Abstract
Acute disseminated encephalomyelitis (ADEM) has been reported after coronavirus disease 2019 (COVID-19). In this review, we systematically included worldwide reported cases on this association. We included 30 case reports (pediatric and adults) and explored epidemiological and clinical evidence. We described time to diagnosis, clinical, imaging, and laboratory features, response to treatment regimens, and differences regarding severity. Also, an original case report was presented. Neurologists must be alert to the occurrence of multifocal neurological symptoms with or without encephalopathy in patients recovered from COVID-19. Timely MRI studies should be performed to establish the diagnosis and to consider early corticosteroid-based treatment.
Keywords: Acute disseminated encephalomyelitis (ADEM), Coronavirus disease 2019 (COVID-19), SARS-Cov-2, Demyelinating disease, Central nervous system (CNS), Autoimmune disease
1. Introduction
Acute disseminated encephalomyelitis (ADEM) is a monophasic, multifocal, demyelinating, autoimmune disease that affects the central nervous system (CNS). It has been described in adults and children (Pohl et al., 2016; Schwarz et al., 2001). It usually occurs after a systemic infection, usually viral, including certain coronavirus infections (Yeh et al., 2004). Coronavirus disease 2019 (COVID-19) is a new entity caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is known to cause respiratory complications, from mild upper respiratory symptoms to acute respiratory failure. Since the beginning of the pandemic, many neurologic complications have been reported, including ADEM (Mao et al., 2020). However, the clinical spectrum of this association is not clear whether it constitutes an inflammatory postinfectious process. Although, previous reviews explored the occurrence of ADEM in COVID-19 cases, most of the studies included other neurological complications of COVID-19 (Bodro et al., 2021), did not use formal evidence assessment approaches (narrative synthesis and risk of bias assessment of case reports) (Almqvist et al., 2020; Correia et al., 2020), and did not described the clinical spectrum nor associations between severity and outcomes; thus, current synthesized evidence identified only a subgroup of the published cases and addressed tangentially this association. Therefore, we performed a systematic review focused exclusively on ADEM including worldwide reported cases to qualitatively describe and assess its relationship with COVID-19, and the associations between disease severity and health outcomes. Furthermore, we added to the literature a case report, from our hospital, who developed ADEM after SARs-CoV-2 infection.
2. Methods
The study aim was to identify all published case reports, case series, or observational studies on ADEM in COVID-19 patients. Moreover, we aimed to contribute to the current literature with an original case report of this association from our hospital. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Liberati et al., 2009) guidelines for the systematic review. We received informed consent from the reported patient and the CARE guidelines were followed (Riley et al., 2017). The study was approved by the Institutional Review Board of Instituto Nacional de Ciencias Neurológicas.
2.1. Systematic search and study selection
We conducted a comprehensive systematic search in PubMed/Medline, EMBASE, and LILACS from inception to February 5, 2021. The search strategy consisted of keywords related to “acute disseminated encephalomyelitis” and “coronavirus infection” (complete search strategy: Table e-1). Additionally, we searched in pre-print repositories (bioRXiv, arXiv, and PeerJ), and manually searched references from included articles and narrative reviews on the topic.
The inclusion criteria were: 1) observational studies, case reports, or case series of ADEM in patients with confirmed COVID-19 infection (by laboratory tests: PCR from respiratory specimens, serum antigen test, or IgM serology positive for SARS-CoV-2), without age restriction; 2) reports providing individual or aggregate sociodemographic and clinical data (including neuroimaging, CSF, antibodies panel findings, and management/prognosis data); and 3) availability of the full-text in any language (in case of identifying a case report in non-English language, we used the Cochrane task exchange platform to contact native speakers whom can help with translation tasks). We excluded papers with 1) different manuscript type (reviews, opinion papers, or editorials); 2) papers with unclear COVID-19 and/or ADEM diagnosis; 3) inconsistent temporal relationship (i.e., COVID-19 in patients with ADEM); 4) patients with acute necrotizing encephalopathy or acute hemorrhagic leukoencephalitis (AHLE, Weston-Hurst syndrome), owing to their potential different prognosis and pathophysiology (Pirko et al., 2008) (i.e., different type of CNS infiltrates, lymphocytes in ADEM and neutrophils in AHLE) that suggest different disease spectrum (Neilson, 2010). For the diagnosis of ADEM in children, we reviewed the criteria of Krupp et al. (2013) However, as COVID-19 patients may have a fever for more than 7 days, we decided to allow those cases in which fever was not the best explanation for encephalopathy.
2.2. Quality assessment
We used the tool proposed by Murad et al. (2018). This tool evaluates the methodological quality of case reports/series assessing 8 questions about four domains. Since our systematic review is not focusing on cases of adverse events, we select only 6 questions for our assessment: 1) selection (a. does the patient(s) represent(s) the whole experience of the investigator (center) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported?); 2) ascertainment (b. was the exposure adequately ascertained? c. was the outcome adequately ascertained?); 3) causality (d. were other alternative causes that may explain the observation ruled out? e. was follow-up long enough for outcomes to occur?); and 4) reporting (f. is the case(s) described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice?). As suggested by the authors, we summed up the scores of the six-binary responses into an aggregate score (six points at maximum).
2.3. Data extraction and narrative synthesis
We used EndNote X9 software to remove duplicates. Subsequently, two authors (LZR & KPB) independently selected the reports and extracted the data in Microsoft Excel 2019. In case of inconsistencies, a third author decided whether to include the article (ROS). The extracted data included: name of the first author, year of publication, sex, age, clinical presentation, COVID-19 diagnosis and severity, laboratory finding (serological and CSF), neuroimaging description, treatment, and prognosis. We describe the categorical variables using frequencies and percentages, and the numerical through medians and interquartile ranges.
A narrative approach to synthesizing the evidence was performed by two reviewers (LZR & KPB). Narrative synthesis is an approach to systematically organize the findings from multiple studies that depends primarily on using words and text to explain and summarize the evidence. We used a four-stage process based on previous guidelines (Popay et al., 2006): 1) developing a theory of how/why the association can be justified (pathophysiological and clinical plausibility); 2) developing a preliminary list of synthesis categories (COVID-19 features, time interval between exposure and outcome, ADEM clinical manifestations, neuroimaging and CSF features, and treatment response and prognosis data); 3) exploring the relationships between and within the included case studies; and 4) presenting the synthesis by a summary table.
2.4. Statistical analysis
We reported the findings by descriptive statistics, using mean and ranges to summarize quantitative variables and percentages for categorical variables. Additionally, we created 2-by-2 tables to assess the following associations: 1) COVID-19 severity (moderate/severe = 1; mild/asymptomatic = 0)(WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection, 2020) and clinical outcome at discharge (favorable or partially favorable [functional improvement] = 1; unfavorable [functional deterioration or death] = 0). 2) ADEM severity (mild = 0; non-mild [coma, severe motor deficit, or brain stem/extensive spinal cord involvement] = 1) and clinical outcome at discharge. 3) COVID-19 severity and ADEM severity. 4) Treatment type (steroids alone = 1; combined therapies [steroids plus IVIg, Tocilizumab, or Rituximab] =0) and clinical outcome at discharge. We calculated the odds ratios (OR) with 95% confidence interval (exact method), and Fisher's exact test to evaluate association. We considered a p-value less than 0.05 as statistically significant.
3. Results
3.1. Case report
A previously healthy 35-year-old man, presented to our institution complaining of weakness, dizziness, tingling and numbness in the four limbs, gait instability, dysarthria, loss of hearing, tinnitus, and double vision. His symptoms began slowly two weeks before admission. He had a history of dry cough and fatigue three weeks before admission, which resolved in a few days. At admission he was alert and oriented. During the neurological examination he presented: multidirectional nystagmus, hypermetric saccades, moderate dysarthria, hypotonia, tetra paresis 4/5 on the Medical Research Council (MRC), hyperreflexia in lower limbs, ataxia, and gait impairment. Examination of the cranial nerves showed bilateral VI cranial nerve palsy, asymmetric sensorineural hearing loss, and absent gag reflex.
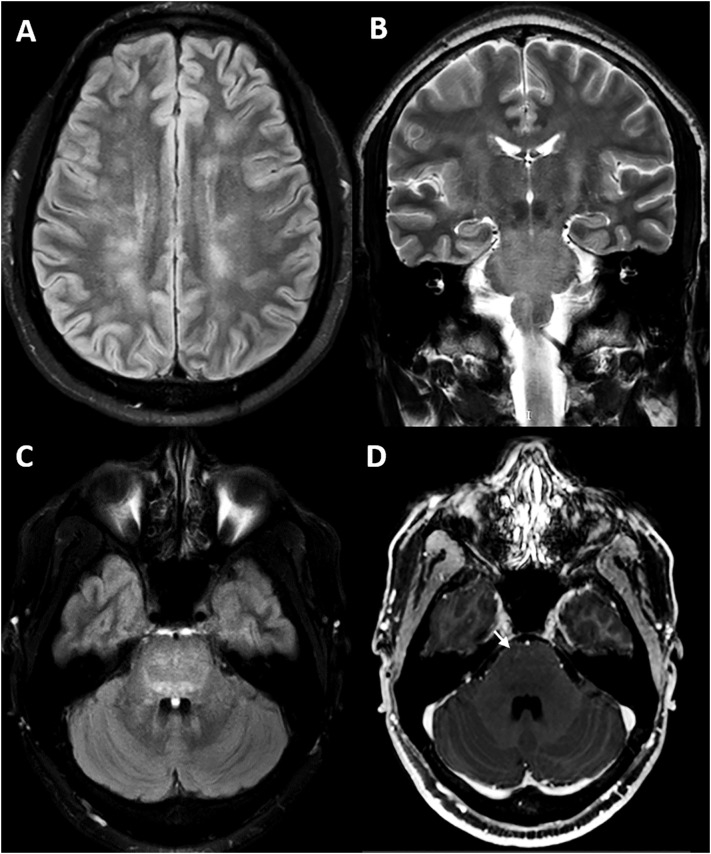
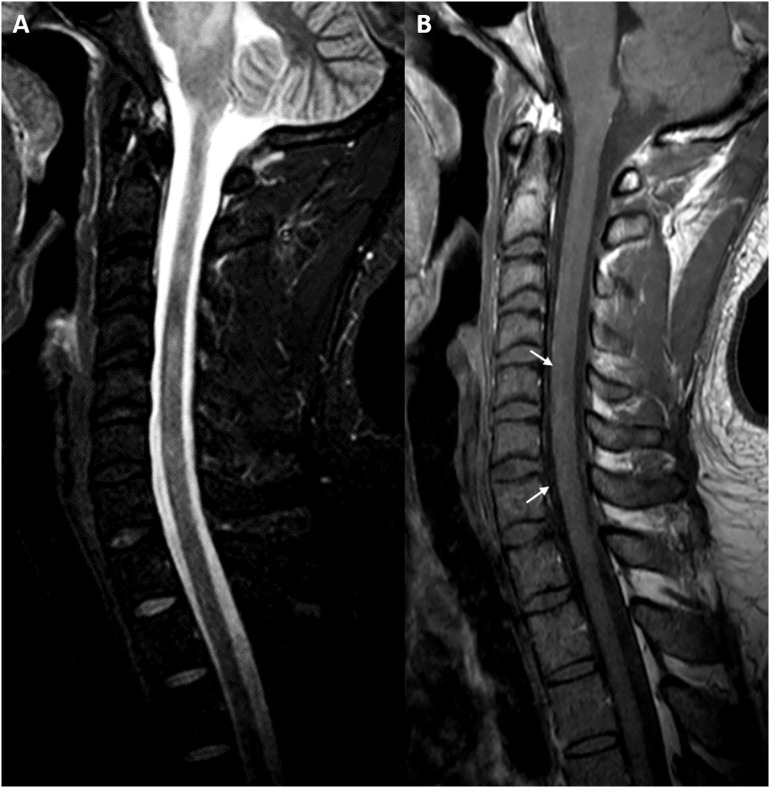
Routine laboratory testing resulted within normal ranges. COVID-19 serum antibody test was positive for both IgM and IgG antibodies. Cerebrospinal fluid (CSF) analysis revealed high proteins count (47 mg/dl, reference range = 15–45 mg/dl), normal glucose and cellularity. We did not test SARS-CoV-2 in CSF due to limited resources. Brain magnetic resonance imaging (MRI) showed white matter areas of decreased signal in T1, increased signal in T2-weighted and Fluid-Attenuated Inversion Recovery (FLAIR) images with minimal enhancement in both the supratentorial and infratentorial structures. Supratentorial lesions were rounded, bilateral and asymmetrical located in the subcortical white matter. Infratentorial lesions were diffuse and mainly involved the pontine tegmentum (Fig. 1 ). MRI of the spinal cord showed diffuse, confluent intramedullary lesions. Gadolinium enhanced T1-weighted image showed faint enhancement between C5-C7 (Fig. 2 ). None of the lesions showed a mass effect. Serum aquaporin-4 antibody (AQP-4) and myelin oligodendrocyte glycoprotein antibody (MOG) were negative. Additional CSF studies carried out on rat brain by avidin biotin peroxidase technique (NMDAR, AMPA, GABA-b, GABA-a, mGluR5, mGluR1, DPPX, IgLON5, LGI1, CASPR2, AQP-4 antibodies) were also negative.
Fig. 1.
(A) FLAIR-weighted axial MRI shows multiple bilateral hyperintense ovoid-like lesions in the white matter of the semioval center and radiated corona. T2-weighted and FLAIR (B and C respectively) MRI shows hyperintense lesions in the bilateral pontine tegmentum extending to the midbrain and spinal cord. (D) Gadolinium-enhanced T1-weighted image shows faint linear enhancement at the pons level (arrow).
Fig. 2.
(A) Sagittal T2-weighted cervical MRI shows hyperintense lesions with a patchy appearance between C2 and C7. (B) Gadolinium-enhanced T1-weighted image shows faint enhancement between C5-C7 (arrow).
Due to the multifocal neurologic signs and symptoms, ADEM was suspected and treatment with methylprednisolone was initiated (two cycles of 1 g/day for 5 days tapered with oral prednisone). The patient showed improvement of diplopia, paresthesia, weakness, and coordination after 10 days. The case report timeline is presented in Figure e-1.
3.2. Systematic review and narrative synthesis
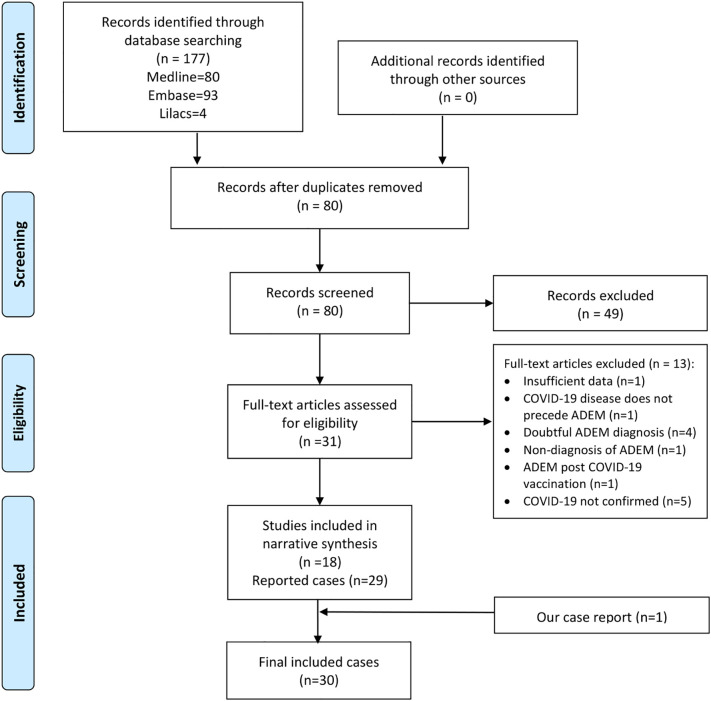
The search strategy identified 177 documents, 80 were duplicates. During the review of titles and abstracts, we selected 31 potential studies and excluded 49 studies for not meeting the inclusion criteria.
After full text assessment, we excluded 13 studies (the reasons of exclusion are detailed in the Table e-2). Only one study was identified in pre-print repositories, but it was excluded due to repeated publication. Thus, a total of 18 documents (30 cases) were included (Fig. 3 ).
Fig. 3.
Search flow diagram (study selection).
3.2.1. Quality assessment
The average quality score was 3.84 (SD = 1.30, range: 1 to 5). None of the studies reached a perfect score (six points). The exposure ascertainment and long follow-up domains were the sections with more missing information. Only eight studies reported follow-up data (longest follow-up were six months). The clinical characteristics or reported outcomes (recovery after discharge) were not associated with the case report quality, the only differences were the certainty level of COVID-19 case confirmation (PCR was most frequently used in high quality reports) and the reported details of clinical features (MRI and CSF viral panel were reported in high quality reports). The complete assessment of the included studies is reported in Table A.1.
3.2.2. Worldwide reported cases
Including our original case report, we identified 30 patients (pediatric = 09, adults = 21) diagnosed with ADEM related with COVID-19 from 18 documents (Abdel-Mannan et al., 2020; Abdi et al., 2020; Assunção et al., 2020; De Miranda Henriques-Souza et al., 2021; Dominguez-Rojas et al., 2020; El Beltagi et al., 2021; Espíndola et al., 2021; Freire-Álvarez et al., 2020; Kumar et al., 2020; Langley et al., 2020; Lindan et al., 2020; Lopes et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020; Utukuri et al., 2020). Only one document was reported in non-English language (Spanish). The narrative synthesis summary is presented in Table A.2.
In pediatric cases, nine patients with a mean age of 6.8 years (range: 0.17 to 15 years) were reported (Abdel-Mannan et al., 2020; De Miranda Henriques-Souza et al., 2021; Dominguez-Rojas et al., 2020; Lindan et al., 2020). Non sex predominance was observed. Data on time from COVID-19 infection to ADEM diagnosis was not available. Five patients registered COVID-19 symptoms, all of them mild or asymptomatic (De Miranda Henriques-Souza et al., 2021; Dominguez-Rojas et al., 2020; Lindan et al., 2020). Comorbidities were reported only in two cases (asthma and obesity)(Abdel-Mannan et al., 2020; Lindan et al., 2020). Seven cases (77.8%) had moderate/severe ADEM clinical presentation. The COVID-19 diagnosis was made using a PCR test or an antigen test in eight cases (Abdel-Mannan et al., 2020; De Miranda Henriques-Souza et al., 2021; Lindan et al., 2020) and by serum antibody test (IgM and IgG) in one case (Dominguez-Rojas et al., 2020). The result of the PCR test for COVID-19 in CSF was reported only in two cases, both negative. Most patients developed encephalopathy (77.8%) (N = 7/9) (Abdel-Mannan et al., 2020; De Miranda Henriques-Souza et al., 2021; Dominguez-Rojas et al., 2020; Lindan et al., 2020). Other neurological findings were pyramidal signs (44.4%) (N = 4/9)(Abdel-Mannan et al., 2020; De Miranda Henriques-Souza et al., 2021; Dominguez-Rojas et al., 2020; Lindan et al., 2020), brainstem signs (11.1%) (N = 1/9) (De Miranda Henriques-Souza et al., 2021), cerebellar signs (22.2%) (N = 2/9) (Abdel-Mannan et al., 2020; Dominguez-Rojas et al., 2020), seizures (33.3%) (N = 3/9) (Lindan et al., 2020), and peripheral nerve compromise (11.1%) (N = 1/9) (Abdel-Mannan et al., 2020). On the MRI, patients presented lesions in the white matter(77.8%) (N = 7/9) (Abdel-Mannan et al., 2020; De Miranda Henriques-Souza et al., 2021; Lindan et al., 2020; Dominguez-Rojas et al., 2020), deep gray matter (44.4%) (N = 4/9) (Lindan et al., 2020; Dominguez-Rojas et al., 2020), spinal cord (44.4%) (N = 4/9) (Dominguez-Rojas et al., 2020; Lindan et al., 2020), brainstem (44.4%) (N = 4/9) (Lindan et al., 2020) and cerebellum (22.2%) (N = 2/9) (Assunção et al., 2020; Lindan et al., 2020). Other isolated findings were microthrombi in cerebrum (Abdel-Mannan et al., 2020) and optic chiasm and cortex involvement (Lindan et al., 2020). Likewise, 55.5% of cases had at least two affected territories: 88.9% had supratentorial involvement and 33.3% infratentorial involvement. Eight cases reported treatment data, with 37.5% receiving only high-dose corticoids (N = 3/8) (De Miranda Henriques-Souza et al., 2021; Lindan et al., 2020; Dominguez-Rojas et al., 2020) and 37.5% receiving human immunoglobulin alone (N = 3/8) (Abdel-Mannan et al., 2020; Lindan et al., 2020). All cases reported follow-up data, 77.8% (N = 7/9) reported a fully favorable outcome,(Abdel-Mannan et al., 2020; Lindan et al., 2020; Dominguez-Rojas et al., 2020), and 22.2% (N = 2/9)developed a partially favorable outcome (De Miranda Henriques-Souza et al., 2021; Lindan et al., 2020).Relapses were not reported.
In adults, we observed a male predominance (73.68%, 14 cases) (Abdi et al., 2020; Assunção et al., 2020; El Beltagi et al., 2021; Freire-Álvarez et al., 2020; Langley et al., 2020; Lopes et al., 2020; McCuddy et al., 2020; Paterson et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020; Utukuri et al., 2020). The mean age was 49.8 years (25 to 70 years). The mean number of days since COVID-19 infection to ADEM diagnosis was 23.2 days (4 to 60 days). The comorbidity most frequently described was hypertension (Lopes et al., 2020; McCuddy et al., 2020; Umapathi et al., 2020), followed by diabetes (Lopes et al., 2020; McCuddy et al., 2020). Regarding the COVID-19 symptoms, 66.7% were severe (N = 12/18)(Assunção et al., 2020; Langley et al., 2020; Lopes et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Umapathi et al., 2020), 22.2% mild (N = 4/18) (El Beltagi et al., 2021; Freire-Álvarez et al., 2020; Kumar et al., 2020) and 11.1% asymptomatic (N = 2/18)(Abdi et al., 2020; Utukuri et al., 2020). The COVID-19 diagnosis was made using a PCR test in 20 cases (Abdi et al., 2020; Assunção et al., 2020; El Beltagi et al., 2021; Espíndola et al., 2021; Freire-Álvarez et al., 2020; Kumar et al., 2020; Langley et al., 2020; Lopes et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020; Utukuri et al., 2020) and a serum antibody test (IgM and IgG antibody) in our case. Results of PCR tests for COVID-19 in CSF were available from 15 patients, with only one being positive (6.7%) (Espíndola et al., 2021). Thirteen cases (68.42%) had moderate/severe ADEM clinical presentation. The most frequent clinical characteristics were encephalopathy (84.2%, N = 16/19)(Abdi et al., 2020; Assunção et al., 2020; Espíndola et al., 2021; Freire-Álvarez et al., 2020; Kumar et al., 2020; Langley et al., 2020; Lopes et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020; Utukuri et al., 2020), pyramidal signs (motor deficits such as hemiparesis or quadriparesis, hyperreflexia, or pathological reflexes such as Babinski) (63.1%, N = 12/19)(Abdi et al., 2020; El Beltagi et al., 2021; Langley et al., 2020; Lopes et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020), brainstem involvement (15.7%, N = 3/19) (Espíndola et al., 2021; Paterson et al., 2020), ataxia (15.7%, N = 3/19) (El Beltagi et al., 2021; Utukuri et al., 2020), peripheral nerve involvement (confirmed by electromyography) (10.5%, N = 2/19) (Kumar et al., 2020; Lopes et al., 2020) and seizures (5.3%, N = 1/19) (Abdi et al., 2020). Most of the images found on the MRI, were hyperintensities in T2 and FLAIR. All cases presented white matter compromise (N = 20/20). Lesions in the subcortical white matter were multiple and less frequent in the periventricular area. Other frequent findings were brainstem lesions (45%, N = 9/20) (Abdi et al., 2020; Assunção et al., 2020; El Beltagi et al., 2021; Kumar et al., 2020; McCuddy et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020), hemorrhagic lesions (35%, N = 7/20) (Langley et al., 2020; Lopes et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Umapathi et al., 2020), spinal cord lesions (20%, N = 4/20) (El Beltagi et al., 2021; Umapathi et al., 2020; Utukuri et al., 2020), deep gray matter compromise (25%, N = 5/20) (Abdi et al., 2020; Freire-Álvarez et al., 2020; Lopes et al., 2020; Pilotto et al., 2021) and cerebellum involvement (15%, N = 3/20) (Lopes et al., 2020; McCuddy et al., 2020; Umapathi et al., 2020). Likewise, 70% of cases had at least two affected territories, 100% had supratentorial involvement and 55% infratentorial involvement. All the lesions appeared at the same evolutionary stage.
The most frequently described CSF alteration was increased protein levels, reported in 11 cases (mean protein level: 64.7 mg/dl; range: 15 to 198) (El Beltagi et al., 2021; Espíndola et al., 2021; Freire-Álvarez et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020). Pleocytosis was reported only in five cases (El Beltagi et al., 2021; Espíndola et al., 2021; Freire-Álvarez et al., 2020; Umapathi et al., 2020; Utukuri et al., 2020). The presence of oligoclonal bands was reported in 15 patients, 2 were positive in the CSF and 3 were positive both in the CSF and at the serum level.The serum aquaporin-4 antibody (AQP-4) and the myelin oligodendrocyte glycoprotein antibody (MOG) were tested in 7 cases and all of them with negative results (Kumar et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Umapathi et al., 2020). Seven patients received only high-dose corticosteroids (Abdi et al., 2020; Espíndola et al., 2021; Langley et al., 2020; McCuddy et al., 2020; Paterson et al., 2020; Pilotto et al., 2021), six received corticosteroids followed by human immunoglobulin (Kumar et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Utukuri et al., 2020), one patient also received plasma exchange (Kumar et al., 2020) and two received only human immunoglobulin (Freire-Álvarez et al., 2020; Umapathi et al., 2020).
The clinical outcome at discharge (clinical and/or radiological) was completely favorable in 5.6% (N = 1/18) (Espíndola et al., 2021), partially favorable in 72.2% (N = 13/18) (Freire-Álvarez et al., 2020; Langley et al., 2020; Lopes et al., 2020; McCuddy et al., 2020; Parsons et al., 2020; Paterson et al., 2020; Pilotto et al., 2021; Umapathi et al., 2020; Utukuri et al., 2020) and unfavorable in 22.2% (N = 4/18) (Abdi et al., 2020; Kumar et al., 2020; Lopes et al., 2020; McCuddy et al., 2020). Two deaths were reported (Abdi et al., 2020; Lopes et al., 2020).
3.2.3. Association results from 2-by-2 tables
We created the 2-by-2 tables for adults' cases. We were unable to create the tables for pediatric cases due to missing data. We found no association for COVID-19 severity and clinical outcomes at discharge (OR = 6.75, 95% CI: 0.39 to 123.27; Fisher's exact p-value = 0.24). However, we found a statistically significant association between ADEM severity and clinical outcome at discharge (OR = 0.14, 95% CI: 0.25 to 0.61; Fisher's exact p-value = 0.02). Thus, a non-mild (moderate/severe) ADEM was associated with smaller odds of favorable outcome. Finally, we found no association among COVID-19 severity and ADEM severity (OR = 1.33, 95% CI: 0.08 to 17.43; Fisher's exact p-value = 0.99), or treatment type and clinical outcome at discharge (OR = 1.6, 95% CI: 0.06 to 106.01; Fisher's exact p-value = 0.99).
4. Discussion
In this systematic review we summarize the worldwide ADEM cases associated with COVID-19 disease. Including our original case report, we found 30 cases (21 adults and 9 children), with predominance of adult men. Previous reviews on neurological manifestations of COVID-19 found 1 to 13 cases of ADEM (Correia et al., 2020; Maury et al., 2021). The ADEM diagnosis was made even after asymptomatic and mild COVID-19 cases. We reported a usual clinical and neuroimaging presentation, but with more frequent moderate/severe ADEM cases.
ADEM is an inflammatory and demyelinating condition of acute or subacute onset. This entity can occur at any age, but is more common in children than in adults (Tenembaum et al., 2002). However, this distribution it is different in the cases preceded by SARS-COV-2 infection since we found a predominance of adult cases (21 adult cases vs 9 pediatric cases). This discrepancy may be due to the fact that children are generally less prone to COVID-19 infection and usually they develop an asymptomatic or mild disease (Mehta et al., 2020). However, although at the beginning of the pandemic it was reported that children were little affected by COVID-19 (He et al., 2021), severe cases of COVID-19 and even neurological manifestations have recently been reported (Kim et al., 2020; Lindan et al., 2020). It is interesting that pediatric ADEM cases reported to date represent 30% of ADEM associated with COVID-19 (Table 2), which suggests that although it is a rare event, physicians should assess carefully neurological symptoms as part of the post-COVID-19 follow-up in children. Another point to highlight is that ADEM-like cases have been described in the context of an entity called MIS-C (Abdel-Mannan et al., 2020; Lindan et al., 2020; Mehra et al., 2020) reported to appear between 10 and 54 days after COVID-19 infection (Cheung et al., 2020; Coll-Vela et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020). However, these MIS-C cases that have presented ADEM-like lesions do not have a confirmatory test for COVID-19 (they only have positive IgG serology) and were not considered in this review. MIS-C has similarities with Kawasaki disease and toxic shock syndrome, which has suggested that it is an immune-mediated postinfectious complication (Coll-Vela et al., 2020). Based on recent reports (Feldstein et al., 2020), most of MIS-C cases had cardiac, gastrointestinal, and hematologic clinical presentation while less than 2% had associated mild to moderate neurological symptoms (headache, altered mental status, and confusion). Thus, the linked pathophysiology between MIS-C and ADEM is not clear yet, but it is plausible that both are part of a common clinical spectrum with a shared immune-mediated phenomenon.
Regarding adult ADEM cases, the average age was around 50 years, this is higher than previous reports of ADEM where the average age was between 33 and 41 years (Ketelslegers et al., 2011; Koelman et al., 2016; Schwarz et al., 2001). This could be explained by the higher frequency of COVID-19 cases in older people (Shahid et al., 2020). Also, it could suggest an influence of COVID-19 severity on the development of ADEM.A larger number of severe cases occur in older adults who often have multiple comorbidities (Helms et al., 2020), which can be associated with a defective and reactive immune system facilitating immune post-infectious condition. However, we found that ADEM can occur in patients with mild COVID-19or even in asymptomatic patients (Abdi et al., 2020; Utukuri et al., 2020) and there was not an association between severe COVID-19 and severe ADEM. Therefore, we hypothesize that the development of COVID-19 related ADEM is multifactorial, not only dependent of the degree of previous viral exposure or the intensity of the initial immune response (that determines the COVID-19 severity).
Regarding sex as biological factor for ADEM, a male predominance has been classically described in pediatric ADEM (male/female ratio 1.8:1) (Tenembaum et al., 2002); however, we did not find this pattern in our included pediatric cases. Regarding adults, no predominance has been described previously (Koelman et al., 2016). However, we found a male/female ratio of 2.8:1 in this population. This is likely due to the fact that men are more affected by COVID 19 as reported in previous studies (Grasselli et al., 2020; Peckham et al., 2020).
Encephalopathy is a frequent manifestation of ADEM in children and being part of the diagnostic criteria. It must not explained by fever, systemic disease or post-ictal symptoms (Krupp et al., 2013). However, we consider that in the context of COVID-19 disease it is expected to find fever, as occurred in 2 of the cases reviewed; therefore, in children with encephalopathy, history of COVID-19 exposure, and fever, ADEM should be considered as part of the differential diagnosis. In contrast, encephalopathy occurs less frequently in adults (19 to 56% of cases) (Koelman et al., 2016; Schwarz et al., 2001). Contradictory, in the included studies, the most common neurological presentation in adults was encephalopathy. Its presence is clinically important since it helps to identify people who are less likely to have a different disease, such as multiple sclerosis, and are more likely to be a classic ADEM (Koelman et al., 2016; Schwarz et al., 2001). In many of these cases it was only evident after removing sedation or post extubation. Hence, as this is the most frequent manifestation, there may be a delay in the diagnosis of ADEM because cases of severe COVID-19 require sedation, mechanical ventilation, and other ICU interventions that difficult the encephalopathy assessment.
The presence of pyramidal signs is extremely important because its identification is feasible in patients with severe COVID-19 who are in the ICU (by searching Babinski's sign, osteotendinous hyperreflexia, or an asymmetric motor response). It reinforces the importance of an adequate and continuous neurological evaluation in patients who are on mechanical ventilation with a prolonged stay. Likewise, as was suggested by Lopes et al. (2020), one must also be alert to identify patients who present a delayed awakening once the sedation has been withdrawn. In both cases, an MRI should be requested as it allows us to establish an earlier diagnosis.
MRI plays a key role in the diagnosis of ADEM and should be performed as soon as it is suspected. The typical findings are identified as lesions with signal hyper-intensity in FLAIR and T2 sequences, they are usually multiple, asymmetric, irregular, poorly defined, and greater than 2 cm. In general, the white matter is affected, although it may involve the deep gray matter, the brainstem, the cerebellum, and the spinal cord (Tenembaum et al., 2007). In our included cases, various involvement patterns were observed, predominantly a white matter compromise. Although the MRI pattern does not appear to influence prognosis, it may be useful to consider differential diagnoses (Tenembaum et al., 2002). Brainstem involvement, for example, may suggest brainstem encephalitis or anti-MOG disease, as initially suggested in our case. Although the association between anti-MOG disease and COVID-19 is unclear (Lindan et al., 2020; Pinto et al., 2020; Sawalha et al., 2020). Furthermore, infratentorial involvement was common in our adult ADEM cases and should be within the differential diagnosis of brainstem lesions. Another useful tool when ADEM is suspected is the study of CSF, which allows us to obtain evidence of inflammation and exclude other infectious or inflammatory causes. CSF findings in ADEM are variable, from normal results to nonspecific findings such as lymphocytic pleocytosis (15–60%) or mildly elevated proteins (25–65%) (Filippi and Rocca, 2020). This was reflected in the included cases of ADEM associated with COVID-19 and can guide the diagnosis, especially when an MRI study is not possible.
The pathogenesis of ADEM associated with COVID-19 is unknown. Although neuro-invasion is well described (Baig et al., 2020), there is a lack of detection in CSF in most cases besides evident inflammation (pleocytosis and hyperproteinemia). This raises the possibility that the majority of ADEMs associated with COVID-19 could be the result of immune-mediated mechanisms or molecular mimicry which generates an aberrant neuro-inflammatory loop, so the virus does not need to cross the blood-brain barrier to cause damage to the CNS (Franke et al., 2020). This same mechanism has been postulated for ADEM cases associated with other causes, including other coronaviruses such as MERS-CoV (Arabi et al., 2015). However, one case isolated SARS-CoV-2 in the CSF, which raises the differential diagnosis of encephalitis associated with COVID-19 with a direct damage mechanism of the virus. Still, not all reported cases of COVID-19-associated encephalitis isolated SARS-CoV-2 in the CSF (Ellul et al., 2020; Lewis et al., 2021). Differences exist between encephalitis associated with COVID-19 and ADEM associated with COVID-19, one of them is temporality. Unlike ADEM, neurological symptoms usually appear simultaneously with respiratory symptoms in COVID-19-associated encephalitis. Brain inflammation expressed by pleocytosis is more frequent in encephalitis associated with COVID-19 (Ellul et al., 2020) and our reported case of ADEM with SARS-CoV-2 in CSF did not present pleocytosis. Finally, there appears to be a pattern of imaging damage typical of COVID-19-associated encephalitis that differs from the pattern of lesions in ADEM (Moriguchi et al., 2020). Therefore, we do not expect to find SARS-CoV-2 in the CSF of ADEM associated with COVID-19, its presence does not rule out the diagnosis and its immune-mediated mechanism. In any case, the entire clinical, laboratory, and imaging context must be assessed.
Lindan et al. (2020) recently reported two cases that had ADEM-type presentations. One of them tested positive for anti-MOG antibodies and the other for anti-NMDAR antibodies, both could cause neurological diseases appearing after viral infections; which, as they indicate, reinforces the theory of immune-mediated damage by COVID-19 to the CNS. Other theories indicate that the virus could be mainly attached to cells and transmitted from one cell to another through a retrograde neuronal pathway, without entering the CSF compartment (Pezzini and Padovani, 2020) or that the virus was present in the CSF at concentrations below the detection level of the test method (Filippi and Rocca, 2020).
The possibility that SARS-COV-2 infection may be involved in triggering the appearance of ADEM is supported by the fact that this exposure was identified through PCR testing in most cases, that the infection precedes ADEM development, and no other potentially responsible agents were found. Additionally, most cases carried out studies to rule out other differential diagnoses such as AQP4 positive optic neuromyelitis, MOG-IgG associated disorders, autoimmune encephalitis, infectious encephalitis, among others.
Regarding clinical outcomes at discharge, we found similar pattern that previous studies where adults have worse prognosis compared to children (Koelman et al., 2016; Schwarz et al., 2001). We found a favorable outcome of 77.8% versus 5.6% in children and adults, respectively. Based on our included studies, the historical COVID-19 severity is not related with poor prognosis, but a moderate/severe ADEM at hospitalization is associated with a poor clinical outcome at discharge. Interestingly, we found no differences among treatment regimens (steroids alone versus combined therapies), it suggests that the use of intravenous methylprednisolone and a correct tapering protocol with oral corticosteroids are the most cost-effective treatment option. Finally, ADEM is an evolving entity, in which long-term follow-up is often required to differentiate monophasic ADEM from a first episode of multiple sclerosis or other demyelinating diseases (Schwarz et al., 2001). Although no relapses were reported due to the short time of COVID-19 in the world and the short follow-up time of published cases, this cannot be confirmed yet.
This study has some limitations. First, it is important to note that it is not easy to identify a history of COVID-19 disease because the polymerase chain reaction (PCR) test could become negative after a few weeks (Kucirka et al., 2020) and serological tests are not frequently performed. Therefore, the reported association could be underestimated in the literature. Second, the average quality of the included reports was moderate (3 from 6 points), with important limitations in case definition and follow-up. Thus, we reported a close temporal association between ADEM and COVID-19, rather that probing a causal relationship. Further studies are needed to test this hypothesis. Third, we should recognize the limited evidence in which we based our conclusions on prognosis association and treatment regimens (non-randomized and non-controlled evidence). Due to the rarity of the disease, it is unlikely that a randomized controlled trial could be performed in this population, therefore, our current evidence summary is valuable for evidence-based diagnosis and treatment of ADEM related to COVID-19.
5. Conclusions
We summarize, for the first time, the worldwide ADEM cases associated with COVID-19. The clinical and neuroimaging presentation was similar to pre-COVID-19 reports, but with more frequent severe ADEM cases, and some demographics changes due to the influence of COVID-19 epidemiology (older adults and men). We found no association between COVID-19 severity and ADEM severity at hospitalization, suggesting an immune-mediated mechanism independent of the intensity of the initial immune response. Recovery rate was very low in adults but high in children,. We found no difference between treatment protocols in terms of clinical outcome at discharge. Thus, corticosteroid-only regimens seem to be as equally effective as combined treatments, but less expensive, which could constitute the best treatment option in low- and middle-income areas. Patients with different COVID-19 severities (even asymptomatic) could develop ADEM, hence neurologists must be alert to the occurrence of multifocal neurological symptoms associated or not with encephalopathy in patients recovered from COVID-19 disease. Timely MRI studies should be performed to establish the diagnosis and considering early corticosteroid-based treatment. Further prospective cohorts are needed to assess potential relapses and differentiate monophasic ADEM from a first episode of multiple sclerosis or other demyelinating diseases.
The following are the supplementary data related to this article.Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
Appendix A
Table A.1.
Quality assessment of included papers.
| Study | Selection |
Ascertainment |
Causality |
Reporting |
|||
|---|---|---|---|---|---|---|---|
| a. Does the patient(s) represent(s) the whole experience of the investigator (center) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported? | b. Was the exposure adequately ascertained? | c. Was the outcome adequately ascertained? | d. Were other alternative causes that may explain the observation ruled out? | e. Was follow-up long enough for outcomes to occur? | f. Is the case(s) described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice? | Total Score | |
| Abdi et al., 2020 | YES | YES | YES | YES | NO | YES | 5 |
| Abdel-Mannan et al., 2020 | YES | YES | YES | YES | NO | NO | 4 |
| Assunção et al., 2020 | YES | NO | YES | NO | NO | NO | 2 |
| De Miranda Henriques-Souza et al., 2021 | YES | YES | YES | YES | NO | YES | 5 |
| Dominguez-Rojas et al., 2020 | YES | NO | YES | YES | NO | YES | 4 |
| El Beltagi et al., 2021 | YES | YES | YES | NO | NO | NO | 3 |
| Espíndola et al., 2021 | NO | YES | NO | YES | NO | NO | 2 |
| Freire-Álvarez et al., 2020 | YES | YES | YES | YES | NO | YES | 5 |
| Kumar et al., 2020 | YES | YES | YES | YES | NO | YES | 5 |
| Langley et al., 2020 | YES | YES | YES | NO | NO | YES | 4 |
| Lindan et al., 2020 | YES | NO | YES | NO | NO | NO | 2 |
| Lopes et al., 2020 | YES | YES | YES | NO | NO | NO | 3 |
| McCuddy et al., 2020 | YES | YES | YES | YES | NO | YES | 5 |
| Parsons et al., 2020 | YES | YES | YES | YES | NO | YES | 5 |
| Paterson et al., 2020 | NO | NO | NO | YES | NO | NO | 1 |
| Pilotto et al., 2021 | YES | YES | YES | YES | NO | NO | 4 |
| Umapathi et al., 2020 | YES | YES | YES | YES | NO | YES | 5 |
| Utukuri et al., 2020 | YES | YES | YES | YES | NO | YES | 5 |
| Present Case | YES | NO | YES | YES | NO | YES | 4 |
Table A.2.
. Cases published on Acute Disseminated Encephalomyelitis in patients with COVID-19 included in systematic review with a summary of the results.
| Study | Age (years) / Sex | Comorbidities | COVID-19 severity | ADEM severity | Interval between Covid-19 symptoms and ADEM diagnosis (Days) | Predominant Neurological Clinic | MRI | COVID-19 diagnostic test | PCR COVID-19 in CSF | CSF: Cells (cells/mm3) & Proteins (mg/dL) |
Other studies carried out | ADEM Treatment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pediatric cases | |||||||||||||
| Abdel-Mannan et al., 2020 | 9 / Male | None | NA | Mild | NA | Encephalopathy, cerebellar signs, proximal leg weakness, urinary retention | WM | PCR (+) | N | C: 2000 P:19 |
Negative culture and virology results, OCB (-) | None | Favorable |
| Abdel-Mannan et al., 2020 | 15 / Female | Obese | NA | Moderate/Severe | NA | Encephalopathy, global proximal weakness, reduced reflexes | WM Microthrombi in cerebrum, brainstem, cerebellum. |
PCR (+), Serum Test (+) ¶ | NA | NA | NA | IVIg 1g/kg, 1 dose | Favorable |
| De Miranda Henriques-Souza et al., 2021 | 12 / Female | None | Mild | Moderate/Severe | 5 | Encephalopathy, oculocephalic, corneal, cough, and gag reflexes were abolished, pyramidal signs. | WM, SC | PCR (+) | N | C: 0 P: 18 |
PCR for arbovirus (-) | MP for 5 days (2 cycles) | Partially favorable |
| Dominguez-Rojas et al., 2020 | 13 / Male | None | Asymptomatic | Moderate/Severe | Indeterminate | Encephalopathy, ataxia, paraparesis | WM, DGM, SC | Serum IgG, IgM Test (+) | NA | C: 40 P: 43 |
PCR enterovirus and herpesvirus (-) | MP 30 mg / Kg /dose for 5 days | Favorable |
| Lindan et al., 2020 | 0.17 / Male | None | Mild | Mild | NA | Seizures | DGM | PCR or antigen test (+) | NA | NA | NA | Supportive measures, Anti-epileptic drugs | Favorable |
| Lindan et al., 2020 | 1.17 / Female | None | NA | Moderate/Severe | Indeterminate | Encephalopathy, dystonic posturing, seizures | DGM, WM, BS | PCR or antigen test (+) | NA | NA | NA | Supportive measures, Anti-epileptic drugs | Favorable |
| Lindan et al., 2020 | 9 / Male | Mild asthma | NA | Moderate/Severe | Indeterminate | Encephalopathy, photophobia, phonophobia, seizures | DGM, WM, Optic chiasm, cerebellum, cortex, BS | PCR or antigen test (+) | NA | NA | NA | IVIg | Favorable |
| Lindan et al., 2020 | 0.25 / Male | None | Mild | Moderate/Severe | 20 | Pyramidal signs, urinary retention | BS, SC | PCR (+) | NA | NA | NA | IVIg | Partially favorable |
| Lindan et al., 2020 | 1.58 / Female | None | Asymptomatic | Moderate/Severe | Indeterminate | Irritability, gait impairment, constipation. | WM SC |
PCR (+) | NA | NA | NA | High dose steroids | Favorable |
| Adult cases | |||||||||||||
| Abdi et al., 2020 | 58 / Male | NA | Asymptomatic | Moderate/Severe | Indeterminate | Encephalopathy, Pyramidal signs, seizure | BS, WM, DGM, cortex | PCR (+) | N | C: 0 P: 15 |
OCB (-), HSV (-), VZV (-), CMV (-), EBV (-) | Dexamethasone 8mg TDS | Unfavorable |
| Assunção et al., 2020 | 49 / Male | None | Severe | Moderate/Severe | 30 | Encephalopathy | BS, WM | PCR (+) | N | NA | NA | NA | NA |
| El Beltagi et al., 2021 | 25 / Male | NA | Mild | Moderate/Severe | 21 | Quadriparesis, ataxia, myalgia | WM, SC, BS | PCR (+) | NA | C: Raised P: Raised |
CSF culture, viral serology panel, TB PCR and autoimmune work-up were all negative | NA | NA |
| Espíndola et al., 2021 | NA | NA | NA | Mild | 4 | Encephalopathy, dysarthria and intracranial hypertension | WM | PCR (+) | P | C: Normal P: Normal |
PCR for arbovirus and herpesvirus (-) | MP 1 g daily for 5 days | Favorable |
| Espíndola et al., 2021 | NA | NA | NA | NA | NA | NA | NA | PCR (+) | N | C: Raised P: Raised |
PCR for arbovirus and herpesvirus (-) | NA | NA |
| Freire-Álvarez et al., 2020 | 39 / Male | NA | Mild | Mild | 14 | Encephalopathy, neck stiffness and paraphasia | WM,DGM,cortex | PCR (+) | N | C: 20 P: 198 |
HSV (-), VZV (-), CMV (-), EBV (-), HHV-6 (-), enterovirus (-), human parechovirus (-). | IVIg 0.4 g /kg / day for 5 days/ Tocilizumab 400 mg/24 hours for 3 days |
Partially favorable |
| Kumar et al., 2020 | 35 / Female | Gastric bypass, iron deficiency anemia | Mild | Moderate/Severe | 60 | Symmetric distal neuropathy, encephalopathy | WM, BS | PCR (+) | NA | C: 1 P: 22 |
AQP4(-), anti-MOG (-); OCB (-), negative meningitis - encephalitis panel (E. coli, H. flu, Listeria, N. Meningitis, S. Agalactaia, S. Pneumo, CMV, Enterovirus, HSV1y2, HHV6, VZV y criptococo) | MP 1 mg/kg for 5 days IVIg 2 g/kg divided over 3 days TPE 5 days |
Unfavorable |
| Langley et al., 2020 | 53 / Male | None | Severe | Mild | 59 | Encephalopathy, hemiparesis | WM, HL | PCR (+) | NA | C: NA P: Normal |
OCB were present in the CSF and serum, CSF bacterial culture was negative, PCR panel (including HSV, VZV, EBV, adenovirus and CMV) were negative | MP 1g/d for 3 days and tapered with oral prednisolone | Partially favorable |
| Lopes et al., 2020 | 59 / Female | HT | Severe | Moderate/Severe | Indeterminate | Encephalopathy, hemiparesis, Bilateral Babinski | WM, DGM, Cerebellum | PCR (+) | N | C: Normal P: Normal |
H1N1(-) | NA | Unfavorable |
| Lopes et al., 2020 | 41 / Male | Diabetes, HT, obesity, smoking | Severe | Moderate/Severe | 20 | Encephalopathy, quadriparesis, moderate subacute sensorimotor polyneuropathy | WM, DGM, HL | PCR (+) | N | C: Normal P: Normal |
OCB (-), microbiological analysis in CSF was negative | NA | Partially favorable |
| McCuddy et al., 2020 | 40 / Female | Diabetes, HT, obesity, pregnancy | Severe | Moderate/Severe | 22 | Pyramidal signs | BS, WM | PCR (+) | N | C: 2 P: 95 |
OCB (-), Meningitis / encephalitis panel negative | Dexamethasone 20mg IV for 5days and 10mg for 5days | Partially favorable |
| McCuddy et al., 2020 | 60 / Male | Diabetes, HT, asthma, chronic kidney disease | Severe | Moderate/Severe | 20 | Encephalopathy, hyporeflexia | Cerebellum, WM | PCR (+) | N | C: 1 P: 55 |
OCB (-) | MP 1 g/d for 5 days IVIg 25g/day for 3 days |
Unfavorable |
| McCuddy et al., 2020 | 70 / Female | Diabetes, HT, chronic kidney disease, obesity, neuropathy | Severe | Moderate/Severe | 16 | Encephalopathy, Pyramidal signs | BS, WM | PCR (+) | N | C: 0 P: 63 |
OCB:3, Meningitis / encephalitis panel negative | MP 1g/d for 5 days IVIg 25g/day for 3 days |
Partially favorable |
| Parsons et al., 2020 | 51 / Female | NA | Severe | Moderate/Severe | 24 | Encephalopathy, hypotonia, hyporeflexia, pyramidal signs | HL, WM | PCR (+) | N | C: 1 P: 62 |
AQP4(-); OCB:4 (both serum and CSF), PCR panel (including HSV, VZV, EBV, and CMV) were negative | MP 1 g daily for 5 days IVIg 0.4 g/kg daily for 5 days |
Partially favorable |
| Paterson et al., 2020 | 52 / Male | NA | Severe | Mild | 9* | Encephalopathy, Pyramidal signs, facial diplegia, areflexia, dysphagia, ophthalmoplegia, | WM, HL | PCR (+) | N | C: 0 P: Raised |
AQP4(-), anti-MOG (-); NMDAR (-), LGI1(-), GAD (-) | MP 1g/daily for 5 days IVIg |
Partially favorable |
| Paterson et al., 2020 | 52 / Male | NA | Severe | Mild | 9* | Encephalopathy, Pyramidal signs | WM, HL | PCR (+) | NA | NA | AQP4(-), anti-MOG (-); NMDAR (-), LGI1 (-), GAD (-), OCB (-) | Supportive | Partially favorable |
| Paterson et al., 2020 | 60/ Male | NA | Severe | NA | 9* | NA | WM, HL | PCR (+) | N | NA | AQP4(-), anti-MOG (-); NMDAR (-), LGI1(-), GAD (-), OCB (-) , viral PCR(-) | MP 1 g for 3 days | Partially favorable. |
| Pilotto et al., 2021 | 65 / Male | NA | NA | Moderate/Severe | 44 | Encephalopathy, motor deficit | WM, BS, DGM | PCR (+) | NA | C: 2 P: 63 |
OCB (-), CSF screening for bacteria and virus infection was negative | high-dose MP | Partially favorable mRS:2 |
| Umapathi et al., 2020 | 59 / Male | HT | Severe | Moderate/Severe | 14 | Encephalopathy, quadriparesis, hyperreflexia, ocular flutter | WM, BS, SC, HL, Cerebellum | PCR (+) | N | C: 6 P: 56 |
AQP4(-), anti-MOG (-); OCB (-), Negative autoimmune encephalitis panel, HSV (-), VZV (-), CMV (-) | IVIG doses were administered for 7 days, then cycles of 2 g/kg at 3-week intervals. | Partially favorable |
| Utukuri et al., 2020 | 44 / Male | None | Asymptomatic | Mild | Indeterminate | Encephalopathy, spinal cord involvement, ataxia | WM, SC | PCR (+) | N | C: 6 P: 36 |
CSF bacterial and viral PCR tests were negative | MP IVIg |
Partially favorable |
| Present Case | 35 / Male | None | Mild | Moderate/Severe | 21 | Pyramidal signs, dysarthria, ophthalmoplegia, sensorineural hearing loss, absent gag reflex, cerebellar symptoms (ataxia). | WM, BS, SC | Serum IgG, IgM Test (+) | NA | C: 1 P: 47 |
AQP4(-), anti-MOG(-); NMDAR(-), AMPA (-), GABAb(-), GABAa(-), mGluR5 (-), mGluR1(-), DPPX(-), IgLON5(-), LGI1(-), CASPR2(-), AQP-4 (-) | MP 1g daily for 5 days (2 cycles) tapered with oral prednisone | Partially favorable |
Table A. 2. Cases published on Acute Disseminated Encephalomyelitis in patients with COVID-19 included in systematic review with a summary of the results. HT: Arterial Hypertension; BS: Brainstem; NA: not available; WM: White matter; DGM: deep gray matter; HL: hemorrhagic lesions, SC: Spinal cord; PCR: Polymerase chain reaction; P & (+): positive, N & (-): negative, ND: Not done, MP: methylprednisolone, IVIg: intravenous immunoglobulin, TPE: therapeutic plasma exchange. C: cell, P: Proteins, *: Average days according to author.
References
- Abdel-Mannan O., Eyre M., Löbel U., Bamford A., Eltze C., Hameed B., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdi S., Ghorbani A., Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020;416:117001. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almqvist J., Granberg T., Tzortzakakis A., Klironomos S., Kollia E., Öhberg C., et al. Neurological manifestations of coronavirus infections – a systematic review. Ann. Clin. Transl. Neurol. 2020;7:2057–2071. doi: 10.1002/acn3.51166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção F.B., Fragoso D.C., Scoppetta T.L.P.D., Maia A.C.M. COVID-19-associated acute disseminated encephalomyelitis–like disease. Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Bodro M., Compta Y., Sánchez-Valle R. Presentations and mechanisms of CNS disorders related to COVID-19. Neurol. Neuroimmunol. Neuroinflammation. 2021;8 doi: 10.1212/NXI.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in new York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll-Vela L.E.D., Zamudio-Aquise M.K., Nuñez-Paucar H., Bernal-Mancilla R.R., Schult-Montoya S.C., Ccorahua-De La Paz M., et al. COVID-19-associated multisystem inflammatory syndrome in children: case series at a pediatric hospital in Peru. Rev. Peru. Med. Exp. Salud Publica. 2020;37:559–565. doi: 10.17843/rpmesp.2020.373.6126. [DOI] [PubMed] [Google Scholar]
- Correia A.O., Feitosa P.W.G., Moreira J.L., Nogueira S.Á.R., Fonseca R.B., Nobre M.E.P. Neurological manifestations of COVID-19 and other coronaviruses: a systematic review. Neurol. Psychiatry Brain Res. 2020;37:27–32. doi: 10.1016/j.npbr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miranda Henriques-Souza A.M., De Melo A.C.M.G., De Aguiar Coelho Silva Madeiro B., Freitas L.F., Sampaio Rocha-Filho P.A., Gonçalves F.G. Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology. 2021;63:141–145. doi: 10.1007/s00234-020-02571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Rojas J., Alvarado-Gamarra G., Del Águila O., Stapleton-Herbozo A., Seminario-Aliaga R., Reyes-Florian G., et al. Manifestaciones Extrapulmonares COVID-19 en pediatría: reporte tres casos hospital Edgardo Rebagliati Martins - Perú. Intensivos. 2020;13(3) [Google Scholar]
- El Beltagi A.H., Vattoth S., Abdelhady M., Ahmed I., Paksoy Y., Abou Kamar M., et al. Spectrum of neuroimaging findings in COVID-19. Br. J. Radiol. 2021;94:20200812. doi: 10.1259/bjr.20200812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola O.M., Brandão C.O., Gomes Y.C.P., Siqueira M., Soares C.N., Lima M.A.S.D., et al. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021;102:155–162. doi: 10.1016/j.ijid.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A. Acute Disseminated Encephalomyelitis. White Matter Dis. 2020:109. doi: 10.1007/978-3-030-38621-4_5. [DOI] [Google Scholar]
- Franke C., Ferse C., Kreye J., Reincke S.M., Sanchez-Sendin E., Rocco A., et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-Álvarez E., Guillén L., Lambert K., Baidez A., García-Quesada M., Andreo M., et al. COVID-19-associated encephalitis successfully treated with combination therapy. Clin. Infect. Pract. 2020;7:100053. doi: 10.1016/j.clinpr.2020.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. Critical Care Medicine. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Guo Y., Mao R., Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J. Med. Virol. 2021;93:820–830. doi: 10.1002/jmv.26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelslegers I.A., Visser I.E.R., Neuteboom R.F., Boon M., Catsman-Berrevoets C.E., Hintzen R.Q. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult. Scler. Houndmills Basingstoke Engl. 2011;17:441–448. doi: 10.1177/1352458510390068. [DOI] [PubMed] [Google Scholar]
- Kim L., Whitaker M., O’Halloran A., Kambhampati A., Chai S.J., Reingold A., et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, march 1-July 25, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelman D.L.H., Chahin S., Mar S.S., Venkatesan A., Hoganson G.M., Yeshokumar A.K., et al. Acute disseminated encephalomyelitis in 228 patients: a retrospective, multicenter US study. Neurology. 2016;86:2085–2093. doi: 10.1212/WNL.0000000000002723. [DOI] [PubMed] [Google Scholar]
- Krupp L.B., Tardieu M., Amato M.P., Banwell B., Chitnis T., Dale R.C., et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult. Scler. Houndmills Basingstoke Engl. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Olivera A., Mueller N., Howard J., Lewis A. Delayed SARS-COV-2 leukoencephalopathy without severe hypoxia. J. Neurol. Sci. 2020;418:117146. doi: 10.1016/j.jns.2020.117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley L., Zeicu C., Whitton L., Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-239597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A., Frontera J., Placantonakis D.G., Lighter J., Galetta S., Balcer L., et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J. Neurol. Sci. 2021;421:117316. doi: 10.1016/j.jns.2021.117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health Care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindan C.E., Mankad K., Ram D., Kociolek L.K., Silvera V.M., Boddaert N., et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc. Health. 2020;0 doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C.C.B., Brucki S.M.D., Passos Neto C.E.B., Corazza L.A., Baima J.P.S., Fiorentino M.D., et al. Acute disseminated encephalomyelitis in COVID-19: presentation of two cases and review of the literature. Arq. Neuropsiquiatr. 2020;78:805–810. doi: 10.1590/0004-282X20200186. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury A., Lyoubi A., Peiffer-Smadja N., de Broucker T., Meppiel E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: a narrative review for clinicians. Rev. Neurol. (Paris) 2021;177:51–64. doi: 10.1016/j.neurol.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuddy M., Kelkar P., Zhao Y., Wicklund D. Acute demyelinating encephalomyelitis (ADEM) in COVID-19 infection: a case series. Neurology. 2020 doi: 10.1101/2020.07.15.20126730. [DOI] [PubMed] [Google Scholar]
- Mehra B., Aggarwal V., Kumar P., Kundal M., Gupta D., Kumar A., et al. COVID-19-associated severe multisystem inflammatory syndrome in children with encephalopathy and neuropathy in an adolescent girl with the successful outcome: an unusual presentation. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2020;24:1276–1278. doi: 10.5005/jp-journals-10071-23685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N.S., Mytton O.T., Mullins E.W.S., Fowler T.A., Falconer C.L., Murphy O.B., et al. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71:2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid.-Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson D.E. The interplay of infection and genetics in acute necrotizing encephalopathy. Curr. Opin. Pediatr. 2010;22:751–757. doi: 10.1097/MOP.0b013e3283402bfe. [DOI] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020;267:2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham H., De Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Masciocchi S., Volonghi I., Crabbio M., Magni E., De Giuli V., et al. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2-related encephalitis: the ENCOVID multicenter study. J. Infect. Dis. 2021;223:28–37. doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A.A., Carroll L.S., Nar V., Varatharaj A., Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol. Neuroimmunol. Neuroinflammation. 2020;7 doi: 10.1212/NXI.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirko I., Suidan G.L., Rodriguez M., Johnson A.J. Acute hemorrhagic demyelination in a murine model of multiple sclerosis. J. Neuroinflammation. 2008;5:31. doi: 10.1186/1742-2094-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl D., Alper G., Van Haren K., Kornberg A.J., Lucchinetti C.F., Tenembaum S., et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87:S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- Popay J., Roberts H., Sowden A., Petticrew M., Arai L., Rodgers M., et al. 2006. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC methods Programme. [DOI] [Google Scholar]
- Riley D.S., Barber M.S., Kienle G.S., Aronson J.K., von Schoen-Angerer T., Tugwell P., et al. CARE guidelines for case reports: explanation and elaboration document. J. Clin. Epidemiol. 2017;89:218–235. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- Sawalha K., Adeodokun S., Kamoga G.-R. COVID-19-induced acute bilateral optic neuritis. J. Investig. Med. High Impact Case Rep. 2020;8 doi: 10.1177/2324709620976018. 2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Mohr A., Knauth M., Wildemann B., Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56:1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- Shahid Z., Kalayanamitra R., McClafferty B., Kepko D., Ramgobin D., Patel R., et al. COVID-19 and older adults: what we know. J. Am. Geriatr. Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenembaum S., Chamoles N., Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- Tenembaum S., Chitnis T., Ness J., Hahn J.S., International Pediatric MS Study Group Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathi T., Quek W.M.J., Yen J.M., Khin H.S.W., Mah Y.Y., Chan C.Y.J., et al. Encephalopathy in COVID-19 patients; viral, parainfectious, or both? eNeurologicalSci. 2020;21:100275. doi: 10.1016/j.ensci.2020.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utukuri P.S., Bautista A., Lignelli A., Moonis G. Possible acute disseminated encephalomyelitis related to severe acute respiratory syndrome coronavirus 2 infection. Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6714. Ajnr;ajnr.A6714v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet Lond. Engl. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet infect. Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article.Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4