Abstract
Background & Methods: We conducted an online COVID-19 survey as the vaccines became available, utilising the UK MS Register, to understand people with multiple sclerosis (pwMS) views on COVID-19 vaccination and the subsequent vaccine uptake rates.
Results & Conclusion: 94.4% of 3191 pwMS surveyed indicated they would get a COVID-19 vaccine, while 5.6% would not. PwMS who have previously had an influenza vaccine, increasing age and the perception of having sufficient information about the vaccine were associated with increased likelihood of getting a vaccine. 51.7% of 3191 pwMS completed a follow-up survey indicating they received at least 1 dose of a COVID-19 vaccine. The proportion having had the vaccination based on their prior opinions was 53.2% in ‘Yes’ group and 27.0% in ‘No’ group, the latter reflecting a change based on their initial views. More information on COVID-19 vaccine safety in pwMS would be helpful for people to make informed decisions.
Keywords: Multiple sclerosis, COVID-19, vaccine; vaccination, UK MS Register
1. Introduction
Vaccination is the primary long-term strategy to manage the global impact of the SARS-CoV-2, a novel coronavirus declared as a global pandemic in March 2020. However, there are concerns whether various vaccinations in multiple sclerosis (MS) has been associated with worsening of the disease (Mailand and Frederiksen, 2017, Kelly et al., 2021). Scientific consensus favours vaccinations in people with MS (pwMS), with exceptions including pwMS on specific immunosuppressive disease-modifying treatments (DMTs) and receiving ‘live’ vaccines around the time of treatment (Riva et al., 2020).
It is essential to understand the acceptability of such an approach to maximise uptake among the public but also in those who have specific risks such as pwMS. Acceptability of COVID-19 vaccines varies widely by country, ethnicity and age (Lazarus et al., 2020, Williams et al., 2021) but crucially over time, as the balance of disease risks versus the real or perceived risks of the vaccination evolves (Williams et al., 2021). Using the UK MS Register (UKMSR), we launched a survey after the approval of the first COVID-19 vaccine in December 2020 (Page, 2020), aiming to examine whether pwMS would get a COVID-19 vaccine and what factors may be playing a role in this decision process. We invited this cohort of pwMS to answer a follow-up survey if they received a COVID-19 vaccine.
2. Materials and methods
PwMS on the UKMSR (Ethics:16/SW/0194, explicit consent gained) were invited by email to complete a COVID-19 vaccine online questionnaire between 7 December 2020 and 19 January 2021. Questionnaires were linked to age, gender, highest educational attainment (‘University’ or not), MS disease type (‘progressive’ – primary progressive and secondary progressive MS, ‘non-progressive’ – relapsing remitting MS, benign and others), whether currently on a disease-modifying treatment (DMT), anxiety was assessed using the Hospital Anxiety and Depression Scale (HADS) score (>=8) and disability was measured using the MS Impact Scale (MSIS-29) normalised physical subscore. Further factors considered include previous childhood, adolescent, travel and influenza vaccinations history and whether pwMS felt they have enough information on the COVID-19 vaccines. PwMS are stratified as an at-risk priority group [group 6 or above] by the Joint Committee of Vaccination and Immunisation (JCVI) in the UK (England, 2020) and should have been offered a COVID-19 vaccine by March 2021. Therefore, pwMS were invited to complete a follow-up questionnaire between 19 December 2020 and 20 May 2021, confirming if they have received at least 1 dose of a COVID-19 vaccine.
Statistical analysis was performed using the R statistical programming language (version 3.5.1). Univariate (t-test, Chi-squared and Kruskall-Wallis test) and logistic regression analyses were used to compare whether the above factors were associated with a difference in likelihood of getting a COVID-19 vaccine. Free text responses were categorised independently by two assessors (YH and RN).
3. Results
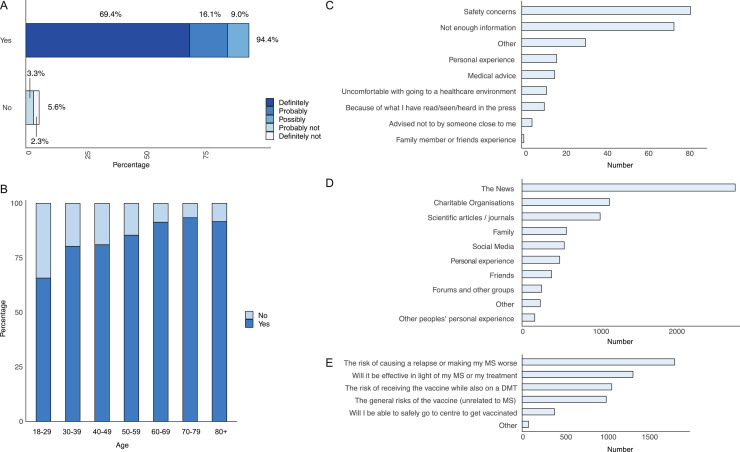
3191 out of 5189 pwMS contacted completed the questionnaire (61.5% response rate). 94.4% (3013/3191) of pwMS indicated they would get the vaccine (69.4% ‘definitely’, 16.1% ‘probably’, 9.0% ‘possibly’) and 5.6% (178/3191) would not (3.3% ‘probably not’ and 2.3% ‘definitely not’) (Figure 1 A).
Figure 1.
A) Bar graph of the responses of pwMS to getting a COVID-19 vaccine (n = 3191), expressed as a percentage of the total number. This was grouped into ‘Yes’ responses, including definitely, probably and possibly to a COVID-19 vaccine; and ‘No’ responses, including probably not and definitely not to a COVID-19 vaccine. B) Bar graph showing the proportion of ‘Yes’ and ‘No’ responses to a COVID-19 vaccine by age group, n = 3189. C-E) Bar graphs showing the frequency of the multiple-choice answers from the survey. C) Reasons why pwMS would not take a COVID-19 vaccine. Total number of participants = 178, total number of responses = 249. D) The sources pwMS receive information about the COVID-19 vaccines. Total number of participants = 3191, total number of responses = 7960. E) Areas where pwMS would like more information about the COVID-19 vaccines. Total number of participants = 3191, total number of responses = 5729.
Univariate analysis (Table 1 ) showed factors including increasing age, male gender, pwMS who had adolescent vaccines, the influenza vaccine and pwMS who feel they have enough information about COVID-19 vaccines were associated with higher proportion of ‘Yes’ responses to a COVID-19 vaccine. Childhood and travel vaccinations were excluded due to the limited numbers declared taken. Logistic regression (Table 1) found increasing age was associated with increased likelihood of a ‘Yes’ response (Figure 1B). In contrast to the univariate analysis, pwMS with a university level education were 1.89 times and those on DMTs were 1.73 times more likely to have the COVID-19 vaccine. PwMS who had an influenza vaccine previously were 7.08 times and pwMS who felt they have sufficient information were 2.03 times more likely to get a COVID-19 vaccine.
Table 1.
Univariate and logistical regression analysis, comparing whether the variables are associated with a difference in the likelihood of getting a COVID-19 vaccine. For univariate analysis, Age is expressed as mean and standard deviation for each response. Reference groups for the subsequent variables are: Gender – female; Education – no university level education; On DMT - No; Progressive - No; Anxiety - No; Motor disability – normalised MSIS-29 physical score <59; Adolescent Vaccine: Yes; Have you ever had an influenza vaccine: No; Do you have enough information: No. The percentage is the variable that answered ‘No’ or ‘Yes’ expressed as a proportion of the total numbers that answered ‘No’ or ‘Yes’ in each category.
| Variable | Univariate analysis | Logistic regression analyses | |||
| No | Yes | p | OR 95% CI[lower, upper], p | n | |
| Age: years | 49.06 (11.33) | 53.66 (11.43) | <0.001 | 1.05 [1.02, 1.07], <0.001 | 3189 |
| Gender: Male | 34 (19.21%) | 811 (26.94%) | 0.029 | 1.17 [0.68, 2.12], 0.579 | 3187 |
| Education: University | 32 (23.36%) | 690 (31.15%) | 0.068 | 1.89 [1.07, 3.55], 0.037 | 2352 |
| On DMT: Yes | 54 (30.34%) | 1093 (36.28%) | 0.127 | 1.73 [1.01, 3.02], 0.047 | 3191 |
| Progressive: Yes | 58 (32.58%) | 1068 (35.46%) | 0.485 | 0.86 [0.46, 1.6], 0.638 | 3190 |
| Anxiety: Yes | 43 (43.00%) | 691 (35.84%) | 0.178 | 0.81 [0.48, 1.37], 0.441 | 2028 |
| Motor Disability - normalised MSIS-29 score: upper quartile >59 | 29 (27.62%) | 497 (25.47%) | 0.707 | 1.00 [0.98, 1.01], 0.648 | 2056 |
| Adolescent Vaccines: No | 10 (5.62%) | 51 (1.69%) | 0.003 | 0.74 [0.18, 5.29], 0.714 | 3191 |
| : Unsure | 10 (5.62%) | 145 (4.81%) | 1.68 [0.55, 7.48], 0.421 | ||
| Have you ever had an influenza vaccine: Yes | 118 (66.29%) | 2853 (94.69%) | <0.001 | 7.08 [4.14, 11.92], <0.001 | 3191 |
| Do you have enough information: Yes | 46 (25.84%) | 1306 (43.35%) | <0.001 | 2.03 [1.19, 3.58], 0.011 | 3191 |
The top two reasons for not getting a COVID vaccine (n=178) are safety concerns and not enough information (Figure 1C). Free text analysis (165/178) found 47.3% (78/165) required further information and were concerned about the unknown long term side effects, and the speed COVID-19 vaccines have been developed and approved.
57.6% (1839/3191) of pwMS felt they do not have enough information about the COVID-19 vaccines. PwMS who received information (Figure 1D) from the news (88.1% [2812/3191]), charitable organisations (36.1% [1153/3191]) and family (18.3% [585/3191]) were 4.79 (3.35,6.82,p=0), 2.31 (1.61,3.38, p<1 × 10−5) and 2.03 (1.21,3.55, p<0.0096) times more likely to get a COVID-19 vaccine. In contrast, pwMS who received information from social media (17.5% [557/3191]) and scientific articles (32.3% [1030/3191]) were less likely to get a COVID-19 vaccine (0.58 [0.4,0.87, p<0.0069] and 0.61 [0.44,0.86, p<0.0046] respectively). Only 7.2% (229/3191) have received advice about vaccination from their MS team and 2.8% (89/3191) from their GP. 72.4% (2311/3191) would like more advice on the risk of causing a relapse and whether the vaccine will be effective with DMTs (Figure 1E).
Logistic regression analysis showed younger age group (0.97 [0.96,0.98], p<0.0001), female gender (0.77 [0.61,0.97], p=0.0027), no university-level degree (0.65 [0.52,0.82], p=0.0003), not progressive MS (0.58 [0.45, 0.74], p<0.0001), more disabled (1.40 [1.08, 1.82], p=0.0012) and never having had an influenza vaccine (0.58 [0.37, 0.90], p=0.017) were associated with increased perception of insufficient information.
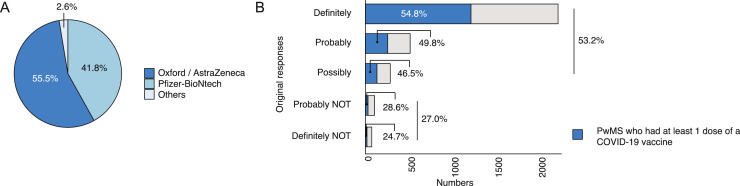
The follow-up survey found 51.7% (1650/3191) of pwMS who had completed the initial survey had received at least one dose of a COVID-19 vaccine by 20th May 2021, a median of 67 days after the initial survey. The majority (55.5%) of pwMS received the Oxford / AstraZeneca vaccine (Figure 2 A) and 50.0% received the vaccine at the vaccination centre. Of those who said they would get a COVID-19 vaccine, 53.2% (1602/3013) had received it and in those who initially indicated they would not get a vaccine, 27.0% (48/178) had received it (Figure 2B).
Figure 2.
A) A pie chart of which COVID-19 vaccine pwMS received. B) A bar plot of the proportion of pwMS who received at least one dose of a COVID-19 vaccine, based on their original response to whether they will get a COVID-19 vaccine if offered. N=1650.
4. Discussion
This online survey performed in the early phases of vaccine availability showed 94.4% of pwMS would accept a COVID-19 vaccine. This is a higher rate compared to online surveys conducted around similar time period, the UK general public (67%) (Web Page: YouGov 2020), patients with autoimmune diseases from Netherland (61%) (Boekel et al., 2021), pwMS from portugal (80.9%) (Serrazina et al., 2021) and pwMS from US in April 2020 (66%) (Ehde et al., 2021). In common with previous surveys, increasing age (Web Page: YouGov 2020, Boekel et al., 2021, Serrazina et al., 2021, Murphy et al., 2021), higher level of education (Web Page: YouGov 2020, Ehde et al., 2021, Murphy et al., 2021) and in those who have previously had an influenza vaccine (Fisher et al., 2020) had an increased likelihood of getting a COVID-19 vaccine. Interestingly, while there are now concerns around vaccination efficacy in those on DMTs (Achiron et al., 2021), pwMS on DMTs were 1.7 times more likely to take a COVID-19 vaccine.
A key message was that if pwMS had sufficient information, they were twice as likely to get the vaccine. The perception of receiving sufficient information is a significant factor in the decision process for 58% of those surveyed. Most pwMS receive information about COVID-19 vaccines from sources associated with a higher level of COVID-19 vaccine acceptance [the news, charitable organisations] compared to social media (Murphy et al., 2021). However, pwMS who received information from scientific articles were also less likely to receive a COVID-19 vaccine, which may reflect the general lack of scientific evidence for vaccine safety in pwMS in scientific publications during the early phase of vaccine availability. Overall, vaccine hesitancy is predominately due to a desire to gain more information to make informed decisions. We characterised the groups who need to be targeted with more information, it appears to be those at lower risk from COVID-19 e.g. younger, non-progressive MS and female gender; those less likely to have a university-level degree; those who are more disabled and those who have never had an influenza vaccine.
The follow-up survey had a response rate of 51.9% and found that 53.2% of pwMS who initially said ‘Yes’ to a COVID-19 vaccine received at least one dose by 20th May 2021. This could be an underestimate if pwMS did not answer the follow-up survey or were not offered a COVID vaccine due to local availability. Interestingly, 27.0% of pwMS who initially said ‘No’, went on to have a COVID-19 vaccine. The difference between the initial surveyed opinion in the early phases of vaccine availability and the final decision to receive a COVID-19 vaccine reflects the complex, multi-factorial and continuously evolving perception of disease risks versus the risks and benefits of vaccination over time (Williams et al., 2021). However, with further information initially hesitant pwMS decided to proceed with vaccination. A limitation of this study is the response rates of 61.5% in the initial survey and 51.9% in the follow-up survey, which may reflect responses from people who are more health conscious or have stronger opinions on health-related issues.
5. Conclusions
The majority of pwMS are willing to have a COVID-19 vaccine when made available. The primary reasons for not having a COVID-19 vaccine include safety concerns and insufficient information. Specifically, pwMS would like more information on the impact of a COVID-19 vaccine on MS disease activity and potential interactions with MS treatments. The final decision to receive a COVID-19 vaccine changes over time with evolving perceived risks versus benefits.
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
Acknowledgments
Funding
This work was supported by Multiple Sclerosis Society [grant number: MSREG-001].
Acknowledgements
We thank Alasdair Coles, Martin Duddy, Emma Gray and Phil Anderson from the MS Society and all the participants of this survey.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.103175.
Appendix. Supplementary materials
References
- Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017;264(6):1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- Kelly H, Sokola B, Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J. Neuroimmunol. 2021;356 doi: 10.1016/j.jneuroim.2021.577599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A, Barcella V, Benatti SV, Capobianco M, Capra R, Cinque P, et al. Vaccinations in patients with multiple sclerosis: A Delphi consensus statement. Mult. Scler. 2020 doi: 10.1177/1352458520952310. [DOI] [PubMed] [Google Scholar]
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020:1–4. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Flowers P, McLeod J, Young D, Rollins L. The Catalyst Project T. Social Patterning and Stability of Intention to Accept a COVID-19 Vaccine in Scotland: Will Those Most at Risk Accept a Vaccine? Vaccines (Basel) 2021;9(1) doi: 10.3390/vaccines9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page Web. 2020. Medicines and Healthcare products Regulatory Agency Regulatory approval of Pfizer/BioNTech vaccine for COVID-19.https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19 Access date: 3 February 2021. [Google Scholar]
- England PH. 2020. COVID-19: the Green book November; p. 27. Chapter 14a. [Google Scholar]
- Web Page: YouGov . 2020. How many Britons are willing to take a coronavirus vaccine?https://yougov.co.uk/topics/health/articles-reports/2020/11/16/how-many-britons-are-willing-take-coronavirus-vacc Access date: 3 February 2021. [Google Scholar]
- Boekel L, Hooijberg F, van Kempen ZLE, Vogelzang EH, Tas SW, Killestein J, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3(4) doi: 10.1016/S2665-9913(21)00037-0. e241-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrazina F, Sobral Pinho A, Cabral G, Salavisa M, Correia AS. Willingness to be vaccinated against COVID-19: An exploratory online survey in a Portuguese cohort of multiple sclerosis patients. Mult Scler Relat Disord. 2021;51 doi: 10.1016/j.msard.2021.102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehde DM, Roberts MK, Herring TE, Alschuler KN. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord. 2021;49 doi: 10.1016/j.msard.2021.102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Vallières F, Bentall RP, Shevlin M, McBride O, Hartman TK, et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021;12(1):29. doi: 10.1038/s41467-020-20226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes Toward a Potential SARS-CoV-2 Vaccine : A Survey of U.S. Adults. Ann. Intern. Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A, Mandel M, Dreyer-Alster S, Harari G, Magalashvili D, Sonis P, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.