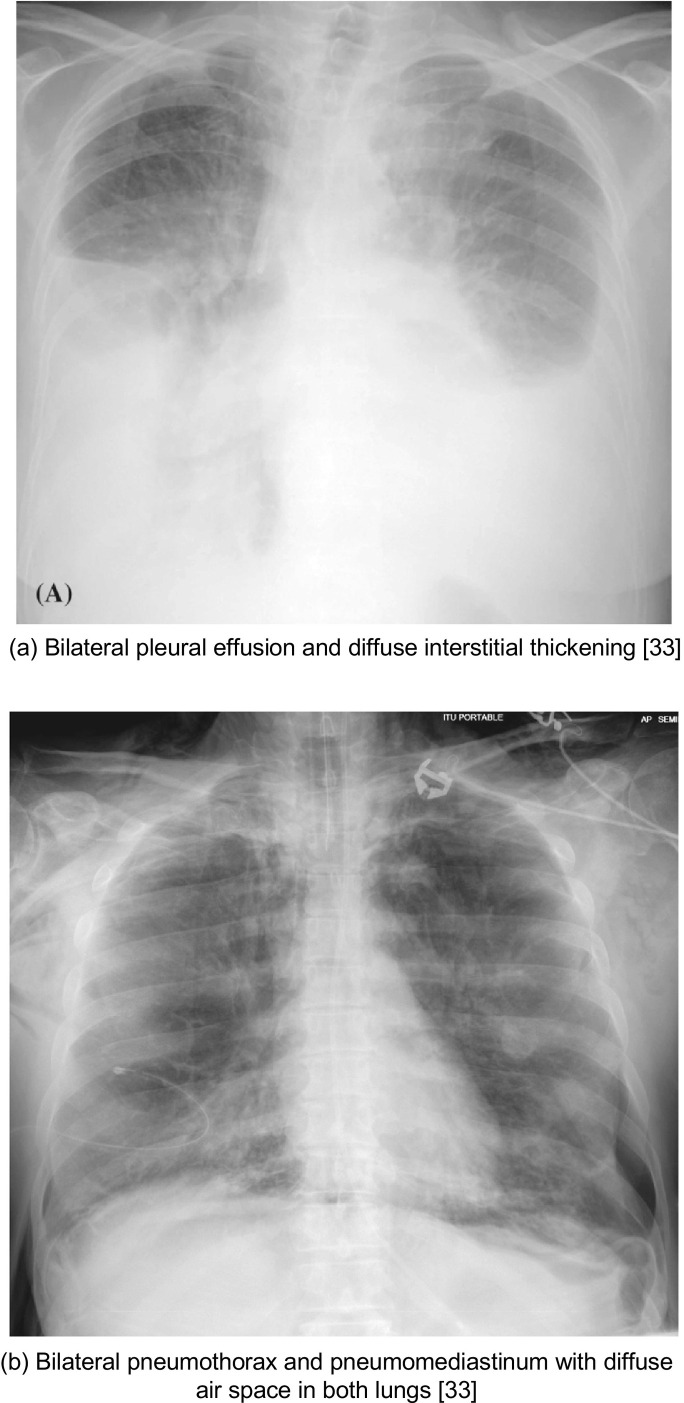
Consolidation and ground-glass opacities (GGOs) were reported in 83.3% (10/12) of the studies; they were the most frequent, atypical abnormalities (Table 1). The pooled prevalence of both consolidation (28%, 95% CI: 8–54%) and GGO (29%, 95% CI: 10–53%) were similar (Table 4). The other two studies did not report any data on these features16., 17.. Besides for consolidation and GGOs, other lung abnormalities were reported in 75.0% (8/12) of these studies, including less common findings like pleural effusion (6%, 95% CI: 1–16%; Fig. 5a) and pneumothorax (1%, 95% CI: 0–3%; Fig. 5b).
Fig. 5.
Cases illustrative of atypical imaging findings on CXR
(a) Bilateral pleural effusion and diffuse interstitial thickening33
(b) Bilateral pneumothorax and pneumomediastinum with diffuse air space in both lungs33
3.1. COVID-19 imaging features
3.1.1. Consolidation
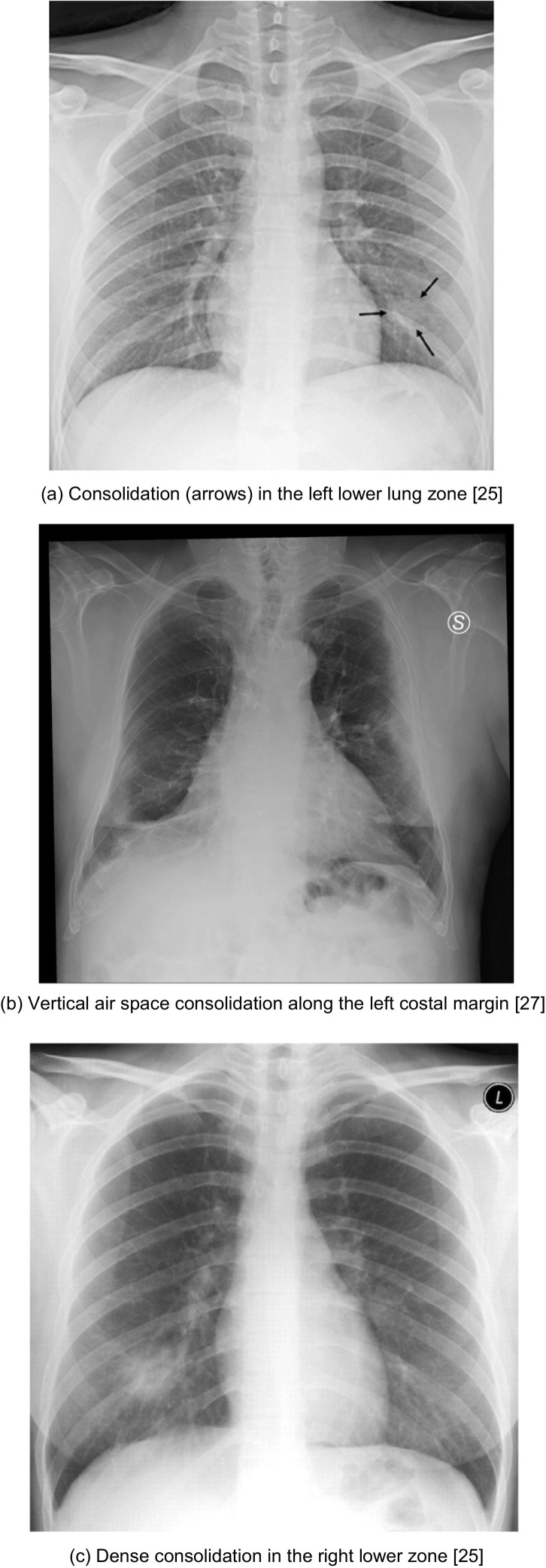
One of the most common abnormalities seen on CXR in COVID-19 patients is consolidation. Consolidation refers to an occupation of the air space that causes filling of the alveolar spaces by pathological products such as water, pus and blood in the lungs. It appears as a homogeneous increase in pulmonary parenchymal attenuation (increased density) that obscures the margins of the vessels and the walls of the airways18. It may present with an air bronchogram, referring to the visualization of the air bronchial lumens within a parenchymal opacity of the lung and, therefore, implies airway patency18. On CXR, it appears to have indistinct margins and presents as fluffy opacities that become confluent over time, so the air that normally surrounds the bronchi becomes opacified and appears white, while the bronchi remain air filled and appear like black tubular structures within the area of consolidation. In Fig. 2a, linear black tubular structures that represent air bronchograms are visible in the left lower lobe and this is one of the key features of consolidation.
Fig. 2.
Cases illustrative of consolidation on CXR
(a) Consolidation (arrows) in the left lower lung zone25
(b) Vertical air space consolidation along the left costal margin27
(c) Dense consolidation in the right lower zone25
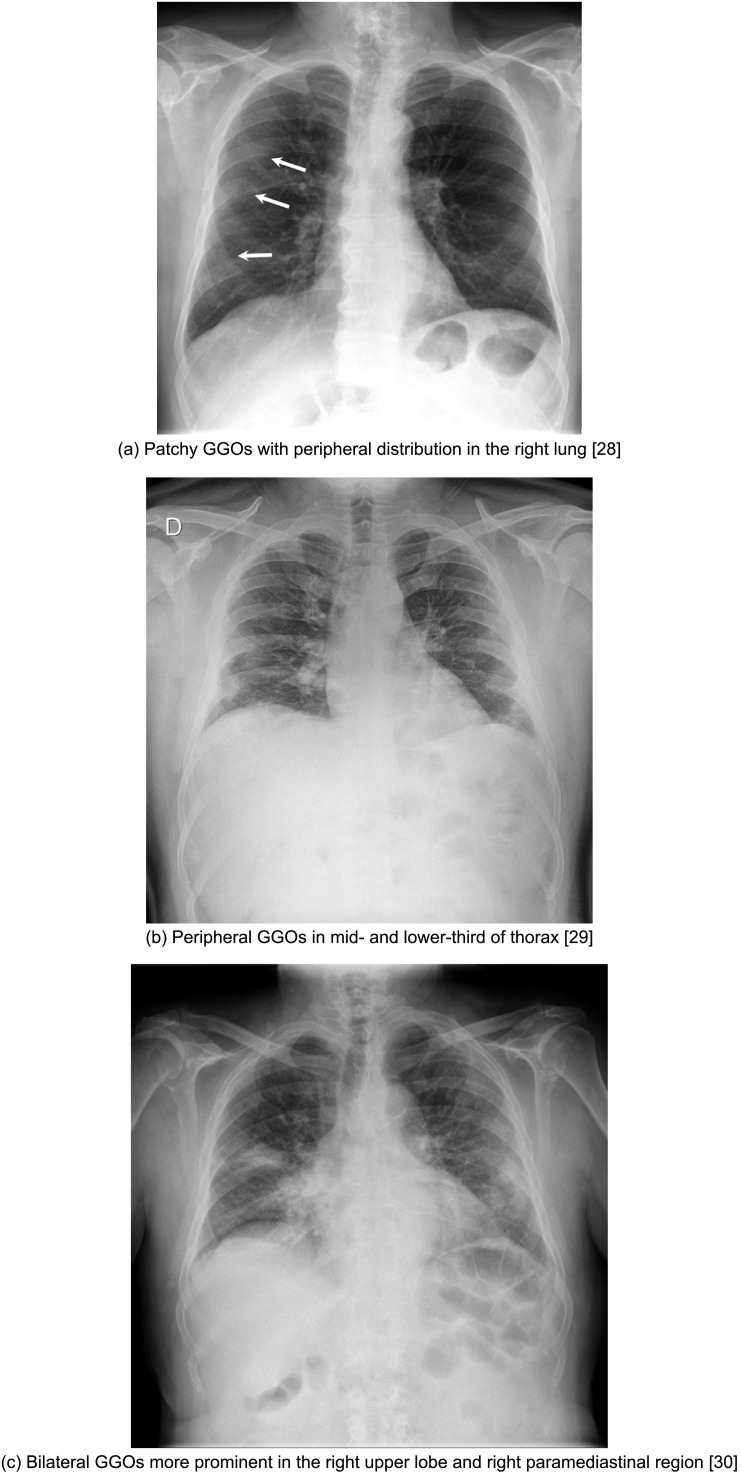
3.1.2. Ground glass opacities
Ground-glass opacities (GGOs) describe the lung parenchymal opacification that produces a minor increase in attenuation for the consolidation (Fig. 3 ). As such, despite the increase in density, the walls of the bronchi and the pulmonary vessels remain differentiated from the affected parenchyma18. GGOs represent a partial occupation of the airspace; they are less opaque than consolidations and, as an important consequence, the CT is more sensitive in its detection than CXR. GGOs are common in earlier presentations and can potentially precede the appearance of consolidation19.
Fig. 3.
Cases illustrative of ground-glass opacity (GGO) on CXR
(a) Patchy GGOs with peripheral distribution in the right lung28
(b) Peripheral GGOs in mid- and lower-third of thorax29
(c) Bilateral GGOs more prominent in the right upper lobe and right paramediastinal region30
3.1.3. Bilateral peripheral air opacities
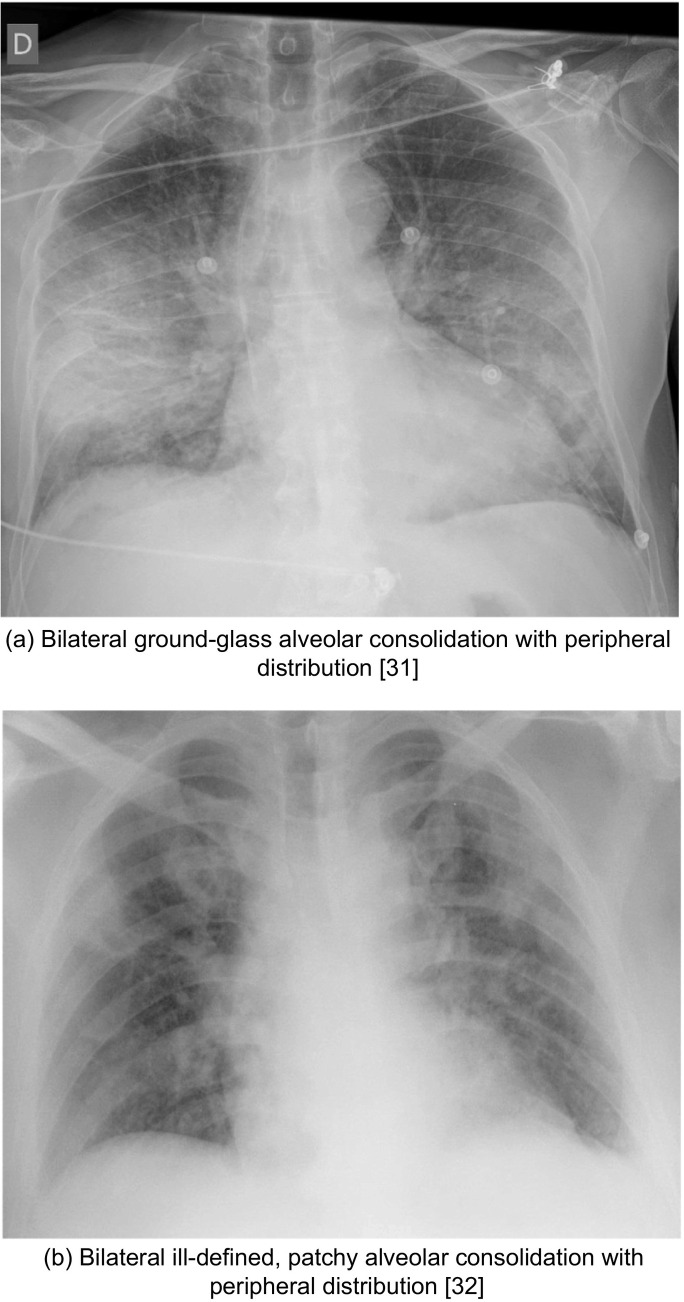
The term “air opacities” is often used in conjunction with “nodules” to denote the filling of air spaces with the products of disease18. Compared to community-acquired bacterial pneumoniae, which typically involve one lobe and tend to be unilateral, COVID-19 similar to other viral pneumoniae usually presents with air opacities greater than a single lobe20. Recognizing multifocal air opacities on CXR is a pertinent imaging feature of COVID-19 pneumonia. Researchers have noted that the air opacities typically tend to have a bilateral, peripheral and predominantly basal distribution4 (Fig. 4 ). Reticular opacities with regions of ground-glass attenuation are usually better visualized on CXR than chest CT19.
Fig. 4.
Cases illustrative of bilateral peripheral air opacities on CXR
(a) Bilateral ground-glass alveolar consolidation with peripheral distribution31
(b) Bilateral ill-defined, patchy alveolar consolidation with peripheral distribution32
3.1.4. Atypical imaging findings
Pleural effusion is considered to be characteristically rare in COVID-19 and its presence may indicate co-existing bacterial pneumonia21. Pleural effusion presents as blunting of the costo-phrenic angle on CXR and often appears as a white area at the lung base. In one study that compared COVID-19 to other viral pneumoniae, pleural effusion was more common in non-COVID-19 viral pneumoniae (Fig. 5 )22. It may also be a poor prognostic indicator in COVID-19 patients23.
Pneumothorax is also a rare finding in COVID-19 patients and presents as a thin, sharp white line at the visceral pleural edge (Fig. 5). The authors of one case report of a COVID-19 patient who had a left-sided tension pneumothorax could not be certain whether this pneumothorax was secondary to COVID-19 or the complication was purely coincidental24. Atelectasis, cardiomegaly, and pneumomediastinum have also been found in COVID-19 patients; yet, it is to be determined if these are findings are unusual manifestations of COVID-19, a sign of dual pathology, or purely incidental25.
4. Discussion
Chest radiographs may be found to be “normal” in early or mild disease, however, COVID-19 patients can later develop radiological or clinical signs of viral pneumonia. Seventy-four percent (74%) (95% CI: 51–92%) of patients with COVID-19 had an abnormal CXR at the initial time of diagnosis or sometime during the disease course. Although the number of participants covered by individual COVID-19 CXR studies is low when compared to similarly designed CT studies, a characteristic set of findings are clear. There is no single feature on CXR that is diagnostic of COVID-19 viral pneumonia. Our findings are consistent with meta-analyses of pertinent COVID-19 features on chest CT18., 21., which suggest that the disease presents as atypical or organizing pneumonia on imaging. The most common abnormalities are consolidation (28%, 95% CI: 8–54%) and ground-glass opacities (29%, 95% CI: 10–53%). The distribution is most frequently bilateral (43%, 95% CI: 27–60%), peripheral (51%, 95% CI: 36–66%), and basal zone (56%, 95% CI: 37–74%) predominant. In contrast to parenchymal abnormalities, pneumothorax (1%, 95% CI: 0–3%) and pleural effusions (6%, 95% CI: 1–16%) are rare.
Our meta-analysis has several strengths. First, the studies, which met the inclusion criteria, occurred in different countries and health centers, providing more generalizable results. Second, the total number of cases was somewhat large for 6 months of early publications, generating an accumulation of evidence to evaluate the diagnosis of COVID-19 by CXR. Third, a variety of different imaging features was gathered, including the distribution patterns in the lungs as well as specific imaging features. However, there are also several limitations. First, the reported imaging findings may not be consistent across studies because of different interpreting radiologists. Second, most of the included studies did not differentiate between COVID-19 patients presenting as clinically mild, moderate, or severe cases. Third, some COVID-19 patients may have had chronic diseases and comorbidities like hypertension and diabetes that could have affected CXR findings.
CXR imaging can play a role in triaging patients due to long PCR turnaround times. Moreover, CXR has several advantages over CT such as cost and practical considerations, which limits CT's utility. The American College of Radiology has noted that CT decontamination, which is required after scanning COVID-19 patients, can disrupt radiology service availability and has suggested that portable CXR be used to reduce the chances of cross-infection5. Common CT findings of lower zone predominance, peripheral distribution and bilateral involvement can also be appreciated on CXR6. As such, CXR is most likely to be the utilized modality for identification of COVID-19 and follow-up of any associated lung abnormalities26.
Possible biases in this meta-analysis may be due to the inclusion criteria only using studies published in English, which may exclude data that show different CXR finding trends and patient demographics that could potentially skew the results. In addition, the CXR pathology findings that were analyzed in this study were chosen based on what we considered to be most pertinent based on the various studies that were included, which may leave out possibly common CXR findings. Another possible bias could be the patient demographics, as most studies reported mostly inpatient cases of COVID-19; this possibly narrows the data to more severe cases of COVD-19.
Our results confirm that CXR will continue to be a useful tool in the evaluation and management of patients diagnosed with COVID-19. Although less sensitive than chest CT, CXR can prove useful in its prognostic predictions such as triaging patients and answering questions in regards to whether a patient should stay home, if they will need the ICU in a few days, if they will respond to a specific treatment such as intubation or some drug, whether they should be taken off a ventilator and their overall chances for survival. With an ever-growing number of suspected cases in this pandemic, CXR should be seen as a quick and easy-to-use modality to assess lung abnormalities.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinimag.2021.06.039.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Coronavirus (COVID-19) events as they happen. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 2.He J.-L., Luo L., Luo Z.-D., et al. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168 doi: 10.1016/j.rmed.2020.105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues J.C.L., Hare S.S., Edey A., et al. An update on COVID-19 for the radiologist - a British Society of Thoracic Imaging statement. Clin Radiol. 2020;75(5):323–325. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong H.Y.F., Lam H.Y.S., Fong A.H.-T., et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019;201160 doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 6.Sun Z., Zhang N., Li Y., Xu X. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg. 2020;10(5):1058–1079. doi: 10.21037/qims-20-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Essentials for radiologists on COVID-19: an update-radiology scientific expert panel. Radiology. 2020:200527. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. BMJ. British Medical Journal Publishing Group; 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement; p. 339.https://www.bmj.com/content/339/bmj.b2535 Accessed August 2, 2020. [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon S.H., Lee K.H., Kim J.Y., et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21(4):494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Lond Engl. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med Mass Med Soc. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng M.-Y., Lee E.Y., Yang J., et al. Imaging profile of the COVID-19infection: radiologic findings and literature review. Radiol Cardiothorac Imaging. 2020;2(1) doi: 10.1148/ryct.2020200034. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7233595/ Accessed July 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ippolito D., Maino C., Pecorelli A., et al. Chest X-ray features of SARS-CoV-2 in the emergency department: a multicenter experience from northern Italian hospitals. Respir Med. 2020;170 doi: 10.1016/j.rmed.2020.106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toussie D., Voutsinas N., Finkelstein M., et al. Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID-19. Radiology. 2020:201754. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim E.S., Chin B.S., Kang C.K., et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35(13) doi: 10.3346/jkms.2020.35.e142. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7131901/ Accessed July 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 19.Jacobi A., Chung M., Bernheim A., Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilar J., Domingo M.L., Soto C., Cogollos J. Radiology of bacterial pneumonia. Eur J Radiol. 2004;51(2):102–113. doi: 10.1016/j.ejrad.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Blachford A. Chest X-Ray findings among urgent care patients with COVID-19 are not affected by patient age or gender: a retrospective cohort study of 636 ambulatory patients. J Urgent Care Med. 2020 https://www.jucm.com/chest-x-ray-findings-among-urgent-care-patients-with-covid-19-are-not-affected-by-patient-age-or-gender-a-retrospective-cohort-study-of-636-ambulatory-patients/ Accessed July 5, 2020. [Google Scholar]
- 22.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; Treasure Island (FL): 2020. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls.http://www.ncbi.nlm.nih.gov/books/NBK554776/ Accessed July 29, 2020. [PubMed] [Google Scholar]
- 23.Litmanovich D.E., Chung M., Kirkbride R., Kicska G., Kanne P.J. Review of chest radiograph findings of COVID-19 pneumonia and suggested reporting language. J Thorac Imaging. 2020;35(6):354–360. doi: 10.1097/RTI.0000000000000541. https://journals.lww.com/thoracicimaging/Abstract/9000/Review_of_Chest_Radiograph_Findings_of_COVID_19.99401.aspx Publish Ahead of Print. Accessed July 4, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Flower L., Lopez J.R., Henry A.M., Carter J.-P.L. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep CP BMJ Specialist J. 2020;13(5) doi: 10.1136/bcr-2020-235861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleverley J., Piper J., Jones M.M. BMJ. Vol. 370. British Medical Journal Publishing Group; 2020. The role of chest radiography in confirming covid-19 pneumonia.https://www.bmj.com/content/370/bmj.m2426 Accessed July 29, 2020. [DOI] [PubMed] [Google Scholar]
- 26.Kooraki S., Hosseiny M., Myers L., Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol JACR. 2020;17(4):447–451. doi: 10.1016/j.jacr.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicoletti D. COVID-19 pneumonia | Radiology Case | Radiopaedia.org. Radiopaedia. https://radiopaedia.org/cases/covid-19-pneumonia-7
- 28.Khan M.I. Post-intubation pneumomediastinum and pneumothorax - background COVID-19 pneumonia | Radiology Case | Radiopaedia.org. Radiopaedia. https://radiopaedia.org/cases/post-intubation-pneumomediastinum-and-pneumothorax-background-covid-19-pneumonia?lang=us
- 29.Patel R. COVID 19 Pneumonia | Radiology Case | Radiopaedia.org. Radiopaedia. https://radiopaedia.org/cases/covid-19-pneumonia-67?lang=us
- 30.Creus A.A. COVID-19 pneumonia | Radiology Case | Radiopaedia.org. Radiopaedia. https://radiopaedia.org/cases/covid-19-pneumonia-22?lang=us
- 31.Lorente E. COVID-19 pneumonia | Radiology Case | Radiopaedia.org. Radiopaedia. https://radiopaedia.org/cases/covid-19-pneumonia-20?lang=us
- 32.Lorente E. COVID-19 pneumonia - evolution over a week | Radiology Case | Radiopaedia.org. Radiopaedia. https://radiopaedia.org/cases/covid-19-pneumonia-evolution-over-a-week-1
- 33.Bell D.J. COVID-19 | Radiology Reference Article | Radiopaedia.org. Radiopaedia. https://radiopaedia.org/articles/covid-19-4
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures