TO THE EDITOR:
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Newly developed vaccines are powerful tools for interrupting the ongoing dissemination of SARS-CoV-2. Because the first vaccines were approved for clinical use within a short period of time, the available data on adverse effects in relation to vaccination for SARS-CoV-2 are still limited.2 One available vaccine is the adenovirus vector–based ChAdOx1 nCov-19 (also known as AZD122) from AstraZeneca.3,4 As this vaccine is considered to be safe,2-4 a new condition named vaccine-induced immune thrombotic thrombocytopenia (VITT) syndrome was reported in relation to previous administration of ChAdOx1 nCov-195-7 and Ad26.COV2.S (Janssen Pharmaceuticals).8
VITT is associated with thrombocytopenia accompanied by thrombosis and antibodies against platelet factor 4 (PF4) in the serum, but it differs from postvaccination immune thrombocytopenic purpura (ITP), a phenomenon associated with both live and inactivated vaccines.9-11 To our knowledge, however, ITP has not yet been described as being associated with administration of ChAdOx1 nCov-19 vaccine. Here, we report our findings in a cohort of 4 patients who presented with severe thrombocytopenia in the absence of thrombosis a short time after receiving a ChAdOx1 nCoV-19 adenoviral vector vaccine at our (single-center) institution.
We conducted retrospective and prospective analyses of patients who received treatment in our institution for ITP associated with ChAdOx1 nCoV19 vaccination within a 19-day period in May 2021. We evaluated patients’ records and confirmed the diagnosis of ITP. Patients’ demographic and clinical characteristics are presented in Table 1. Detailed case descriptions are provided in the supplemental information (available on the Blood Web site). Informed consent was provided by each patient, and monocentric data acquisition was in line with local requirements according to the Hamburg Hospital Act (HmbKHG) §12 and in accordance with the Declaration of Helsinki.
Table 1.
Patient characteristics
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age, y | 72 | 71 | 66 | 64 |
| Sex | Male | Female | Male | Female |
| Preexisting conditions and past medical history | Radioiodine-treated autoimmune thyroiditis | Latent hyperthyroidism, nodular goiter, breast cancer, stroke | Hypertension, mild thrombocytopenia | Hypertension, chronic obstructive pulmonary disease, steatosis hepatis |
| Allergies | Pollen | None | None | None |
| Medication on admission | None | Aspirin, metamizole, denosumab (once every 6 mo) | Amlodipine, valsartan, hydrochlorothiazide, tamsulosin | Candesartan, hydrochlorothiazide, zolpidem, salbutamol, tilidine, tiotropium bromide |
| Time from vaccination to admission, days | 11 | 11 | 2 | 15 |
| Symptoms | Petechiae, epistaxis, headache | Petechiae, hyposphagma | Petechiae | None |
| Platelet count nadir × 109/L | <5 | <5 | <5 | 6 |
| D-dimer peak, mg/L (reference range, 0.21-0.52 mg/L FEU) | 0.83 | 1.16 | 0.35 | 0.51 |
| PF4-polyanion antibodies (ELISA) | Negative | Negative | Negative | Negative |
| Treatment | Intravenous immunoglobulin, glucocorticoids | Intravenous immunoglobulin, glucocorticoids, thrombopoietin receptor agonist | Glucocorticoids | Glucocorticoids |
ELISA, enzyme-linked immunosorbent assay; FEU, fibrinogen equivalent units.
The patients were White women and men between 64 and 72 years of age from Germany. They presented 2 to 15 days after receiving the first dose of ChAdOx1 nCov-19 with severe symptomatic thrombocytopenia of ≤6 × 109/L cells. Patients 1 and 2 had a medical history of thyroid disorders (autoimmune thyroiditis and latent autoimmune hypothyroidism, respectively), patient 3 was previously diagnosed with minor thrombocytopenia (∼60 × 109/L), and patient 4 reported preexisting conditions, including chronic obstructive pulmonary disease and arterial hypertension. Initial symptoms included petechiae (patients 1, 3, and 4), hematomas (patient 1), headaches (patient 2), hyposphagma (patient 3), and epistaxis (patient 4). All patients reported that prior vaccinations against seasonal influenza (all patients), pneumococcus, and rubella (patients 2 and 3) were well tolerated. At admission, all patients were SARS-CoV2 negative according to a polymerase chain reaction test. In addition, patient 2 had a serologic test that was positive for SARS-CoV-2 spike receptor-binding domain and SARS-CoV-2 spike trimer immunoglobulin G (IgG) or IgM antibodies and negative for SARS-CoV2 nucleocapsid IgG or IgM antibodies (Elecsys Anti-SARS-CoV2, electrochemiluminescence immunoassay [ECLIA], Roche), indicating active immune response after vaccination.
The patients did not present with signs or symptoms of thrombotic events, and no antibodies to PF4-polyanion complexes were detected in enzyme-linked immunosorbent assay (Asserachrom HPIA-IgG, Stago), so the patients discussed were not associated with VITT. In addition, a magnetic resonance imaging scan was conducted in patient 2 to rule out intracranial bleeding and cerebral vein or sinus thrombosis because she presented with headaches. A bone marrow biopsy from patient 2 revealed an increased megakaryocyte count with no signs of malignancy. No clinical signs or symptoms of infection were present in any of the patients. On the basis of presentation and after ruling out differential diagnoses, ITP was diagnosed in all 4 patients.12
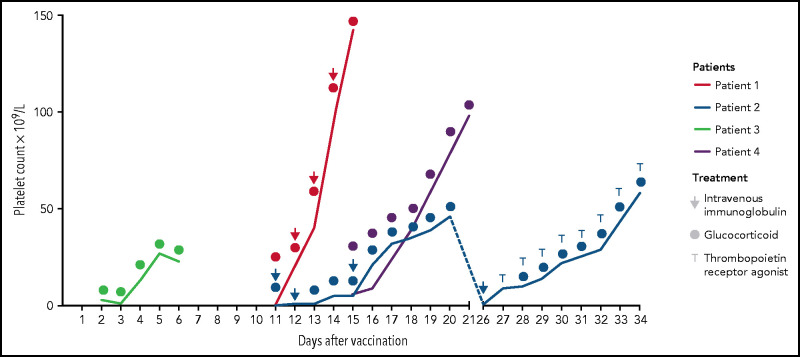
The patients received corticosteroids (prednisolone 100 mg/day; initial bolus of 250 mg in patient 1) as initial treatment (Figure 1). No further treatment was initiated in patients 3 and 4 because of a quick recovery of the platelet counts that increased to 23 × 109/L and 98 × 109/L after 4 and 6 days, respectively. Patients 3 and 4 were discharged for outpatient follow-up with a steroid reduction plan. Intravenous immunoglobulin (IVIG) was administered to patients 1 (0.4 g/kg) and 2 (1 g/kg) (Figure 1). Patient 2 did not respond, so dexamethasone (40 mg) was initiated for 7 more days. Platelet counts increased in patients 1 (142 × 109/L) and 2 (46 × 109/L) as shown in Figure 1, so they were discharged. Seven days later, at the first outpatient visit, patient 2 presented with a platelet count <5 × 109/L and was re-admitted for treatment with corticosteroids (prednisolone 100 mg) and IVIGs (1 g/kg). Thrombopoietin receptor agonist (TPO) eltrombopag was administered to patient 2 (50 mg once per day) due to the refractory course of the disease, and thrombocyte count increased quickly to 58 × 109/L. On follow-up, all patients were recovering with increasing platelet counts of 253 × 109/L (patient 1, after 5 days of follow-up), 71 × 109/L (patient 2, after 5 days of follow-up), 89 × 109/L (patient 3, after 3 days of follow-up), and 121 × 109/L (patient 4, after 6 days of follow-up).
Figure 1.
Platelet count responses to treatment. Patients received glucocorticoids (all patients), intravenous immunoglobulins (patients 1 and 2), and thrombopoietin receptor agonist (patient 2).
We present 4 patients with symptomatic thrombocytopenia associated with previous administration of ChAdOx1 nCov-19. Of note, 3 of 4 patients presented with a medical history known to be associated with the occurence of ITP or thrombocytopenia, such as thyroid disorders or preexisting mild thrombocytopenia. However, platelet counts in all patients were stable before the ChAdOx1 nCov-19 vaccine was administered. Because no patients presented with signs of thrombotic events and antibodies to PF4-polyanion complex, thrombocytopenia was not associated with VITT. One patient with a history of mild thrombocytopenia developed symptoms just 1 day after receiving the vaccination, and his condition may have caused a faster decline of thrombocytes.
To our knowledge, this is the first report in the literature that describes ITP being associated with the COVID-19 vector-based vaccination. Rare cases of ITP after use of messenger RNA (mRNA)–based COVID-19 vaccines have been reported.13-16 All of our patients were older than age 60 years, which might have been influenced by German authorities’ recommendation to use ChAdOx1 nCov-19 only in elderly patients because of the possibly higher risk of VITT in younger adults.
Symptomatic ITP is a severe condition that harbors the risk of fatal hemorrhage and is generally treated with corticosteroids and/or intravenous Ig. In patients with refractory disease, splenectomy or administration of TPO agonists can be considered. However, using TPO agonists earlier might be considered to avoid the use of immunosuppressants shortly after vaccination, which might impair the intended immunity against SARS-CoV-2.17 Considering that nearly 7 million doses of ChAdOx1 nCov-19 have been administered in Germany so far and that the incidence of ITP is about 3.3 per 100 000 adults per year,17 it is possible that our findings are coincidental. However, thrombocytopenia after receiving a vaccination for diseases such as rubella,11 pneumococcus,10 and influenza9 is a known phenomenon and is presumed to be linked to hyperfunction of B cells as seen in ITP.18,19 Moreover, considering the most recent few years, we counted an average of only 1 patient per month being admitted to our department for ITP, meaning a fourfold increase within a time period of only 19 days.
Our observations should prompt clinicians to take secondary ITP into account as an underlying cause in patients presenting with bleeding symptoms or low platelet counts after vaccination with ChAdOx1 nCov-19. Unlike with VITT, the risk of thrombosis with ITP is usually not increased, and anticoagulation, which is a suggested treatment in VITT5,7 should not be considered. All patients received their first vaccination with ChAdOx1 nCov-19, and it has not yet been determined which vaccine should be administered to these patients for their subsequent vaccination because mRNA-based vaccines were associated with ITP as well.13,14 Further investigations are needed to explicate the pathological mechanism and epidemiology of possible secondary ITP associated with COVID-19-vaccination.
Supplementary Material
The online version of this article contains a data supplement.
Authorship
Contribution: F.-O.P., C. Schaefers, and C. Seidel designed the study, analyzed the data, and wrote the manuscript; C.F., F.E.H., U.W. and C.B. provided clinical data; F.L., F.E.H., U.W., and C.B. critically reviewed the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Seidel, University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany; e-mail: c.seidel@uke.de.
References
- 1.Park M, Cook AR, Lim JT, Sun Y, Dickens BL.. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020; 9(4):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM.. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol Rep. 2021;8:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folegatti PM, Ewer KJ, Aley PK, et al. ; Oxford COVID Vaccine Trial Group . Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249): 467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy MN, Minassian AM, Ewer KJ, et al. ; Oxford COVID Vaccine Trial Group . Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S.. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althaus K, Möller P, Uzun G, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2- vaccination associated immune thrombotic thrombocytopenia [published online ahead of print on 20 May 2021]. Haematologica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagasaki J, Manabe M, Ido K, et al. Postinfluenza vaccination idiopathic thrombocytopenic purpura in three elderly patients. Case Rep Hematol. 2016;2016:7913092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng HF, Sy LS, Qian L, et al. Pneumococcal conjugate vaccine safety in elderly adults. Open Forum Infect Dis. 2018;5(6):ofy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black C, Kaye JA, Jick H.. MMR vaccine and idiopathic thrombocytopaenic purpura. Br J Clin Pharmacol. 2003;55(1):107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarawneh O, Tarawneh H.. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol. 2021;96(5):E133-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms JM, Ansteatt KT, Roberts JC, et al. Severe, refractory immune thrombocytopenia occurring after SARS-CoV-2 vaccine. J Blood Med. 2021;12:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N.. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2021;39(25):3329-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN.. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol. 2010;85(3):174-180. [DOI] [PubMed] [Google Scholar]
- 18.Giordano P, Cascioli S, Lassandro G, et al. B-cell hyperfunction in children with immune thrombocytopenic purpura persists after splenectomy. Pediatr Res. 2016;79(2):262-270. [DOI] [PubMed] [Google Scholar]
- 19.Yokomichi H, Tanaka-Taya K, Koshida R, et al. Immune thrombocytopenic purpura risk by live, inactivated and simultaneous vaccinations among Japanese adults, children and infants: a matched case-control study. Int J Hematol. 2020;112(1):105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.