Abstract
As an important indicator of sexual maturity of hens, age at first egg (AFE) is significantly associated with reproduction performance. In this study, 400 hens were divided into 6 groups based on AFE to analyze the difference of reproduction performance, reproduction hormone levels and the characterization of the hypothalamo–pituitary–gonadal axis in chickens. The results showed that the egg production of the hens in the late-maturing groups was significantly lower than that of the ones in other groups and the precocious hens had a lower egg production rate. The hens in late-maturing group had a lower fertility rate, LH levels and shorter duration of peak of egg production (PEP), the precocious hens had lower PRL levels. In addition, the characterization of the hypothalamo–pituitary–gonadal axis showed that the individuals with normal AFE had higher GNRH, GNRHR, ESR1, KITLG, and CYP11A1 expression levels than late-maturing and precocious individuals, which indicated that the chickens with normal AFE advantages on reproduction regulation system.
Key words: age at first egg, hens, reproductive traits, sexual maturity, mRNA expression
INTRODUCTION
The normal reproduction ability is one of the important characteristics of sexual maturity. In hens, beginning to lay eggs is a signal of sexual maturity (Jambui et al., 2017), therefore, the age at first egg (AFE) is an important indicator of reproduction traits of hens. In animals, the puberty plays a key role in regulation of sexual maturity and reproduction capacity (Palmert and Boepple, 2001). As the transient period for animals to reach sexual maturity, puberty is regulated by various genes and pathways (de Vries et al., 2014). During this period, the growth rate and metabolic rate increase and the second sexual characteristics appear, gonads develop rapidly and produce mature gametes, animals start to have fertility (Ei and L., 2016; Vijayakumar et al., 2018). The hypothalamus–pituitary–gonad (HPG) axis participates in this process of puberty to sexual maturity and multiple reproduction hormones with different effect are involved in this mechanism (Kuohung and Kaiser, 2006). The upregulation of gonadotropin-releasing hormone (GnRH) is considered as the main cause of HPG axis activation in prepuberty, the secretion of the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are increased by GnRH, which induce the trigger of puberty (Abreu et al., 2013), GNRHR is the receptor of GnRH. However, as the inhibitor of GnRH, RFamide-related peptides (RFRP) could suppress the expression of GnRH and KISS-1 gene (Tan et al., 2020). Moreover, ovarian function is regulated by KITLG, CYP11A1, etc. genes (Shih et al., 2011; Deepak et al., 2015).
Precocious puberty, which is pathological status of sexual maturity, caused by early activation of pulsatile GnRH secretion (Carel et al., 2004). Precocious puberty has a negative effect on reproduction function of animals. In humans, diet, genetics, and environment, etc. could all be the triggers for precocious puberty. Precocious puberty can affect the development of bones and lead to shorter height in adulthood (Gohil et al., 2020), which also increase the risk of breast cancer and reproduction disorders in women (Klein et al., 2001). Premature estrus will reduce the conception rate of female cows and reduce the milk production. In the milk industry, hormone treatment is usually used to improve estrous detection rates, synchrony of estrus and the pregnancy rate of artificial insemination (Dejarnette et al., 2001; Kim et al., 2003). In pigs, age at first estrus is significantly related to reproduction longevity, which also has effect on the body weight and reproduction traits of sows and early puberty individuals have better reproduction performance than late ones (Patterson et al., 2010; Tart et al., 2013).
Delayed puberty also has negative effect on reproduction system. In humans, environmental contaminants could suppress the function of HPG axis to delay the pubertal development in girls (Selevan et al., 2003). Delayed puberty is induced by the primary gonadal failure and hypergonadotropic hypogonadism in females (Layman et al., 1997). Previous studies proved that the rats with delayed puberty might have reproduction disorders (Zhu and Chan, 2015). However, whether the delayed puberty cause the poor reproduction in hens is unclear.
In chickens, AFE is regulated by duration of light, diet and genetics factors (Lewis et al., 2008). However, there is little study about the effect of early-maturity and late-maturity on reproduction traits in chickens. To study the effect of AFE on chickens, the present study investigated the difference of reproduction performance, reproduction hormone levels, and the expression levels of related genes based on different AFE.
MATERIALS AND METHODS
This study was conducted in accordance with Chinese guidelines for animal welfare and was approved by the animal welfare committee of the Animal Science College, Zhejiang University, the approval number is ZJU20190149.
Grouping and Egg Production Rate Calculation
A total of 400 Zhenning yellow chickens (340 d) used in this study were obtained from Poultry Breeding Center of the Ningbo Zhenning Animal Husbandry Co., Ltd in Zhejiang province. Every individual was from the same hatching batch and was fed under the same condition. All birds had free access to feed and water and were kept in single cages to facilitate the statistics of the number of eggs with a 8- to 16-h dark-light cycle, and the environment maintained 60% humidity and 21°C temperature, feed is supplied in accordance with NYT33-2004 standards. All hens were divided into 6 groups based on age at first egg (AFE) which including group A (134–138 d), B (139–143 d), C (144–148 d), D (149–153 d), E (154–158 d), and F (>159 d) (Table 2). The egg production rate (EPR) of every chicken was calculated according to records using the model: EPR = EP/ (Age-AFE), the EPR higher than 70% was considered as the peak of egg production (PEP). The EPR curves of each group were drawn respectively. The age at that half of the total individuals started laying eggs was considered as age at first egg of population (AFEP).
Table 2.
Grouping based on age at first egg and population information.
| Group | Period | Mean | Mode | Quantity | 1AFEP |
|---|---|---|---|---|---|
| A | 134–138 | 135.43 | 134 | 42 | / |
| B | 139–143 | 141.39 | 142 | 36 | / |
| C | 144–148 | 147.52 | 148 | 61 | / |
| D | 149–153 | 150.59 | 149 | 126 | / |
| E | 154–158 | 155.68 | 152 | 68 | / |
| F | >159 | 162.09 | 159 | 67 | / |
| Total | 134–167 | 150.47 | 148 | 400 | 151 |
AFEP = age at first egg of population.
Evaluation of Reproduction Traits
Reproduction traits of all chickens were evaluated including fertilization rate (FR), hatching rate of fertilized eggs (FEHR), hatching rate of hatching eggs (HEHR), egg production at 340 d of age (EP340). In addition, 10 chickens were randomly chosen from each group based on AFE respectively, a total of 60 chickens were slaughtered to collect tissues which including hypothalamus, pituitary, ovaries, and granulosa layer for investigating the expression levels of different genes mRNA, and the quantity of hierarchical follicles (HF) and small yellow follicles (SYF) were counted after ovaries were extracted. Furthermore, the blood samples for hormone levels detection were collected from jugular vein when 60 hens were slaughtered.
Determination of Reproduction Hormone Levels
Serum was isolated from blood samples by centrifugation at 3,200 × g for 10 min. Levels of follicle-stimulating hormone (FSH), estrogen (E2), luteinizing hormone (LH), and prolactin (PRL) were analyzed using the ELISA kits (Zeyu Biological Technology Co., Ltd., Jiangsu, China) according to the recommendations of the manufacturer.
RNA Extraction
Total RNA was isolated from frozen tissue samples using TRNzol-A+ Reagent (TIANGEN, Beijing, China) according to the instructions. The RNA purity and quality were evaluated by spectrophotometry and agarose gel electrophoresis. The qualified RNA was stored at −20°C.
Reverse Transcription PCR
RNA reverse transcription was performed using a PrimeScript RT reagent kit with gDNA Eraser (TAKARA, Beijing). Following the manufacturer's instructions, each reaction mixture A was assembled to a total of 10 L, which contained 2 L of 5 × gDNA eraser buffer, 1 L of gDNA eraser, 1 L of total RNA, and 6 L of RNase free water. This reaction was preheated to 42°C for two minutes. Each reaction mixture B was assembled to a total of 10 L, which contained 1 L of PrimeScript RT Enzyme Mix 1, 1 L of RT Primer Mix, 4 L of PrimeScript Buffer 2, and 4 L of RNase free water. The 20-L total reaction mixture was incubated in a PCR amplification instrument (Eppendorf AG 22,331, Germany) in a PCR tube for 15 min at 37°C and 5 s at 85°C and was subsequently held at 4°C.
Quantitative Real-Time PCR
Real-time PCR was performed on a StepOnePlus Real-time PCR System (Applied Biosystems, Foster City, CA) using TB SYBR Premix Ex Taq II (TAKARA, Beijing). The 20-L reaction system included 10 L of SYBR Premix Ex Taq II, 0.8 L of PCR Forward Primer and 0.8 L of PCR Reverse Primer, 0.4 L of ROX Reference Dye (50 ×), 2 L of cDNA, and 6 L of dH2O. The entire process contained 2 stages: 30 s at 95°C for predenaturation, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. All primers of 10 genes were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) from the National Center of Biotechnology Information (NCBI) based on chicken genes sequences (Table 1).
Table 1.
Primer of genes for QPCR.
| Primers | NCBI NO | Sequences (5′-3′) | Tm (°C) | Product size(bp) |
|---|---|---|---|---|
| GnRH-F | NC_052553.1 | CGGGAAGAGTTGGAGCGATT | 60.11 | 142 |
| GnRH-R | CGGGAAGAGTTGGAGCGATT | 60.03 | ||
| RFRP-F | NC_052533.1 | GGCCGAGTGCTTATTTGCCT | 59.75 | 177 |
| RFRP-R | CTTCCCGAATCTCTGTGGCA | 59.38 | ||
| GnRHR-F | NC_052541.1 | GGTACTGGTTCTGTCCACGA | 59.04 | 108 |
| GnRHR-R | CATAGGTGATGGGGTCCAGG | 59.23 | ||
| RFRPR-F | NC_052537.1 | CAATGGCAGCTGGGCTAATG | 59.61 | 151 |
| RFRPR-R | CAGGATGTTGCCGATCATGC | 59.69 | ||
| FSHR-F | NC_052534.1 | GCCTCTGTGAAGGCAGGATA | 59.17 | 135 |
| FSHR-R | CTGTGAAAGCTCCCTTCGGA | 59.68 | ||
| LHR-F | NC_052534.1 | GGGCTTTCCCAAGCCTACAT | 60.03 | 133 |
| LHR-R | TGGTGTCTTTATTGGCGGCT | 59.96 | ||
| PRLR-F | NC_052572.1 | AGGAGTTACAGCCTGGGATGA | 60.27 | 124 |
| PRLR-R | TCACATCAAGGGCTCACGAAA | 59.93 | ||
| ESR1-F | NC_052534.1 | GCGACATGTACGTGGAAAGC | 59.91 | 145 |
| ESR1-R | AGGCTGCTTGACCCAAAAGA | 59.81 | ||
| KITLG-F | NC_052532.1 | AGCGCTGCCATTCCTTATGA | 59.82 | 121 |
| KITLG-R | ATCTGTCACTGGATTCCCGC | 59.82 | ||
| CYP11A1-F | NC_052541.1 | CGTGGACACGACTTCCATGA | 59.96 | 175 |
| CYP11A1-R | GAGAGTCTCCTTGATGGCGG | 60.04 | ||
| β-Actin-F | NC_052545.1 | CATTGTCCACCGCAAATGCT | 59.76 | 108 |
| β-Actin-R | AGCCATGCCAATCTCGTCTT | 59.75 |
Statistical Analysis
All statistical analysis for factor measurements of the difference between groups were conducted using ANOVA analysis available with the SPSS 20.0 (SPSS, Chicago, IL). The data were presented as means ± SEM, and the P < 0.05 was considered statistically significant.
Results
Grouping and Population Information
As shown in Table 2, 400 hens were divided into 6 groups based on AFE. Age at first egg of population was 151 d, the mode and the mean of total individuals was 148 d and150.47 d, so hens in group C and D were considered as the individuals with normal AFE, group A were considered as the precocious individuals, hens in group F were considered as the late-maturing individuals.
The Effect of AFE on Reproduction Performance
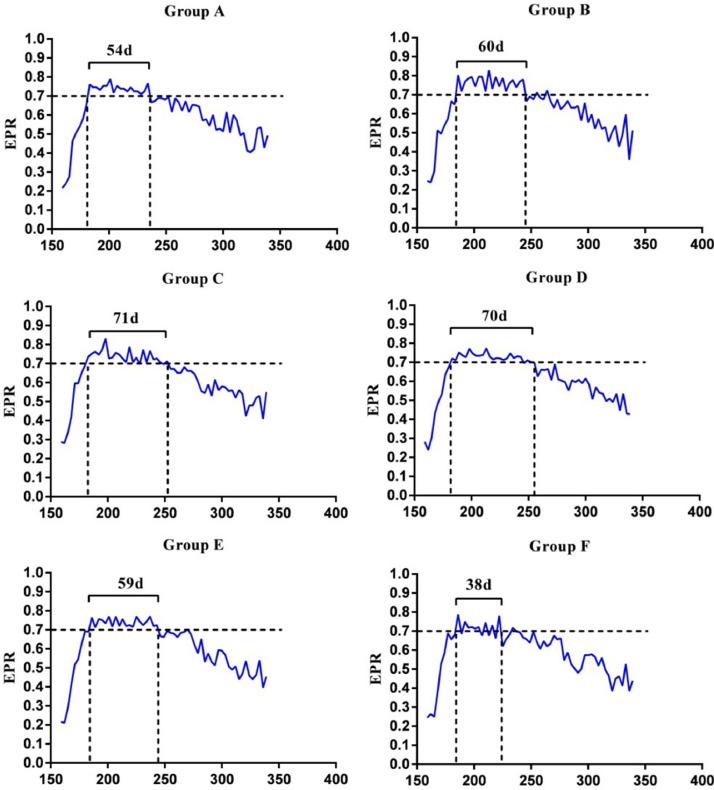
The EPR curves of each group were shown in Figure 1, the beginning time of PEP of all groups was around 183 d. Hens in group C had the longest duration of PEP and group F had the shortest PEP. The result of reproduction performance comparison among groups was showed in Table 3. The EP340 of group A, B, C, and D was significantly higher than that of group E and F (P < 0.01), group B had higher EP340 than group A (P < 0.05) and group E had higher EP340 than group F (P < 0.05). The EPR340 of group A was lower than that of other 5 groups (P < 0.05). The FR of group F was lower than that of group A, C, D, and E (P < 0.05).
Figure 1.
Egg production rate curve of 6 groups during 160–340 d. The two vertical dotted lines in each figure represent the start and end time of PEP. The curve above horizontal dotted line represent the EPR was higher than 70%. The number of days above the curve represent the duration of PEP. Abbreviations: EPR, egg production rate; PEP, peak of egg production.
Table 3.
Effect of AFE on reproduction traits.
| Traits (Mean ± SE) |
|||||
|---|---|---|---|---|---|
| Group | 1EP340(n) | 2EPR340 | 3FR | 4FEHR | 5HEHR |
| A | 116.4 ± 1.475Ab | 0.578 ± 0.007b | 0.984 ± 0.011a | 0.949 ± 0.019 | 0.947 ± 0.021 |
| B | 120.4 ± 2.176Aa | 0.606 ± 0.019a | 0.973 ± 0.013ab | 0.966 ± 0.015 | 0.937 ± 0.021 |
| C | 119.6 ± 0.873A | 0.618 ± 0.004a | 0.985 ± 0.007a | 0.952 ± 0.019 | 0.938 ± 0.020 |
| D | 117.3 ± 0.791A | 0.621 ± 0.004a | 0.983 ± 0.006a | 0.940 ± 0.011 | 0.929 ± 0.012 |
| E | 113.4 ± 1.206B,a | 0.616 ± 0.006a | 0.987 ± 0.007a | 0.933 ± 0.018 | 0.921 ± 0.018 |
| F | 108.7 ± 1.409B,b | 0.605 ± 0.008a | 0.946 ± 0.016b | 0.928 ± 0.020 | 0.897 ± 0.020 |
a,b,A,BMeans of different groups at the same locus with the different uppercase letters were extremely significant (P < 0.01), and the different lowercase letters was significant (P < 0.05); The difference between the same letters was not significant (P > 0.05).
EW300 = egg weight at 300 d.
EP300 = egg production at 300 d.
FR = fertilization rate.
FEHR = hatching rate of fertilized eggs.
HEHR = hatching rate of hatching eggs.
Effects of AFE on Follicles Quantity and Hormone Levels
As shown in Table 4, group D had higher HF than the other five groups (P < 0.05).The LH level of group F was lower than that of group B, C, and D (P < 0.05). The PRL level of group E and F was higher than that of group A (P < 0.05).
Table 4.
Effects of AFE on hormone levels and follicles quantity.
| Traits (Mean ± SE) |
||||||
|---|---|---|---|---|---|---|
| Group | 1HF (n) | 2SYF (n) | 3E2 (pmol/L) | 4FSH (U/L) | 5LH (ng/L) | 6PRL (ng/L) |
| A | 4.00 ± 0.333b | 12.00 ± 1.838 | 95.12 ± 5.650c | 1.92 ± 0.086 | 44.53 ± 2.022a,b | 44.11 ± 3.353b |
| B | 4.00 ± 0.433b | 10.90 ± 1.418 | 96.19 ± 5.468 | 1.71 ± 0.106 | 49.88 ± 2.623a | 49.04 ± 2.730a,b |
| C | 4.60 ± 0.221b | 11.00 ± 0.683 | 93.39 ± 3.152 | 1.70 ± 0.118 | 49.71 ± 2.312a | 46.53 ± 1.711a,b |
| D | 5.20 ± 0.327a | 10.90 ± 1.069 | 93.08 ± 5.202 | 1.90 ± 0.099 | 50.22 ± 2.146a | 50.34 ± 2.434a,b |
| E | 4.60 ± 0.379b | 12.80 ± 1.162 | 83.55 ± 4.824 | 1.76 ± 0.062 | 46.33 ± 2.694a,b | 53.33 ± 2.276a |
| F | 4.20 ± 0.657b | 11.00 ± 1.807 | 85.83 ± 5.626 | 1.79 ± 0.097 | 41.23 ± 2.825b | 53.79 ± 3.013a |
a,b,cAt the same locus, the difference between groups with different lowercase letters was significant (P < 0.05), the difference between the same letters was not significant (P > 0.05).
HF = hierarchical follicles.
SYF = small yellow follicles.
E2 = estrogen.
FSH = follicle-stimulating hormone.
LH = luteinizing hormone.
PRL = prolactin.
Expression Levels of RFRP and GnRH mRNA in Hypothalamus
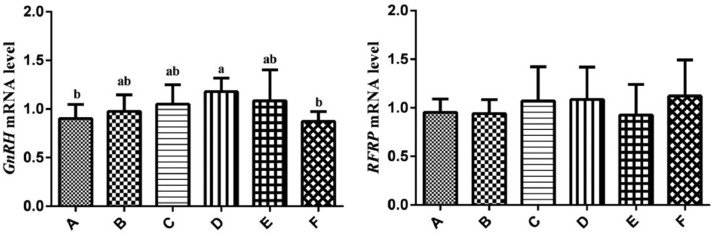
The mRNA expression levels of RFRP and GnRH in hypothalamus were showed in Figure 2. The expression levels of GnRH in group D were higher than that in group A and F (P < 0.05). There was no difference in the expression levels of RFRP mRNA among groups (P > 0.05).
Figure 2.
Relative expression levels of the GnRH and RFRP genes in hypothalamus of hens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SEM. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Bars without superscript was not significant (P > 0.05). GnRH, gonadotropin-releasing hormone; RFRP, RFamide-related peptides.
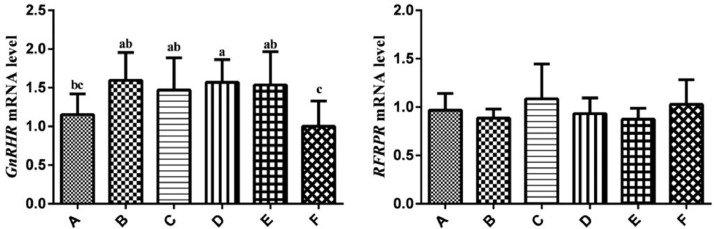
Expression Levels of GnRHR and RFRPR Gene mRNA in Pituitary
The results of GnRHR and RFRPR mRNA expression levels analysis in pituitary were showed in Figure 3. The mRNA expression levels of GnRHR in group F were lower than that in group B, C, D and E (P < 0.05), moreover, GnRHR mRNA expression levels in group A were lower than that in group D (P < 0.05). There was little difference in RFRPR expression levels among groups (P > 0.05).
Figure 3.
Relative expression levels of the GnRHR and RFRPR genes in pituitary of hens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SEM. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Bars without superscript was not significant (P > 0.05).
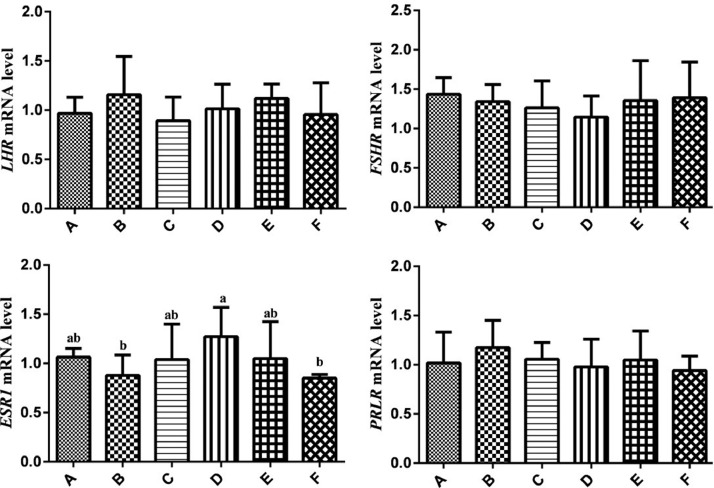
Expression Levels of LHR, FSHR, ESR1, and PRLR Gene mRNA in Ovary
The results of LHR, FSHR, ESR1, and PRLR mRNA expression levels analysis in ovary were showed in Figure 4. The mRNA expression levels of ESR1 in group D were higher than that in group B and F (P < 0.05). There was little difference in LHR, FSHR, PRLR expression levels among groups (P > 0.05).
Figure 4.
Relative expression levels of the LHR, FSHR, ESR1, and PRLR genes in ovary of hens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SEM. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Bars without superscript was not significant (P > 0.05).
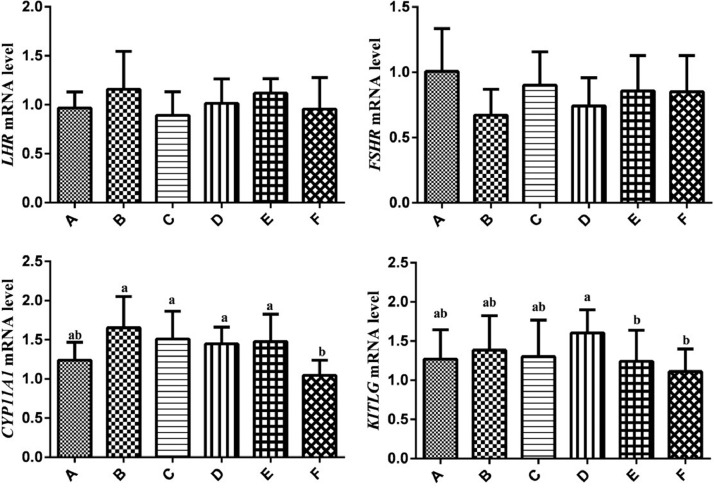
Expression Levels of FSHR, LHR, CYP11A1, and KITLG Gene mRNA in Granulosa Layer
The Expression levels of LHR, FSHR, CYP11A1, and KITLG gene mRNA were detected in granulosa layer cells. As shown in Figure 5, CYP11A1 gene mRNA expression levels in group F were lower than that in group B, C, D, and E (P < 0.05), the expression levels of KITLG in group D were higher than that in group E and F (P < 0.05), There was little difference in FSHR and LHR expression levels among groups (P > 0.05).
Figure 5.
Relative expression levels of the FSHR, LHR, KITLG and CYP11A1 genes in granulosa cells of chickens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SEM. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Bars without superscript was not significant (P > 0.05).
DISCUSSION
In the poultry industry, AFE is usually regarded as one of the important reproduction indicator in chicken (Haiping et al., 2011). Although there are many studies on the regulatory factors of AFE, study about the specific impact of AFE on reproduction performance of chickens is few. The present study investigated the impact of AFE on reproduction performance through the comparative analysis of numerous traits and indicators.
According to the EPR curves of each group, we found something interesting that the PEP beginning time of all groups was almost the same at about 183 d, the duration of PEP in group F was shorter and it indicated that the ovulation ability of the late-maturing hens was poor, which also implied that the ovarian function of late-maturing individuals would aging earlier and it was not good for poultry industry. It has been proven that the delayed puberty has a negative effect on female reproduction system, especially on the ovarian function (Han et al., 2016), moreover, the late-maturing hens had shorter laying cycle, so that the EP340 of group F was the worst. However, it seemed like that the EPR showed an opposite situation, the precocious hens had lower EPR instead of late-maturing ones, the main reason for this issue was that precocious hens had a poor laying performance at early laying stage (134–150 d) and it was proven that precocious rats probably enter estrus but not ovulate at the early stage (Risma et al., 1997). We could speculate that the EPR of the precocious individuals was low due to the abnormal ovulation function at early stage and such negative effects will affect the entire egg production cycle (Fuqua, 2013). The FR was also affected by AFE. Sperm quality is often considered as the main factor affecting FR (Saacke et al., 2000).In the present study, the FR of the late-maturing individuals was significantly lower than that of earlier-maturing individuals and the difference of HEHR was also close to statistical significance (P = 0.0515). We may speculate that the hatching rate of the individuals with delayed puberty was lower and the specific mechanism of the impact needs further study.
The number of hierarchical follicles is an indicator of ovulation ability, which has an important impact on egg production, and it also indicated that hens with normal AFE had advantages in follicles selection (Waddington and Walker, 1988). In terms of reproduction hormone levels, LH plays a vital role in follicle development and ovulation and it is also a crucial reproduction hormone that regulates the HPG axis and affects the development of animal reproduction systems (Demeestere et al., 2005; Cao et al., 2018). Previous studies proved that PRL has a negative effect on the function of the HPG axis. It has been proved that high concentrations of PRL levels could cause a reduction in the egg production of hens, reduce the synthesis of steroids even inhibit ovulation (Reddy et al., 2002). In this study, group F had lower LH level and higher PRL level, which was consistent with the poor laying performance.
Finally, 10 related genes expression levels in four tissues were analyzed separately. GNRH and RFRP are synthesized and secreted by the hypothalamus, promoting or inhibiting the production of gonadotropins in the pituitary and participating in the regulation of the HPG axis and affect the fertility (Lopez-Gatius and Garcia-Ispierto, 2020). GNRH plays a key role in triggering puberty and promotes the synthesis and secretion of LH and FSH (Herbison, 2016). In contrast to GNRH, RFRP could suppress the function of the HPG axis, inhibit gonad development, and suppresses the trigger of puberty by suppressing the KISS-1 gene (Tsutsui et al., 2012). GNRHR is a key receptor for GNRH normal physiological functions (Limonta et al., 2012). In the present study, the GNRH and GNRHR mRNA expression of hens with normal AFE was higher, which may also indicated that the hens with normal AFE (149–153 d) had better reproduction performance. In poultry, granulosa cells had an important secretory function in the ovary and participates the process of follicle selection, development, and apoptosis (Kunitomi et al., 2019; Liang et al., 2020). In granulosa cells, there was no significant difference in the expression levels of FSHR and LHR. KITLG gene is a key regulatory gene for the development of granulosa cells. The expression level of KITLG gene in granulosa cells of apoptosis follicles is significantly downregulated relative to normal granulosa cells (Lima et al., 2012). The lower KITLG gene expression levels implied the follicles development is poor. CYP11A1 is a crucial gene that mediates the conversion of cholesterol to pregnenolone and it is further converted to progesterone (O'Hara et al., 2014). CYP11A1 expression level of hens in group F meant that the late-maturing individuals had weaker steroid synthesis ability and reproduction performance. The characterization of the hypothalamo–pituitary–gonadal axis was largely consistent with reproductive performance.
CONCLUSIONS
This study explored the effects of sexual maturity time on the reproductive performance of hens from three levels which include traits, blood biochemical indicators, and related gene expression. It revealed the specific negative effects of precocious puberty and late sexual maturity on the reproductive performance of hens, it also provided a theoretical basis for the necessity of controlling AFE and some reproductive diseases.
Acknowledgments
ACKNOWLEDGMENTS
The research was supported by the Major Science and Technology Project of Zhejiang province: New Variety Breeding Livestock and Poultry (NO. 2016C02054-15).
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Abreu A.P., Dauber A., Macedo D.B., Noel S.D., Brito V.N., Gill J.C., Cukier P., Thompson I.R., Navarro V.M., Gagliardi P.C., Rodrigues T., Kochi C., Longui C.A., Beckers D., de Zegher F., Montenegro L.R., Mendonca B.B., Carroll R.S., Hirschhorn J.N., Latronico A.C., Kaiser U.B. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 2013;368:2467–2475. doi: 10.1056/NEJMoa1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Ding Y., Kong X., Feng G., Xiang W., Chen L., Yang F., Zhang K., Chu M., Wang P., Zhang B. Reproductive role of miRNA in the hypothalamic-pituitary axis. Mol. Cell Neurosci. 2018;88:130–137. doi: 10.1016/j.mcn.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Carel J.C., Lahlou N., Roger M., Chaussain J.L. Precocious puberty and statural growth. Hum. Reprod. Update. 2004;10:135–147. doi: 10.1093/humupd/dmh012. [DOI] [PubMed] [Google Scholar]
- de Vries L., Gat-Yablonski G., Dror N., Singer A., Phillip M. A novel MKRN3 missense mutation causing familial precocious puberty. Hum. Reprod. 2014;29:2838–2843. doi: 10.1093/humrep/deu256. [DOI] [PubMed] [Google Scholar]
- Deepak P., Leena R., Neeta S., Sher A., Permyakov E.A. Cross talk between KGF and KITLG proteins implicated with ovarian folliculogenesis in buffalo bubalus bubalis. Plos One. 2015;10 doi: 10.1371/journal.pone.0127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejarnette J.M., Salverson R.R., Marshall C.E. Incidence of premature estrus in lactating dairy cows and conception rates to standing estrus or fixed-time inseminations after synchronization using GnRH and PGF2α. Anim. Reprod. Sci. 2001;67:27–35. doi: 10.1016/s0378-4320(01)00107-5. [DOI] [PubMed] [Google Scholar]
- Demeestere I., Centner J., Gervy C., Englert Y., Delbaere A. Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents. Reproduction. 2005;130:147–156. doi: 10.1530/rep.1.00648. [DOI] [PubMed] [Google Scholar]
- Ei T., L. F.D. Neurobiological mechanisms of the onset of puberty in Primates*. Endocrine Rev. 2016;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Fuqua J.S. Treatment and outcomes of precocious puberty: an update. J. Clin. Endocrinol. Metab. 2013;98:2198–2207. doi: 10.1210/jc.2013-1024. [DOI] [PubMed] [Google Scholar]
- Gohil, A., E. A. J. E. Eugster, and M. C. o. N. America. Delayed and precocious puberty: genetic underpinnings and treatments.2020. 49:741-757. [DOI] [PMC free article] [PubMed]
- Haiping Xu, Hua Zeng, Chenglong Luo, Dexiang Zhang, Qian, Wang Genetic effects of polymorphisms in candidate genes and the QTL region on chicken age at first egg. BMC Genet. 2011;12:33. doi: 10.1186/1471-2156-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W., Zhu Y., Su Y., Li G., Liu H. High-throughput sequencing reveals circulating miRNAs as potential biomarkers for measuring puberty onset in chicken (Gallus gallus) PLoS One. 2016;11 doi: 10.1371/journal.pone.0154958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison A.E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016;12:452–466. doi: 10.1038/nrendo.2016.70. [DOI] [PubMed] [Google Scholar]
- Jambui M., Honaker C.F., Siegel P.B. Correlated responses to long-term divergent selection for 8-week body weight in female White Plymouth Rock chickens: sexual maturity. Poult. Sci. 2017;96:3844–3851. doi: 10.3382/ps/pex224. [DOI] [PubMed] [Google Scholar]
- Kim I.-H., Suh G.-H., Son D.-S. A progesterone-based timed AI protocol more effectively prevents premature estrus and incomplete luteal regression than an Ovsynch protocol in lactating Holstein cows. Theriogenology. 2003;60:809–817. doi: 10.1016/s0093-691x(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Klein K.O., Barnes K.M., Jones J.V., Feuillan P.P., Cutler G.B., Metabolism Increased final height in precocious puberty after long-term treatment with LHRH agonists: the National Institutes of Health Experience. J. Clin. Endocrinol. 2001;86:4711–4716. doi: 10.1210/jcem.86.10.7915. [DOI] [PubMed] [Google Scholar]
- Kunitomi C., Harada M., Takahashi N., Azhary J.M.K., Kusamoto A., Nose E., Oi N., Takeuchi A., Wada-Hiraike O., Hirata T., Hirota Y., Koga K., Fujii T., Osuga Y. Activation of endoplasmic reticulum stress mediates oxidative stress–induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol. Hum. Reprod. 2019;26:40–52. doi: 10.1093/molehr/gaz066. [DOI] [PubMed] [Google Scholar]
- Kuohung W., Kaiser U.B. GPR54 and KiSS-1: role in the regulation of puberty and reproduction. Rev. Endocr. Metab. Disord. 2006;7:257–263. doi: 10.1007/s11154-006-9020-2. [DOI] [PubMed] [Google Scholar]
- Layman L.C., Lee E.J., Peak D.B., Namnoum A.B., Vu K.V., van Lingen B.L., Gray M.R., McDonough P.G., Reindollar R.H., Jameson J.L. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N. Engl. J. Med. 1997;337:607–611. doi: 10.1056/NEJM199708283370905. [DOI] [PubMed] [Google Scholar]
- Lewis P.D., Tyler N.C., Gous R.M., Dunn I.C., Sharp P.J. Photoperiodic response curves for plasma LH concentrations and age at first egg in female broiler breeders. Anim. Reprod. Sci. 2008;109:274–286. doi: 10.1016/j.anireprosci.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Liang A., Plewes M.R., Hua G., Hou X., Blum H.R., Przygrodzka E., George J.W., Clark K.L., Bousfield G.R., Butnev V.Y., May J.V., Davis J.S. Bioactivity of recombinant hFSH glycosylation variants in primary cultures of porcine granulosa cells. Mol. Cell Endocrinol. 2020 doi: 10.1016/j.mce.2020.110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima I.M., Celestino J.J., Faustino L.R., Magalhaes-Padilha D.M., Rossetto R., Brito I.R., Donato M.A., Lopes C.A., Campello C.C., Peixoto C.A., Figueiredo J.R., Rodrigues A.P. Dynamic medium containing kit ligand and follicle-stimulating hormone promotes follicular survival, activation, and growth during long-term in vitro culture of caprine preantral follicles. Cells Tissues Organs. 2012;195:260–271. doi: 10.1159/000325150. [DOI] [PubMed] [Google Scholar]
- Limonta P., Montagnani Marelli M., Mai S., Motta M., Martini L., Moretti R.M. GnRH receptors in cancer: from cell biology to novel targeted therapeutic strategies. Endocr. Rev. 2012;33:784–811. doi: 10.1210/er.2012-1014. [DOI] [PubMed] [Google Scholar]
- Lopez-Gatius F., Garcia-Ispierto I. Treatment with an elevated dose of the GnRH analogue dephereline in the early luteal phase improves pregnancy rates in repeat-breeder dairy cows. Theriogenology. 2020;155:12–16. doi: 10.1016/j.theriogenology.2020.06.011. [DOI] [PubMed] [Google Scholar]
- O'Hara L., York J.P., Zhang P., Smith L.B. Targeting of GFP-Cre to the mouse Cyp11a1 locus both drives cre recombinase expression in steroidogenic cells and permits generation of Cyp11a1 knock out mice. PLoS One. 2014;9:e84541. doi: 10.1371/journal.pone.0084541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmert M.R., Boepple P.A. Variation in the timing of puberty: clinical spectrum and genetic investigation. J. Clin. Endocrinol. Metab. 2001;86:2364–2368. doi: 10.1210/jcem.86.6.7603. [DOI] [PubMed] [Google Scholar]
- Patterson J.L., Beltranena E., Foxcroft G.R. The effect of gilt age at first estrus and breeding on third estrus on sow body weight changes and long-term reproductive performance. J. Anim. Sci. 2010;88:2500–2513. doi: 10.2527/jas.2008-1756. [DOI] [PubMed] [Google Scholar]
- Reddy I.J., David C.G., Sarma P.V., Singh K. The possible role of prolactin in laying performance and steroid hormone secretion in domestic hen (Gallus domesticus) Gen. Compar. Endocrinol. 2002;127:249–255. doi: 10.1016/s0016-6480(02)00034-5. [DOI] [PubMed] [Google Scholar]
- Risma K.A., Hirshfield A.N., Nilson J.H. Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology. Endocrinology. 1997;8:3540–3547. doi: 10.1210/endo.138.8.5313. [DOI] [PubMed] [Google Scholar]
- Saacke R.G., Dalton J.C., Nadir S., Nebel R., Bame J.H. Relationship of seminal traits and insemination time to fertilization rate and embryo quality. Anim. Reprod. Sci. 2000;60:663–677. doi: 10.1016/s0378-4320(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Selevan S.G., Rice D.C., Hogan K.A., Euling S.Y., Pfahles-Hutchens A., Bethel J. Blood lead concentration and delayed puberty in girls. N. Engl. J. Med. 2003;348:1527–1536. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- Shih M., Chiu Y.N., Hu M.C., Guo I.C., Chung B.C. Regulation of steroid production: analysis of Cyp11a1 promoter. Mol. Cell. Endocrinol. 2011;336:80–84. doi: 10.1016/j.mce.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Tan Y.G., Xu X.L., Cao H.Y., Mao H.G., Yin Z.Z. RFamide-related peptides gene expression, polymorphism and their association with reproductive traits in chickens. Poult. Sci. 2020;100:488–495. doi: 10.1016/j.psj.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tart J.K., Johnson R.K., Bundy J.W., Ferdinand N.N., McKnite A.M., Wood J.R., Miller P.S., Rothschild M.F., Spangler M.L., Garrick D.J., Kachman S.D., Ciobanu D.C. Genome-wide prediction of age at puberty and reproductive longevity in sows. Anim. Genet. 2013;44:387–397. doi: 10.1111/age.12028. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Ubuka T., Bentley G.E., Kriegsfeld L.J. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. Gen. Comp. Endocrinol. 2012;177:305–314. doi: 10.1016/j.ygcen.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Op de Macks Z., Shirtcliff E.A., Pfeifer J.H. Puberty and the human brain: insights into adolescent development. Neurosci. Biobehav. Rev. 2018;92:417–436. doi: 10.1016/j.neubiorev.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington D., Walker M.A. Distribution of follicular growth, atresia and ovulation in the ovary of the domestic hen (Gallus domesticus) at different ages. J. Reprod. Fertil. 1988;84:223–230. doi: 10.1530/jrf.0.0840223. [DOI] [PubMed] [Google Scholar]
- Zhu J., Chan Y.M. Fertility issues for patients with hypogonadotropic causes of delayed puberty. Endocrinol. Metab. Clin. North Am. 2015;44:821–834. doi: 10.1016/j.ecl.2015.07.011. [DOI] [PubMed] [Google Scholar]