Highlights
-
•
Results of a breast cancer planning study with 4 cases conducted in the Netherlands are reported.
-
•
A guideline for evaluation of breast cancer radiotherapy treatment plans has been established.
-
•
The dosimetric parameters D98%, Dmean and D2% are adopted for target volume dose evaluation.
-
•
For the heart and lungs, Dmean is adopted as the most important dosimetric parameter.
Keywords: Breast cancer, Radiotherapy, Treatment planning, Consensus, Guideline, National
Abstract
Purpose
The aim was to reach consensus in The Netherlands on which parameters should be used to evaluate breast cancer radiotherapy (RT) plans.
Materials and methods
A Benchmark Case with delineated planning target volumes (PTVs) and Organs At Risk (OARs) was sent to all Dutch radiotherapy centres in combination with a questionnaire, with the request to generate RT plans prescribing 15 times 2.67 Gy for four different treatment indications according to the institutional irradiation technique. The plans and accompanying questionnaire answers were analysed using descriptive statistics. These results, together with a harmonisation proposal, were sent to all centres. The proposal was discussed at a meeting of the Dutch Society of Radiation Oncology breast cancer platform. Distinct parameters were accepted if consensus on them was reached.
Results
19 out of 20 Dutch departments participated in this study. PTV coverage varied considerably, with D98% between 63% and 99% for the breast and between 37% and 97% for the internal mammary nodes (IMN). Also substantial OAR dose differences were observed, with e.g. mean heart doses ranging between 1.85 Gy and 5.42 Gy in case the IMN were included in the PTV. For evaluation of the PTVs D98%, D2% and Dmean were chosen to report on, with target values of ≥ 95% (90% for the PTV_IMN), ≤ 107%, and 99–101%, respectively. For OARs, consensus was reached on the parameters to be evaluated, without target values: Dmean of the heart, Dmean and V5% of the lungs, and in case of periclavicular radiotherapy V30Gy of the thyroid gland. For patients younger than 40 years a contralateral mean breast dose of ≤ 1 Gy was agreed upon.
Conclusion
A new Dutch consensus guideline for evaluation of breast cancer RT plans has been established.
Introduction
Postoperative breast cancer radiotherapy (RT) reduces the risk of local recurrence with a factor of 3–4 [1]. In The Netherlands the standard schedule for breast radiotherapy has been 15 fractions of 2.67 Gy since 2013, based on 10-year follow-up results of the START B trial [2]. When designing an RT plan, a continuous trade off between optimal dose to the target versus optimal sparing of organs at risk (OAR) needs to be made. As the relationship between dose-volume parameters, local control and side-effects is not very clear, and because the acceptance of a local recurrence risk and side effects differ per physician and per patient, a wide practice variation exists in the trade-offs made. To be able to adequately compare the quality of RT plans, there is a need for consensus about dose-volume parameters to be evaluated for both target volumes and organs at risk. There is however a reasonable consensus about delineation of target volumes and OARs for breast cancer patients [3], [4], [5].
In treatment plan comparison studies, often the volume receiving 95% (V95%) or 107% (V107%) of the prescribed dose is used as a criterion to evaluate the RT plan [6], while the dose received by 98% (D98%) and 2% (D2%) of the volume [7] or the dose to 99% and to maximally 2 cc [8] can also be used. Some studies use the homogeneity index (HI) and conformity index (CI) as the main target volume parameters to compare plans [8], [9] or a combination of HI, V95% and V107% [10].
In addition, the exact target volume for which dose evaluation criteria are reported varies; some studies report the dose to the breast and lymph nodes combined, others separately report the dose to the breast and dose to the lymph nodes [10], while some report the dose to the breast and to each individual lymph node level.
Furthermore, clinical factors like age or co-morbidity, are often implicitly taken into account during clinical plan evaluation. This variety of contributing factors may introduce interobserver variation in plan evaluations. Such factors should be taken into account in plan comparison studies as they might help in the development of even more patient specific tailored treatments.
Therefore, the aim of the current work was to develop a national consensus guideline on which dose volume and clinical parameters should be used in the evaluation of breast cancer radiotherapy plans irrespective of the irradiation technique used, using 15 fractions of 2.67 Gy, as this was the national consensus fractionation at that time.
Materials and methods
Outline of the study
First, a questionnaire was developed to gain insight into local protocols and local trade-offs that are applied for RT plan-optimization and evaluation. Second, target volumes were delineated for a CT-scan of a randomly selected left-sided breast cancer patient who had undergone a lumpectomy. Both the questionnaire and the delineated CT scan were subsequently sent out to all 20 Dutch RT centres with the request to make four different RT plans for four different target volumes, according to their local protocols and using their own RT technique, without altering the delineation. After analysis of the RT plans and questionnaires, a concept-protocol was developed for RT plan evaluation. Finally, a consensus meeting was organized with the Dutch Society of Radiation Oncology Breast cancer platform which led to a finalized consensus protocol.
Questionnaire
The questionnaire included a table in which those volumes considered for RT plan optimization and evaluation, and DVH criteria used for these volumes could be filled in. In addition, four questions were asked related to possible compromises in target coverage that might be made in clinical practice (Supplementary Questionnaire A1).
Benchmark cases
Target volume delineation of the breast, regional lymph nodes and tumour bed was performed based on the ESTRO guidelines [3]. The heart, lungs, thyroid, humeral head and contralateral breast were delineated as OAR. The participating RT centers were requested to submit the following four photon treatment plans, without altering the delineations: Case A: irradiation of the breast only with a fractionation of 15*2.67 Gy. Case B: irradiation of the breast including an integrated boost of 20*2.18/2.67 Gy. Case C: irradiation of the breast and the regional axillary and periclavicular lymph nodes (level 1 to 4 and the interpectoral lymph nodes) with a fractionation of 15*2.67 Gy. Case D was equivalent to case C, but also including the left sided internal mammary lymph nodes (IMN). Clinical details of patient and tumour characteristics were also provided (Supplementary Table A1). For all cases, the skin was not considered to be at risk for invasion and thus not part of the CTV.
Treatment plan results were centrally collected and processed using Raystation v.8b (Raysearch, Stockholm). Several DVH, average dose and conformity data were thereafter imported in Microsoft Excel 2013 to generate descriptive statistics (e.g. median, range) over all plans per submitted case. Among others, D98%, D2%, Dmean and the van ‘t Riet conformity index [11] were evaluated for the PTVs.
Consensus meeting
The DVH analyses, in combination with data from literature and the results of the questionnaire, were used to formulate a consensus proposal. The results and the proposal were sent to all RT centres in the Netherlands with the request to analyse the proposal and collect feedback from the colleagues in their own department.
The proposal was thereafter discussed at a consensus meeting of the Dutch Society of Radiation Oncology breast cancer platform, in which representatives from all RT centres in the Netherlands participated. The platform consists of both radiation oncologists as well as medical physicists of all radiotherapy centres in the Netherlands specialized in breast cancer radiotherapy. During this meeting the proposal and suggestions for adaptations were discussed per evaluation item and per target volume and OAR. Distinct evaluation parameters were accepted if consensus on them was reached, i.e. if no one objected. The revised proposal was thus formulated and formally approved by the platform.
Results
Results of the questionnaire
19 out of 20 Dutch departments participated in the questionnaire and the benchmark study. One institute did not participate due to time limitations. A wide variation between local protocols was seen: institutes cropped Clinical target volumes (CTVs) and planning target volumes (PTVs) with various margins beneath the skin, and PTV_Breast was sometimes also cropped to exclude the lungs, again with various margins.
Institutes used a variety of PTVs combinations for evaluation, varying from one PTV for all target volumes, to evaluation of each lymph node level and PTV_Breast separately. The PTV evaluation parameters used clinically also varied considerably, as nine different dose/volume criteria for PTV coverage were reported.
Of the OARs, the heart and lungs were used most often. The mean dose was regularly used as an evaluation criterion for these OARs, while the corresponding constraint varied strongly between centres (Supplementary Table A2). For the lungs, sometimes one or more other DVH criteria were used, which also differed per institute. Relatively few institutes included the thyroid, spinal cord, humeral head or brachial plexus as OAR [12].
All institutes replied that sometimes compromises were made in target coverage. Half of the institutes take clinical factors into consideration when delineating the CTV. The main clinical factor considered during CTV delineation is tumour location, but also cardiac and pulmonary risk factors are considered. Smoking history and gene-mutations were rarely reported as a reason to compromise PTV coverage. Clinical factors were generally not considered in the expansion of the CTV to PTV. When compromises in target coverage were made, this was usually done without predefined criteria (See Supplementary Table A1 for more details).
Results of the benchmark cases
The overall results of the planning study are given in Table 1. The results for the individual institutes are given graphically in the appendix (Supplementary Figs. A1 to A4).
Table 1.
Results of the benchmark cases performed by 19 centres, presented according to the parameters as agreed upon during the consensus meeting together with the conformity index.
| Median (min–max):out of tolerancea | Median (min–max):out of tolerance | Median (min–max):out of tolerance | Median (min–max):out of tolerance | ||
|---|---|---|---|---|---|
| Volume | Criterion | Case A | Case B | Case C | Case D |
| PTV_Breastb | D98% (%) ≥ 95% |
96 (63–98): 3 | 95 (66–98): 2 | 95 (87–100): 6 | 95 (90–98): 3 |
| Dmean (%) 100%± 1% |
100 (98–101): 1 | 107 (106–109) | 100 (99–102): 4 | 101 (100–103): 5 | |
| D2% (%) ≤ 107% |
104 (102–105): 0 | 125 (122–128) | 104 (102–106): 0 | 105 (103–110): 1 | |
| Conformity | 0.76 (0.72–0.81) | 0.72 (0.66–0.80) | NA | NA | |
| Lungs | Dmean (cGy) | 205 (150–226) | 287 (256–385) | 505 (363–770) | 680 (565–910) |
| V5Gy (%) | 8 (5–9) | 13 (10–23) | 20 (17–27) | 26 (24–40) | |
| Heart | Dmean (cGy) | 150 (81–192) | 178 (107–275) | 179 (115–352) | 310 (185–542) |
| Contralateral breast | Dmean (cGy) | 35 (13–77) | 48 (15–165) | 64 (23–113) | 169 (37–501) |
| Thyroid | V30Gy (%) | 0 (0–0) | 0 (0–0) | 1 (0–15) | 1 (0–6) |
| PTV_Boost | D98% (%) ≥ 95% |
NA | 95 (94–98): 1 | NA | NA |
| Dmean (%) | NA | 100 (98–101): 1 | NA | NA | |
| D2% (%) | NA | 103 (101–106): 0 | NA | NA | |
| Conformity | NA | 0.79 (0.70–0.88) | NA | NA | |
| PTV_N1n2pectc | D98% (%) ≥ 95% |
NA | NA | 94 (89–99): 8 | 95 (89–98): 7 |
| Dmean (%) | NA | NA | 100 (98–102) | 100 (99–102) | |
| D2% (%) | NA | NA | 103 (101–106) | 103 (101–106) | |
| PTV_N3n4d | D98% (%) ≥ 95% |
NA | NA | 94 (79–98): 12 | 95 (79–97): 7 |
| Dmean (%) | NA | NA | 100 (98–103) | 100 (98–102) | |
| D2% (%) | NA | NA | 105 (102–108) | 104 (102–107) | |
| PTV_IMNe | D98% (%) ≥ 90% |
NA | NA | NA | 89 (37–97): 8 |
| Dmean (%) | NA | NA | NA | 99 (96–102) | |
| D2% (%) | NA | NA | NA | 105 (103–107) | |
: Number of plans that did not meet the evaluation criterion as agreed upon during the consensus meeting.
: All target volumes are expansions of their respective CTV by 5 mm and are clipped 5 mm below the skin.
PTV_N1n2pect: PTV of the lymph node levels 1 and 2 and the interpectoral lymph nodes.
PTV_N3n4: PTV of the lymph node levels 3 and 4.
PTV_IMN: PTV of the internal mammary lymph nodes.
The D98% for the PTV of the locoregional lymph node volumes was in general somewhat lower than for PTV_Breast, and was the lowest for PTV_IMN. For case B, a large variation in boost dose conformity values was found (range: 0.70 to 0.88). The median dose did not differ much from the mean dose, with an average difference of 0.5%, 0.2%, 0.4% and 0.2% for the dose to PTV_Breast for cases A to D, respectively.
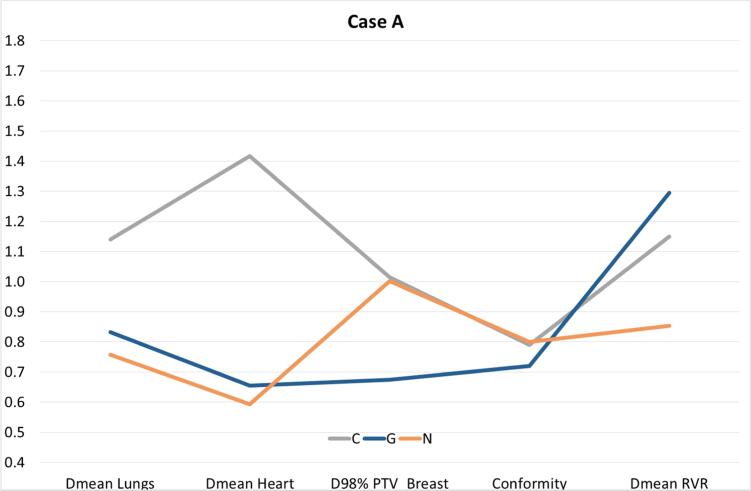
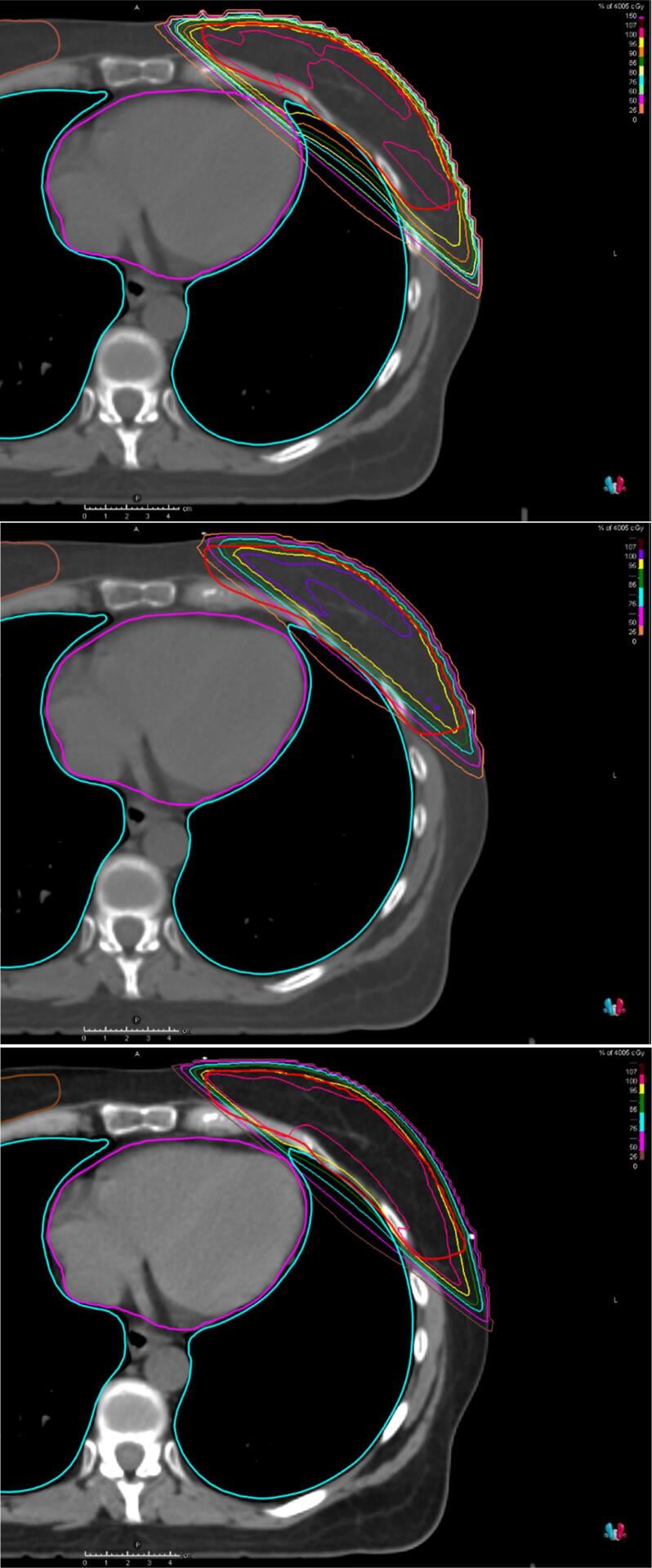
In Fig. 1, Fig. 2, the results for case A are given for three institutes which had either a relatively poor or good treatment plan to illustrate the variety in treatment plan quality. Institute C had higher OAR doses with almost equal target coverage as the institutes N and G, indicating that institute C could improve its treatment plan. Both institutes N and G had a relatively low heart and lung dose, but the PTV coverage (D98%) of institute G was much lower than the average D98% over all centres. This was caused by blocking the heart with a margin of 5 mm in the tangential fields. In addition, in institute G the dose to the target volume was evaluated using a PTV which excludes the 5 mm closest to the heart seen from the beams-eye-view of the tangential fields. As such, they do not report the lower DVH value of the original PTV.
Fig. 1.
The results for Case A are given for the 3 institutes that had either a relatively poor or good treatment plan. The values given in this figure are the relative values as compared to the average values found over all institutes. For example, institute C had a 1.41 times higher mean heart dose than the mean heart dose averaged over all 19 institutes. RVR: Remaining Volume at Risk.
Fig. 2.
Axial CT slice showing the dose distribution for Case A for respectively institutions C, G and N.
The institutions were actually requested to repeat the benchmark 4 months after consensus was reached. For completeness, results of the second benchmark are given in Table 2. Not much difference was observed between the first and second benchmark.
Table 2.
Results of the repeat Benchmark Case performed by 17 centres (centres F and P did not repeat the Benchmark Case). Some institutes improved their treatment plan. As a consequence, the number of out-of-tolerance values reduced slightly.
| Median (min–max): out of tolerancea | Median (min–max): out of tolerance | Median (min–max): out of tolerance | Median (min–max): out of tolerance | ||
|---|---|---|---|---|---|
| Volume | Criterion | Case A-Repeat | Case B-Repeat | Case C-Repeat | Case D-Repeat |
| PTV_Breastb | D98% (%) | 96 (63–98): 1 | 96 (66–98): 1 | 96 (87–98): 3 | 95 (90–97): 2 |
| Dmean (%) | 101 (98–102): 2 | 108 (106–110) | 100 (99–103): 4 | 101 (99–103): 2 | |
| D2% (%) | 104 (102–106): 0 | 126 (123–128) | 104 (102–106): 0 | 104 (103–107): 0 | |
| Conformity | 0.76 (0.72–0.82) | 0.71 (0.67–0.80) | NA | NA | |
| Lungs | Dmean (cGy) | 194 (142–235) | 275 (232–391) | 493 (406–563) | 628 (531–910) |
| V5Gy (%) | 8 (6–10) | 12 (9–23) | 21 (17–23) | 25 (20–40) | |
| Heart | Dmean (cGy) | 137 (80–189) | 169 (104–233) | 179 (125–267) | 290 (186–542) |
| Contralateral breast | Dmean (cGy) | 38 (13–68) | 45 (15–164) | 64 (13–210) | 143 (32–501) |
| Thyroid | V30Gy (%) | 0 (0–0) | 0 (0–0) | 1 (0–15) | 1 (0–3) |
| PTV_Boost | D98% (%) | NA | 95 (95–99): 0 | NA | NA |
| Dmean (%) | NA | 100 (98–103): 3 | NA | NA | |
| D2% (%) | NA | 104 (102–105): 0 | NA | NA | |
| Conformity | NA | 0.76 (0.64–0.88) | NA | NA | |
| PTV_N1n2pectc | D98% (%) | NA | NA | 96 (89–99): 4 | 96 (92–98): 2 |
| Dmean (%) | NA | NA | 100 (99–101) | 100 (98–103) | |
| D2% (%) | NA | NA | 104 (102–105) | 104 (102–105) | |
| PTV_N3n4d | D98% (%) | NA | NA | 95 (91–98): 9 | 96 (93–97): 4 |
| Dmean (%) | NA | NA | 100 (99–102) | 101 (99–102) | |
| D2% (%) | NA | NA | 104 (102–107) | 105 (101–107) | |
| PTV_IMNe | D98% (%) | NA | NA | NA | 92 (0.77–0.95): 4 |
| Dmean (%) | NA | NA | NA | 100 (98–102) | |
| D2% (%) | NA | NA | NA | 105 (102–107) | |
: Number of plans that did not meet the evaluation criterion.
: All target volumes are expansions of their respective CTV by 5 mm and are clipped 5 mm below the skin.
PTV_N1n2pect: PTV of the lymph node levels 1 and 2 and the interpectoral lymph nodes. dPTV_N3n4: PTV of the lymph node levels 3 and 4.
PTV_IMN: PTV of the internal mammary lymph nodes.
Results of the consensus meeting
At the consensus meeting, the results of the planning study and the proposal for DVH criteria for plan evaluation were discussed. DVH criteria for which consensus was reached are summarized in Table 3, Table 4; the parameters for which no consensus was reached are given in Table A3 in the Appendix.
Table 3.
National consensus on dosimetric parameters and target volume names to be used in the evaluation of a breast cancer RT-plan.
| D98% | Dmean | D2% | |
|---|---|---|---|
| PTV_Breast | ≥ 95% | 99–101%a | ≤ 107% |
| PTV_Boost | ≥ 95% | 100%b | ≤ 107% |
| PTV_N1n2pectc | ≥ 95% | No target value given | No target value given |
| PTV_N3n4c | ≥ 95%d | No target value given | No target value given |
| PTV_IMNe | ≥ 90%c | No target value given | No target value given |
: With the exception of plans including a boost volume.
: 100% is given as target value but may differ per patient. No consensus was reached what range of values would be acceptable.
: These node levels can be jointly evaluated.
: In case this PTV includes lung, a concession to this target value is allowed.
: D98% should be ≥95% for CTV_IMN, also taking into account set-up uncertainty.
Table 4.
National consensus dosimetric parameters and OAR volume names.
| Parameter | Remark | |
|---|---|---|
| Lungs | Dmean and V5Gy | No threshold value specified |
| Heart | Dmean | No threshold value specified |
| Contralateral breast | Dmean ≤ 1 Gy | Only for patients < 40 years. Delineation if needed. |
| Thyroid | V30Gy | Only if lymph node levels 3 and 4 are included in the target volume. No threshold value specified. |
Consensus for target volumes
It was agreed that both CTV and PTV should be clipped 5 mm below the skin for reporting purposes. It also became clear that the breast CTV is sometimes deliberately delineated smaller than prescribed by the guidelines, in order to facilitate sparing of the OARs while still reaching the required target coverage. While this might lead to an acceptable plan, this hampers proper reporting of the actually planned dose to the breast if it would have been defined according to the guideline. It was therefore agreed to adhere to the delineation guidelines and, in case of underdosage, to report the reason why this was accepted.
For all cases, consensus was obtained to use D98%, Dmean and D2% as parameters to evaluate the near minimum, mean and near maximum dose, as D98% and D2% are also recommended by ICRU 91 [13]. There was some discussion regarding whether it would be better to evaluate Dmedian, as recommended by ICRU 91, instead of Dmean. As the general consensus was that these two values would not differ much and that Dmean is reported in most treatment planning systems, contrary to Dmedian, it was decided to choose Dmean as parameter.
It was agreed that D98% should be ≥95% and D2% should be ≤107%, largely matching with the ICRU recommendation. There was also consensus to strive for a Dmean within plus or minus 1% from the prescription dose for the breast in plans without a boost.
In the draft, the suggestion was made to optionally evaluate the dose values for the CTV, to make plan comparisons with proton plans easier, as proton plans usually report dose to CTV and not to PTV. However, as photon plans would need to be based on a probability based planning for a proper comparison with proton plans, this motion was not supported.
In case of breast with boost plans, it was agreed upon that Dmean should be evaluated for the boost PTV (PTV_Boost). However, striving to contain this value within 1% of the prescription dose was deemed neither needed nor desired.
A proposal was offered to report Dmean and D2% for PTV_Breast excluding the PTV_Boost (PTV_Breast-PTV_Boost). This proposal was not adopted as such values would vary considerably between patients.
For scientific reasons, it would provide most information when for regional radiotherapy the dose would be evaluated for all lymph node levels separately. However, to limit work in clinical practice, where often levels 1 and 2 and interpectoral nodes, and likewise levels 3 and four, are combined, it was agreed upon to report dose to these two combined volumes and to the IMN separately.
For the coverage of PTV_IMN, a D98% of >90% was deemed sufficient as long as the D98% to CTV_IMN would remain >95%. This consensus was based on the fact that the dorsal part of PTV_IMN often includes lung, resulting in reduced build-up and thus underdosage. If one would still try to reach a dose of 95% in this part of PTV_IMN, this would lead to a relatively high increase in lung and heart dose. Because the dose to CTV_IMN would probably only change slightly if the optimal dose level for PTV_IMN would be set at 90%, further increasing the coverage and therefore the lung and heart dose was considered unacceptable.
Consensus for organs at risk
For the OARs several proposals were discussed. For the lungs and the heart, it was agreed upon to evaluate the mean dose, as this is the most used parameter in literature [14], [15], [16], [17], [18], as well as in the Dutch institutes. The suggestion to include V5Gy for the lungs was approved, as it was considered that this dose level could correlate with the possible effects of switching from tangential techniques to VMAT techniques, which may cause a larger volume of the lungs to be irradiated to lower dose levels. As there was consensus that the lung and heart dose can differ considerably between patients due to their varying anatomy, no threshold values for heart and lung doses were yet specified. Presently, contralateral breast cancer induction is mainly reported for patients < 40 years with a mean contralateral breast dose > 1 Gy [19]. Thus, consensus was reached that delineation of the contralateral breast should be performed in those individual patients < 40 years in which this dose level might be reached. For the thyroid, it was concluded that V30Gy should be evaluated when lymph node levels 3 and 4 are part of the target volume [20].
Consensus on clinical factors
Besides the dosimetric parameters presented in Table 3, Table 4, it was agreed upon that apart from tumour related parameters, also (risk factors for) cardiac morbidity, smoking history and age should be considered in the decision whether or not to irradiate, or to do concessions to target coverage. Furthermore, when concessions are made to target coverage in order to spare organs at risk, both the stage of the disease as well as the location of the tumour should be considered. The new national guideline was approved in November 2019 and is presented in Table 3, Table 4.
Discussion
A breast cancer radiotherapy benchmark study with four clinical cases was conducted in 19 out of 20 Dutch RT centres. We did not only find considerable variation in target dose coverage and dose to OARs, but also a wide variation in dose volume histogram parameters used to evaluate the treatment plans. Based on the results of the benchmark study and a consensus meeting, a new guideline for evaluation of breast cancer RT planning was established.
A next step could be to further harmonise the criteria for plan optimisation for patients where compromises need to be made. The further development and validation of NTCP and TCP models might make this process easier in the future.
There are only a few published (national) guidelines involving breast radiotherapy treatment plan evaluation criteria. Duma et al. recently published a recommendation on heart sparing techniques of the breast cancer expert panel of the German society of radiation oncology (DEGRO) [21]. They restated their recommendation for dose constraints for the heart and highly encouraged reporting of a wide number of parameters: mean dose to heart and left ventricle (LV) and Left Anterior Descending Artery (LAD) as well as V5GyLV, V23GyLV, V30GyLAD and V40GyLAD. Although we agree that reporting all these parameters may lead to a better knowledge on toxicity, we proposed a limited number of essential parameters to encourage its use by all institutes in The Netherlands as much as possible. DEGRO also gave upper limits for these criteria. In our consensus meeting we discussed that this would not be optimal, as we formulated that the dose should be as low as possible for each individual patient and due to the widely varying anatomy, these values are highly patient dependent. Further studies are required to investigate whether strict upper limits may still be defined for the OARs for different target volumes to be irradiated.
In a consensus statement of the Royal College of Radiologists (UK) dating from 2016, besides treatment indications for breast radiotherapy, also some DVH constraints were given [22]. For example, for patients receiving IMN treatment: heart V17Gy < 10%, ipsilateral lung V17Gy < 35% and mean contralateral breast dose < 3.5 Gy. They included a target mean heart dose as this would help departments to implement breath-hold techniques. This argument is not valid for the Dutch guideline, as all Dutch institutes already have implemented breath-hold for left-sided breast radiotherapy.
Nielsen et al. [23] also reported dose constraints for breast cancer patients, given 25 fractions of 2 Gy. They used specific DVHs points to report on the heart and lung dose, while we prefer to use the mean heart and lung dose as these are commonly reported on in the literature and correlate with toxicity [14], [15]. Furthermore, they included constraints for the spinal cord and maximum dose of 54 Gy outside the PTV or in the plexus brachialis. We did not include this standardly in our evaluation, as our prescription dose in the benchmark cases was much lower.
This Dutch consensus guideline is based on the current scientific knowledge and available technology in the Netherlands. As such, this consensus should be periodically updated.
In conclusion, utilizing the results of a benchmark and a questionnaire, a new guideline for treatment plan evaluation for breast cancer patients was generated. This guideline is one of the most detailed national consensus statements up until now concerning breast radiotherapy plan evaluation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors highly appreciate all the discussions that have taken place and have contributed to the generation of the consensus guideline. We acknowledge all the work the radiation oncologists, clinical physicists and especially RTTs have done to provide us with the planned benchmark cases. The critical English review by An-Sofie Verrijsen is also highly appreciated. The project was sponsored by Stichting Kwaliteitsgelden Medisch Specialisten.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2021.06.004.
Contributor Information
Coen Hurkmans, Email: coen.hurkmans@cze.nl.
Cindy Duisters, Email: cindy.duisters@catharinaziekenhuis.nl.
Mieke Peters-Verhoeven, Email: Mieke.Peters-Verhoeven@radboudumc.nl.
Liesbeth Boersma, Email: liesbeth.boersma@maastro.nl.
Karolien Verhoeven, Email: karolien.verhoeven@maastro.nl.
Nina Bijker, Email: n.bijker@amsterdamumc.nl.
Koen Crama, Email: K.F.Crama@lumc.nl.
Tonnis Nuver, Email: T.Nuver@radiotherapiegroep.nl.
Maurice van der Sangen, Email: maurice.vd.sangen@catharinaziekenhuis.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Darby S., McGale P., Correa C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 3.Offersen B.V., Boersma L.J., Kirkove C. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 2016;118:205–208. doi: 10.1016/j.radonc.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Kong F.M., Ritter T., Quint D.J. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81:1442–1457. doi: 10.1016/j.ijrobp.2010.07.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vikstram J., Hjelstuen M.H., Wasba E., Mjaaland I., Dybvik K.I. A comparison of conventional and dynamic radiotherapy planning techniques for early-stage breast cancer utilizing deep inspiration breath-hold. Acta Oncol. 2018;57:1325–1330. doi: 10.1080/0284186X.2018.1497294. [DOI] [PubMed] [Google Scholar]
- 7.Wang J., Hu W., Yang Z. Is it possible for knowledge-based planning to improve intensity modulated radiation therapy plan quality for planners with different planning experiences in left-sided breast cancer patients? Radiat Oncol. 2017;12:85–0822. doi: 10.1186/s13014-017-0822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goddu S.M., Chaudhari S., Mamalui-Hunter M. Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys. 2009;73:1243–1251. doi: 10.1016/j.ijrobp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Popescu C.C., Olivotto I., Patenaude V., Wai E., Beckham W.A. Inverse-planned, dynamic, multi-beam, intensity-modulated radiation therapy (IMRT): a promising technique when target volume is the left breast and internal mammary lymph nodes. Med Dosim. 2006;31:283–291. doi: 10.1016/j.meddos.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.van Duren-Koopman M.J., Tol J.P., Dahele M. Personalized automated treatment planning for breast plus locoregional lymph nodes using Hybrid RapidArc. Pract Radiat Oncol. 2018;8:332–341. doi: 10.1016/j.prro.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 11.van't RA, Mak AC, Moerland MA, Elders LH, van der ZW. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 1997;37:731-736. doi: 10.1016/s0360-3016(96)00601-3. [DOI] [PubMed]
- 12.Surmann K, van der LJ, Branje T et al. Elective breast radiotherapy including level I and II lymph nodes: a planning study with the humeral head as planning risk volume. Radiat Oncol 2017;12:22 doi: 10.1186/s13014-016-0759-7. [DOI] [PMC free article] [PubMed]
- 13.ICRU report 91 prescribing, recording and reporting of stereotactic treatments with small photon beams. 2014. ICRU.
- 14.Taylor C., Correa C., Duane F.K. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 16.Bongers E.M., Botticella A., Palma D.A. Predictive parameters of symptomatic radiation pneumonitis following stereotactic or hypofractionated radiotherapy delivered using volumetric modulated arcs. Radiother Oncol. 2013;109:95–99. doi: 10.1016/j.radonc.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Defraene G., Schuit E., De R.D. Development and internal validation of a multinomial NTCP model for the severity of acute dyspnea after radiotherapy for lung cancer. Radiother Oncol. 2019;136:176–184. doi: 10.1016/j.radonc.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Jacob S., Camilleri J., Derreumaux S. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: a dosimetric evaluation based on individually-determined radiation dose (BACCARAT study) Radiat Oncol. 2019;14:29. doi: 10.1186/s13014-019-1234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stovall M., Smith S.A., Langholz B.M. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72:1021–1030. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haciislamoglu E., Canyilmaz E., Gedik S. Effect of dose constraint on the thyroid gland during locoregional intensity-modulated radiotherapy in breast cancer patients. J Appl Clin Med Phys. 2019;20:135–141. doi: 10.1002/acm2.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duma M.N., Baumann R., Budach W. Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO) Strahlenther Onkol. 2019;195:861–871. doi: 10.1007/s00066-019-01495-w. [DOI] [PubMed] [Google Scholar]
- 22.Royal College of Radiologists. Postoperative radiotherapy for breast cancer: UK consensus statements; 2016. [DOI] [PubMed]
- 23.Nielsen M.H., Berg M., Pedersen A.N. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013;52:703–710. doi: 10.3109/0284186X.2013.765064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.