Abstract
The novel coronavirus pandemic has radically changed the landscape of normal surgical practice. Lifesaving cancer surgery, however, remains a clinical priority, and there is an increasing need to fully define the optimal oncologic management of patients with varying stages of lung cancer, allowing prioritization of which thoracic procedures should be performed in the current era. Healthcare providers and managers should not ignore the risk of a bimodal peak of mortality in patients with lung cancer; an imminent spike due to mortality from acute coronavirus disease 2019 (COVID-19) infection, and a secondary peak reflecting an excess of cancer-related mortality among patients whose treatments were deemed less urgent, delayed, or cancelled.
The European Association of Cardiothoracic Anaesthesiology and Intensive Care Thoracic Anesthesia Subspecialty group has considered these challenges and developed an updated set of expert recommendations concerning the infectious period, timing of surgery, vaccination, preoperative screening and evaluation, airway management, and ventilation of thoracic surgical patients during the COVID-19 pandemic.
Key Words: Thoracic anesthesia, lung separation, personal protective equipment, coronavirus, COVID-19
IN EARLY 2020, the members of the Thoracic Subspecialty Committee of the European Association of Cardiothoracic Anaesthesiology and Intensive Care (EACTAIC) released preliminary recommendations on the perioperative care of patients with suspected or diagnosed coronavirus disease 2019 (COVID-19) who undergo thoracic surgery.1 Since the time of writing these preliminary recommendations, there have been subsequent surges of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across several regions in the world.2 As of May 12, 2021, the number of confirmed cases and deaths increased around the world by +228% and +98%, respectively, since the date of acceptance of publishing the EACTAIC recommendations on March 30, 2020.3 , 4
After the publication of the recommendations last year, there have been a huge number of studies, recommendations, and guidelines (many of which have been updated themselves), benefiting from new learning, and, in places, contradicting preliminary reports on diagnosis and management of patients with SARS-CoV-2 throughout the last year. Current practice also might be affected by the widespread rollout of vaccination. There is, therefore, the need to update the preliminary EACTAIC recommendations for perioperative management of patients undergoing thoracic surgery during the multiple waves of COVID-19 (Table 1 , Box A ).
Table 1.
Changes Between the 2020 Recommendations and 2021 Update
| 2020 | 2021 |
|---|---|
| Most elective surgeries are postponed. | There are guidelines and algorithms regarding elective surgeries, based on better evidence and improved understanding compared with 2020. |
| Not to include anesthesiologists at high risk for COVID-19 infection, such as those of older age (>60 y), immunosuppressed, pregnant, or having serious chronic comorbidities in the intubating team. | This may not be practical during subsequent waves of the COVID-19 pandemic because of staff shortage, particularly with the need for quarantining of staff exposed to infected patients, and an anticipated increased volume of surgical cases after widespread cancellation of elective surgery during the first wave. |
| Two attendants in the red zone inside the operating room and a second doctor to help administer drugs and monitor the patient, and to be available in case of unanticipated difficulty. | Not supported by any evidence so far; may not be appropriate. |
| FOB should be used but can be a part of contagion; rational solutions should be applied if the use of FOB has to be avoided. | FOB should not be compromised. |
| (If no FOB) a DLT can be used with clinical evaluation of the position. | (If no FOB) a DLT can be used with clinical evaluation of the position, although this has low sensitivity and poor diagnostic accuracy. |
| (If no FOB) an EZ-blocker can be used. | (If no FOB) an EZ-blocker can be inserted, although that requires experience and careful clinical evaluation to exclude malposition. |
| HFNO should be avoided. | The possible benefits of HFNO appear to overcome its risks. There is currently no convincing evidence that HFNO increases the risk of cross-infection to healthcare workers. |
NOTE. There are no changes in some recommendations such as the following (not exhaustive)*:
PPE rules
Donning and doffing habits
Airway trolley
Use of videolaryngoscope
Use of DLT with an embedded camera (especially if FOB is not available)
All patients should be considered COVID-suspected.
If a negative-pressure operating room is not possible, increase the PPE level
Appropriate preoxygenation, no facemask ventilation, rapid sequence induction
BB in its special indications
Nonintubated (or awake) surgery only in very exceptional cases
Avoid awake intubation
Two filters (HEPA or HMA) during OLV
General extubation rules including avoidance of coughing
Some recommendations were not existing in 2020 and are new in 2021, such as the following (not exhaustive):
So-called post-COVID sequelae; approach to patients with post-COVID sequelae
Timing after infection (new algorithms regarding urgency of operation, severity of the infection, and other factors)
Vaccination: Timing of operation after vaccination of the patients, possible problems associated to vaccination; no change in PPE guidance even if the staff are vaccinated
Intraoperative mechanical ventilation regarding preoperative evaluation (eg, based on CT findings, different phenotypes, based on point-of-care lung ultrasound evaluation)
Abbreviations: BB, beta-blocker; COVID, coronavirus disease; CT, computed tomography; DLT, double-lumen tube; FOB, fiberoptic bronchoscope; HEPA, high-efficiency particulate air; HFNO, high-frequency nasal oxygen; HME, heat and moisture exchanger; OLV, one-lung ventilation; PPE, personal protection equipment.
*Recommendations with no or minimum changes; some of them are kept in the 2021 update to maintain the integrity.
Box A.
 |
Consequently, the EACTAIC expert thoracic anesthesia panel implemented a living guideline model to provide an update to their guidance on the perioperative management of patients with suspected or diagnosed COVID-19 undergoing thoracic surgery. Considering the challenges of recurring peaks and new variants/mutations, the authors developed an updated set of expert recommendations concerning the infectious period, timing of surgery, vaccination, preoperative screening and evaluation, airway management, and ventilation of thoracic patients during the COVID-19 era.
Methods
The consensus was built on four sources. The authors electronically searched all major databases for literature concerning the management of patients with COVID-19. The authors electronically searched major databases (eg, MEDLINE) and online (eg, Google) to identify recent consensus recommendations, guidelines, relevant systematic reviews, randomized controlled trials, observational studies, and case series. These electronic searches were performed looking for studies published in English until May 12, 2021. Studies performed in patients undergoing thoracic surgery and those with results that could be extrapolated for thoracic anesthesia practice were evaluated by a writing committee, including six experts from the EACTAIC Thoracic Committee. The articles included were chosen through an agreement among the writing committee, including six experts of the EACTAIC Thoracic Committee (M.S., M.R.T., B.S., L.L.Z., F.P., and M.-J.L.). All authors then were asked if they agreed with the recommendations and newly added references through a simple survey without ranking the importance of the articles chosen. The authors also included any additional articles proposed by any of the other authors at this point.
The authors electronically searched specialist thoracic anesthesia societies’ and groups’ previous recommendations on the perioperative management principles for patients with potential SARS-CoV-2 infection, focusing on unique aspects of thoracic anesthesia.5, 6, 7, 8, 9
The thoracic subcommittee of EACTIC has performed a survey exploring changes in clinical practice occurring during the COVID-19 era to better understand how practice has evolved and to aid development of further general recommendations on the perioperative management of patients with suspected or diagnosed COVID-19 undergoing thoracic surgery (Szegedi L et al, 2021). This survey included a 36-item questionnaire to explore the changes in daily practice during the COVID-19 pandemic, emphasizing the general aspects of anesthesia, airway management and lung isolation, ventilation, and postoperative analgesia. The survey was sent by e-mail to 4,060 subscribers to the EACTAIC newsletters from each of the different EACTAIC subspecialties including cardiac, thoracic, vascular, intensive care, and perfusion, starting from August 3, 2020, until February 2, 2021. A total of 414 responses (10%) were received after sending nine reminders; 341 (82.4%) of them were returned with complete responses.
Finally, members of the subcommittee have discussed these three sources to define their recommendations. The subcommittee members had the opportunity to review, discuss, and edit all recommendations, agree on the references used, and suggest inclusion of additional references through exchange of evolving manuscript versions. Additionally, the subcommittee members discussed any point of debate through a closed WhatsApp group and through email exchanges.
Here, the authors underline some important notes. First, although there were a huge number of studies published in one year about the management of patients with COVID-19, there are still very few studies that can be considered as providing evidence-based knowledge on which to base practice. Second, the authors observed that the differences among different countries and among different centers within the same country often were based on local logistics rather than the scientific background. Indeed, the long-term impacts of the SARS-CoV-2 disease have led to vast differences in practice (eg, the regulations surrounding use of personal protection equipment [PPE] or preoperative screening of patients for COVID-19 infection).
The recommendations in this publication might be adapted to suit local clinical conditions, and available equipment and facilities, provided this can be done in a safe and controlled manner. As the goal was to update the initial consensus recommendations to guide all thoracic teams in their day-to-day clinical practice, the authors acknowledge limitations of the adopted methodology. This document perhaps, therefore, should be considered as a basis for future Task Force discussions, seeking to develop a multisociety consensus, taking into appropriate consideration new evidence uncovered during the COVID-19 pandemic.
General Considerations and Principles
Duration of the Infectious Period of SARS-CoV-2
It is crucial to determine when a patient confirmed to have SARS-CoV-2 is no longer infectious to allow decision-making on the optimal timing of procedures after recovery. Although there is a consensus among major surgical and anesthesia societies that “in order to minimize infection spread, all known or suspected COVID-19–positive patients requiring surgical intervention must be treated as positive until proven otherwise,”10 the panel acknowledges that such consensus may not be consistent across different regions of the world. The Centers for Disease Control and Prevention provides guidance to decide when transmission-based precautions (eg, isolation, use of PPE and engineering controls) may be discontinued for hospitalized patients or home isolation may be discontinued for outpatients (Online Supplement 1).
Timing of Surgery After Contracting SARS-CoV-2 Infection
Surgical mortality and complications are higher in patients with active or recent COVID-19 infection compared with patients without COVID-19, which highlights the need to consider postponing elective surgery after COVID-19 infection. Patients with ongoing symptoms of SARS-CoV-2 infection had higher mortality than patients whose symptoms had resolved or who had been asymptomatic (6.0% [95% CI 3.2-8.7] v 2.4% [95% CI 1.4-3.4] v 1.3% [(95% CI 0.6-2.0], respectively), even when elective surgery was delayed for ≥seven weeks.11 The COVIDSurg Collaborative and GlobalSurg Collaborative recommend delaying surgery for at least seven weeks after SARS-CoV-2 infection whenever possible.11 However, this seven-week interval needs to be considered carefully in the light of the following potential influential factors.
The Severity of SARS-CoV-2 Infection
In general, patients with SARS-CoV-2 infection can be classified into five categories according to the severity of presenting symptoms as shown in Online Supplement 2. There is an increasing proportion of globally asymptomatic SARS-CoV-2 infections being described.12 Patients who test positive for SARS-CoV-2 infection with no or mild symptoms at baseline are expected to have a shorter time to recovery than those with more severe symptoms.13 In contrast, severely ill SARS-CoV-2 patients requiring high-flow nasal oxygen (HFNO), noninvasive ventilation (NIV), or invasive mechanical ventilation during their hospital stay have been shown to have extensive lung diffusion impairment at follow-up six months after illness onset (Online Supplement 2).14
The American Society of Anesthesiologists (ASA) and Anesthesia Patient Safety Foundation developed a joint consensus on the suggested waiting time before surgery for COVID-19-recovered patients using both symptoms- and severity-based categories as follows15:
-
•
Four weeks for an asymptomatic patient or recovery from only mild, nonrespiratory symptoms
-
•
Six weeks for a symptomatic patient (eg, cough, dyspnea) who did not require hospitalization
Eight-to-ten weeks for a symptomatic patient who has diabetes, is immunocompromised, or was hospitalized
-
•
Twelve weeks for a patient who was admitted to an intensive care unit (ICU) due to SARS-CoV-2 infection
These timelines should not be considered definitive; each patient's preoperative risk assessment should be individualized, taking into consideration the surgical necessity, patient comorbidities, and the benefit/risk ratio of further delaying surgery.
The Urgency of Lung Cancer Surgery
The number of newly diagnosed patients with lung cancer in the United States decreased by 91% in the period from March to April 2020, compared with the same period in 2019,16 indicating the negative impact of national lockdowns and restrictions on seeking healthcare advice, including lack of outpatient clinics and access to diagnostic tests (eg, computed tomography [CT] scans, positron emission tomography and bronchoscopy).
Measures aimed at increasing healthcare capacity for patients with COVID-19 were implemented after a notable 60% decrease in cancer-directed surgery in Canada.17 The reduction in cancer-related surgery has two explanations; first, the delay in new cancer diagnoses, and second, delays due to cancellation in light of overburdened healthcare resources. These delays can negatively affect the care and outcome of cancer patients. These hard lessons illustrate the need for careful consideration of delaying lung cancer surgery in patients infected with SARS-CoV-2.
Although delaying lung cancer surgery for >seven weeks in patients infected with SARS-CoV-2 might be advantageous, allowing better preoperative preparation including prehabilitation, nutrition, and correction of anemia, it also could be associated with a deterioration in oncologic outcomes. Thoracic surgery and oncologic boards have published specific guidelines to triage elective cases considering urgency of procedure, potential alternative treatments, and the burden of patients with COVID-19 on hospital resources. The United States National Comprehensive Cancer Network recommends scheduling elective cancer resection within eight weeks after completion of clinical staging to prevent upstaging if the burden of local COVID-19 cases is low. In contrast, delaying curative surgery beyond eight weeks is acceptable and associated with similar long-term outcomes if the burden of COVID-19 is high. Importantly, suspected tumors with low malignant potential (adenocarcinoma in situ, minimally invasive adenocarcinoma, or ground-glass opacities) can be deferred safely with imaging to reassess for progression at three-to-six--month intervals.18 , 19 The authors recommend multidisciplinary team discussion to define the optimum timing of lung cancer surgery, with consideration of vaccination status,20 history of SARS-CoV-2 infection, and results of preoperative screening for SARS-CoV-2 disease.
Post–COVID-19 Sequelae
Several published reports demonstrated persistent and prolonged symptoms and sequelae after SARS-CoV-2 infection affecting a range of body systems. These are summarized in Table 2 . Careful preoperative evaluation to identify the presence of post–COVID-19 sequelae might help to individualize the optimal timing of thoracic surgery.
Table 2.
Post–COVID-19 Sequelae
| System | Sequelae |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: COVID-19, coronavirus disease 2019; ECGtb1fn1, electrocardiogram; SARS-COV-2, severe acute respiratory syndrome coronavirus 2.
Preoperative Screening
COVID-19 Pathways
It is preferable for hospital organizations to have separate perioperative pathways for non-COVID-19 and COVID-19-positive patients, 21 although this is not always feasible in some regions.
Perioperative Testing for COVID-19 Infection
In general, every patient should be screened clinically and virologically for SARS-CoV 2 infection by pharyngeal or nasal swab before surgery.22 , 23 Testing should be performed as close to surgery as possible (preferably fewer than 48 hours, not exceeding five days) to decrease the risk that a patient becomes positive while waiting for the surgical procedure.23 , 24 A computerized CT should be considered for patients with clinical symptoms suggestive of SARS-CoV-2 infection with a negative PCR test result23 , 25
Retesting of Patients Recovered From SARS-CoV-2 Infection23
The Centers for Disease Control and Prevention have developed a recommended approach to the management of patients undergoing surgery after contracting coronavirus, as follows.23
-
•
Retesting is not recommended in asymptomatic patients who have recovered from laboratory-confirmed SARS-CoV-2 infection within 90 days of their initial diagnosis.23
-
•An adult does not require repeated testing or quarantine for SARS-CoV-2 in the context of the new exposure to someone with suspected or confirmed COVID-19 if he or she:
-
1.Has recovered from illness from laboratory-confirmed SARS-CoV-2 infection and already has met criteria to end isolation (Online Supplement 1), and
-
2.Is within the first 90 days after the onset of symptoms of their initial laboratory-confirmed SARS-CoV-2 infection or within the first 90 days of their first positive SARS-CoV-2 test result if they were asymptomatic during initial infection, and
-
3.Has remained asymptomatic since the new exposure.
-
1.
-
•
If an adult has a new exposure to a person with suspected or confirmed SARS-CoV-2 infection and meets the first two above criteria but has or develops new symptoms consistent with SARS-CoV-2 infection within 14 days of the new exposure, consultation with infectious disease or infection control experts may be necessary.
-
•
If an alternative cause of the symptoms cannot be identified readily, retesting for SARS-CoV-2 infection may be warranted.
Preoperative Evaluation and Telemedicine
The World Health Organization defines telemedicine “as the provision of healthcare services via the use of communication technology for the diagnosis and treatment of diseases and for continuing education of healthcare providers.” Utilization of secured internet networks and video cameras has allowed specialists in distant geographic locations to perform complete clinical and physical examinations.26 During the COVID-19 pandemic, there was a substantial increase in telemedicine use across the specialty of thoracic surgery.27 Similarly, the ASA recognizes that many anesthesiologists may have increased or explored the use of telehealth and telemedicine visits during the COVID-19 pandemic.
Preanesthesia evaluation must be performed by a qualified anesthesiologist. Although telemedicine precludes physical examination of a patient, it does allow gathering of information before a patient's admission, including evaluation of the severity and progression of the presenting disease, identifying other comorbidities, defining the urgency of the surgical treatment, and selecting the preferable analgesic technique. It also can help in screening and triaging patients with suspected or established SARS-CoV-2 infection.28
Protection of Healthcare Providers
Operating Room and Equipment Management
Asymptomatic patients tested negative and recovered from COVID-19 infection (Table 3)
Table 3.
Recommendations for the Levels of Personal Protective Equipment (PPE)
| Asymptomatic Tested Negative | Recovered Patients From COVID-19 Infection | Asymptomatic, Untested | Asymptomatic, Tested Positive | A Patient Under Investigation (Symptomatic, but Without a Test Result) | The Symptomatic Patient Who Has Tested Positive | |
|---|---|---|---|---|---|---|
| Aerosol-generating procedure | Highest level of PPE available | Highest level of PPE available | Full PPE | Full PPE | Full PPE | Full PPE |
| Anesthesiologists | Highest level of PPE available | Highest level of PPE available | Full PPE | Full PPE | Full PPE | Full PPE |
| Other operating room staff | A lower level of PPE | A lower level of PPE | Full PPE | Full PPE | Full PPE | Full PPE |
Abbreviations: COVID-19, coronavirus disease 2019.
Transmission-based precautions, including droplet precautions, should be considered by the operating room (OR) staff for all patient care, including those testing negative for SARS-CoV-2 infection, because false negatives may occur in those with suspected or confirmed infection without prior testing. Additionally, healthcare providers should use an N95 mask, eye protection, gloves, and a long-sleeve fluid-resistant gown before performing an aerosol-generating procedure in these groups of patients.21 , 23 The Task Force acknowledges the wide variation in practice across different countries and regions based on the transmission threat level, and so here the authors advise anesthesiologists to follow local/national public health guidance.
Patients with suspected or diagnosed with COVID-19 infection or those who are considered to still be infectious
This group of patientes includes: (a) asymptomatic, untested; (b) asymptomatic, tested positive; (c) a patient under investigation (symptomatic, but without a test result); and (d) the symptomatic patient who has tested positive (Table 3 ) (Online Supplement 1)
Ideally, the operating or dedicated anesthesia room should be an isolated negative-pressure room with >12 air changes/hour. Only half of the 198 respondents to an unpublished survey from the EACTAIC-Society of Cardiovascular Anesthesiology reported that they have a negative-pressure room at their facilities, and 43% of the half who have negative-pressure rooms have just one-to-five negative-pressure rooms. This highlights the importance of the preliminary recommendations for practice in circumstances in which a negative-pressure room is not available:
-
•
The level of PPE should be increased (eg, respirators in place of masks, face shield, or helmet).
-
•
Alternatively, intubation can be performed in a negative-pressure room followed by transfer to the OR, such as in an isolation ward or ICU. The benefits of such an approach, however, need to be judged against its disadvantages and possible complications.
-
•
In rooms with positive pressure, the room can be put under the least possible positive pressure with the rest of the surrounding unit under higher positive pressure, and the doors kept closed, so that the high exchange rate of air in operating rooms limits dispersion of aerosols outside the room, despite the positive pressure.
The OR temperature level should be reduced to 18°C-to-20°C29 and humidity kept between 40% and 60%.23 , 30 A closed system for tracheal and endobronchial suction should be used.23 Displays of laryngoscopes, bronchoscopy, and anesthesia machines, computers, anesthesia trolley, etc, could be covered with disposable transparent plastic sheets.23 The breathing circuit should be checked as is standard practice. Antiviral filters like high-efficiency particulate air or heat and moisture exchanger filters should be attached between the face mask/tracheal tube and the Y-shaped connector, and at the expiratory outlet of the breathing circuit. The CO2 sample line should remain near the Y-connector but be situated on the ventilator side of the filter to avoid contamination.
Staff Planning
Patients with suspected or diagnosed with COVID-19 infection or those who are considered to still be infectious (Online Supplement 1)
-
•
Medical staff involved in tracheal intubation should be limited to those with essential roles.
-
•
The authors previously recommended not to include anesthesiologists at high risk for COVID-19 infection, such as those of older age (>60 years), immunosuppressed, pregnant, or having serious chronic comorbidities, in the intubating team. The authors acknowledge, however, this may not be practical during subsequent waves of the COVID-19 pandemic because of staff shortage; particularly with the need for quarantining of staff exposed to infected patients, and an anticipated increased volume of surgical cases after widespread cancellation of elective surgery during the first wave. The authors recommend, however, having discussions within work units to make appropriate decisions on the involvement of high-risk individuals in teams operating on patients with suspected or diagnosed infection with SARS-CoV-2.23
-
•
The previous recommendation of having two attendants in the “red zone” inside the OR and a second doctor to help administer drugs and monitor the patient, and to be available in case of unanticipated difficulty, is not supported by any evidence so far.1 Additionally, this criterion probably also is not practical to achieve in many centers. There must, however, be a “runner” physician available directly outside the room in “yellow zone” with full donned PPE, in case of need for help. Though it is desirable to have an observer outside the dedicated OR “white zone,” to monitor the PPE donning/doffing processes, this also may not be practically possible at many centers, particularly out-of-hours with limited staff numbers available. The surgical, anesthesia, nursing, and paramedical staffs who are not involved with airway management should not enter the operating room until after the airway has been secured.
-
•
Staff turnover within the OR should be minimized (ie, the same anesthesia personnel should remain in the OR for the entirety of the case, if possible).23
-
•
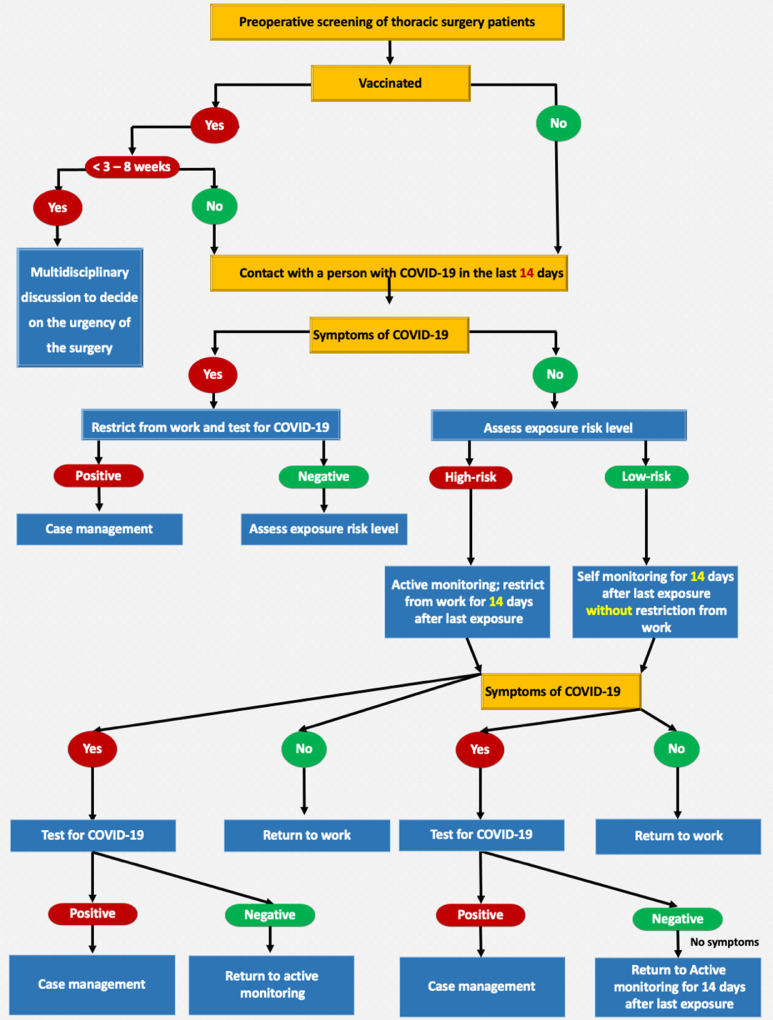
A stepwise approach reproduced from the Centers for Disease Control and Prevention's interim operational consideration is recommended for preoperative screening of patients undergoing thoracic surgery (Fig 1 ).
Fig. 1.
A stepwise approach reproduced from the CDC's interim operational consideration is recommended for the preoperative screening of patients undergoing thoracic surgery. CDC, Centers for Disease Control and Prevention.
Personal Protective Equipment (PPE)
Asymptomatic, tested negative, and recovered patients from COVID-19 infection (Table 3)
Due to the reasons explained earlier, the number of patients who should be considered to have suspected COVID-19 infection definitely is higher than the positive group, and because it is logistically, rationally, and also psychologically not possible to consider all patients as COVID-19- suspected, it is suggested that anesthesiologists should work with the highest level of PPE available, particularly during aerosol-generating procedures. The other OR staff might wear a lower level of PPE.
Patients with suspected or diagnosed with COVID-19 infection or those who are considered to still be infectious include
(a) asymptomatic, untested; (b) asymptomatic, tested positive; (c) a patient under investigation (symptomatic, but without a test result); and (d) the symptomatic patient who has tested positive (Table 3).
All OR staff, including anesthesiologists, surgeons, and nurses, must wear full PPE for all suspected or COVID-19–positive patients for aerosol-generating procedures and other surgical and interventional procedures, including masks (N95, FFP2), eye protection, double nonsterile gloves, gowns, and hair, and shoe cover as per the regularly updated World Health Organization recommendations.23 In light of perceived shortages of N95 and FFP masks occurring during the multiple waves of SARS-CoV-2 infections, the authors acknowledge that in some centers, resterilization and reuse of these masks might be needed.31 , 32 The authors recommend fit testing for masks that are being used. Most healthcare workers are vaccinated in many countries. Nonetheless, the task force recommends exercising the same precautions surrounding PPE use even in vaccinated staff, because the protective effects of different vaccines are not yet proven.
Mental and Physical Welfare
Providing perioperative care to patients with SARS-CoV-2 infection for long periods while wearing PPE is challenging and emotionally demanding. There is a high prevalence of burnout among healthcare workers during the COVID-19 pandemic.33 In a survey including 5,030 healthcare workers in the United States health system, the challenge of providing childcare for participants with children was repeatedly highlighted. Women with children were identified as being at increased risk of needing to leave the workforce and/or reduce hours.34
Psychological counseling should be available (virtually if possible) to support the emotional health of staff.23 , 35 , 36 Anesthesiologists are vulnerable to experiencing contact dermatitis, erythema, maceration, and fissures from prolonged use of occlusive gloves, frequent hand cleansing, and repeated use of alcohol-based hand gels.37 Staff should consider their hydration and use of the toilet before starting the long cases.23 Staff should be given appropriate break times between cases.23 Additional urgent cases may be performed best by a new team if possible.23
Attention should be paid to the environmental safety of restrooms and restaurants in hospitals to allow physical and psychological relief of the staff during break times. Several strategies can reduce the risk of exposure of staff to SARS-CoV-2 infection, such as using masks, providing adequate ventilation or outdoor dining if possible, promoting frequent hand hygiene, and making environmental modifications that promote physical distancing.38
Protection of Patients
Vaccination and Timing of surgery after vaccination
There are several different vaccines available with differing dosing schedules and time intervals between initial and subsequent doses. Preoperative SARS-CoV-2 vaccination could support safer elective surgery, particularly in patients aged 70 years or more needing cancer surgery.39 Prioritizing vaccination should be considered for any clinically extremely vulnerable group, which should include those undergoing cancer surgery and patients older than 70.
The vaccine itself may result in some systemic effects, such as fever and chills, in the first one-to-two days after vaccination, but these symptoms generally resolve soon after; it has been reported these normally resolve completely within a week. Such fever is uncommon after the first dose but occurs in about 15% after second dose. There are reported findings of venous thrombosis and thrombocytopenia five-to-16 days after vaccination40 , 41; there is minimal experience with the unwarranted effects of different vaccines. These adverse effects and complications could, however, affect the outcome of any surgery.
The authors advise waiting for three weeks after vaccination before undergoing elective surgery, both to avoid diagnostic confusion regarding the cause of any symptoms such as fever that may be attributed to the consequences of either vaccination or the surgery itself, and also to permit a sufficient time for antibody formation.42 At this time, it is not clear how long optimal protection lasts after vaccination; the authors recommend that the optimal timing of surgery after vaccination would be between three and eight weeks.42 Further studies are needed to support these recommendations. Any waiting period for surgery (after either the infection or the vaccination) should be used to facilitate sufficient preparation and prehabilitation.
Perioperative Thromboprophylaxis
Thrombosis prophylaxis using a standard prophylactic dose of enoxaparin (40 mg/d) under anti-Xa activity monitoring is recommended for acutely and critically ill hospitalized patients with SARS-CoV-2 infection.23 , 43, 44, 45 In the absence of bleeding, the strict application of thrombosis prophylaxis is recommended for all suspected or diagnosed SARS-Co-V-2 patients after surgery.23
General Recommendations
The previous recommendations discussed the standard contents of the intubation area, which should be prepared to achieve safe and successful airway control and establishment of controlled ventilation without compromising the high-risk patient, and while providing maximal protection to the healthcare team.1 The authors again recognize that some of the materials, devices, and drugs cited here are not available in some countries and centers. In this case, these recommendations should be read as a pathfinder to adapted guidelines.
Tracheal Intubation in Patients With COVID-19 for Thoracic Surgery
A. Asymptomatic and recovered patients from COVID-19 infection.
The authors advise clinicians to follow the local regulations as explained earlier.
B. Patients with suspected or diagnosed with COVID-19 infection or those who are considered still to be infectious
-
•
Aerosol-generating procedures include surgery with high-speed devices, intubation and extubation procedures, bronchoscopy, sputum induction, manual ventilation, airway suctioning, cardiopulmonary resuscitation, tracheotomy and tracheostomy procedures, noninvasive ventilation, high-flow oxygen therapy, breaking closed ventilation systems, nebulized or aerosol therapy, and high-frequency oscillatory ventilation.46
-
•
Tracheal intubation47 and tracheostomy in patients with COVID-19 undergoing thoracic surgery are high-risk procedures for the anesthesia team because of the risks of aerosol transmission of infection during placement of the airway device and bronchoscopic check. It is also a risk period for patients with severe COVID-19 who may not tolerate long periods of apnea or inadequate oxygenation in case of delayed or failed tracheal intubation.
-
•
An elective intubation should be preferred, if possible, as emergency intubation may compromise protective procedures and also could increase the patient's risk.
-
•
Tracheostomy should be considered in patients with COVID-19 when prolonged mechanical ventilation is anticipated. However, there is insufficient evidence for recommending a specific timing for tracheostomy.47 , 49 The authors recommend leaving the decision on the relative merits of open versus percutaneous tracheostomy to the physician's discretion and institutional experience.50
-
•
Intubation and tracheostomy should be performed by the most experienced physician to minimize delay or related complications.
C. Intubation for thoracic anesthesia:
-
•
Preparation: These recommendations have been outlined in the previous article.1 Since its publication, there have been some changes, such as recommendations that barrier enclosures should not be used as a substitute for adequate PPE until validating their efficacy and safety in future studies.51 , 52
-
•Preoxygenation53:
-
○Appropriate preoxygenation is crucial as it can prevent/decrease the need for mask ventilation before securing the airway.
-
○Placing an oxygen face mask over the surgical mask on the patient's mouth and nose during preoxygenation could reduce the risk of viral transmission without compromising the inspired fraction of oxygen.54
-
○Preoxygenation should be performed using a well-fitting face mask and a Mapleson C (Waters) or anesthetic circuit.
-
○Face mask ventilation should be avoided unless needed. If necessary, a two-person, low-flow, low-pressure technique should be used with attention to minimizing leak wherever possible.
-
○Target an end-tidal oxygen concentration (EtO2) of 90% if possible.53
-
○
-
•Induction:
-
•Intubation1
-
○Intubation should be performed using videolaryngoscopy,52 preferably via a laryngoscope with a single-use blade if available and a separate remote screen. The latter would extend the distance between the airway of the patient and the anesthesiologist to minimize or avoid airborne spread.
-
○The pathway for dealing with an unanticipated difficult airway in thoracic anesthesia is similar to that of general airway management.11
-
○The ETT cuff or the cuff of the tracheal lumen of the double-lumen tube (DLT) should be inflated to seal the airway before starting ventilation and the depth should be noted and recorded. The cuff pressure should be kept at least 5-10 cmH2O above the maximum airway pressure, using an inflatable manometer. This is to ensure adequacy of cuff seal and minimize the risks of aerosol generation.
-
○
-
•
DLT or bronchial blocker (BB)1
The attending anesthesiologist should be aware of the indications for and the differences between lung separation and isolation.1
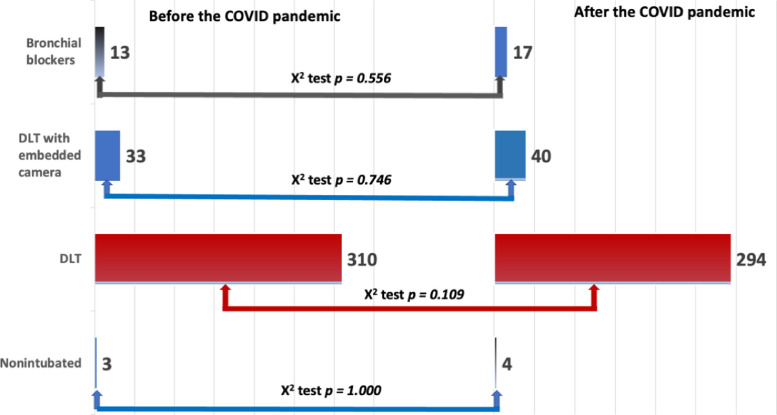
The majority of 362 respondents who completed the EACTAIC survey reported the use of DLTs with or without an embedded camera. Few respondents reported using BBs or nonintubated surgery. There was no statistically significant difference in the use/preference for DLT or bronchial blocker between the period before and after the onset of the COVID-19 pandemic (Fig 2 ).
Fig. 2.
The results of the EACTAIC Survey from 362 completed responses on the changes to current practice concerning the use of bronchial blocker, double-lumen tube (DLT) with and without an embedded camera, or nonintubated surgery before and during the COVID-19 pandemic. X2 test was used to compare data before and during the COVID-19 pandemic; p < 0.05 represents a statistically significant difference. COVID-19, coronavirus disease 2019. EACTAIC, European Association of Cardiothoracic Anaesthesiology and Intensive Care.
Bronchial blockers
-
○Lung separation with a single-lumen endotracheal tube (ETT) and BB may be preferred particularly:
-
1.In patients already intubated (this approach would avoid the risk of aerosolization during tube exchange)
-
2.In patients with a difficult airway (a “difficult” airway for ETT can be even more difficult for DLT)
-
3.In patients with tracheostomy
-
4.In short procedures
-
5.In patients in whom mechanical ventilation will be continued in the postoperative period (to avoid the need for tube exchange at the end of the surgery, which can be more difficult because of edema of the airways and be an additional mechanism of contagion)
-
1.
-
○
It is suggested to use an ETT swivel-connector with a valve. Before opening the valve of the swivel and introducing the bronchoscope, the anesthesia ventilator should be paused. If saturation is critical, preoxygenation can be performed in advance. During bronchoscopy, ventilation may be resumed, but it is important to ensure that the valve of the swivel fits snugly enough such that there is no leakage. Otherwise, bronchoscopy should be performed during apnea. The same procedure should be carried out when the bronchoscope is withdrawn from the tube. Other openings of the airway, for example, suctioning, also should be performed under apnea.
-
○
If a BB is to be used, the trachea of the patient should be intubated with the largest-possible standard ETT to allow enough room for the insertion of both the bronchial blocker and the fiberoptic bronchoscope.
-
○
Tracheal intubation should be confirmed with continuous waveform capnography.
-
○
In patients intubated with ETTs and BBs, the position of the BB (and the tube) should be confirmed with a flexible bronchoscope (FOB) or an ETT with an embedded camera. The authors suggest using a disposable FOB to avoid contagion, if available.
Double-lumen tubes
-
○
The position of the DLT should be confirmed with a disposable FOB; use of a DLT with an embedded camera can minimize the requirement for a bronchoscope and avoid the need to open the airway.
-
○
Ideally, disposable bronchoscopes are the best option to avoid the need for decontamination after the procedure. If disposable devices are not available, reusable bronchoscopes also can be used with strict adherence to cleaning regulations.
-
○
The authors share the recommendations for clinical practice endorsed by the Association for Cardiothoracic Anesthesia and Critical Care and the Society for Cardiothoracic Surgery in Great Britain and Ireland on the need for careful storage of the contaminated bronchoscope after use in a designated area, disposing of the outer set of gloves worn by the operator and performing hand hygiene after bronchoscope use.9
-
○
In any case, use of a bronchoscope (either disposable or reusable) where necessary should not be compromised in the face of a perceived increased risk of transmission of infection; only tubes (ETT or DLT) with embedded cameras can replace the need for position confirmation by bronchoscopy. Even when using tubes with embedded cameras, bronchoscopes occasionally still can be necessary. The authors strongly maintain the philosophy that fiberoptic bronchoscopy is a crucial part of thoracic anesthesia; if, however, a bronchoscope is not available at all or it is preferred to avoid its use for any (mostly logistic) reason:
-
○
A DLT with an embedded camera can be used.
-
○
A DLT can be used with clinical evaluation of the position, although this has low sensitivity and poor diagnostic accuracy.55
-
○
An EZ-blocker can be inserted, although this requires experience and careful clinical evaluation to exclude malposition.56
Difficult intubation
-
○
Airway management may take longer in case of difficult intubation, exposing healthcare workers to increased risk. The same or greater level of PPE should be considered in all airway procedures in SARS-CoV-2 patients with a difficult airway.53
-
○
Awake intubation should be avoided when possible and should be limited to strict indications in patients with an anticipated difficult airway because of the risk of aerosol or vaporization used for airway topicalization.53
-
○
In case of the need for awake intubation in patients with difficult airways and SARS-CoV-2 infection, topical techniques like injecting local anesthetics into the larynx and trachea via a FOB or holding the patient's tongue with a gauze pad as a local anesthetic is trickled into the pharynx can be considered.53 However, procedures with a high risk of coughing, such as transtracheal or translaryngeal injection of local anesthetics, should be avoided.53
-
○
Supplemental oxygen can be delivered using face masks that allow endoscope passage (eg, endoscopy mask), use of low- or high-flow nasal cannulae, and via bronchoscope adaptors during the use of an intubation conduit (eg, supraglottic device, channeled laryngoscope).53
-
○
Titrated sedation with an infusion pump and close monitoring of the depth of sedation are essential.53
-
○
For intubation, a flexible (preferably disposable) endoscope with a separate remote screen should be used.53
-
○
Rescue intubation through an intubating supraglottic airway device or early front-of-neck access can be necessary and equipment, therefore, should be ready before the intubation attempt.
After intubation
-
○
If necessary, a nasogastric tube can be placed immediately after intubation.
-
○
If the diagnosis of COVID-19 is suspected but not already confirmed, a deep tracheal aspirate for virology can be taken using closed suction.
-
○
The patient should remain connected to the breathing circuit as much as possible. A closed system with infraglottic catheter tip should be used for suction.57 , 58 If a disconnection from the breathing circuit is inevitably necessary, the ventilator should be switched to standby, and the endotracheal tube should be clamped.
-
○
After tracheal intubation, any airway equipment that has been contaminated but that may be needed again during the procedure should be stored in the operating room in a designated box with a sign clearly indicating contamination. After the surgical procedure, the equipment can be discarded or appropriately decontaminated.
-
○
Disposable equipment should be discarded appropriately and reusable equipment should be placed immediately inside sheaths and decontaminated according to the manufacturer's recommendations.
-
○
Doffing should be performed according to the prescribed sequence and be monitored meticulously by an observer.
-
○
If the intubation room is separate from the OR, this room should be cleaned 20 minutes after intubation (and after all similar aerosol-generating procedures).
-
○
Personal protective equipment should be worn until the end of the surgery, after immediately changing the outer gloves. Otherwise, hand hygiene must be performed before and after all patient contact. For tracheal extubation, caution should be exercised in view of the risks of aerosol transmission with coughing or the need for reintubation. The whole donning and doffing procedure should be repeated as described.
Intraoperative Anesthesia Management
Patients with suspected or diagnosed with COVID-19 infection or those who are considered to still be infectious
Volatile versus total intravenous anesthetics
The use of volatile anesthetics for sedation of ventilated patients infected with SARS-CoV-2 has been associated with a shorter duration of ventilation with improved gas exchange.59 Volatile anesthetics also might have beneficial antiinflammatory effects through their immune-modulating properties either by direct effects on immune cells or indirect effects on cellular protective pathways.60
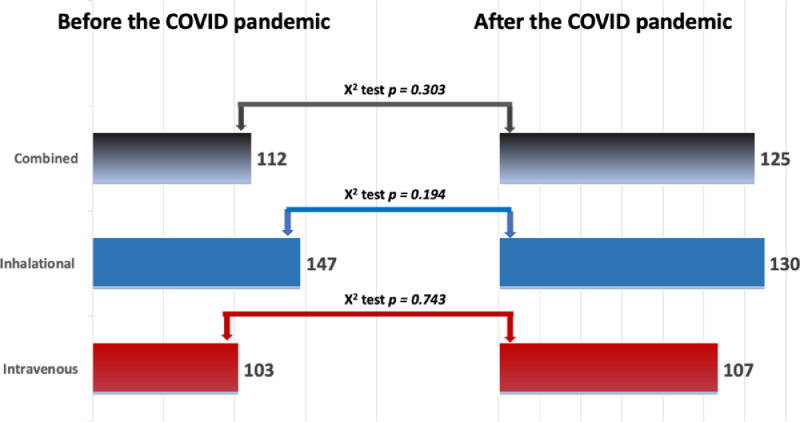
Interestingly, the 362 completed responses to the EACTAIC survey showed no statistically significant changes in thoracic anesthesia practice before and after the COVID-19 pandemic in preference for volatile anesthetics, TIVA, or both techniques together (Fig 3 ).
Fig. 3.
The results of the EACTAIC Survey from 362 completed responses on the changes to current practice concerning the use of volatile and intravenous anesthetics before and during the COVID-19 pandemic. X2 test was used to compare data before and during the COVID-19 pandemic; p < 0.05 represents a statistically significant difference. COVID-19, coronavirus disease 2019.
The authors recommend leaving the choice of anesthetic for thoracic surgery to the decision and expertise of the practitioners using it until a supportive body of evidence is available.
Low-flow anesthesia
A short survey for the opinions of the expert panel on the use of low-flow volatile anesthesia showed no changes in practice compared to the period before the COVID-19 pandemic. Most of the respondents report using a gas flow rate of 0.8-to-2 L/min and incorporating an antiviral filter into the breathing circuit.
Nonintubated thoracic surgery
The EACTAIC survey, however, showed no statistical difference between the use of nonintubated surgery before or during the COVID-19 pandemic (Fig 2). Although some guidelines for the management of other clinical conditions during the COVID-19 pandemic advocate regional anesthesia to avoid general anesthesia and the need for airway management, the authors do not suggest this approach for thoracic surgery. Donning of the same level of PPE should be considered during regional anesthesia for infected patients with SARS-CoV-2 because the airway is left open to the room for the duration of the procedure, with the associated risk of aerosol generation. There is no supporting evidence nor previous reports describing the use of the nonintubated technique in patients with highly contagious diseases. Intraoperative coughing and the risk of needing urgent intubation in a complex clinical scenario during nonintubated surgery are considered a contraindication to performing this technique during the pandemic to reduce the exposure of healthcare workers to droplet spread.61 Even in the non-COVID-19 population, nonintubated thoracic surgery is a novel, less well-described approach; which, contrary to some beliefs, is more challenging for the anesthesiologist. In the new clinical situation with SARS-CoV-2, there may be some exceptional patients who would benefit from this approach, but overall, it cannot be recommended.
All techniques of noninvasive ventilation (NIV) also are associated with an increased risk of aerosol spread. Cautious use of NIV, therefore, is recommended, with full airborne level PPE donned and the patient preferably treated in an isolated environment. There is currently no convincing evidence that HFNO increases the risk of cross-infection to healthcare workers. It is recommended, therefore, to weigh the risk/benefit ratio of using HFNO in patients with SARS-CoV-2 infection.53 , 62 Face mask oxygenation is likely to deliver better preoxygenation than NIV and HFNO, particularly for the difficult airway.53 , 62
Ventilation and One-Lung Ventilation
Patients with suspected or diagnosed with COVID-19 infection or those who are recovering or considered to still be infectious
Distinct clinical phenotypes of COVID-19 infection exist as described by Gattinoni et al.
Phenotype L is characterized by (1) low elastance normal compliance, (2) low ventilation-to-perfusion (VA/Q) ratio, (3) low lung weight with only ground-glass densities present on CT scan located subpleurally and along the lung fissures, and (4) low lung recruitability because of the very low amount of nonaerated tissue.63
Phenotype H is characterized by (1) high elastance and low compliance due to increased edema, (2) high right-to-left shunt due to the fraction of cardiac output perfusing the nonaerated tissue, (3) high lung weight (> 1.5 kg) resembling the acute respiratory distress syndrome, and (4) high lung recruitability because of the increased amount of nonaerated tissue.63
Reviewing the chest xrays can be helpful to define the needed ventilation settings. The chest CT scan is scored as having 0%, 25%, 50%, 75%, or 100% involvement, and the chest radiograph is scored as having opacities in one, two, three, or four quadrants.64
Point-of-care ultrasound offers additional benefits in the clinical management of patients with SARS-CoV-2 infection.65 There are two distinctive sonographic lung ultrasound patterns to differentiate patients who may respond to high positive end-expiratory pressure (PEEP) from those who would benefit from moderate PEEP.
-
•
Pattern one shows diffuse or coalescent B-line artifact descending from the pleural line to the bottom of the scan sector without fade, moving in concert with the sliding pleura. This appearance is thought to be caused by local subpleural inflammation/interstitial edema (ground-glass lesions) on CT and correlates with the L phenotype described by Gattinoni et al.63 Using moderate levels of PEEP (8-10 cmH2O) and higher tidal volumes (7-8 mL/kg predicted body weight) would be an appropriate initial strategy for those patients with preserved respiratory system compliance. In contrast, high PEEP levels of 10-to-15 cmH2O or alveolar recruitment maneuvers could lead to overdistention and cardiovascular instability.
Pattern two shows significant basal consolidation in the posterior lateral zone referred to as “lung hepatization” due to extensive atelectasis or a pneumonic process, and signs of aeration in the anterior zones. This pattern resembles the H phenotype, which may benefit from higher levels of PEEP.
-
•
The reports about different patterns or different phenotypes should alert the anesthesiologist to identify the patient's respiratory condition and subsequently decide about the appropriate conduct of intraoperative mechanical ventilation. One of the most important conclusions of these reports should be that COVID-19 has different pulmonary characteristics in clinical practice.
During one-lung ventilation (OLV), another high-efficiency particulate air or heat and moisture exchanger filter should be applied to the end of the DLT lumen corresponding to the operative lung, which is disconnected during one-lung ventilation.
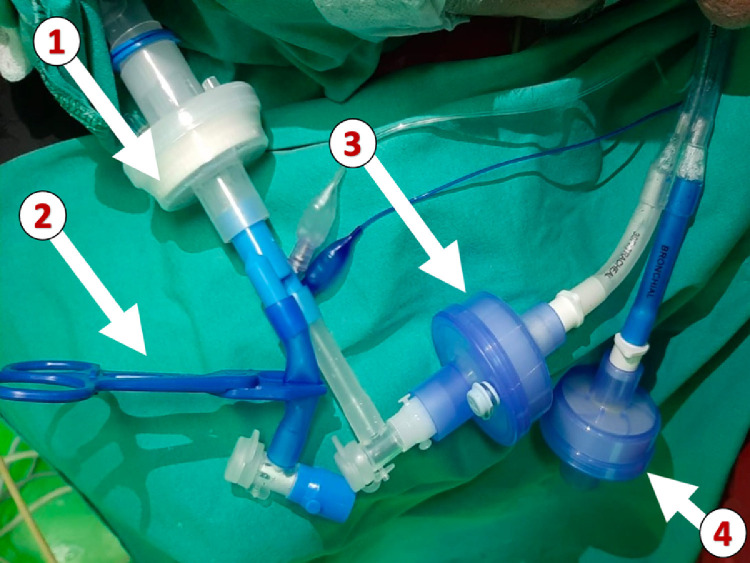
Other techniques have been suggested to isolate the lumen to the operative lung from the operating roomenvironment, such as applying a continuous suction or a sealed empty surgical glove. This might avoid (or decrease) the risk of aerosolization through the disconnected lumen (Fig 4 ). This probably is one of the most important and avoidable risks of thoracic anesthesia in SARS-CoV-2 patients.
Fig. 4.
Minimization of atmospheric contamination using antiviral filters during one-lung ventilation: (1) a filter connected to the Y-piece of the circuit, (2) a clamped connector for the nonventilated lumen of the double-lumen tube (DLT), (3) a filter connected to the end of the lumen corresponding to the ventilated lung, and (4) a filter connected to the end of the lumen corresponding to the nondependent lung. (Permission to reproduce was obtained from Professor Mert Senturk, Istanbul University, Turkey).
Because the oxygenation of SARS-CoV-2 patients already is compromised, one-lung ventilation could be more challenging, and a higher incidence of hypoxemia during one-lung ventilation might be expected. Generic recommendations for the conduct of OLV using lung-protective ventilation also can be considered valid in these patients49:
-
•
Volume-controlled or pressure-controlled ventilation.64
-
•
The authors avoid suggesting a FIO2 of 1.0; instead, the authors suggest applying the most appropriate FIO2 to ensure normoxia, as both hypoxia and hyperoxia can be harmful, especially in a critical case.
-
•
Low tidal volume 4-to-6 mL/kg predicted body weight.
-
•
Some degree of hypercapnia can be permitted by adjusting the respiratory frequency (pH kept above 7.2).
-
•
The required PEEP will vary widely according to the respiratory system compliance.64 A PEEP titration strategy is suggested, but this should be performed very cautiously so as not to compromise cardiac output at higher levels of PEEP.64 , 66
-
•
Targeting plateau pressures less than 30 cm H2O66 and a driving pressure of 11-to-16 cmH2O64 are essential. Highinflation pressures should be used with caution because of the risks of pneumomediastinum in ventilated patients with SARS-CoV-2 infection.67
-
•
Patients may benefit from the application of a staircase alveolar recruitment maneuver,66 and a trial is recommended. However, any recruitment strategy can impair hemodynamic stability in a more extended way than in healthy patients.49
Clearly, in some patients with active lung disease, maintenance of OLV may be impossible due to oxygenation problems. In such patients, without obligatory indications for lung isolation (eg, airway leakage, unilateral bleeding), the price to continue the OLV must be to never compromise oxygenation. This general rule must be adhered to even more strictly in challenging cases such as SARS-CoV-2 patients.
If the application of continuous positive airway pressure is deemed necessary, the authors recommend incorporating an antiviral filter to the operative lung.7 , 9 In some cases, application of inhaled nitric oxide or even extracorporeal assist systems (for oxygenation and/or carbon dioxide removal) can be indicated. These cases are beyond the scope of this review.
VATS or Open Thoracotomy
Patients with suspected or diagnosed with COVID-19 infection or those who are considered to still be infectious
The relative merits in safety of open thoracotomy versus thoracoscopy in patients with SARS-CoV-2 infection have been the subject of much debate. To the best of the authors’ knowledge, there is no strong supporting body of evidence on the superiority of one surgical approach over the other.
A multicenter observational cohort study demonstrated that the risk of nosocomial COVID-19 contamination did not appear to be higher with robotic-assisted thoracic surgery for different oncologic surgeries.68 Sixty-three percent of 343 respondents to the EACTAIC survey reported using VATS, 28% used open thoracotomy, 2% used robotic-assisted thoracic surgery, and 7% used other surgical approaches, including sternotomy for thoracic surgery, in their institutions before the COVID-19 pandemic. Interestingly, only 10% of the 415 respondents reported changes to the surgical approach selected during the COVID-19 pandemic.
Potential risks from high-concentration bursts of smoke from the body cavity when exchanging instruments, removing specimens, or desufflating the abdomen have been described during laparoscopic surgery compared with open surgery. Such a risk requires consideration and may be mitigated in part by the donning of PPE by surgeons, keeping the port sites and instruments clear of body fluids, avoiding the use of two-way insufflation devices, using lower insufflation pressures and the lowest power settings for electrosurgical dissection, evacuation of carbon dioxide before extracting the excised tissues or specimen, and using air filters for the gas.69 , 70 A systematic review and meta-analysis concluded that there is insufficient evidence to recommend the laparoscopic approach over the open one for abdominal surgery in the COVID-19 era.71 In VATS, carbon dioxide insufflation is not used, so the risk of aerosol generation may be even lower than in laparoscopic surgery.
Extubation (as illustrated in Figure 5 in the previous version)1
Patients with suspected or diagnosed with COVID-19 infection or those who are considered to still be infectious
The authors assume that in almost all intubated SARS-CoV-2 patients requiring thoracic surgery, mechanical ventilation may need to be continued after the surgery. However, they acknowledge that extubation should not be delayed for the risk of reintubation.49 If a BB was used, it simply can be removed at the end of the surgery. If a DLT was used, it should be changed to a normal ETT under the guidance of a video laryngoscope53 using an appropriate tube exchanger (caveat: specific tube exchangers for DLT should be used). In such cases, regulations for PPE (donning and doffing) should be repeated step by step.
In patients who are to be extubated:
-
•Before extubation, aspiration via a closed system followed by a recruitment maneuver is suggested.
-
•Patients should be ready for extubation onto a face mask. Air flow to the surrounding area should be avoided as much as possible; a tight-fitting face mask is, therefore, essential.
-
•A mask-over-tube extubation is recommended.72 The anesthesiologist positions the ETT or DLT to the side of the mouth closest to the assistant and places a face mask over the patient's mouth and nose with a second antiviral filter using a two-handed technique to ensure a good seal.72 After deflation of the tube cuff, extubation is performed at end-expiration while maintaining the face mask seal.72
-
•Again, aerosolizing procedures (eg, NIV, HFNO, nebulizing bronchodilators) only should be used if needed to treat hypoxemia or bronchoconstriction after extubation, and only should be performed while donning the same level of PPE, conditions, and logistics, as applied during intubation.62
-
•Any maneuver that risks precipitating coughing should be avoided: oral suctioning (if any) should be very gentle; patients should not be asked to cough. In difficult airway cases, using an extubation catheter (eg, with a soft, thin tip) can be possible, but in these cases, keeping the patient intubated is more important.
-
•Use of medication known to effectively lower the incidence of coughing (eg, dexmedetomidine, lidocaine) and postoperative nausea and vomiting is advocated.53
-
•Placing a surgical mask or N95 respirator on the patient while providing supplementary oxygen via face mask or nasal prongs after extubation could prevent hypoxemia and reduce risk of environmental viral spreading.54
-
•Using a simple antiviral filter with bubbling chest drains is recommended to reduce in-hospital spread of SARS-CoV-2.73
-
•Intrahospital transfer of extubated patients should follow local regulations.
-
•
After Extubation
Patients with suspected or diagnosed with COVID-19 infection or those who are considered to be still infectious
-
•
The breathing circuit should be changed.
-
•
Airway breathing system/anesthetic gas scavenging system and soda lime canisters should be decontaminated.
-
•
All disposable material should be discarded; reusable material should be sent for appropriate decontamination.
-
•
A waiting period of 20 minutes is necessary before disinfection with a 3%-to-5% chlorine solution.
Postoperative Analgesia
Thirty-six percent of 343 respondents to the EACTAIC survey reported using thoracic epidural analgesia, 22% used paravertebral block, 16% used local anesthetic infiltration, 11% used erector spinae block, and 11% used intravenous analgesia for thoracic surgery in their institutions before the COVID-19 pandemic. Interestingly, only 5% of the 415 respondents reported changing analgesic methods during the COVID-19 pandemic. The authors cannot recommend changes to institutional practice for perioperative analgesia and regional blockade in light of the lack of supporting evidence. However, the authors recommend following the recommendations of the Joint Statement by the American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anesthesia and Pain Therapy.74
Conclusion
The COVID-19 pandemic undoubtedly has become one of the most important challenges for the human race in recent memory. Health personnel in all likelihood will have to deal with a wide range of COVID-19 patients undergoing different surgeries.
Observing the changes that multiple waves of the COVID-19 pandemic already have caused, the authors can foresee that routine life of daily practice in the authors’ hospitals will become radically different, with all materials used for anesthesia potentially subject to shortage in time.
Although these updated recommendations have been prepared with expert opinions, unpublished results of the EACTAIC survey, and best evidence available to date, they cannot claim to be entirely evidence- based. Nonetheless, the authors hope that they can be helpful to colleagues, not only for thoracic anesthesia but also to organize general management of this challenging patient group.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.jvca.2021.07.027.
Appendix. Supplementary materials
References
- 1.Senturk M, El Tahan MR, Szegedi LL, et al. Thoracic anesthesia of patients with suspected or confirmed 2019 novel coronavirus infection: Preliminary recommendations for airway management by the European Association of Cardiothoracic Anaesthesiology Thoracic Subspecialty Committee. J Cardiothorac Vasc Anesth. 2020;34:2315–2327. doi: 10.1053/j.jvca.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cacciapaglia G, Cot C, Sannino F. Multiwave pandemic dynamics explained: How to tame the next wave of infectious diseases. Sci Rep. 2021;11:6638. doi: 10.1038/s41598-021-85875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report –70. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200330-sitrep-70-covid-19.pdf?sfvrsn=7e0fe3f8_4. Accessed June 18, 2021
- 4.World Health Organization . 2021. World Health Organization.https://covid19.who.int Available at. Accessed June 18. [Google Scholar]
- 5.Fiorelli S, Menna C, Piccioni F, et al. The cutting edge of thoracic anesthesia during the coronavirus disease 2019 (COVID-19) outbreak. J Cardiothorac Vasc Anesth. 2020;34:3203–3210. doi: 10.1053/j.jvca.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccioni F, Droghetti A, Bertani A, et al. Recommendations from the Italian intersociety consensus on Perioperative Anesthesia Care in Thoracic surgery (PACTS) part 1: Preadmission and preoperative care. Perioper Med (Lond) 2020;9:37. doi: 10.1186/s13741-020-00168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccioni F, Droghetti A, Bertani A, et al. Recommendations from the Italian intersociety consensus on Perioperative Anesthesa Care in Thoracic surgery (PACTS) part 2: Intraoperative and postoperative care. Perioper Med (Lond) 2020;9:31. doi: 10.1186/s13741-020-00159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reebye R, Finlayson H, May C, et al. Practical guidance for outpatient spasticity management during the coronavirus (COVID-19) pandemic: Canadian Spasticity COVID-19 Task Force. Can J Neurol Sci. 2020;47:589–593. doi: 10.1017/cjn.2020.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton M, Reid D, Shelley B, et al. Management of the airway and lungisolation for thoracic surgery during the COVID-19 pandemic: Recommendations for clinical practice endorsed by the Association for Cardiothoracic Anaesthesia and Critical Care and the Society for Cardiothoracic Surgery in Great Britain and Ireland. Anaesthesia. 2020;75:1509–1516. doi: 10.1111/anae.15112. [DOI] [PubMed] [Google Scholar]
- 10.Coccolini F, Perrone G, Chiarugi M, et al. Surgery in COVID-19 patients: Operational directives. World J Emerg Surg. 2020;15:25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Boghdadly K, Cook TM, Goodacre T, et al. SARS-CoV-2 infection, COVID-19 and timing of elective surgery: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. Anaesthesia. 2021;76:940–946. doi: 10.1111/anae.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren R, Zhang Y, Li Q, et al. Asymptomatic SARS-CoV-2 infections among persons entering China From April 16 to October 12, 2020. JAMA. 2021;325:489–492. doi: 10.1001/jama.2020.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect Dis. 2020;20:911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The American Society of Anesthesiology (ASA) and Anesthesia Patient Safety Foundation (APSF) joint consensus . 2021. ASA and APSF Joint Statement on Elective Surgery and Anesthesia for Patients after COVID-19 Infection.https://www.asahq.org/about-asa/newsroom/news-releases/2020/12/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection Available at. Accessed June 18. [Google Scholar]
- 16.Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskander A, Li Q, Hallet J, et al. Access to cancer surgery in a universal health care system during the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passaro A, Addeo A, Von Garnier C, et al. ESMO management and treatment adapted recommendations in the COVID-19 era: Lung cancer. ESMO Open. 2020;5(Suppl 3) doi: 10.1136/esmoopen-2020-000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shipe ME HD, Deppen SA, Kozower BD, et al. Modeling the impact of delaying the diagnosis of non-small cell lung cancer during COVID-19. Ann Thorac Surg. 2021;112:248–254. doi: 10.1016/j.athoracsur.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korompoki E, Gavriatopoulou M, Kontoyiannis DP. COVID-19 vaccines in patients with cancer—A welcome addition, but there is need for optimization [e-pub ahead of print] JAMA Oncol. 2021 May 13 doi: 10.1001/jamaoncol.2021.1218. [DOI] [PubMed] [Google Scholar]
- 21.Donatelli F, Miceli A, Glauber M, et al. Adult cardiovascular surgery and the coronavirus disease 2019 (COVID-19) pandemic: The Italian experience. Interact Cardiovasc Thorac Surg. 2020;31:755–762. doi: 10.1093/icvts/ivaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel V, Jimenez E, Cornwell L, et al. Cardiac surgery during the coronavirus disease 2019 pandemic: Perioperative considerations and triage recommendations. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarracino F, Shernan SK, Tahan ME, et al. EACTA/SCA Recommendations for the Cardiac Anesthesia Management of Patients With Suspected or Confirmed COVID-19 Infection: An Expert Consensus from the European Association of Cardiothoracic Anesthesiology and Society of Cardiovascular Anesthesiologists with Endorsement from the Chinese Society of Cardiothoracic and Vascular Anesthesiology. J Cardiothorac Vasc Anesth. 2021;35:1953–1963. doi: 10.1053/j.jvca.2021.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman DT, Lother S, George I, et al. Adult cardiac surgery and the COVID-19 pandemic: Aggressive infection mitigation strategies are necessary in the operating room and surgical recovery. J Thorac Cardiovasc Surg. 2020;160:447–451. doi: 10.1016/j.jtcvs.2020.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao JM, Ayuso SA, Deerenberg EB, et al. A systematic review of CT chest in COVID-19 diagnosis and its potential application in a surgical setting. Colorectal Dis. 2020;22:993–1001. doi: 10.1111/codi.15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullen-Fortino M, Rising KL, Duckworth J, et al. Presurgical assessment using telemedicine technology: Impact on efficiency, effectiveness, and patient experience of care. Telemed J E Health. 2019;25:137–142. doi: 10.1089/tmj.2017.0133. [DOI] [PubMed] [Google Scholar]
- 27.Chao GF, Li KY, Zhu Z, et al. Use of telehealth by surgical specialties during the COVID-19 pandemic [e-pub ahead of print] JAMA Surg. 2021 doi: 10.1001/jamasurg.2021.0979. 2021 Mar 26Accessed June 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mihalj M, Carrel T, Gregoric ID, et al. Telemedicine for preoperative assessment during a COVID-19 pandemic: Recommendations for clinical care. Best Pract Res Clin Anaesthesiol. 2020;34:345–351. doi: 10.1016/j.bpa.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He H, Zhao S, Han L, et al. Anesthetic management of patients undergoing aortic dissection repair with suspected severe acute respiratory syndrome COVID-19 infection. J Cardiothorac Vasc Anesth. 2020;34:1402–1405. doi: 10.1053/j.jvca.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quraishi SA, Berra L, Nozari A. Indoor temperature and relative humidity in hospitals: Workplace considerations during the novel coronavirus pandemic. Occup Environ Med. 2020;77:508. doi: 10.1136/oemed-2020-106653. [DOI] [PubMed] [Google Scholar]
- 31.Cassorla L. Decontamination and reuse of N95 filtering facepiece respirators: Where do we stand? Anesth Analg. 2021;132:2–14. doi: 10.1213/ANE.0000000000005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sickbert-Bennett EE, Samet JM, Clapp PW, et al. Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemic. JAMA Intern Med. 2020;180:1607–1612. doi: 10.1001/jamainternmed.2020.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuo T, Kobayashi D, Taki F, et al. Prevalence of health care worker burnout during the coronavirus disease 2019 (COVID-19) pandemic in Japan. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delaney RK, Locke A, Pershing ML, et al. Experiences of a health system's faculty, staff, and trainees’ career development, work culture, and childcare needs during the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller AE, Hafstad EV, Himmels JPW, et al. The mental health impact of the covid-19 pandemic on healthcare workers, and interventions to help them: A rapid systematic review. Psychiatry Res. 2020;293 doi: 10.1016/j.psychres.2020.113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro J, McDonald TB. Supporting clinicians during Covid-19 and beyond – Learning from past failures and envisioning new strategies. N Engl J Med. 2020;383:e142. doi: 10.1056/NEJMp2024834. [DOI] [PubMed] [Google Scholar]
- 37.Spence NZ, Lu ME, Larson AR, Ortega R. COVID-19 and occupational skin hazards for anaesthetists. Br J Anaesth. 2020;125:e476–e478. doi: 10.1016/j.bja.2020.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guy GP, Jr, Massetti GM, Sauber-Schatz E. Mask mandates, on-premises dining, and COVID-19. JAMA. 2021;325:2199–2200. doi: 10.1001/jama.2021.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.COVIDSurg Collaborative GC. SARS-CoV-2 vaccination modelling for safe surgery to save lives: Data from an international prospective cohort study [e-pub ahead of print]. Br J Surg 2021 Mar 24. doi: 10.1093/bjs/znab101.40 [DOI] [PMC free article] [PubMed]
- 40.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: A timeline of immunological insights. Nat Rev Immunol. 2021;21:245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson T, Deibert D, Wyatt G, et al. Classification of aerosol-generating procedures: A rapid systematic review. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Boghdadly K, Wong DJN, Owen R, et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: A prospective international multicentre cohort study. Anaesthesia. 2020;75:1437–1447. doi: 10.1111/anae.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamb CR, Desai NR, Angel L, et al. Use of tracheostomy during the COVID-19 pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology Program Directors Expert Panel Report. Chest. 2020;158:1499–1514. doi: 10.1016/j.chest.2020.05.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasa P, Azoulay E, Khanna AK, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021;25:106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiesa-Estomba CM, Lechien JR, Calvo-Henríquez C, et al. Systematic review of international guidelines for tracheostomy in COVID-19 patients. Oral Oncol. 2020;108 doi: 10.1016/j.oraloncology.2020.104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorbello M, Rosenblatt W, Hofmeyr R, et al. Aerosol boxes and barrier enclosures for airway management in COVID-19 patients: A scoping review and narrative synthesis. Br J Anaesth. 2020;125:880–894. doi: 10.1016/j.bja.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong DJN, El-Boghdadly K, Owen R, et al. Emergency airway management in patients with COVID-19: A prospective international multicenter cohort study [e-pub ahead of print] Anesthesiology. 2021 doi: 10.1097/ALN.0000000000003791. 2021 Apr 13Accessed June 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foley LJ, Urdaneta F, Berkow L, et al. Difficult airway management in adult COVID-19 patients: Statement by the Society of Airway Management [e-pub ahead of print] Anesth Analg. 2021 doi: 10.1213/ANE.0000000000005554. 2021 Mar 12Accessed June 18. [DOI] [PubMed] [Google Scholar]
- 54.Binks AC, Parkinson SM, Sabbouh V. Oxygen: Under or over a surgical facemask for COVID-19 patients? Anaesthesia. 2020;75:1691–1692. doi: 10.1111/anae.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szegedi LL, Bardoczky GI, Engelman EE, et al. Airway pressure changes during one-lung ventilation. Anesth Analg. 1997;84:1034–1037. doi: 10.1097/00000539-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 56.Mourisse J, Liesveld J, Verhagen A, et al. Efficiency, efficacy, and safety of EZ-blocker compared with left-sided double-lumen tube for one-lung ventilation. Anesthesiology. 2013;118:550–561. doi: 10.1097/ALN.0b013e3182834f2d. [DOI] [PubMed] [Google Scholar]
- 57.Cook TM, El-Boghdadly K, McGuire B, et al. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorbello M, El-Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: Recommendations from clinical practice. Anaesthesia. 2020;75:724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 59.Jerath A, Ferguson ND, Cuthbertson B. Inhalational volatile-based sedation for COVID-19 pneumonia and ARDS. Intensive Care Med. 2020;46:1563–1566. doi: 10.1007/s00134-020-06154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nieuwenhuijs-Moeke GJ, Jainandunsing JS, Struys M. Sevoflurane, a sigh of relief in COVID-19? Br J Anaesth. 2020;125:118–121. doi: 10.1016/j.bja.2020.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piccioni F, Di Gregorio G, Rosboch GL, et al. Sometimes less is worse: A recommendation against nonintubated video-assisted thoracoscopy during the COVID-19 pandemic. J Cardiothorac Vasc Anesth. 2020;34:2859–2861. doi: 10.1053/j.jvca.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei H, Jiang B, Behringer EC, et al. Controversies in airway management of COVID-19 patients: Updated information and international expert consensus recommendations. Br J Anaesth. 2021;126:361–366. doi: 10.1016/j.bja.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Botta M, Tsonas AM, Pillay J, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir Med. 2021;9:139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conway H, Lau G, Zochios V. Personalizing invasive mechanical ventilation strategies in coronavirus disease 2019 (COVID-19)-associated lung injury: The utility of lung ultrasound. J Cardiothorac Vasc Anesth. 2020;34:2571–2574. doi: 10.1053/j.jvca.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alhazzani W, Evans L, Alshamsi F, et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First update. Crit Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 67.Suwanwongse K, Shabarek N. Pneumomediastinum in mechanically ventilated coronavirus disease 2019 patients. J Cardiothorac Vasc Anesth. 2021;35:686–688. doi: 10.1053/j.jvca.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blanc T, Pinar U, Anract J, et al. Impact of the COVID-19 pandemic on oncological and functional robotic-assisted surgical procedures. J Robot Surg. 2021;28:1–8. doi: 10.1007/s11701-021-01201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vigneswaran Y, Prachand VN, Posner MC, et al. What is the appropriate use of laparoscopy over open procedures in the current COVID-19 climate? J Gastrointest Surg. 2020;24:1686–1691. doi: 10.1007/s11605-020-04592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leow L, Ramanathan K, Kofidis T, et al. Organization of thoracic surgical services during the COVID pandemic. Surgeon. 2021;19:e1–e8. doi: 10.1016/j.surge.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patterson TJ, Currie PJ, Beck J, et al. A systematic review of viral transmission risk to healthcare staff comparing laparoscopic and open surgery. Surgeon. 2020;18:e72–e77. doi: 10.1016/j.surge.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D'Silva DF, McCulloch TJ, Lim JS, et al. Extubation of patients with COVID-19. Br J Anaesth. 2020;125:e192–e195. doi: 10.1016/j.bja.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duffy C, Kidd A, Francis S, et al. Chest drain aerosol generation in COVID-19 and emission reduction using a simple anti-viral filter. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uppal V, Sondekoppam RV, Landau R, et al. Neuraxial anaesthesia and peripheral nerve blocks during the COVID-19 pandemic: A literature review and practice recommendations. Anaesthesia. 2020;75:1350–1363. doi: 10.1111/anae.15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.