Abstract
Background
This study aimed to evaluate the accuracy of pulse oximetry-derived oxygen saturation (SpO2) on room air, determined at hospital admission, as a predictor for the need for mechanical ventilatory support in patients with Coronavirus Disease-2019 (COVID-19).
Methods
In this retrospective observational study, demographic and clinical details of the patients were obtained during ICU admission. SpO2 and respiratory rate (RR) on room air were determined within the first 6 h of hospital admission. As all measurements were obtained on room air, we calculated the simplified respiratory rate‑oxygenation (ROX) index by dividing the SpO2 by the RR. Based on the use of any assistance of mechanical ventilator (invasive or noninvasive), patients were divided into mechanical ventilation (MV) group and oxygen therapy group. The accuracy of the SpO2, CT score, and ROX index to predict the need to MV were determined using the Area under receiver operating curve (AUC).
Results
We included 72 critically ill patients who tested COVID-19-positive. SpO2 on the room air could predict any MV requirement (AUC [95% confidence interval]: 0.9 [0.8–0.96], sensitivity: 70%, specificity 100%, cut-off value ≤78%, P < 0.001). Within the MV group, the use of noninvasive ventilation (NIV) was successful in 37 (74%) patients, whereas 13 patients (26%) required endotracheal intubation. The cut-off ROX value for predicting early NIV failure was ≤1.4, with a sensitivity of 85%, a specificity of 86%, and an AUC of 0.86 (95% confidence interval of 0.73–0.94, P < 0.0001).
Conclusions
A baseline SpO2 ≤78% is an excellent predictor of MV requirement with a positive predictive value of 100%. Moreover, the ROX index measured within the first 6 h of hospital admission is a good indicator of early NIV failure.
Keywords: COVID-19, Peripheral oxygen saturation, ROX index, Mechanical ventilatory support
1. Introduction
Coronavirus disease-2019 (COVID-19) represents a major global health threat. One of the significant factors related to the increased mortality rate observed in patients with COVID-19 is the high infectivity, which has led to an overwhelming load of admissions exceeding the capacity of the healthcare system in some countries [[1], [2], [3]]. Therefore, early triaging and identification of critical patients with COVID-19 are essential during the initial assessment of patients. Several clinical, laboratory, and radiological risk factors had been evaluated early detection of critical cases which require highly-equipped centers Computed tomography (CT) score upon admission showed good ability to predict severe cases among patients with COVID-19 [4,5]. However, routine CT imaging for patients with COVID-19 remains a debatable issue as it carries the risk of spreading the virus inside the healthcare facility. Other laboratory investigations were also included in risk stratification [5,6]. Laboratory investigations might sometimes be time-consuming and not feasible in resource-shortage situations. The use of simple bedside parameters would be easier and more economic especially during the peaks of the pandemic. Noninvasive ventilation (NIV) had received wide acceptance as a key-step in the management of COVID-19. However, there is still a considerable number of patients who fail on NIV and require timely initiation of invasive ventilation. Delaying invasive mechanical ventilation (MV) in those patients is associated with poor outcomes [7]. Thus, there is an urgent need for early indicators of NIV failure.
The use of parameters that are derived from simple clinical data such as the respiratory rate (RR) and the pulse oximetry-derived oxygen saturation (SpO2) had been previously reported in non-COVID-19 patients. The SpO2/fraction of inspired oxygen (FiO2) ratio was found to predict the outcome in non-COVID-19 patients with acute respiratory distress syndrome (ARDS) [8]. The respiratory rate‑oxygenation (ROX) index is another parameter that is derived from the RR and oxygenation and was used for predicting the success of the high flow nasal cannula in non-COVID-19 patients. In the current study, we aimed to evaluate the accuracy of the SpO2 on room air upon hospital admission as a single variable and in combination with the RR in predicting the need for mechanical ventilatory support and to identify the failure of NIV therapy in patients with COVID-19.
2. Methods
In this retrospective observational study, we included patients with laboratory-confirmed, moderate-to-severe COVID-19 [9] who were admitted to the intensive care unit (ICU) of our University Hospital during the period from May 14, 2020, to July 25, 2020. The present study was approved by the institutional research ethics board (N-98-2020). Written consent was not required due to the purely observational and retrospective nature of the study. Upon admission to the ICU, all patients were treated according to our standardized respiratory and hemodynamic protocols [10,11]. The oxygen flow was adjusted to maintain an SpO2 of 92%–96%. If the RR did not fall below 30 breaths/min and/or the SpO2 did not reach the target, NIV was initiated. The following features were considered as NIV failure: worsening of dyspnea, worsening or lack of improvement of hypoxemia (defined as SpO2 < 90%), the persistence of RR >35 breaths/min, the appearance of respiratory acidosis (defined as pH <7.3 and arterial carbon dioxide tension >50 mmHg), circulatory shock (defined as the use of vasopressor to maintain the mean arterial pressure at >65 mmHg), or altered sensorium. A patient who developed any feature of NIV failure was qualified to receive invasive mechanical ventilation. When NIV failure occurred within 48 h of NIV, it was defined as early NIV failure, whereas failure occurring after 48 h of NIV was defined as late NIV failure [12].
2.1. Data collection
Based on the use of any assistance of mechanical ventilator (invasive or noninvasive), the study patients were divided into the MV group and the oxygen therapy group. Demographic and clinical details were obtained during ICU admission. SpO2 obtained using a pulse oximeter on room air and RR were ascertained within the first 6 h of hospital admission. The ROX index was calculated by dividing SpO2 / FiO2 to RR. As all measurements were obtained on room air, the calculation of the ROX index was simplified as SpO2/RR.
2.2. Chest CT severity score
Bilateral lungs were divided into the following five zones according to the anatomical structure of the lung: left upper lobe, left lower lobe, right upper lobe, right middle lobe, and right lower lobe. Each lung lobe was assigned a score that was based on the following criteria: score 0, 0% involvement; score 1, <5% involvement; score 2, 5% to <25% involvement; score 3, 25% to <50% involvement; score 4, 50% to <75% involvement; and score 5, ≥75% involvement. The summation of scores provided a semiquantitative evaluation of overall lung involvement (the maximum CT score for both lungs was 25 [13]. All patients underwent CT imaging at hospital admission, and the images were scored by an experienced radiologist who was blinded to the clinical data.
2.3. Study outcome
The primary outcome of this study was to determine the predictive ability of SpO2 for the need of mechanical ventilatory support as a single variable and after being adjusted for Acute Physiology and Chronic Health Evaluation II (APACHE II) score, age, and gender. The secondary outcome was to compare the performance of SpO2 in predicting the outcome of patients compared with the CT score. In addition, the ROX index at admission was determined to predict patients at risk of early NIV failure.
2.4. Statistical analysis
Data were reported as median and quartile or mean and standard deviation values as appropriate and analyzed using the Mann–Whitney test. Categorical variables were summarized as counts and percentages and analyzed using the Chi-squared or Fisher's exact test as appropriate. Receiver-operating characteristic curves were constructed, and the area under the curve (AUC) was calculated for the SpO2. The best cut-off value was calculated using the Youden index. Multivariate analysis was performed to obtain adjusted odds ratio (OR) and 95% confidence intervals (CI) for SpO2 and the ROX index as risk factors for the need to MV and the failure of NIV. Statistical analysis was conducted using MedCalc, version 19 (MedCalc Software by, Ostend, Belgium).
3. Results
A total of 72 critically ill patients were included in this study, of whom 50 (69%) patients required ventilatory support and 22 (31%) did not. The mean (standard deviation) age of patients in the oxygen therapy group was 53 [12] years, which was significantly lower than that [61 [13] years] of patients in the MV group (P = 0.001). Other demographic and clinical characteristics were comparable between the two groups (Table 1 ). Upon admission to the ICU, the median SpO2 on room air and the ROX index were significantly lower in the MV group than in the oxygen therapy group (Table 2 ). After exclusion of patients who required oxygen therapy only, the baseline inflammatory markers were comparable between patients who succeeded and those who failed on NIV (Table 3 ). The optimal cut-off value of SpO2 for predicting ventilatory requirement according to the maximum Youden index was ≤78%. This cut-off value showed a sensitivity of 70% and a specificity of 100%, and the AUC was 0.9 (95% CI 0.8–0.96, P < 0.0001). After adjusting for age, APACHE II score, and gender, SpO2 on room air remained independently associated with the need for mechanical ventilatory support (OR [95% CI]: 0.7 [0.6–0.8], P < 0.0001).
Table 1.
Patient characteristics, respiratory status, ICU stay, and hospital mortality. Data are presented as mean (standard deviation), median (quartiles), or number (%).
| Characteristics | All patients (N = 72) |
Oxygen therapy group (N = 22) | MV group (N = 50) |
P-value |
|---|---|---|---|---|
| Sex, Male (%) | 49 (68%) | 16(72%) | 33(66%) | 0.3 |
| Age (years) | 59(13) | 53(12) | 61(13) * | 0.001 |
| Weight (kg) | 95(20) | 101(21) | 90(20) | 0.4 |
| APACHE II score | 10(4) | 9(3) | 11(5.5) | 0.2 |
| Coexisting disorder | ||||
| Chronic cardiac disease (%) | 17(23%) | 4(18%) | 13(26%) | 0.5 |
| Chronic pulmonary disease (%) | 5 (7%) | 0 | 5(10%) | 0.1 |
| Chronic kidney disease (%) | 7 (10%) | 2(9%) | 5(10%) | 0.9 |
| Chronic diabetes (%) | 39 (54%) | 11(50%) | 28(56%) | 0.6 |
| Chronic hypertension (%) | 46 (64%) | 13(59%) | 33(66%) | 0.9 |
| Smoking (%) | 9(16.4%) | 3(14%) | 6(12%) | 0.8 |
| Obesity (%) | 18(25%) | 6(27%) | 12(24%) | 0.6 |
| Smoking (%) | 9(12%) | 3(14%) | 6(12%) | 0.7 |
| Respiratory variables | ||||
| SpO2 on room air (%) | 80(70–85) | 88(85–89) | 75(65–82) * | <0.001 |
| RR on room air (breaths/min) | 39(30–45) | 35(30–40) | 40(33–45) * | 0.025 |
| CT score | 14(10–19) | 10(8–12) | 18(13−20) * | <0.0001 |
| Outcome variable | ||||
| ICU stay (days) | 6(3–8) | 3(2–4) | 7(4–8) * | <0.0001 |
| Mortality (%) | 16(22%) | 0 | 16(32%)* | 0.002 |
APACHE II: Acute Physiology and Chronic Health Evaluation II, CT: Computed tomography, ICU: Intensive care unit. SpO2: peripheral oxygen saturation, RR: respiratory rate, MV: mechanical ventilation * denotes significance compared to the other group, P < 0.05.
Table 2.
Respiratory variables, ICU stay, and hospital mortality. Data are presented as mean (standard deviation), median (quartiles), or number (%).
| Characteristics | All patients (N = 72) | Oxygen therapy group (N = 22) | MV group (N = 50) | P-value |
|---|---|---|---|---|
| SpO2 on room air (%) | 80(70–85) | 88(85–89) | 75(65–82) * | <0.0001 |
| RR on room air (breaths/min) | 39(30–45) | 35(30–40) | 40(33–45) * | 0.025 |
| ROX index | 2(1.6–2.6) | 2.5(2.2–3) | 1.8(1.4–2.6)* | <0.0001 |
| CT score | 14(10–19) | 10(8–12) | 18(13–20) * | <0.0001 |
| Outcome variable | ||||
| ICU stay (days) | 6(3–8) | 3(2–4) | 7(4–8) * | <0.0001 |
| Mortality (%) | 17(23%) | 0 | 16(34%)* | 0.002 |
CT: Computed tomography, ICU: intensive care unit, MV: mechanical ventilation, ROX: respiratory rate‑oxygenation, RR: respiratory rate, SpO2: peripheral oxygen saturation, * denotes significance compared to the other group, P < 0.05.
Table 3.
Patient characteristics and inflammatory data in patients who required mechanical ventilatory support. Data are presented as meadian (quartiles).
| Variables | NIV successful (N = 37) | NIV failure (N = 13) |
P-value | |
|---|---|---|---|---|
| Early failure (N = 7) | Late failure (N = 6) | |||
| Age | 65(58–69) | 62(45–72) | 70(62–75) | 0.3 |
| Admitting leucocytic count | 9(6–12) | 6(5–11) | 6.8(4–12) | 0.32 |
| Admitting Lymphocytes | 0.8(0.5–1.2) | 0.6(0.5–1.4) | 0.5(0.4–0.8) | 0.36 |
| Admitting Ferritin | 966(690–1783) | 710(640–2300) | 1226(890–1890) | 0.6 |
| Admitting LDH | 652(488–833) | 630(496–1171) | 410(341–743) | 0.2 |
| Admitting CRP | 168(88–208) | 147(69–226) | 103(24–188) | 0.28 |
CRP: C-reactive protein, LDH: lactate dehydrogenase, NIV: non-invasive ventilation.
Among patients in the MV group, the use of NIV was successful in 37 (74%) patients, whereas 13 patients (26%) required endotracheal intubation. The median (quartiles) time for the duration of NIV was 3 [[2], [3], [4], [5]] days. In patients with NIV failure, the median time to intubation was 2 [[2], [3], [4], [5]] days. Early NIV failure (within the first 48 h) was observed in 7 patients because of refractory hypoxemia, whereas 6 patients had late NIV failure, i.e., after 48 h. The cause of delayed failure was sepsis in 5 patients and the development of stroke in 1 patient. Both the SpO2 and ROX indices were able to predict early NIV failure. Although not statistically significant, the AUC of the ROX index tended to be higher than that of the SpO2. The cut-off value of the ROX index for predicting early NIV failure was ≤1.4, with a sensitivity of 85%, a specificity of 86%, and an AUC of 0.86 (95% CI 0.73–0.94, P < 0.0001). The cut-off value of the SpO2 for predicting early NIV failure was ≤60%, with a sensitivity of 57%, a specificity of 84%, and an AUC of 0.76 (95% CI 0.6–0.86, P < 0.007). The multivariate analysis revealed that the ROX index remained independently associated with early NIV failure after adjusting for age, P = 0.013.
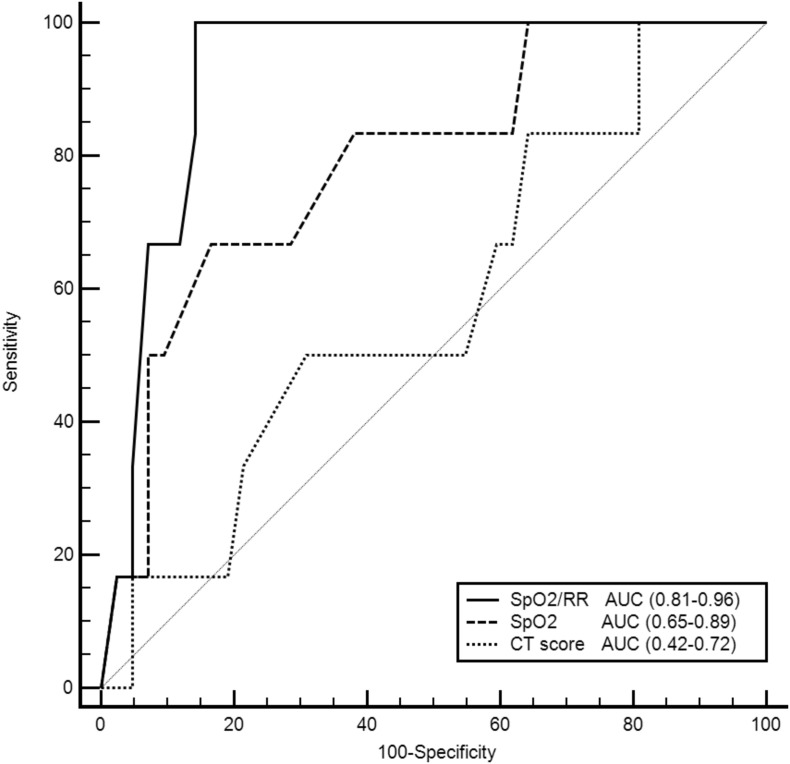
The median (quartiles) of the CT score at ICU admission in the oxygen therapy group was 10 [[7], [8], [9], [10], [11], [12]], which was significantly lower than that [18 [[13], [14], [15], [16], [17], [18], [19], [20]]] in the MV group (P < 0.0001). A significant negative correlation (r = −0.6) was detected between SpO2 on room air and the CT score (P < 0.0001). The optimal CT score to predict the need for mechanical ventilatory support was ≥13 (sensitivity 80%, specificity 73%, AUC 0.9). No significant difference was observed between the AUC of both SpO2 and CT score for predicting the requirement of MV support. However, both the ROX index and SpO2 were significantly better than the CT score in predicting early NIV failure (Fig. 1 ).
Fig. 1.
Receiver-operating characteristic curves comparing the ability of SpO2, ROX index, and CT score to predict early failure of noninvasive ventilation. AUC: area under the curve, CT: Computed tomography, ROX: respiratory rate‑oxygenation, SpO2: peripheral oxygen saturation.
During the study period, 17/72 (23%) patients died, and all belonged to the MV group (Table 2). A statistically significant, unadjusted association was found between admission SpO2 on room air and hospital mortality (P = 0.01). After adjusting for age, gender, and APACHE II score, SpO2 remained independently associated with hospital mortality (OR [95% CI]: 0.94 [0.91–0.97], P = 0.02). This model showed good discriminatory ability, with an AUC of 0.8.
4. Discussion
In the present study, we demonstrated that SpO2 on the room air, as a marker of impaired oxygenation at the time of hospital admission, was an excellent predictor for the need of MV support. Furthermore, the ROX index measured on room air before the initiation of mechanical ventilatory support could be used as a marker of early NIV failure.
Several studies have reported that the SpO2/FiO2 ratio is a valid predictor of the severity of ARDS and can guide the management of critically ill patients who require ventilatory support [8,10]. In the present study, we evaluated SpO2 on the room air rather than on oxygen because the determination of FiO2 remains inaccurate and influenced by minute ventilation, air leak, and breathing pattern in patients receiving oxygen through a facemask. Few studies had evaluated the validity of baseline SpO2 as an important risk factor in COVID-10; however, most of these studies used SpO2 as a component of multivariate prediction models [[14], [15], [16]] and did not use the need to ventilatory support as the primary outcome. Pre-hospital lowest SpO2 had been evaluated for triaging of patients with COVID-19. Lancet et al. had reported that patients with pre-hospital SpO2 < 90% as more likely to need hospital admission [17]. Dillon et al. had reported that pre-hospital SpO2, particularly the lowest recorded value, independently predicts death in these patients [18]. Our study goes in line with the current impression that low SpO2 is a strong risk factor in patients with COVID-19 l furthermore, it adds a special focus on the need for ventilatory support; moreover, we strictly included hospitalized patients with moderate-to-severe symptoms and calculated the cutoff value using AUC analysis.
The ROX index was initially formulated to predict the success of high-flow nasal oxygen in patients with severe pneumonia and it was calculated by dividing SpO2/FiO2 by RR [19]. To the best of our knowledge, the ROX index has never been tested as a marker of NIV success in COVID nor non-COVID patients. In the present study, we calculated the ROX index while the patients were breathing room air by dividing SpO2 by RR before the initiation of any ventilatory support, and we identified that a value ≤1.4 is highly predictive of early NIV failure. Low ROX index remained independently associated with early NIV failure even after adjustment for age. In a recent study, it was observed that an ROX index <5.4 measured within the first 4 h of HFNO initiation could be used as a marker of invasive mechanical ventilation requirement [20].
In our study, a CT score ≥ 13 was a predictor for the need for MV support. Our finding was consistent with that reported by Mahdjoub E et al. who demonstrated that an admission CT score ≥ 13 was an independent predictor of mechanical ventilation and/or death in patients with COVID-19 [1]. Interestingly, the AUC of SpO2 did not significantly differ from that of the CT in predicting the need of MV support. Furthermore, both SpO2 and SpO2/RR performed better than the CT score in predicting early NIV failure.
The pandemic pattern of COVID-19 resulted in major shortage in medical supplies in many countries which increased the rate of mortality [3]. Thus, it is essential to prioritize the patients as early as possible and to pick up the sickest who should be referred to higher equipped hospitals. It is also warranted to perform this triaging using the most simple and economic methods which can perform in situation with low resources.
5. Limitations
Our study had few important limitations, including the small sample size and the retrospective design. Nevertheless, our study does indicate that certain simple parameters (such as SpO2 and SpO2/RR) could be used reliably to predict the outcome of critically ill patients with COVID-19 presenting with respiratory failure.
6. Conclusion
In conclusion, SpO2 measured on room air in patients with COVID-19 at hospital admission is a reliable tool in predicting the outcome of patients compared with CT scan. A baseline SpO2 ≤78% on room air could predict the need for MV support with a positive predictive value of 100%. Furthermore, the ROX index measured within the first 6 h of hospital admission is a good indicator of early NIV failure.
Funding
None.
Declaration of interest
None.
Availability of data and material
Data are available from the authors upon reasonable request after permission of Cairo University.
References
- 1.Lemos D.R.Q., D’Angelo S.M., Farias L.A.B.G., et al. Health system collapse 45 days after the detection of COVID-19 in Ceará, Northeast Brazil: a preliminary analysis. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0354-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carenzo L., Costantini E., Greco M., et al. Hospital surge capacity in a tertiary emergency referral Centre during the <scp>COVID</scp> −19 outbreak in Italy. Anaesthesia. 2020;75:928–934. doi: 10.1111/anae.15072. [DOI] [PubMed] [Google Scholar]
- 3.Hasanin A., de Vasconcellos K., Abdulatif M. COVID-19 in Africa: current difficulties and future challenges considering the ACCCOS study. Anaesth Crit Care Pain Med. 2021;40:100912. doi: 10.1016/j.accpm.2021.100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahdjoub E., Mohammad W., Lefevre T., et al. Admission chest CT score predicts 5 - day outcome in patients with COVID - 19. Intensive Care Med. 2020:19–21. doi: 10.1007/s00134-020-06118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cecconi M., Piovani D., Brunetta E., et al. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for Covid-19 infection in Lombardy, Italy. J Clin Med. 2020;9:1548. doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aly M.H., Rahman S.S., Ahmed W.A., et al. Indicators of critical illness and predictors of mortality in COVID-19 patients. Infect Drug Resist. 2020;13:1995–2000. doi: 10.2147/IDR.S261159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobler C.C., Murad M.H., Wilson M.E. Noninvasive positive pressure ventilation in patients with COVID-19. Mayo Clin Proc. 2020;95:2594–2601. doi: 10.1016/j.mayocp.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Festic E., Bansal V., Kor D.J., et al. SpO 2 /FiO 2 ratio on hospital admission is an indicator of early acute respiratory distress syndrome development among patients at risk. J Intensive Care Med. 2015;30:209–216. doi: 10.1177/0885066613516411. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 10.Mukhtar A., Lotfy A., Hasanin A., et al. Outcome of non-invasive ventilation in COVID-19 critically ill patients: a retrospective observational study. Anaesth Crit Care Pain Med. 2020;39:579–580. doi: 10.1016/j.accpm.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasanin A., Mostafa M. Evaluation of fluid responsiveness during COVID-19 pandemic: what are the remaining choices? J Anesth. 2020;34:758–764. doi: 10.1007/s00540-020-02801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan J., Wang S., Liu P., et al. Early prediction of noninvasive ventilation failure in COPD patients: derivation, internal validation, and external validation of a simple risk score. Ann Intensive Care. 2019;9:108. doi: 10.1186/s13613-019-0585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang A.Z., Ehrman R., Bucca A., et al. Can we predict which COVID-19 patients will need transfer to intensive care within 24 hours of floor admission? Acad Emerg Med. 2021;28:511–518. doi: 10.1111/acem.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berenguer J., Borobia A.M., Ryan P., et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: the COVID-19 SEIMC score. Thorax. 2021;0:1–10. doi: 10.1136/thoraxjnl-2020-216001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger J., McGuire F., Risa E., et al. Emergency department characteristics and associations with intensive care admission among patients with coronavirus disease 2019. J Am Coll Emerg Physicians open. 2021;2 doi: 10.1002/emp2.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancet E.A., Gonzalez D., Alexandrou N.A., et al. Prehospital hypoxemia, measured by pulse oximetry, predicts hospital outcomes during the new York City COVID-19 pandemic. J Am Coll Emerg Physicians open. 2021;2 doi: 10.1002/emp2.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon K., Hook C., Coupland Z., et al. Pre-hospital lowest recorded oxygen saturation independently predicts death in patients with COVID-19. Br Paramed J. 2020;5:59–65. doi: 10.29045/14784726.2020.09.5.3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roca O., Caralt B., Messika J., et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 20.Zucman N., Mullaert J., Roux D., et al. Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46:1924–1926. doi: 10.1007/s00134-020-06177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon reasonable request after permission of Cairo University.