Introduction
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an uncommon nonvasculitic neutrophilic dermatosis.1 SS is categorized as classic Sweet syndrome, malignancy-associated (MSS), or drug-induced. SS may be further divided into phenotypic and histopathologic variants. Here, we present a patient with multiple simultaneous histopathologic and phenotypic variants of SS.
Case report
The inpatient dermatology service was consulted to evaluate a 41-year-old woman who was recently admitted with newly diagnosed acute monocytic leukemia and undergone chemotherapy induction. The patient had developed fever and an indurated, tender lesion on her right breast 7 days previously. She subsequently developed similar areas elsewhere on her trunk and extremities, which did not improve with empiric intravenous antibiotic, antiviral, and antifungal treatment. Examination was notable for indurated, pink, and exquisitely tender plaques on the right shoulder, right medial breast, and right abdomen. A nonblanchable violaceous plaque was also noted on the left shin. There was symmetric, tender, pre-auricular adenopathy.
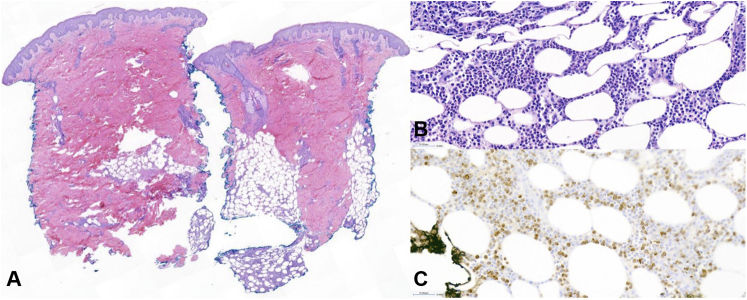
A punch biopsy of the right shoulder lesion demonstrated papillary dermal edema and a dense lobular infiltrate of polymorphonuclear leukocytes and admixed scattered mononuclear precursors within the deep dermal and subcutaneous tissue, consistent with subcutaneous Sweet syndrome (SSS) (Fig 1).
Fig 1.
Histopathology of subcutaneous Sweet syndrome, punch biopsy from the shoulder. There is dermal edema and a dense infiltrate in the subcutis (A, hematoxylin-eosin stain; original magnification: ×14), composed of polymorphonuclear leukocytes and mononuclear precursors (B, hematoxylin-eosin stain; original magnification: ×100). The mononuclear precursors were highlighted with MPO immunohistochemical stain (C, hematoxylin-eosin stain; original magnification: ×100).
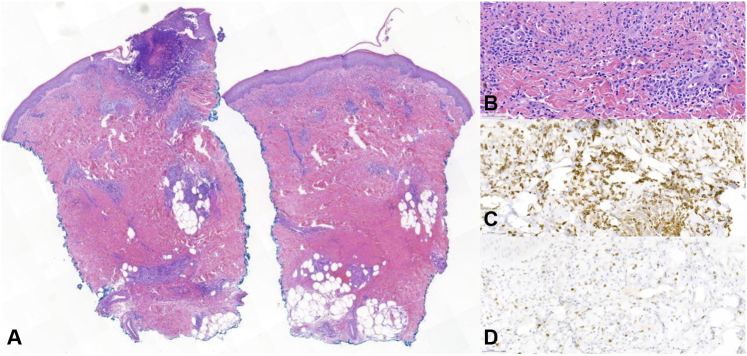
A punch biopsy of the left shin revealed a predominately mononuclear infiltrate in the dermis, focal ulceration with suppuration, and papillary edema. Mononuclear cells represented >50% of the infiltrate and were highlighted by both CD68 and myeloperoxidase (MPO) immunohistochemical stains, consistent with histiocytoid Sweet syndrome (HSS) (Fig 2).
Fig 2.
Histopathology of histiocytoid Sweet syndrome, punch biopsy from the shin: Superficial and deep infiltrate with dermal edema and focal overlying ulceration with suppuration was obeserved (A, hematoxylin-eosin stain; original magnification: ×14). The infiltrate was predominantly composed of mononuclear cells (B, hematoxylin-eosin stain; original magnification: ×100), which were highlighted with both CD68 (C, CD68 stain; original magnification: ×100) and MPO (D, MPO stain; original magnification: ×100) immunohistochemical stains.
No fungal, bacterial, or acid fast-organisms were identified on Grocott methenamine silver, Gram, or Fite stains. One day later, the patient developed a violaceous edematous plaque in the right antecubital fossa at a previous intravenous site (Fig 3) and also reported dyspnea and sharp right-sided chest pain. Methylprednisolone was initiated at 1 mg/kg/day for suspected SS, and the patient experienced an immediate resolution of her fever overnight.
Fig 3.
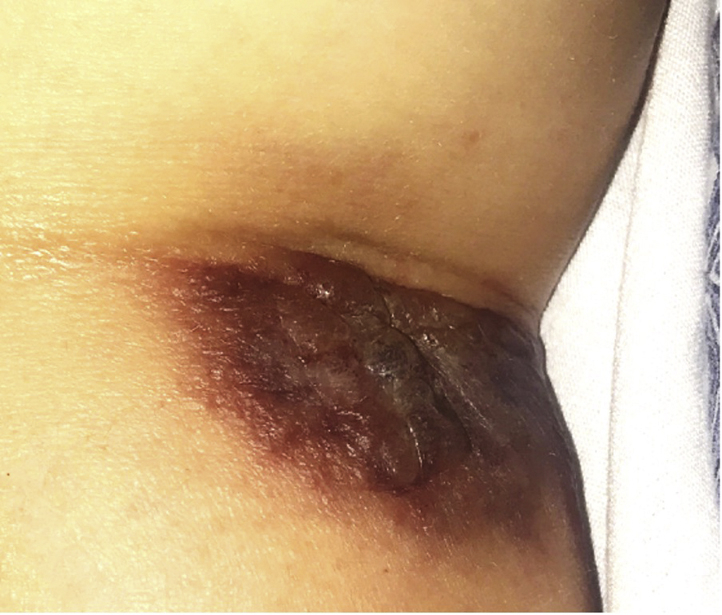
Vesiculobullous Sweet syndrome: Violaceous edematous plaque in the antecubital fossa at a previous intravenous access site, representative of pathergy.
Over the next 48 hours, the patient developed acute respiratory failure and shock requiring emergent intubation and pressor support. Bronchoscopy and bronchoalveolar lavage did not reveal any cardiac or infectious etiologies. Due to concern for pulmonary extracutaneous SS, intravenous pulsed methylprednisolone 1000 mg was restarted. The patient had continued rapid resolution of skin lesions, her respiratory status improved, and she was extubated successfully. The patient subsequently died several weeks later after declining further intervention for her malignancy.
Discussion
SS is a prototypic example of the neutrophilic dermatoses (ND), a group of cutaneous inflammatory diseases characterized by a sterile dermal neutrophilic infiltrate.1 The diagnosis of SS is made in patients with the abrupt development of characteristic skin lesions, histopathologic findings consistent with one of the subtypes of SS, and at least 2 of the following:1,2
-
1.
Lesions preceded by an associated infection, vaccination; accompanied by an associated malignancy or inflammatory disorder; associated with drug exposure or pregnancy
-
2.
Presence of fever >38°C and/or constitutional signs and symptoms
-
3.
Evidence of 3 or more of the following abnormal laboratory values at presentation: erythrocyte sedimentation rate >20 mm/hr, positive C-reactive protein, >8000 leukocytes, >70% neutrophils
-
4.
Excellent response to systemic corticosteroid treatment
In addition to etiology, clinical morphology, and histopathology may be used to further subclassify SS (Table I).
Table I.
Clinical and histopathologic subtypes of Sweet syndrome
| Classification | Findings |
|---|---|
| Clinical | |
| Classic | Rapid development of painful, erythematous nodules and/or plaques |
| Giant cellulitis-like | Large erythematous vesicular, bullous, or hemorrhagic plaques |
| Necrotizing | Erythematous lesions with deep neutrophilic invasion and soft tissue necrosis |
| Pustular/bullous | Vesicles/pustules with or without coalescence into bullae |
| Histopathologic | |
| Neutrophilic | Primary neutrophilic infiltrate within the dermis |
| Cryptococcoid | Degenerating inflammatory cells with resemblance to Cryptococcus capsule and acellular bodies resembling yeast |
| Histiocytoid | Primary mononuclear infiltrate in the dermis composed of MPO-positive histiocyte-like mononuclear cells |
| Lymphocytic | Primary lymphocytic infiltrate into dermis with immature granulocytes |
| Subcutaneous | Dense neutrophilic infiltrate within subcutis in lobular or septal patterns |
MPO, Myeloperoxidase.
MSS accounts for 3%-67% of SS cases, with up to 87% of these hematologic (most commonly acute monocytic leukemia).1, 2, 3, 4 HSS and subcutaneous Sweet syndrome (SSS) are more frequently associated with malignancy than neutrophilic SS (NSS).1,5 Atypical clinical morphologies have historically been associated with MSS, but recent studies found no difference in the frequency of vesicles, bullae, or pustules between classic Sweet syndrome and MSS.4,6,7 The clinical presentation of MSS may vary between malignancies: Arthralgia may occur more commonly in acute monocytic leukemia-associated SS than in myelodyplasia-associated SS.8 In one case, a patient was reported to develop NSS following HSS resolution.6 At least 6 reported cases involved subcutaneous HSS, a subtype with histiocytes interspersed among the septal or lobular neutrophilic infiltrate of SSS.9 This spectrum of histologic findings is also seen in other ND. SS has also been reported in cases with simultaneous pyoderma gangrenosum, neutrophilic eccrine hidradenitis, and other ND.1,2 This may be explained by the growing body of evidence for the shared pathophysiologic role of cytokines in ND.1,3 Our patient had multiple simultaneous clinical (classic Sweet syndrome and bullous SS) and histopathologic (SSS and HSS) variants.
Our patient also developed extracutaneous SS involvement, specifically pulmonary SS and noninfectious shock. Common extracutaneous symptoms of SS include episcleritis, myalgias, and arthralgias.2 Severe extracutaneous disease is associated with MSS, may develop in any organ system, and is a rare cause of death.2,3 Pulmonary SS, the most frequently reported extracutaneous finding, may present with symptoms ranging from dyspnea to acute respiratory failure.2,7
To our knowledge, this is the first report of SS occurring with multiple simultaneous clinical and histopathologic variants in a single patient. Our case demonstrates that SS may occur with a spectrum of varying clinical and histopathologic findings, even in a single patient. Finally, in patients with unusual cutaneous findings and underlying malignancy, the development of rapid-onset dyspnea or hemodynamic collapse may represent extracutaneous SS.
Conflicts of interest
None declared.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Nelson C.A., Stephen S., Ashchyan H.J., James W.D., Micheletti R.G., Rosenbach M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79(6):987–1006. doi: 10.1016/j.jaad.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P.R. Sweet's syndrome – a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marzano A.V., Borghi A., Wallach D., Cugno M. A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol. 2018;54(1):114–130. doi: 10.1007/s12016-017-8621-8. [DOI] [PubMed] [Google Scholar]
- 4.Nelson C.A., Noe M.H., McMahon C.M. Sweet syndrome in patients with and without malignancy: a retrospective analysis of 83 patients from a tertiary academic referral center. J Am Acad Dermatol. 2018;78(2):303–309.e4. doi: 10.1016/j.jaad.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Haber R., Feghali J., El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal. J Am Acad Dermatol. 2020;83(2):661–663. doi: 10.1016/j.jaad.2020.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Morgan K.W., Callen J.P. Sweet's syndrome in acute myelogenous leukemia presenting as periorbital cellulitis with an infiltrate of leukemic cells. J Am Acad Dermatol. 2001;45(4):590–595. doi: 10.1067/mjd.2001.119032. [DOI] [PubMed] [Google Scholar]
- 7.Kinser K.N., Panach K., Dominguez A.R. Recurrent malignancy-associated atypical neutrophilic dermatosis with noninfectious shock. Am J Med Sci. 2017;354(6):626–632. doi: 10.1016/j.amjms.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Merlant M., Lepelletier C., Battistella M. Acute myeloid leukemia and myelodysplastic syndrome–associated Sweet syndrome: a comparative multicenter retrospective study of 39 patients. J Am Acad Dermatol. 2021;84(3):838–840. doi: 10.1016/j.jaad.2020.09.089. [DOI] [PubMed] [Google Scholar]
- 9.Lee J., Cornejo K.M., Rork J., Rothman K., Deng A. Subcutaneous histiocytoid Sweet syndrome in a patient with relapsed acute myeloblastic leukemia. Am J Dermatopathol. 2018;40(6):459–462. doi: 10.1097/DAD.0000000000000855. [DOI] [PubMed] [Google Scholar]