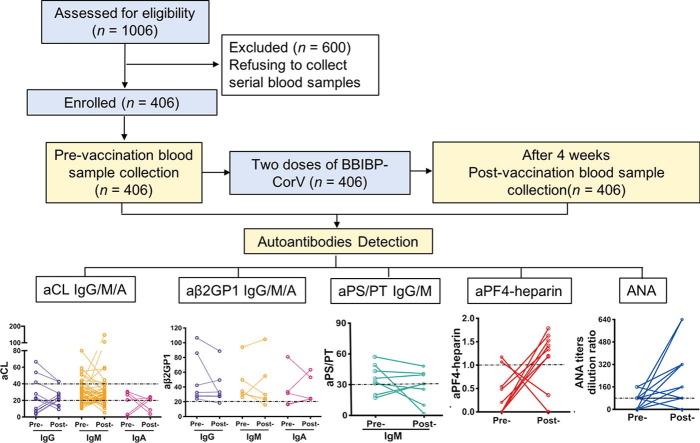
Graphical abstract
Keywords: Inactivated COVID-19 vaccine, Antiphospholipid antibody, Anti-PF4-heparin antibody, Thrombosis, Thrombocytopenia
Abstract
The presence of antiphospholipid antibodies was shown to be associated with thrombosis in coronavirus disease 2019 (COVID-19) patients. Recently, according to reports from several studies, the vaccine-induced immune thrombotic thrombocytopenia is mediated by anti-platelet factor 4 (PF4)-polyanion complex in adenovirus-vectored COVID-19 vaccine recipients. It is impendent to explore whether inactivated COVID-19 vaccine widely used in China influences prothrombotic autoantibody production and induces thrombosis. In this prospective study, we recruited 406 healthcare workers who received two doses, 21 days apart, of inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (BBIBP-CorV, Sinopharm). Paired blood samples taken before vaccination and four weeks after the second vaccination were used in detecting prothrombotic autoantibodies, including anticardiolipin (aCL), anti-β2 glycoprotein I (aβ2GP1), anti-phosphatidylserine/prothrombin (aPS/PT), and anti-PF4-heparin. The seroconversion rate of SARS-CoV-2 specific antibodies was 95.81% (389/406) four weeks after vaccination. None of the subjects had spontaneous thrombosis or thrombocytopenia over a minimum follow-up period of eight weeks. There was no significant difference in the presence of all ten autoantibodies between samples collected before and after vaccination: for aCL, IgG (7 vs. 8, P = 0.76), IgM (41 vs. 44, P = 0.73), IgA (4 vs. 4, P = 1.00); anti-β2GP1, IgG (7 vs. 6, P = 0.78), IgM (6 vs. 5, P = 0.76), IgA (3 vs. 5, P = 0.72); aPS/PT IgG (0 vs. 0, P = 1.00), IgM (6 vs. 5, P = 0.76); aPF4-heparin (2 vs. 7, P = 0.18), and antinuclear antibody (ANA) (18 vs. 21, P = 0.62). Notably, seven cases presented with anti-PF4-heparin antibodies (range: 1.18–1.79 U/mL) after vaccination, and none of them exhibited any sign of thrombotic disorder. In conclusion, inactivated SARS-CoV-2 vaccine does not influence the profile of antiphospholipid antibody and anti-PF4-heparin antibody nor increase the risk of thrombosis.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused 162 million cases of coronavirus disease 19 (COVID-19), with more than three million deaths as of May 8, 2021 [1]. Exposure to SARS-CoV-2 can result in a range of clinical outcomes, varying from asymptomatic infection to multiorgan system involvement and death. Abnormal coagulation correlates with COVID-19 severity. Several studies reported the presence of antiphospholipid antibodies (aPLs), such as anticardiolipin (aCL), anti-β2 glycoprotein I (aβ2GPI), lupus anticoagulant (LAC), and anti-phosphatidylserine/prothrombin (aPS/PT) in COVID-19 patients, which were related to thromboembolic complications [2], [3], [4], [5].
Antiphospholipid antibodies are a group of heterogeneous procoagulant autoantibodies, including autoantibodies to phospholipids and phospholipid-binding proteins, and are the pathogenic driver of the antiphospholipid syndrome (APS), which is the leading cause of acquired thrombophilia. The persistent presence of anticardiolipin antibodies and anti-β2 glycoprotein I antibodies are included in the classification criteria for APS [6]. Besides, phosphatidylserine/prothrombin (aPS/PT) autoantibodies are associated with an increased prevalence of thrombotic events in patients with APS [7]. These autoantibodies can activate platelets, neutrophils, and endothelial cells, interfere with complement activation and induce activated protein C to participate in the formation of thrombosis, leading to the pathological process of the thrombotic antiphospholipid syndrome [8]. According to previous studies, antiphospholipid antibodies are associated with other viral infections, including hepatitis C virus, cytomegalovirus (CMV), and the human immunodeficiency virus [9]. The presence of aPLs in COVID-19 patients strengthens the hypothesis that SARS-CoV-2 can cause coagulation disorder mediated through the autoimmune mechanism. Moreover, the anti-PF4-heparin antibody, which triggers platelet activation to form dangerous clots in heparin‐induced thrombocytopenia (HIT), was also presented with high titers in COVID-19 patients, possibly having a significant impact on the clinical course [10], [11].
As the COVID-19 pandemic persists globally, the need for a safe and effective vaccine has never been more urgent. Increasing evidence has identified the efficiency of the COVID-19 vaccine based on neutralizing antibody production [12], [13], [14], [15], [16], [17]. However, recent reports of severe but rare thrombotic events after receiving the COVID-19 vaccine based on adenovirus vectors brought concerns about the vaccine’s safety. Anti-PF4-heparin antibodies were detected in these patients with thrombosis and thrombocytopenia, indicating vaccine-induced immune thrombotic thrombocytopenia development. The clinical and laboratory manifestations resembled heparin-induced thrombocytopenia (HIT) [18], [19], [20]. Although the precise mechanism is still unclear, the rise of the antibody could be attributed to the errant immune reaction post-vaccine administration.
Vaccines are still the most successful and cost-effective way to prevent COVID-19 infection. The WHO approved the Sinopharm inactivated vaccine for emergency use on May 7, 2021. Up to May 16, over 406 million doses of inactivated vaccines have been used in China [21]. It is impendent to explore the effect of COVID-19 vaccination on the prothrombotic autoantibody production and risk of thrombosis.
2. Materials and methods
2.1. Study design and participants
In this study, healthcare workers who were able to voluntarily receive two doses of inactivated SARS-CoV-2 vaccine (BBIBP-CorV, Sinopharm, Beijing, China), administered intramuscularly 21 days apart were enrolled. Eligible individuals in good health were from 18 to 59 years. Exclusion criteria were fever, cough, sore throat, diarrhea, and dyspnea within 14 days before vaccination; pregnancy or lactation; allergy to any ingredient included in the vaccine; a history of seizures or mental illness; inability to comply with the study schedule. Paired blood samples were collected before vaccination and four weeks after receiving the second dose of the inactivated SARS-CoV-2 vaccine defined as pre-vaccination and post-vaccination, separately. The phlebotomists in the hospital conducted blood collection in Ruijin Hospital and serum was separated using centrifugation after blood clotting and kept frozen until the usage time. The cohort was recruited from January 14 to March 10, 2021, with a minimum follow-up period of eight weeks. The study was reviewed and approved by the Institutional Review Board of Ruijin Hospital (approval ID: 2021–12), Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from individuals as part of a more extensive observational cohort study (ID: NCT04795414). All procedures conducted in studies involving human participants followed the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki.
2.2. Study procedures
Liver function and blood count were tested before the first dose of vaccination and after the second dose of vaccination. SARS-CoV-2 antibodies were detected four weeks after the second dose of vaccination. Paired serum samples were used to detect the ten autoantibodies in the same batch to avoid measurement errors inherent to detection between batches. Testing was conducted by well-trained specialists with more than three years of experience following strict protocols. All planned tests were completed with no invalid or missing data.
2.3. SARS-CoV-2 antibody detection
We measured SARS-CoV-2 specific antibodies in all enrolled individuals using a chemiluminescence kit (Wantai BioPharm, Xiamen, China) following the manufacturer’s instructions. The assay manufacturers supplied thresholds for positivity.
2.4. Antinuclear antibody measurement
Antinuclear antibody was assessed using indirect fluorescence on Hep-2 cells according to the manufacturer’s instructions (Inova Diagnostics, San Diego, USA). Serum was diluted at 1:40 initially, after which serial two-fold dilution was used to stain Hep-2 cells. The diluted serum was incubated on the slides for 30 min. After washing off unbound serum, the slides were incubated with a fluoresceinated goat anti-human IgG secondary antibody before viewing using a fluorescence microscope. The results were reported as the endpoint dilution at which fluorescence was detectable. The suggested cutoff for ANA is 1:80 according to the international guidelines [22], [23].
2.5. Antiphospholipid antibodies testing
Antiphospholipid antibodies, such as aCL IgG, aCL IgM, aCL IgA, anti-β2GPI IgG, anti-β2GPI IgM, anti-β2GPI IgA, aPS/PT IgG, and aPS/PT IgM were measured using the commercial Elisa kit from Inova Diagnostics (Quanta Lite®, Inova Diagnostics) according to the manufacturer’s instructions. Quanta Lite® aPL antibody Elisas (Inova Diagnostics) were used by the antiphospholipid syndrome alliance for clinical trials and international networking (APS ACTION) to test aPLs in registry samples, which is the largest international research cooperation organization created to conduct multicenter studies in persistently aPL positive individuals [24], [25]. The suggested cutoff for aCL IgG/IgM/IgA is ≥ 20 GPL/MPL/APL, for anti-β2GPI IgG/IgM/IgA is ≥ 20 SGU/SMU/SAU, and for aPS/PT antibody is ≥ 30 units according to the manufacturer’s instructions, which have been validated in our local laboratory.
2.6. Anti-platelet factor 4-heparin antibody detection
According to the manufacturer’s instructions, anti-platelet factor 4-heparin antibodies, including all Ig isotypes, were detected using an automated latex immunoturbidimetric assay (HemosIL ® HIT-Ab (PF4-H), Instrumentation Laboratory, Bedford, USA). The cutoff value is > 1 U/mL as determined by the manufacturers.
2.7. Data collection
Data on the demographic, clinical, and laboratory examinations were collected. As of May 5, 2021, clinical data collection was completed.
2.8. Statistical analysis
Statistical analyses were conducted using SPSS software (Version 23.0). Data were expressed in the form of positive numbers, percentages for categorical variables, and median (Q1–Q3) for continuous variables that did not conform to the normal distribution. Pearson Chi-square test or by Fisher’s Exact Test were used to compare the categorical variables, where applicable. A two-sided P value less than 0.05 was considered significant.
3. Results
3.1. Baseline characteristics
Healthcare workers in Shanghai Ruijin Hospital registered for voluntary vaccination. From the registration order, we approached 1006 healthcare workers who applied for vaccine administration with eligibility for this study. All the 1006 individuals consented for long-term follow-up; among them, 406 individuals consented for the collection of serial blood samples. All 406 individuals with paired blood samples in our study were enrolled (Fig. 1 ). The cohort’s median age was 36 years (Q1–Q3: 29–44), and 76.8% (312/406) were women.
Fig. 1.
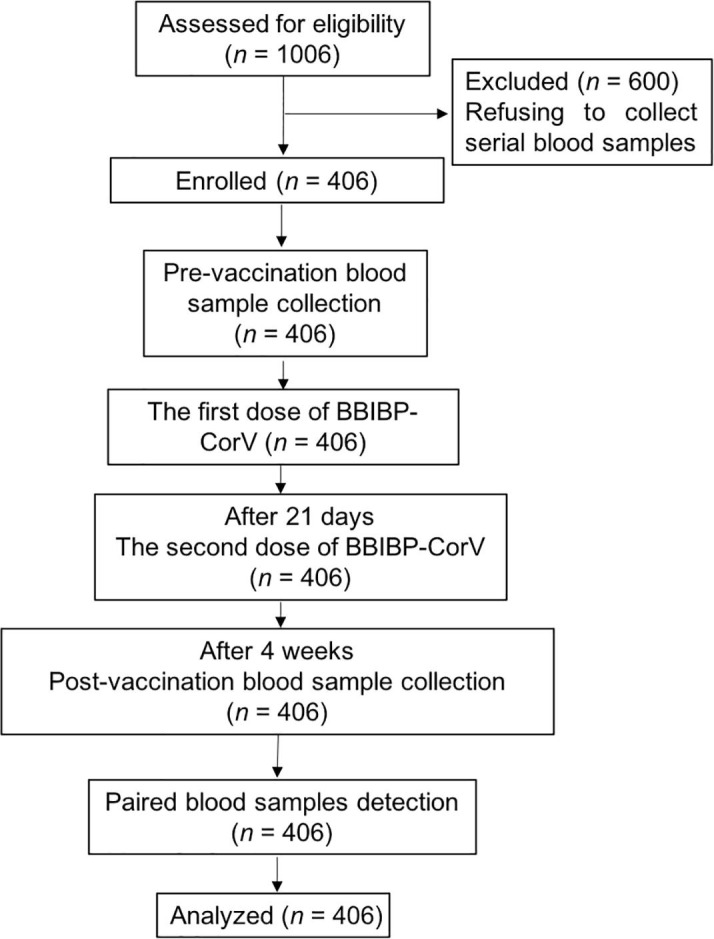
Flowchart of the current study.
We assayed SARS-CoV-2 antibody in samples collected before vaccination and four weeks after administration of the second dose of inactivated SARS-CoV-2 vaccine. As none of the enrolled individuals reported exposure to known patients with COVID-19, no serological response to SARS-CoV-2 was detected in the pre-vaccination samples. Four weeks after the second vaccination, 95.81% (389/406) of samples tested positive with SARS-CoV-2 antibody assay.
Notably, none of the enrolled subjects exhibited symptoms of spontaneous thrombosis or thrombocytopenia over a minimum follow-up period of eight weeks. Within four weeks after vaccination, adverse reactions were reported in 225 (55.42%) of the 406 participants of the study. The most common adverse systemic reaction was fatigue (25.62%), followed by dizziness and headache (13.79%: 56/406). The most common local adverse reaction was pain and tenderness, which was reported by 29.56% (120/406). All adverse reactions were mild, transient, and self-limiting. No abnormal laboratory tests were found after the COVID-19 vaccination (Table 1 ).
Table 1.
Demographic and laboratory characteristics of enrolled individuals.
| Pre-vaccination | Post-vaccination | Reference | |
|---|---|---|---|
| Age (median, Q1–Q3, years) | 36 (29–44) | ||
| Sex (F/M) | 312/94 | ||
| Laboratory assay | |||
| Leukocytes (× 109/L) | 6.27 (5.17–7.40) | 6.37 (5.30–7.40) | 3.69–9.16 |
| Red blood cell (× 1012/L) | 4.45 (4.21–4.80) | 4.46 (4.17–4.80) | 3.68–5.13 |
| Hemoglobin (g/L) | 133 (125–143) | 133 (124–143) | 113–151 |
| Platelet count (× 109/L) | 255 (220–290) | 259 (222–293) | 101–320 |
| Aspartate aminotransferase (IU/L) | 22 (20–27) | 20 (16–25) | 8–40 |
| Alanine aminotransferase (IU/L) | 16 (13–23) | 17 (3–25) | 10–64 |
| Total bilirubin | 8.2 (6.4–10.4) | 7.8 (6.1–10.2) | 4.7–24.0 |
| Albumin | 45 (43–47) | 43 (31–45) | 35–55 |
| Seroconversion rate of SARS-CoV-2 specific antibodies | 0 (0%) | 389 (95.81) | |
| Adverse reactions, n (%) | / | 225 (55.42) | |
| Systemic adverse reactions | / | 155 (38.18) | |
| Fever | / | 11 (2.71) | |
| Dizziness and headache | / | 56 (13.79) | |
| Fatigue | / | 104 (25.62) | |
| Diarrhea | / | 25 (6.16) | |
| Constipation | / | 0 | |
| Nausea and vomiting | / | 20 (4.93) | |
| Noninoculation site muscle and joint pain | / | 6 (1.48) | |
| Abnormal skin and mucosa | / | 13 (3.20) | |
| Local adverse reactions | / | 133 (32.76) | |
| Pain and tenderness | / | 120 (29.56) | |
| Lumps and swelling | / | 28 (6.90) | |
| Rash | / | 7 (1.72) | |
| Redness | / | 40 (9.85) | |
| Other adverse events | / | 26 (6.40) |
3.2. Autoantibody prevalence in vaccine recipients and the correlation with clinical features
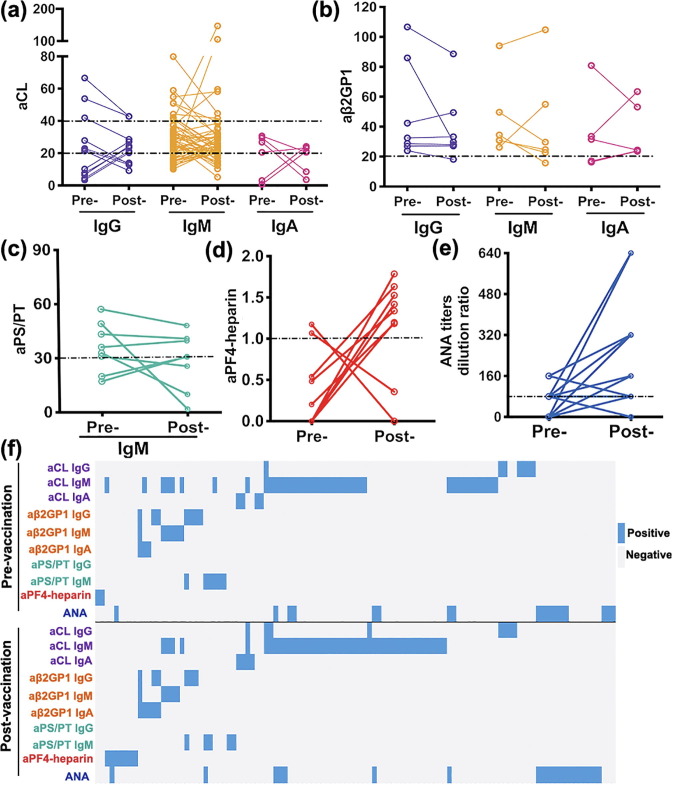
The prevalence of positivity in all ten autoantibodies were similar between the samples collected before vaccination and four weeks after the administration of the second dose of inactivated SARS-CoV-2 vaccine: for anticardiolipin antibody, IgG (7 vs. 8, P = 0.76), IgM (41 vs. 44, P = 0.73), IgA (4 vs. 4, P = 1.00); anti-β2GP1 antibody, IgG (7 vs. 6, P = 0.78), IgM (6 vs. 5, P = 0.76), IgA (3 vs. 5, P = 0.72); aPS/PT IgG (0 vs. 0, P = 1.00), IgM (6 vs. 5, P = 0.76); aPF4-heparin (2 vs. 7, P = 0.18), and ANA (18 vs. 21, P = 0.62) (Table 2 ). Among them, low titers of autoantibodies were more frequently detected. The antibody levels among the ten autoantibodies tend to fluctuate slightly after vaccination, changes in the autoantibody profiles after inactivated COVID-19 vaccination is presented in Fig. 2 (Fig. 2a, aCL; Fig. 2b, aβ2GP1; Fig. 2c, aPS/PT; Fig. 2d, aPF4-heparin; Fig. 2e, ANA). The distribution of all ten autoantibodies among paired samples is shown in the heat map (Fig. 2f). Notably, seven cases without heparin exposure history presented low levels of anti-PF4-heparin antibodies (range: 1.18–1.79 U/mL) four weeks after receiving the second dose of inactivated SARS-CoV-2 vaccine, and none of them exhibited symptoms of the thrombotic disorder (Table 3 ).
Table 2.
Prevalence of autoantibodies in serum before vaccination and four weeks after receiving the second dose of COVID-19 vaccine.
| Autoantibodies | Pre-vaccination, n (%) | Post-vaccination, n (%) | P |
|---|---|---|---|
| aCLa IgG | 7 (1.72) | 8 (1.97) | 0.76 |
| Low titere | 4 (0.99) | 6 (1.48) | 0.75 |
| Medium-high titerf | 3 (0.74) | 2 (0.49) | 1.00 |
| aCL IgM | 41 (10.10) | 44 (10.84) | 0.73 |
| Low titere | 30 (7.39) | 38 (9.36) | 0.31 |
| Medium-high titerf | 11 (2.71) | 6 (1.48) | 0.22 |
| aCL IgA | 4 (0.99) | 4 (0.99) | 1.00 |
| aβ2GPIb IgG | 7 (1.72) | 6 (1.48) | 0.78 |
| aβ2GPI IgM | 6(1.48) | 5 (1.23) | 0.76 |
| aβ2GPI IgA | 3 (0.74) | 5 (1.23) | 0.72 |
| aPS/PTc IgG | 0 (0.00) | 0 (0.00) | 1.00 |
| aPS/PT IgM | 6 (1.48) | 5 (1.23) | 0.76 |
| aPF4-heparin complex | 2 (0.49) | 7 (1.72) | 0.18 |
| ANAd | 18 (4.43) | 21 (5.17) | 0.62 |
Manufacturer’s cutoff: aCL IgG, IgM, IgA ≥ 20 GPL/MPL/APL; aβ2GPI IgG, IgM, IgA ≥ 20 SGU/SMU/SAU; aPS/PT IgG, IgM ≥ 30 units, aPF4-heparin complex > 1.
aCL, anticardiolipin antibodies;
aβ2GPI, anti-beta-2 glycoprotein I antibodies;
aPS/PT, anti-phosphatidylserine/prothrombin antibodies;
ANA, antinuclear antibodies;
Low titer of aCL IgG/IgM means aCL IgG/IgM isotype in serum present in titers >20 GPL/MPL but <40 GPL/MPL;
Medium-high titer of aCL IgG/IgM means aCL IgG/IgM isotype present in titers > 40 GPL/MPL.
Fig. 2.
Changes of autoantibody profiles after inactivated COVID-19 vaccination. (a) anticardiolipin (aCL) IgG, IgM, IgA; (b) anti-β2 glycoprotein I (aβ2GPI) IgG, IgM, IgA; (c) anti-phosphatidylserine/prothrombin (aPS/PT) IgM; (d) anti-platelet factor 4-heparin antibody (aPF4-heparin); (e) antinuclear antibody (ANA) titers in paired samples before vaccination and four weeks after the administration of the second dose of inactivated SARS-CoV-2 vaccine. The slope of the trend line between pairs indicated the intensity of changes. The horizontal dashed line represents the cutoff value defined by the manufacturers. (f) The heat map shows the distribution of the ten autoantibodies among paired samples. Pre-: pre-vaccination; Post-: post-vaccination (four weeks after receiving the second dose of inactivated SARS-CoV-2 vaccine).
Table 3.
Characteristics of the individuals presented with anti-PF4/heparin complex antibody four weeks after the second dose of COVID-19 vaccination.
| Individual ID | |||||||
|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Age (year) | 49 | 60 | 41 | 57 | 41 | 26 | 41 |
| Sex | F | F | F | M | F | F | F |
| Preexisting conditions | None | None | None | None | None | None | None |
| Pre-vaccination | |||||||
| Platelet count (× 109/L) | 170 | 226 | 301 | 133 | 285 | 231 | 177 |
| Aspartate aminotransferase (IU/L) | 19 | 21 | 22 | 30 | 15 | 25 | 25 |
| Alanine aminotransferase (IU/L) | 12 | 28 | 12 | 28 | 10 | 17 | 20 |
| Total bilirubin | 23.7 | 14.1 | 6.0 | 10.1 | 6.1 | 5.4 | 11.7 |
| Albumin | 43 | 43 | 47 | 45 | 38 | 45 | 46 |
| Post-vaccination | |||||||
| Platelet count (× 109/L) | 177 | 222 | 257 | 113 | 270 | 198 | 178 |
| Aspartate aminotransferase (IU/L) | 16 | 46 | 19 | 28 | 14 | 21 | 27 |
| Alanine aminotransferase (IU/L) | 13 | 26 | 13 | 23 | 11 | 17 | 20 |
| Total bilirubin | 16.3 | 11.9 | 5.3 | 10.7 | 10.7 | 6.3 | 5.2 |
| Albumin | 39 | 46 | 42 | 41 | 41 | 41 | 45 |
| SARS-CoV-2 specific antibodies (S/COa) | 28.90 | 124.94 | 124.54 | 16.79 | 108.22 | 12.56 | 46.75 |
| Symptoms | None | Fatigue | None | None | Pain (local) | None | Pain (local) |
S/CO: signal to cutoff.
4. Discussion and conclusion
This study explored whether inactivated SARS-CoV-2 vaccine (BBIBP-CorV, Sinopharm) can stimulate the prothrombotic autoantibody production in healthcare workers. Neither changes in autoantibody profile nor related unfavorable clinical manifestations (e.g., thrombosis and thrombocytopenia) after vaccination were observed.
The prompt development of a safe and effective vaccine to reduce the spread of COVID-19 and establish higher levels of herd immunity is a global imperative. As of May 8, 2021, a total of 1.2 billion doses of COVID-19 vaccines were administered globally [1]. In China, over 406 million doses have been administered up to May 16, 2021 [21]. Antiviral vaccines can be classified into four broad categories: inactivated virus vaccines, nucleic acid vaccines, viral-vector vaccines, and protein-based vaccines [27]. Among them, inactivated vaccines are predominantly used in China. The effectiveness of inactivated COVID-19 vaccine (Sinopharm, China) was shown to be 72.8% in terms of prevention of symptomatic COVID-19 based on randomized clinical trial [28]. In this study, four weeks after vaccination, 95.81% (389/406) samples tested positive with SARS-CoV-2 antibody assay among 406 enrolled participants.
Safety is a primary goal for vaccines that are administered to the general population. Recently, the rare but severe side effects characterized by dangerous blood clots and low platelet counts after administration of the adenovirus-vectored COVID-19 vaccine have attracted much attention. It was defined as vaccine-induced immune thrombotic thrombocytopenia based on the presence of the anti-PF4-heparin in vaccine recipients [18], [19]. For us, this meant we must be vigilant about these events; it forced us to explore the effect of the vaccine on anti-PF4-heparin production. In this study, seven cases were presented with low levels of anti-PF4-heparin four weeks after the administration of the second dose of inactivated SARS-CoV-2 vaccine. However, none of them exhibited spontaneous thrombosis or thrombocytopenia during a minimum follow-up period of eight weeks. Alternatively, the autoantibodies may be boosted by the vaccine, but they are kept in check by an immune mechanism known as peripheral tolerance, as 0.3%–0.5% of healthy individuals can harbor PF4 antibodies without related clinical manifestations [29], [30]. It was noted that an observational study reported a low prevalence of anti-PF4-heparin antibodies (6/492) post COVID-19 vaccination, and none of the six cases exhibited symptoms of thrombocytopenia. Considering the cross-sectional nature of the previous study, there is limited information available on the relationship between vaccine and autoantibody production [31].
Notably, the presence of prothrombotic antibodies, such as aCL, anti-β2GP1, LAC, and aPS/PT was related to the hypercoagulation of COVID-19, which influenced the unfavorable clinical outcome [5], [26], [32]. It was found that 31 out of 66 (47%) severely ill SARS-CoV-2-infected patients were presented with β2GPI or/and aCL autoantibodies [33]. Notably, the coagulopathy found in critically ill COVID-19 patients bears some similarities to the catastrophic antiphospholipid syndrome, which presented with multiple-organic thrombosis [34], [35]. According to recent studies, there is a questionable association between aPLs and major thrombotic events considering the transient and low titers of aPLs in some COVID-19 patients [36], [37], [38], [39], [40]. Taha et al. [39] conducted a meta-analysis and a systematic review including 21 studies and found that COVID patients with severe disease presented with a higher prevalence of aPL. However, there was no association between aPL positivity and disease outcomes, including thrombosis [39]. Borghi et al. [40] reported that aPLs displayed different epitope specificity between COVID-19 patients and APS patients. Although whether antiphospholipid antibodies were transient or persistent needs to be on the basis of the multicenter registration of aPLs in a longitudinal study, the potentially pathogenic role of aPL purified from COVID-19 patients was proved in thrombotic mice models [2], [41]. Here, SARS-CoV-2 served as a trigger for the production of antiphospholipid antibodies. Beyond viruses, certain pathogenic elements contained in the tetanus toxoid and influenza vaccine can also lead to aPL production mediated through molecular mimicry [42]. Studies have estimated that the prevalence of aPLs in the general population ranges between 1% and 5%. The positive result of autoantibodies depends on the person’s age (frequency increases with age) and previous infection history [43]. In our previous study, we enrolled 120 healthy controls, which found that the positive rates of aPLs were less than 4%, which were in the same order of magnitude as the current study (less than 10%) [44].
Whether inactivated COVID-19 vaccines can trigger the production of prothrombotic antibodies is still unknown. In our study, the prevalence of all eight antiphospholipid antibodies positivity was similar between the samples collected before vaccination and four weeks after the administration of the second dose of inactivated SARS-CoV-2 vaccine. In the specimens with positive results, low titers of autoantibody were more frequently detected and fluctuated slightly. There were four cases presented with more than doubled levels of antiphospholipid antibody after vaccination without documented thrombosis or thrombocytopenia during the follow-up.
During a minimum follow-up period of eight weeks after the second dose of inactivated COVID-19 vaccine, no thrombosis was observed in this study, while the vaccine-induced immune thrombotic thrombocytopenia was observed within 16 days after the first vaccination with the adenovirus-vectored COVID-19 vaccine [18], [19]. However, long-term follow-up is greatly needed to investigate whether these increased autoantibodies could be responsible for increased thrombotic risk, especially for subjects who developed anti-PF4/heparin antibodies. To better clarify this concern, we will continue to monitor thrombocytopenia and thrombotic events for all enrolled participants. Additionally, a larger cohort based on a multicenter setting is also important to elucidate the relationship between vaccination and prothrombotic autoantibody production in future studies.
To the best of our knowledge, this is the first prospective study evaluating the prevalence of antiphospholipid antibodies and anti-PF4-heparin antibodies in paired samples collected from pre and post-vaccination individuals. In this study, the inactivated vaccine does not change the profile of the antiphospholipid antibody and the anti-PF4-heparin antibody. Notably, none of the subjects developed spontaneous thrombosis or thrombocytopenia after vaccination. According to our data, the inactivated vaccine does not increase the risk of prothrombotic antibody-induced thrombosis.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
Acknowledgments
This work was supported by the Cultivation Project of Shanghai Major Infectious Disease Research Base (20dz2210500), the Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases (20dz2261100), and the National Natural Science Foundation of China (81671589 and 81871272).
Author contributions
Jieming Qu, Erzhen Chen, Xuefeng Wang, Xinxin Zhang, and Chengde Yang conceived and designed the study. Xiaoqi Yu, Dong Wei, and Wenxin Xu contributed to the recruitment of healthcare professionals. Jing Dai, Xinming Shi, Guanqun Xu, Yu Liu, Ce Shi, and Qi Ni carried out the laboratory tests. Tingting Liu, Zhitao Yang, and Yanping Xu led the data collection, data analysis and data interpretation. Tingting Liu and Zihan Tang drafted the manuscript. All authors provided critical review and final approval of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Biographies

Tingting Liu received her Ph.D. degree in Rheumatology and Immunology from Shanghai Jiao Tong University School of Medicine. She is currently a resident at Ruijin Hospital. Her research interest focuses on the clinical characteristics and molecular mechanism of antiphospholipid syndrome.

Jing Dai received her Ph.D. degree in 2015 from Shanghai Jiao Tong University School of Medicine and worked as a visiting scholar in Prof. Mortimer Poncz’s Lab, University of Pennsylvania from 2016–2018. Her research focuses on the basic and clinical studies of bleeding and thrombotic disorders.

Zhitao Yang is a chief physician at the Emergency Department of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. He worked at Necker-Enfants Malades Hospital in Paris as a foreign resident. He is skilled in the clinical treatment of severe infectious disease, sepsis, and critical cardiovascular diseases. He dedicates himself to clinical and scientific research in critical care medicine and infectious disease and has some achievements in studying hepatitis B, COVID-19, and fungal infection.

Xiaoqi Yu obtained her bachelor’s degree from Shanghai Jiao Tong University School of Medicine. She is currently a Ph.D. candidate at the Research Laboratory of Clinical Virology of Ruijin Hospital, majoring in Internal Medicine. As part of her Ph.D. training, she made an academic visit to the Department of Immunology and Virology of INSERM Unit 1052, Lyon, France. Her research mainly focuses on the diagnosis, treatment, and pathogenesis of hepatitis B and emerging infectious diseases.

Yanping Xu is currently a Ph.D. student in Respiratory Disease at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Her doctoral tutor is Prof. Jieming Qu. Her research interest includes the pathogenic epidemiology of the lower respiratory tract infection and its clinical diagnosis and treatment; molecular mechanism underlying the horizontal transmission of virulence plasmid in Klesbiella pneumoniae.

Chengde Yang serves as a professor and director of the Department of Rheumatology and Immunology in Ruijin Hospital; vice president of Chinese Rheumatology Association. His research interest includes the diagnostic, treatment, and pathogenic mechanism of the antiphospholipid syndrome and the adult-onset Still’s disease.

Xinxin Zhang graduated from Shanghai Second Medical University in 1987. She is currently a professor of Infectious Diseases at Ruijin Hospital, an executive member of the Chinese Hepatology Society, and has been a chairman of the Shanghai Medical Virology Society. Her research interest focuses on the diagnostic, treatment, and pathogenesis of viral diseases, especially the mutations of hepatitis B virus and SARS-CoV-2 and their clinical significance.

Xuefeng Wang received his M.D. and Ph.D. degrees in Immunology from Shanghai Jiao Tong University School of Medicine and is currently a professor and the dean of the Department of Laboratory Medicine of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine. His current research interests include the molecular mechanism of blood coagulation and thrombosis and clinical diagnosis and treatment of bleeding and thrombotic disorders, particularly hemophilia and thrombophilia.

Erzhen Chen is an Emergency and Critical Care physician and the vice president of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. He devoted himself to Emergency and Critical Care Medicine for more than 30 years. Currently, he is engaged in clinical and scientific research on sepsis and Multiple Organ Dysfunction Syndrome.

Jieming Qu is a chief physician of Pulmonary and Critical Care Medicine. He currently serves as the president of the Chinese Thoracic Society and deputy president of the Chest Physicians Society of the Chinese Medical Doctor Association. His research interest includes the pathogenic epidemiology of the lower respiratory tract infection and its clinical diagnosis and treatment; the treatment effects and mechanism of mesenchymal stem cells in severe pulmonary infection; the relationship between microbe balance and respiratory disease in the lower respiratory tract.
Footnotes
Supplementary materials to this article can be found online at https://doi.org/10.1016/j.scib.2021.07.033.
Appendix A. Supplementary materials
The following are the Supplementary materials to this article:
References
- 1.WHO. WHO coronavirus disease (COVID-19) dashboard. 2021. https://covid19.who.int/ (accessed May 8, 2021).
- 2.Zuo Y., Estes S.K., Ali R.A. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12:eabd3876. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowles L., Platton S., Yartey N. Lupus anticoagulant and abnormal coagulation tests in patients with COVID-19. N Engl J Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trahtemberg U, Rottapel R, Dos Santos CC, et al. Anticardiolipin and other antiphospholipid antibodies in critically ill COVID-19 positive and negative patients. Ann Rheum Dis 2021. doi: 10.1136/annrheumdis-2021-220206. [DOI] [PMC free article] [PubMed]
- 6.Miyakis S., Lockshin M.D., Atsumi T. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 7.Pengo V., Biasiolo A., Pegoraro C. Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb Haemost. 2005;93:1147–1152. doi: 10.1160/TH04-12-0839. [DOI] [PubMed] [Google Scholar]
- 8.Meroni P.L., Borghi M.O., Raschi E. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011;7:330–339. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 9.Asherson R.A., Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62:388–393. doi: 10.1136/ard.62.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodard J., Kremer Hovinga J.A., Fontana P. COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J Thromb Haemost. 2021;19:1294–1298. doi: 10.1111/jth.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dragonetti D., Guarini G., Pizzuti M. Detection of anti-heparin-PF4 complex antibodies in COVID-19 patients on heparin therapy. Blood Transfus. 2020;18:328. doi: 10.2450/2020.0164-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu F.-C., Guan X.-H., Li Y.-H. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia S., Duan K., Zhang Y. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., Hu Y., Xu M. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folegatti P.M., Ewer K.J., Aley P.K. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin U., Muik A., Derhovanessian E. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 17.Xia S., Zhang Y., Wang Y. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz N.H., Sørvoll I.H., Michelsen A.E. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greinacher A., Thiele T., Warkentin T.E. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scully M., Singh D., Lown R. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Health Commission of the People’s Republic of China. COVID-19 vaccination status. http://www.nhc.gov.cn/ (accessed May 17, 2021).
- 22.Tozzoli R., Bizzaro N., Tonutti E. Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases. Am J Clin Pathol. 2002;117:316–324. doi: 10.1309/Y5VF-C3DM-L8XV-U053. [DOI] [PubMed] [Google Scholar]
- 23.Meroni P.L., Schur P.H. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69:1420–1422. doi: 10.1136/ard.2009.127100. [DOI] [PubMed] [Google Scholar]
- 24.Erkan D., Lockshin M.D. APS ACTION members. APS ACTION–AntiPhospholipid Syndrome Alliance For Clinical Trials and InternatiOnal Networking. Lupus. 2012;21:695–698. doi: 10.1177/0961203312437810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sciascia S., Willis R., Pengo V. The comparison of real world and core laboratory antiphospholipid antibody ELISA results from Antiphospholipid Syndrome Alliance for Clinical Trials & International Networking (APS ACTION) clinical database and repository analysis. Thromb Res. 2019;175:32–36. doi: 10.1016/j.thromres.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Hasan Ali O., Bomze D., Risch L. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580:576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 28.Al Kaabi N., Zhang Y., Xia S. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krauel K, Pötschke C, Weber C, et al. Platelet factor 4 binds to bacteria, inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood. 2011;117:1370-1378. [DOI] [PubMed]
- 30.Hursting M.J., Pai P.J., McCracken J.E. Platelet factor 4/heparin antibodies in blood bank donors. Am J Clin Pathol. 2010;134:774–780. doi: 10.1309/AJCPG0MNR5NGKNFX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørvoll I.H., Horvei K.D., Ernstsen S.L. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost. 2021;19:1813–1818. doi: 10.1111/jth.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollmer O., Tacquard C., Dieudonné Y. Follow-up of COVID-19 patients: LA is transient but other aPLs are persistent. Autoimmun Rev. 2021;20:102822. doi: 10.1016/j.autrev.2021.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao M., Zhang Y., Zhang S. Antiphospholipid antibodies in critically ill patients with COVID-19. Arthritis Rheumatol. 2020;72:1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cervera R., Font J., Gómez-Puerta J.A. Validation of the preliminary criteria for the classification of catastrophic antiphospholipid syndrome. Ann Rheum Dis. 2005;64:1205–1209. doi: 10.1136/ard.2004.025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatto M., Perricone C., Tonello M. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin Exp Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 37.Siguret V., Voicu S., Neuwirth M. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devreese K.M.J., Linskens E.A., Benoit D. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemost. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taha M., Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borghi M.O., Beltagy A., Garrafa E. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol. 2020;11:584241. doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talotta R., Robertson E.S. Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia: the straw that breaks the camel’s back? Cytokine Growth Factor Rev. 2021;60:52–60. doi: 10.1016/j.cytogfr.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martirosyan A., Aminov R., Manukyan G. Environmental triggers of autoreactive responses: induction of antiphospholipid antibody formation. Front Immunol. 2019;10:1609. doi: 10.3389/fimmu.2019.01609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreiber K., Sciascia S., de Groot P.G. Antiphospholipid syndrome. Nat Rev Dis Primers. 2018;4:17103. doi: 10.1038/nrdp.2017.103. [DOI] [PubMed] [Google Scholar]
- 44.Liu T., Gu J., Wan L. “Non-criteria” antiphospholipid antibodies add value to antiphospholipid syndrome diagnoses in a large Chinese cohort. Arthritis Res Ther. 2020;22:33. doi: 10.1186/s13075-020-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.