Abstract
The case of a diver with a history of decompression sickness (DCS) after recreational scuba diving is presented. Cutis marmorata, a subtype of cutaneous DCS, has been consistently associated with the presence of a persistent (patent) foramen ovale (PFO) as a risk factor. Diagnostic uncertainty arose when transthoracic echocardiography with antecubital injection of agitated saline bubbles (ASBs) did not show any significant shunt, but the presence of a large Eustachian valve was counteracted by intra-femoral injection of ASBs, showing a large PFO with spontaneous shunting. The importance of proper echocardiography techniques prior to resorting to intra-femoral injection of ASBs to counteract the haemodynamic effects of the Eustachian valve is emphasised.
Keywords: Bubbles, Decompression illness, Right-to-left shunt
Introduction
The case of a diver with a history of decompression sickness (DCS) after recreational scuba diving is presented. Cutis marmorata, a subtype of cutaneous decompression sickness, has been consistently associated with the presence of a persistent (patent) foramen ovale (PFO) as a risk factor for DCS. The case highlights an interesting point in relation to echocardiography for investigation for PFO.
Case history
The patient gave written consent for details and images pertaining to this case to be published.
A 38-year-old female certified diving instructor was brought to the emergency department. She complained of an itchy rash over her abdomen 30 minutes after surfacing from her single dive of the day. She had a history of 3,996 logged lifetime dives, as well as three prior episodes of DCS, three years (lymphatic), two years (cutaneous) and one year (cutaneous) prior to this presentation. The maximum depth of the dive was 34 metres’ sea water (msw) for an absolute bottom time of 85 minutes on open circuit 31% nitrox, with a total decompression time of 35 minutes, with a gas switch to 50% nitrox at 21 msw and 80% nitrox at 9 msw for accelerated decompression purposes. On the day prior to presentation, she had performed a single dive to 60 msw on open circuit trimix with a total dive time of 67 minutes.
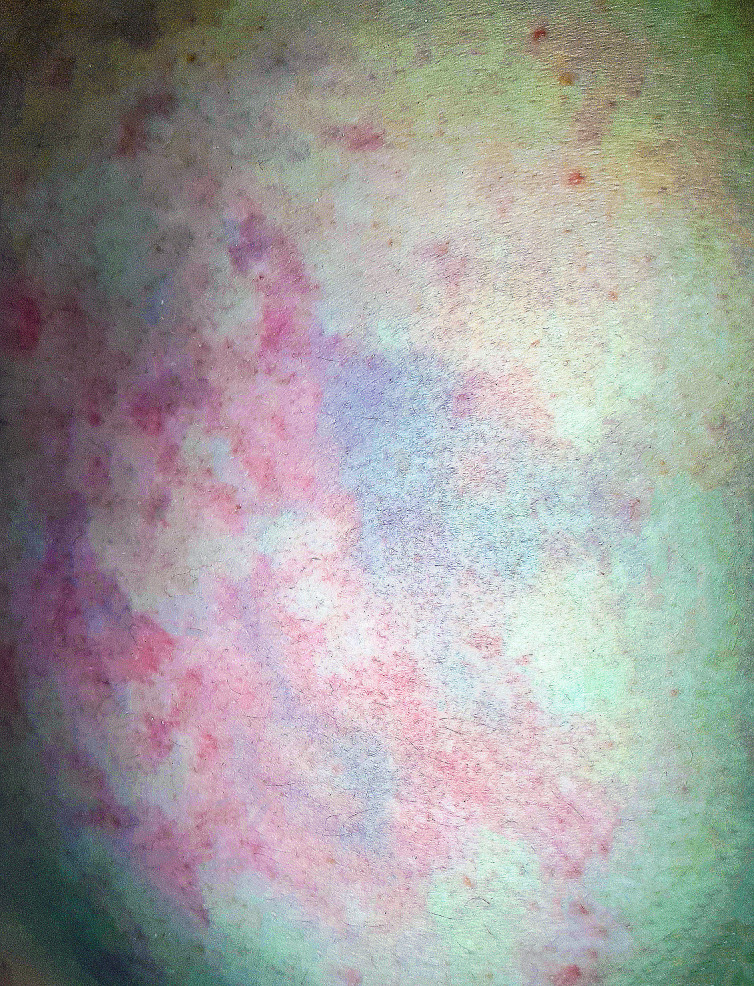
On arrival at the emergency department, the patient was alert and orientated, eupnoeic, afebrile and normotensive. She had a normal full neurological examination. Her body mass index was 24.5 kg·m-2 and lab tests showed blood glucose of 5.6 mmol·L-1 and oxygen saturation of 99% on room air. A macular, pruritic non-blanching rash was present over all quadrants of her abdomen (Figure 1).
Figure 1.
Cutis marmorata rash post-diving
She was administered two litres of intravenous 0.9% sodium chloride prior to being transferred urgently to the hyperbaric unit for recompression therapy. She was treated with a United States Navy Treatment Table 5 with 100% oxygen at a treatment pressure of 282 kPa, with a total therapeutic table time of 135 minutes, excluding descent. This began within 35 minutes from the onset of her symptoms post surfacing. She exhibited full resolution of the rash within 10 minutes of compression to 282 kPa.
Trans-oesophageal echocardiography (TOE) incorporating a bubble contrast study performed in Hungary after the second episode of DCS had shown no evidence of a right-to-left shunt at pulmonary or atrial level. During this procedure contrast had been injected antecubitally, but no provocation measures were performed as the patient was sedated. Trans-thoracic echocardiography (TTE), this time performed in Malta, with antecubital vein injection of two separate boluses of bubble contrast, one at rest and one with provocation manoeuvres after the third episode of DCS, had shown an atrial level shunt with minimal spontaneous shunting. Abdominal compression and prolonged Valsalva augmented flow across the shunt. The diver elected not to change her diving practices seeing the small documented size of the atrial level shunt at this point. TTE performed in Malta eight weeks following the fourth episode of DCS, with intra-femoral injection of two boluses of bubble contrast to avoid the effect of the Eustachian valve, showed a large PFO with manifest spontaneous right-to-left shunting. The diver elected to proceed with percutaneous PFO closure.
Discussion
The foramen ovale is an inter-atrial connection that enables rapid flow of umbilical blood to the brain and vital organs during intra-uterine foetal life. At birth, the foramen ovale flap, the septum primum, is physiologically closed onto the septum secundum when pulmonary vascular resistance and right atrium pressure drop. Fusion, which begins with contact, is completed in the first two years of life, but may remain incomplete in up to 25–30% of the general population.[ 1] While individuals with PFO are generally discovered incidentally during autopsies performed for other indications, antemortem diagnosis is made during the diagnostic workup of clinical scenarios associated with PFO, such as cryptogenic stroke, migraine, sleep apnoea, platypnea-orthopnoea syndrome and DCS. In an autopsy study consisting of 965 people, PFO diameters were measured at between 1–19 mm (4.9 mm on average) and the mean size was 3.4 mm in the first decade and was 5.8 mm in the tenth decade.[ 1] One may thus hypothesise that small PFOs are closed over time and that large ones may remain open. When this process fails, the foramen remains patent and hence allows blood flow across it, while another mechanism which might be at play is dehiscence of a previously fused foramen ovale flap.
The combining hypothesis for the association of PFO with the numerous clinical scenarios mentioned previously is based on the passage of particulate emboli, bubbles or chemical substances from the venous circulation to the systemic circulation, bypassing the lungs through a right-to-left shunt. The left atrial pressure is higher than the pressure in the right atrium, which normally prevents passage by holding down the septum primum flap opposed to the septum secundum. However, situations may arise in which changes in intrathoracic pressure (e.g., during knee bends, straining post-dive, forceful Valsalva manoeuvre, or cough) may result in spikes in right atrial blood loading, increasing the risk of an embolisation process via a PFO.[ 2]
Another issue about PFO-mediated shunting and its implications during the diagnostic process are the blood flow dynamics in the right atrium and their relationship with the fossa ovalis. In the right atrium, the currents from the caval veins do not collide head-to-head, but turn forward and contribute to the rotation of the blood in a clockwise direction. This filling pattern, associated with directing the atrial volume towards the tricuspid valve entry, is extremely important in maintaining the continuous activity of the heart with minimal energy. The ‘semi-lunar groove’, lying next to the fossa ovalis where the PFO is located, needs to be appreciated as the source of turbulent blood flow which may impact the passage of blood through the PFO.[ 2] Venous bubbles injected into a large blood vessel antecubitally may be swept away from the inter-atrial septum by this turbulence and thus be prevented from becoming ‘paradoxical gas emboli’. This may make the detection of a PFO by bubble contrast echocardiography a challenging task and contribute to a ‘false-negative’ result. Knowledgeable cardiologists are aware of this possibility and coach the subject to perform respiratory provocation (a sharp inspiratory sniff), Valsalva manoeuvres and abdominal compression manoeuvres to reliably diagnose a PFO.[ 3]
The PFO-mediated shunt can be determined by different echocardiographic techniques, namely TTE, TOE and transcranial Doppler (TCD). TOE has superior image resolution, and is able to define morphology, as well as the presence, number and size of these accompanying defects. It also allows assessment of the completeness of the septum apart from the defect and the presence of anatomic structures that will affect the placement of a closure device and visualising the three-dimensional appearance of PFO once closure is being considered.[ 4] However, it comes after TTE or TCD in the evaluation hierarchy because it is a semi-invasive procedure with well-defined staff training criteria and potentially life-threatening complications, such as oesophageal haemorrhage and perforation, and it is contraindicated in patients with severe bleeding risk. TTE is thus the most frequently used initial screening test because of its low cost, non-invasive nature and easy accessibility.
These observations lead to the conclusion that while the anatomical caveats of the heart remain unchanged from the times of Vesalius and Galen, it is our understanding of its biomechanical properties and of the variable diagnostic impact of these aforementioned anatomical issues that has changed over the course of time. In 1986, Wilmshurst and colleagues observed neurological decompression sickness in a recreational scuba diver after a 15 minute dive to 38 m, and attributed its cause to venous gas emboli passing through a previously undocumented atrial septal defect.[ 5] While large atrial septal defects such as the one demonstrated in Wilmshurst’s case are rare, small defects such as PFOs are present in up to 30% of the population, and provide a similar route for bubbles to enter the arterial blood. Indeed, several investigators have demonstrated an association between PFO and certain types of DCS, predominantly cerebral, spinal cord, cutaneous and vestibulo-cochlear DCS.[ 6 - 11] In these studies, right-to-left shunts have been demonstrated in as many as 89% of symptomatic divers with vestibulo-cochlear DCS, and 60% of divers in cases of cerebral or spinal cord DCS, compared with 20–30% of control subjects.6–11 A recent study has also elucidated the presence of bubbles in the skin microcirculation underlying cutis marmorata in DCS patients with large right to left shunts.[ 12]
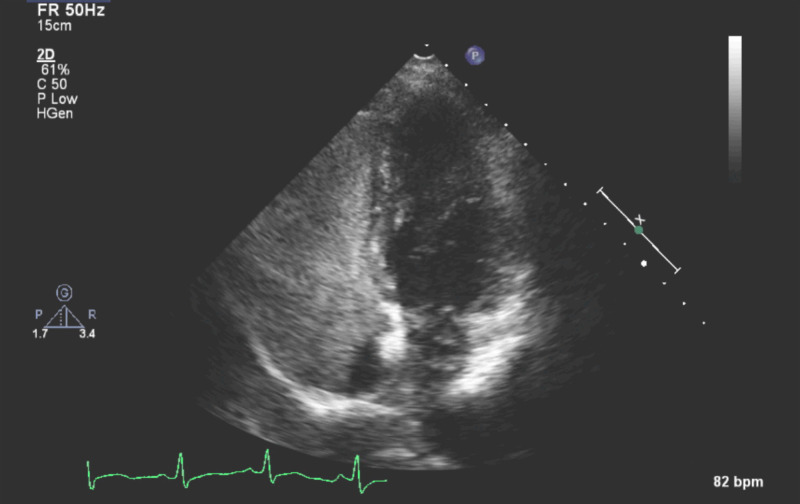
The presence of a persistent large Eustachian valve diverts bubble contrast approaching from the superior vena caval territory away from the interatrial septum. This can be appreciated and actively sought for on an apical 4-chamber transthoracic echocardiogram view, where a contrast-free zone may be visible peri-septally on the right side of the interatrial septum. This can be appreciated from the imaging obtained during the workup of the present case (Figure 2).
Figure 2.
Contrast-free zone peri-septally on the right side of the inter-atrial septum following antecubital bubble contrast injection
This contrast-free zone peri-septally should be seen as an indicator of a potentially false negative TTE for shunt identification, or underestimated shunt magnitude. We would not advocate to immediately change to femoral vein access, given that there are a number of techniques and manoeuvres to alter the flow dynamics and the opacification by bubble contrast of the right atrium, such as changing the positioning of the patient and pressing on the abdomen to reduce IVC inflow (Valsalva and IVC compression aim to reduce right atrial venous return to shrink the heart, so a rapid ingress of blood, opacified with bubbles, can improve diagnostic quality). Even if moving the patient does not alter the peri-septal contrast-free zone and identify the shunt, a sharp inspiratory sniff can improve the opacification and frequently demonstrates the shunt. If this does not work, then a prolonged Valsalva will, after release, cause a sudden increase in venous return and increase in right atrial filling. If this is timed appropriately with bubble contrast injection the entire chamber becomes opacified, and as the left atrial pressure and volume takes a little longer to recover, the septum will swing towards the left side, and if there is an atrial level shunt, bubbles will cross the septum, and be visible on the transthoracic echocardiogram.
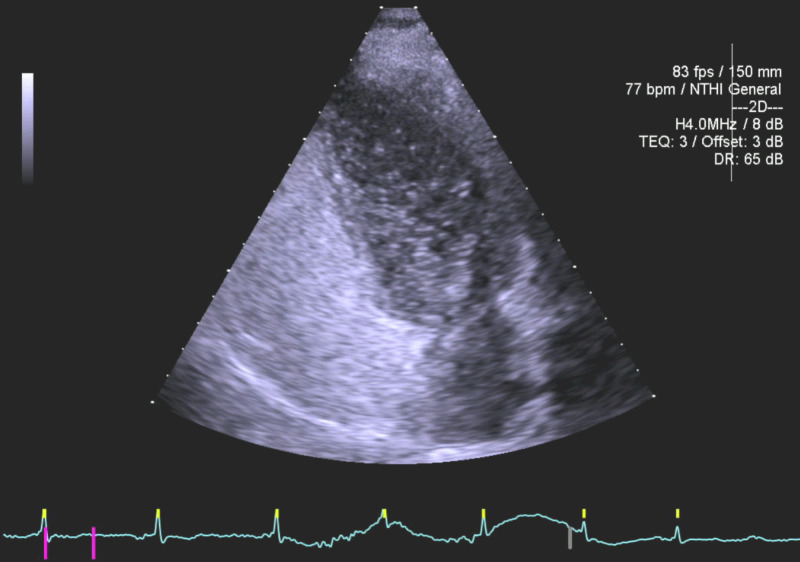
In situations where the diagnosis of an atrial level shunt is still in doubt due to the presence of a Eustachian valve interfering with the injected bubble contrast reaching the inter-atrial septum, injecting bubble contrast by repeating the ante-cubital injection of contrast should be attempted, before resorting to injection via the femoral vein, which should remain the very last resort technique. This will introduce contrast via the inferior vena cava and result in better contact with the interatrial septum (Figure 3). One must take into consideration that femoral vein injection is associated with the risk of producing arterio-venous fistulas and haematomas, but this can be minimised with ultrasound guidance, and thus we must re-iterate our advice to use this technique with utmost clinical judgement and acumen.
Figure 3.
Transfemoral bubble contrast injection in same patient with no contrast-free zone on the right side of the inter-atrial septum; marked right-to-left bubble contrast shunting within 3 cardiac cycles
Conclusion
The anatomical location of the Eustachian valve presents identifiable challenges to the accuracy and the sensitivity of TTE with transcubital injection of bubble contrast for the diagnosis of PFO. We recommend repeating TTE with femoral injection of bubble contrast only as a very last resort in cases with negative, repeated transcubital studies despite the use of provocative manoeuvres such as inspiratory sniff and abdominal compression, and only when elevated clinical suspicion of PFO-associated paradoxical embolism persists clinically. We thus advocate for proper echocardiography techniques during antecubital injection of bubble contrast to counteract the effect of the Eustachian valve during the echocardiographic diagnostic process of PFOs in divers with a history of DCS.
Footnotes
Conflict of interest and funding: nil
Contributor Information
Charles P Azzopardi, Baromedicine Department, Hyperbaric Unit, Mater Dei Hospital, Malta.
Kurt Magri, Baromedicine Department, Hyperbaric Unit, Mater Dei Hospital, Malta.
Alex Borg, Cardiology Department, Mater Dei Hospital, Malta.
Jake Schembri, Mater Dei Hospital, Malta.
Jonathan Sammut, Mater Dei Hospital, Malta.
References
- Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- Balestra C, Germonpré P, Marroni A. Intrathoracic pressure changes after Valsalva strain and other maneuvers: Implications for divers with patent foramen ovale. Undersea Hyperb Med. 1998;25:171–4. [PubMed] [Google Scholar]
- Rodrigues AC, Picard MH, Carbone A, Arruda AL, Flores TF, Klohn J, et al. Importance of adequately performed valsalva maneuver to detect patent foramen ovale during transoesophageal echocardiography. J Am Soc Echocardiogr. 2013;26:1337–43. doi: 10.1016/j.echo.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transoesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anaesthesiologists. J Am Soc Echocardiogr. 2013;26:921–64. doi: 10.1016/j.echo.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Wilmshurst PT, Ellis BG, Jenkins BS. Paradoxical gas embolism in a scuba diver with an atrial septal defect. Br Med J (Clin Res Ed). 1986;293:1277. doi: 10.1136/bmj.293.6557.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmshurst PT, Byrne JC, Webb-Peploe MM. Relation between interatrial shunts and decompression sickness in divers. Lancet. 1989;2:1302–6. doi: 10.1016/s0140-6736(89)91911-9. [DOI] [PubMed] [Google Scholar]
- Germonpré P, Dendale P, Unger P, Balestra C. Patent foramen ovale and decompression sickness in sports divers. J Appl Physiol (1985). 1998;84:1622–6. doi: 10.1152/jappl.1998.84.5.1622. [DOI] [PubMed] [Google Scholar]
- Wilmshurst P, Bryson P. Relationship between the clinical features of neurological decompression illness and its causes. Clin Sci (Lond). 2000;99:65–75. [PubMed] [Google Scholar]
- Cantais E, Louge P, Suppini A, Foster PP, Palmier B. Right-to-left shunt and risk of decompression illness with cochleovestibular and cerebral symptoms in divers: Case control study in 101 consecutive dive accidents. Crit Care Med. 2003;31:84–8. doi: 10.1097/00003246-200301000-00013. [DOI] [PubMed] [Google Scholar]
- Klingmann C, Benton PJ, Ringleb PA, Knauth M. Embolic inner ear decompression illness: Correlation with a right-to-left shunt. Laryngoscope. 2003;113:1356–61. doi: 10.1097/00005537-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Wilmshurst PT, Pearson MJ, Walsh KP, Morrison WL, Bryson P. Relationship between right-to-left shunts and cutaneous decompression illness. Clin Sci (Lond). 2001;100:539–42. [PubMed] [Google Scholar]
- Garcia E, Mitchell SJ. Bubbles in the skin microcirculation underlying cutis marmorata in decompression sickness: Preliminary observations. Diving Hyperb Med. 2020;50:173–7. doi: 10.28920/dhm50.2.173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]