ABSTRACT
Ca2+ influx during oocyte maturation and after sperm entry is necessary to fill the internal Ca2+ stores and for complete egg activation. We knocked out the transient receptor potential vanilloid member 3 (TRPV3) and the T-type channel, CaV3.2, to determine their necessity for maintaining these functions in mammalian oocytes/eggs. Double-knockout (dKO) females were subfertile, their oocytes and eggs showed reduced internal Ca2+ stores, and, following sperm entry or Plcz (also known as Plcz1) cRNA injection, fewer dKO eggs displayed Ca2+ responses compared to wild-type eggs, which were also of lower frequency. These parameters were rescued and/or enhanced by removing extracellular Mg2+, suggesting that the residual Ca2+ influx could be mediated by the TRPM7 channel, consistent with the termination of divalent-cation oscillations in dKO eggs by a TRPM7 inhibitor. In total, we demonstrated that TRPV3 and CaV3.2 mediate the complete filling of the Ca2+ stores in mouse oocytes and eggs. We also showed that they are required for initiating and maintaining regularly spaced-out oscillations, suggesting that Ca2+ influx through PM ion channels dictates the periodicity and persistence of Ca2+ oscillations during mammalian fertilization.
KEY WORDS: Oocyte, Fertilization, Ca2+, Signaling, TRP channel
Summary: Electrophysiology and Ca2+-imaging studies revealed that Ca2+ influx via TRPV3, CaV3.2 and TRPM7 ion channels supports the filling of the Ca2+ stores in mouse oocytes and eggs.
INTRODUCTION
Mammalian egg activation is a widely researched field as it is the first stage of embryo development. During this event, the egg undergoes changes such as resuming and completing meiosis, remodeling its outer cortex to block polyspermy, reorganizing the cytoskeleton and meiotic spindle, undergoing pronuclear formation and DNA synthesis, translating and changing maternal mRNA and protein levels to commence mitotic cycles (Horner and Wolfner, 2008; Florman and Fissore, 2015).
In mammals, fertilization induces egg activation after the sperm fuses to a mature metaphase II (MII) oocyte (egg), and initiates a series of precise Ca2+ oscillations in the concentration of free intracellular Ca2+ {[Ca2+]i}, known as oscillations. The oscillations are ultimately responsible for triggering embryonic development via modification of proteins that regulate the resumption and completion of meiosis (Ducibella et al., 2002; Miyazaki and Igusa, 1981; Ozil et al., 2005). Ca2+ oscillations rely on Ca2+ influx from the extracellular media to replenish the stores (Igusa et al., 1983; Wakai and Fissore, 2013). Currently, the channel(s) responsible for this influx have not yet been completely established.
During oocyte maturation – the process initiated following the surge of luteinizing hormone (LH) – and prior to ovulation and fertilization, the oocyte undergoes a plethora of changes including an increase in the content of the internal Ca2+ stores {[Ca2+]ER}. Ca2+ influx underlies the increase in Ca2+ store content (Wakai et al., 2013, 2011; Whitaker, 2006), and previous studies have demonstrated that [Ca2+]ER content and Ca2+ influx undergo contrasting functional changes during maturation such that while [Ca2+]ER content increases, Ca2+ influx progressively decreases (reviewed in Wakai et al., 2011). This strict regulation is necessary as an excess of Ca2+ content and/or influx can predispose eggs or oocytes to parthenogenetic activation, fragmentation and/or apoptosis (Gordo et al., 2002; Ozil et al., 2005), whereas a deficit might impede cellular functions, including protein synthesis, completion of maturation and initiation of embryonic development. Consequently, oocytes and eggs have several mechanisms to regulate baseline [Ca2+]i and store Ca2+, including pumps, channels and exchangers, where the plasma membrane (PM) Ca2+-ATPase (PMCA) and Na+/Ca2+ exchangers extrude excess Ca2+, and the sarcoendoplasmic reticulum Ca2+ ATPases (SERCAs; also known as ATP2As) reuptake Ca2+ into the endoplasmic reticulum (ER), refilling the stores (Berridge et al., 2000; Jones et al., 1995; Kline and Kline, 1992; Wakai et al., 2011). This complement of molecules is known as the ‘Ca2+ toolkit’, one that every cell type possesses to regulate Ca2+ and trigger crucial processes such as muscle contraction, exocytosis and metabolism, among others (Berridge et al., 2003).
The identification of the channels responsible for Ca2+ homeostasis in mammalian oocytes and eggs is largely incomplete. Among the PM channels, the mammalian transient receptor potential (TRP) family of channels includes six subfamilies and nearly 30 human members that are expressed in multiple cell types and tissues (Wu et al., 2010). We have demonstrated the presence of two family members in oocytes and eggs including TRP vanilloid member 3 (TRPV3) (Carvacho et al., 2013). TRPV3 allows divalent cations such as Sr2+ and Ca2+ into eggs, and although it is essential for mediating parthenogenetic embryonic development caused by exposure to Sr2+, it is not required for normal fertility, as null females are fertile (Carvacho et al., 2013). Another channel expressed in oocytes and eggs is the T-type voltage-gated Ca2+ (CaV) channel, CaV3.2 (Bernhardt et al., 2015; Chen et al., 2003; Kang et al., 2007; Peres, 1987). Cacna1h-null females are only mildly subfertile, which is consistent with early measurements showing minor changes in membrane potential during mouse fertilization (Igusa et al., 1983). Further, the eggs' resting membrane potential in conjunction with the inactivation of CaV3.2 channels at this potential would allow, in theory, only a limited number of CaV channels to be open (Bernhardt et al., 2015; Igusa et al., 1983). Despite that these factors limit the open probability of these channels, we cannot rule out that local and cellular effects might be affecting the Ca2+ influx mediated by these channels (Jaffe and Cross, 1984).
TRPV3 and CaV3.2 channels are differentially expressed in oocytes and eggs. The functional expression of TRPV3 is nearly absent at the beginning of maturation at the germinal vesicle stage (GV) but rises steadily during it with its maximal levels at the MII stage (Carvacho et al., 2013). On the other hand, the functional expression of CaV channels during maturation is unknown, although electrophysiological recordings indicated that GV oocytes display greater Ca2+ voltage-gated currents than ovulated eggs; however, the biophysical properties of the channels expressed in GV oocytes seem to be different from the channel expressed in eggs, identified as CaV3.2 (Peres, 1987, 1986; Kang et al., 2007). The reason for the differential expression and/or regulation of these channels during maturation requires further investigation.
Thus, despite the identification of several channels in mammalian oocytes and eggs, the complete set of channels responsible for filling the internal Ca2+ stores and supporting oscillations has not been determined. Furthermore, the lack of specific pharmacological agents as well as the lack of specific antibodies has delayed progress in this area of gamete physiology. Therefore, the use of genetic models lacking a specific or combined set of channels is necessary to identify all the channel(s) that underlie Ca2+ homeostasis in these cells, and electrophysiology will make the assessment of the specificity of commonly used pharmacological inhibitors possible. To these ends, here we describe the generation of mice lacking both Trpv3 and Cacna1h, which show that these females are subfertile. Most importantly, we found that double-knockout (dKO) oocytes and eggs exhibit altered Ca2+ homeostasis and mount shorter duration Ca2+ oscillations following fertilization with reduced periodicity, which can nevertheless be enhanced/restored by the removal of extracellular Mg2+ {[Mg2+]o}. These results suggest the functional involvement of the TRP melastatin 7 (TRPM7) channel in dKO oocytes and eggs, which has been shown to affect Ca2+ homeostasis in mouse oocytes and eggs (Bernhardt et al., 2018; Carvacho et al., 2016). Our findings, therefore, reveal insights into the Ca2+ channels required to initiate and maintain fertilization-induced [Ca2+]i oscillations in the mouse and possibly in other mammals.
RESULTS
Double-null mice lacking Trpv3 and Cacna1h genes are subfertile
Our first goal was to generate a dKO mouse line lacking the Trpv3 and Cacna1h genes. The rationale stemmed from the finding that both channels are expressed in oocytes and eggs but single-knockout (KO) lines for these genes cause minor perturbation on Ca2+ homeostasis or influx in these cells (Ardestani et al., 2020; Bernhardt et al., 2015; Carvacho et al., 2013). With this genetic model, we would be able to pinpoint the channel or set of channels responsible for the maintenance of Ca2+ homeostasis during oocyte maturation and fertilization.
Single-knockout mice were bred to generate the initial pool of double heterozygotes. Males and females of this generation were bred to generate the parent generation of dKO and wild-type (WT) mice that were used in the following studies (Table S1). Germline deletion of the Trpv3 and Cacna1h alleles was confirmed via PCR analysis (Fig. S1). We first investigated the possibility of obvious differences in ovarian size, ovulation rates and rates of in vitro maturation. We found that there were no significant differences in ovarian weight and the number of eggs ovulated post hormone stimulation between the groups (Fig. S2A,B). Ovarian shape and size were also similar between the two groups (Fig. S2C). There was also no delay in any stage of in vitro maturation in dKO oocytes compared to rates observed in WT oocytes (Fig. S2D). These data reinforce the notion that TRPV3 and CaV3.2 channels are not required for oocyte maturation and ovulation in female mice.
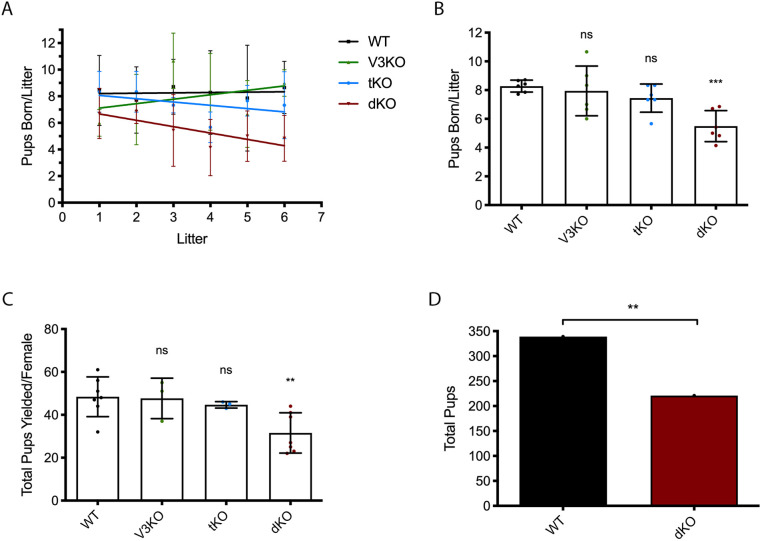
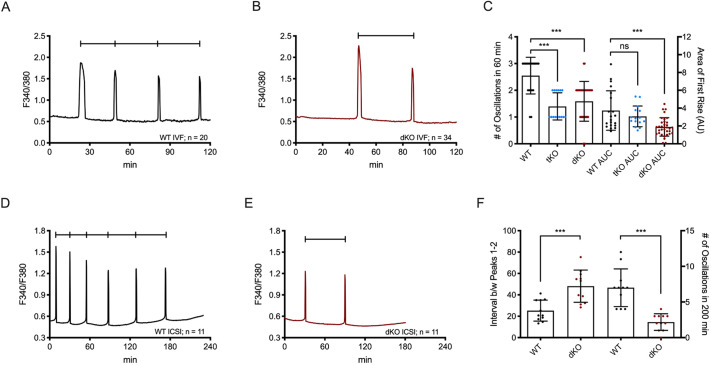
We then sought to evaluate the fertility of dKO females. The single-knockout lines for Trpv3−/− and Cacna1h−/− have previously been shown to be viable and fertile (Bernhardt et al., 2015; Carvacho et al., 2013; Chen et al., 2003; Cheng et al., 2010). WT mice were used as controls. Seven females from each WT and dKO line were bred with seven males of the same genotype for 36 weeks; Trpv3-knockout (V3KO) and Cacna1h-knockout (tKO) mating studies were performed with three pairs of mice. Our results, using data from the first six litters, show that the dKO mice produced fewer numbers of pups, and the number of pups per litter decreased significantly by, or after, the third parturition (dKO: 5.49±1.1 versus WT: 8.28±0.41; P<0.001) (Fig. 1A,B). In contrast, V3KO females yielded a similar number of pups per litter compared to WT females, and although tKO females yielded fewer pups per litter than WT females the differences remained not significant (V3KO: 7.94±1.7; tKO: 7.44±0.98; P>0.05 for both genotypes) (Fig. 1B). Similarly, the total numbers of pups yielded per female were decreased by ∼35% in the dKO line (dKO: 31.6±9.4 versus WT: 48.4±9.3, P>0.05) (Fig. 1C). Importantly, a total of 339 pups were born from WT females, whereas 221 pups were born from the same number of dKO females (P=0.004) (Fig. 1D). It is also worth noting that after the third parturition in dKO females, and with each successive parturition, the number of neonatal deaths became prominent, with ∼40–80% of pups dying per litter. We monitored mating cages once to twice daily from parturition until pups were weaned, and observed that neonatal deaths occurred post-parturition and independent of the mother's behavior. These results demonstrate that these channels are not required for fertilization, although they are necessary for full fertility.
Fig. 1.
dKO females display subfertility. (A) Number of pups born per litter up to six parturitions. Linear regression applied using data from each individual mating pair per genotype (WT, n=7; V3KO, n=3; tKO, n=3; dKO, n=7). (B) Quantification of A; ***P<0.001. (C) Total number of pups yielded per female; **P=0.005. (D) Total number of pups born from the WT and dKO genotypes; **P=0.004. ns, not significant. Chi-square test.
TRPV3 and CaV3.2 currents are absent in dKO females
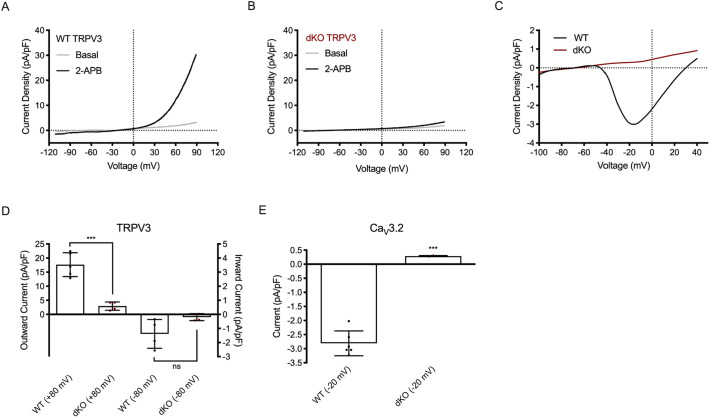
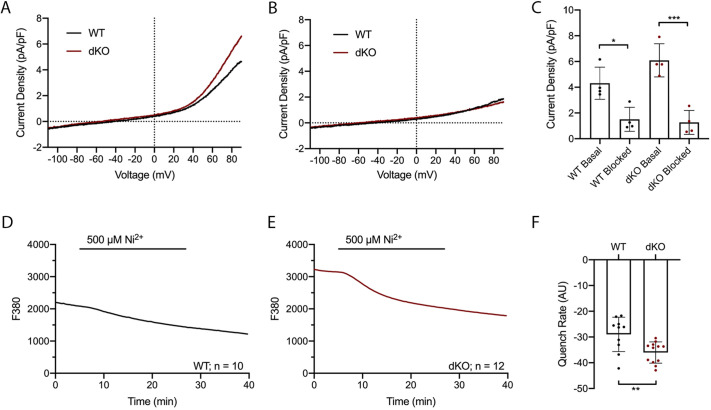
We used whole-cell patch-clamp techniques to record and determine TRPV3 and T-type channel properties in MII eggs. Previous data established the expression of TRPV3 channels in mouse eggs and its potentiation by 2-aminoethoxydiphenyl borate (2-APB) (Carvacho et al., 2013). 2-APB, besides being a non-specific blocker of Ca2+ channels, was also identified as an inhibitor of inositol 1,4,5-triphosphate receptor 1 (IP3R1; also known as ITPR1) (Maruyama et al., 1997). However, at the concentration used here in eggs and for the purpose of evaluating PM channels, 2-APB acts selectively on TRPV3 channels (Lee et al., 2016). In response to a voltage ramp, the addition of 200 µM 2-APB evoked an outwardly rectifying current with properties characteristic of TRPV3 (Hu et al., 2004), and was congruent with Carvacho et al. (2013) (Fig. 2A). At +80 mV, WT eggs displayed a mean current density of 17.7±4.2 pA/pF, whereas this current was absent in dKO eggs (2.94±1.5 pA/pF), which was comparable to dKO basal currents. The inward current in both genotypes was statistically insignificant in the presence and absence of 2-APB (Fig. 2A,B, respectively), thus confirming the identity and absence of the channel. Next, in response to voltage steps from −100 mV to +50 mV, with a holding potential of −80 mV, we observed a current–voltage (I-V) relationship that matches the biophysical characteristics reported for CaV3.2 channel currents (Hille, 2001). The peak of the currents in 20 mM extracellular Ca2+ was at −20 mV (Fig. 2C), which was consistent with previous reports (Carbone et al., 2014; Day et al., 1998; Peres, 1986). This current was absent in dKO eggs (WT: −2.81±0.18 pA/pF versus dKO: 0.29±0.01 pA/pF). To summarize, we show averaged current amplitudes at +80 mV and −80 mV (Fig. 2D), and at −20 mV (Fig. 2E), confirming that dKO oocytes were truly null for both channels. We further confirmed the dKO genotype by measuring CaV3.2 current, and subsequently, TRPV3 current in response to 2-APB in the same egg, and observed the absence of these currents in dKO eggs (Fig. S3), which confirms that these channels are absent from the PM of dKO eggs.
Fig. 2.
TRPV3 and CaV3.2 currents are absent in dKO eggs. (A,B) Ramp protocol from −100 mV to +100 mV to measure TRPV3 current [holding potential (HP): 0 mV]. Mean basal response (gray trace) and mean response to 200 µM 2-APB (black trace). (A) WT eggs, n=5. (B) dKO eggs, n=5. (C) I-V relationship from the voltage step protocol measuring CaV3.2 channel activity. WT mean (black trace, n=5) and dKO mean (red trace, n=4) are shown. (D) Averaged TRPV3 current responses in response to 200 µM 2-APB at +80 mV and −80 mV for WT (black dots) and dKO eggs (red dots). ***P(+80 mV)<0.001; P(−80 mV)>0.05. ns, not significant. (E) Averaged CaV3.2 current at −20 mV in WT vs dKO eggs. ***P<0.001 (Student's t-test).
Ca2+ stores are diminished in dKO females
The subfertility of the dKO mice suggested that the oocytes/eggs may have impaired Ca2+ homeostasis parameters. Little is known about the mechanism by which oocytes accumulate Ca2+ in the stores during this process, although results from our laboratory suggest that the main source of increased [Ca2+]ER is due to the influx of external Ca2+ (Wakai et al., 2013). CaV3.2 channels have also been shown to contribute to the increase in [Ca2+]ER during oocyte maturation (Bernhardt et al., 2015), although the effects of TRPV3 channels have not been examined. Nevertheless, given that TRPV3 and CaV3.2 are important Ca2+ influx channels in oocytes, we hypothesized that the total Ca2+ stores and [Ca2+]ER would be diminished in dKO eggs.
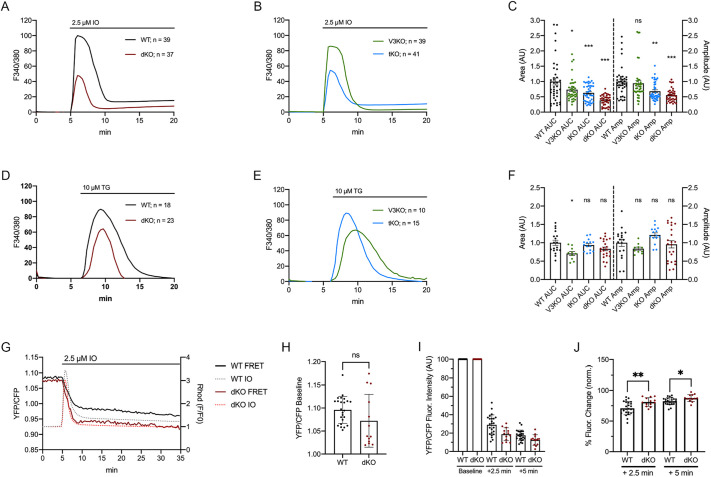
To estimate free cytosolic Ca2+ levels, we first examined baseline Fura2 fluorescence in WT and dKO eggs. We obtained values of 0.639±0.145 and 0.628±0.108 for WTs and dKOs, respectively (P>0.05), suggesting comparable homeostasis in these eggs. We then added the Ca2+ ionophore, ionomycin (IO), to empty the Ca2+ stores (Fig. 3A,B). After normalizing the response curves, the total Ca2+ content as estimated by the area under the curve (AUC), was decreased by ∼61.5% in dKO mice [147.4±66.1 arbitrary units (AU), n=37] compared to WT mice (383.2±271.4, n=39; P<0.001) (Fig. 3C, left y-axis) as was the maximum amplitude (WT: 111.3±54.0 AU; dKO: 61.75±23.6 AU; P<0.001) (Fig. 3C, right y-axis); single-channel KO lines also displayed reduced total stores, but not to the extent of dKOs (Fig. 3B,C). Next, we directly examined the [Ca2+]ER in eggs using thapsigargin (TG), a SERCA inhibitor, which is the pump that fills the ER (Fig. 3D,E) (reviewed in Berridge, 2002). We observed a slight but non-significant decrease in [Ca2+]ER levels in dKO eggs (325.4±95.68 AU, n=23) versus WT eggs (390.1±115 AU, n=18; P>0.05) (Fig. 3F). AUC and amplitude were also not different for tKO eggs, but the AUC of the V3KO eggs was significantly reduced compared to that of the WT eggs but not compared to that of the eggs of the other groups (Fig. 3D–F; P<0.05).
Fig. 3.
Ca2+ stores are reduced in dKO eggs and ER efflux is increased after addition of IO. (A,B) Representative [Ca2+]ER measurements after addition of 2.5 µM IO in WT (A, black trace), dKO (A, red trace), V3KO (B, green trace) and tKO (B, blue trace) MII eggs. (C) Quantification of the area under the curve (AUC) of: WT, n=39; WT versus dKO, n=37, ***P<0.0001; WT versus V3KO, n=39, *P<0.05; WT versus tKO, n=41, ***P<0.0001; and of amplitude: WT versus V3KO, ns, not significant; WT versus tKO, **P<0.001; WT versus dKO, ***P<0.0001. (D,E) Representative [Ca2+]ER measurements after TG addition in WT (D, black trace), dKO (D, red trace), V3KO (E, green trace) and tKO (E, blue trace) MII eggs. (F) AUC of WT, n=18; dKO, n=23, P=0.55; V3KO, n=10, P=0.11; tKO, n=15, P>0.99; amplitude of WT versus dKO, P>0.99; WT versus V3KO, P>0.99; WT versus tKO, *P=0.32. (G) Emission ratios of D1ER (YFP/CFP; left y-axis; WT, black solid trace; dKO, red solid trace) after addition of 2.5 µM IO in Ca2+-free medium. To perform simultaneous measurements of [Ca2+]ER and [Ca2+]i, the latter was recorded using Rhod-2AM (right y-axis; WT, gray dotted line trace; dKO, red dotted line trace). (H) Quantification of baseline YFP/CFP values during the first 5 min of recording (WT, n=24; dKO, n=13, P>0.05). (I) Quantification of baseline-normalized YFP/CFP ratios comparing values at 2.5 min and 5 min after IO. (J) Percentage change in normalized FRET values 2.5 min and 5 min after IO (**P=0.004 and *P=0.01, respectively). ns, not significant (Student's t-test).
To extend the above results and directly evaluate Ca2+ levels in the ER of WT and dKO eggs, we expressed the genetically encoded ER Ca2+ sensor, D1ER (Palmer et al., 2004). Increased [Ca2+]ER and Ca2+ binding to the calmodulin cassette of D1ER leads to an increase in fluorescence resonance energy transfer (FRET) from CFP to YFP (Palmer et al., 2004). Using D1ER in mouse oocytes and eggs, we observed and reported similar changes in FRET in response to a variety of agonists (Wakai et al., 2013). We first used D1ER fluorescence and confocal microscopy to examine the organization of the ER in WT and dKO oocytes/eggs. We found that the absence of TRPV3 and CaV3.2 did not compromise the organization of the ER in dKO oocytes, which displayed the characteristic reticular pattern of organization also observed in WT eggs (Fig. S4A–D; FitzHarris et al., 2007; Wakai et al., 2013). We next examined YFP/CFP ratios in WT and dKO eggs before adding IO to estimate basal [Ca2+]ER levels. As shown in Fig. 3G,H, the YFP/CFP baseline ratios were higher in WT (1.1±0.03) than in dKO eggs (1.07±0.06), although the difference was not significant due to the wide range in the collected values, especially in dKO eggs (P>0.05). Importantly, YFP/CFP ratios underwent a rapid decrease after addition of IO (Fig. 3H,I). The changes in [Ca2+]ER levels were greater and occurred faster in dKO eggs than in WT eggs both at 2.5 min and 5 min after IO (Fig. 3G,I,J), suggesting altered Ca2+ dynamics in dKO eggs and more efflux from the ER in dKO eggs (Fig. 3J; P<0.05). Collectively, these results show that eggs null for two Ca2+ influx channels can uptake Ca2+ into the internal stores during maturation and maintain it in MII eggs, but at a reduced level. Also, upon stimulation, dKO eggs efflux a greater proportion of Ca2+ from the ER than WT eggs, suggesting that the filling and re-filling of the internal Ca2+ stores in these cells require TRPV3 and CaV3.2 channels.
Sr2+ influx and 2-APB responses are abolished in dKO oocytes and eggs
Carvacho et al. demonstrated that TRPV3 channels mediate the Sr2+ influx that induces egg activation, leading to parthenogenesis in rodent eggs (Carvacho et al., 2013). Remarkably, in a subsequent study, the authors reported that, in GV oocytes, the Sr2+ influx occurred through a different channel(s), as oscillations persisted in V3KO GV oocytes (Carvacho et al., 2016). We therefore tested whether exposing WT and dKO GV oocytes and MII eggs to 10 mM SrCl2 induced oscillations. We found that Sr2+ failed to induce oscillations in dKO MII eggs, whereas WT eggs showed robust responses (Fig. S5A–C). Further, dKO eggs incubated with Sr2+-containing media for 2 h did not show any signs of activation, such as extrusion of the second polar body, pronucleus formation or cleavage, whereas WT showed complete egg activation (data not shown). Another way to test for the absence of TRPV3 is by examining the response to 2-APB, as it is the most used activator of TRPV3 (Chung et al., 2004; Hu et al., 2009, 2004). Here, we show that, at 200 µM, 2-APB does not induce a Sr2+ rise in dKO eggs, but it does in WT eggs (Fig. S5A,B). These data confirm and reinforce the finding that 2-APB induces a Sr2+/Ca2+ rise in eggs through the TRPV3 channel, which our dKO mice lack.
The absence of CaV3.2 is harder to test without electrophysiology, as there are no specific agonists for these channels. Nevertheless, recently published results from our laboratory suggest that CaV3.2 may be an important mediator of Sr2+ influx in GV oocytes (Ardestani et al., 2020). This is consistent with previously shown results where 10 mM SrCl2 exposure at the GV stage elicited spontaneous and irregular rises in WT and V3KO oocytes (Carvacho et al., 2016). Importantly, here we show that, unlike WT oocytes, dKO oocytes failed to mount oscillations in the presence of Sr2+ (Fig. S5D,E), confirming our published results. Together, our results show that dKO mice lack functional expression of TRPV3 and CaV3.2, and that their combined expression in oocytes contributes to the influx of divalent cations throughout maturation.
dKO eggs display oscillations with reduced frequency in response to fertilization
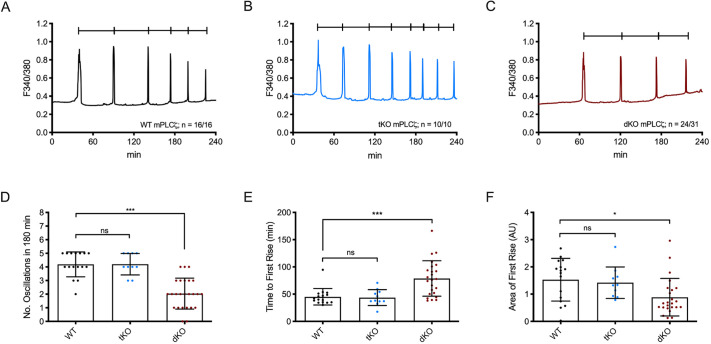
The introduction of PLCz (also known as PLCZ1) following sperm–egg fusion is thought to trigger the fertilization-associated [Ca2+]i oscillations responsible for egg activation (Saunders et al., 2002). As a surrogate of fertilization, we tested the ability of dKO eggs to mount Ca2+ oscillations following the injection of Plcz cRNA (Parrington et al., 1999, 2007; Swann et al., 2006). As shown, the time to initiation of oscillations was longer, and the mean number of Ca2+ transients in the first 180 min was lower, for dKO eggs (2.04±1.14) versus WT eggs (4.20±0.91; P=0.001) and tKO eggs (4.20±0.79) (Fig. 4A–D). The AUC of the first rise was also significantly reduced in dKO eggs versus WT eggs, with tKO eggs resembling WT eggs (Fig. 4F). Furthermore, ∼77% of the dKO injected eggs mounted oscillations compared to 100% of the injected control eggs (Fig. 4A,C; 16/16 eggs versus 24/31). Previous results with V3KO eggs also showed responses comparable to those of controls (Carvacho et al., 2013). Collectively, the diminution of all Ca2+ parameters analyzed suggests that the dKO eggs' inability to influx the necessary amount of Ca2+ to support the filling and refilling of the ER undermines the ability to initiate and maintain timely oscillations (Fig. 4E,F).
Fig. 4.
Plcz cRNA-initiated oscillations are diminished post-Plcz cRNA microinjection. (A–C) Oscillations induced by microinjection of 0.01 µg/µl mouse Plcz cRNA in WT eggs (n=16; A) versus tKO eggs (n=10; B) vs dKO eggs (n=31; C). (D) Number of oscillations within 180 min. WT:dKO, ***P<0.001 and WT:tKO, P>0.05. (E) Time to reach first rise. WT:dKO, ***P<0.001; WT:tKO, P>0.05. (F) AUC of the first rise. WT:dKO, *P<0.05 and WT:tKO, P>0.05. ns, not significant (Student's t-test).
We also evaluated in Plcz cRNA-injected eggs whether individual parameters of subsequent [Ca2+]i rises were comparable between dKO and WT eggs. We hypothesized that significant differences in certain parameters such as rise time and/or amplitude could suggest effects on other components of the Ca2+ toolkit such as IP3R1s. To test this, we chose the third Ca2+ transient and examined the rise time by measuring the slope between the first point of persistent increase in baseline Ca2+ until the value before reaching maximum amplitude (Deguchi et al., 2000). This parameter was not significantly different between groups, nor was the AUC for this rise (Fig. S6A,B). Further, to rule out that the longer time to the first rise and the subsequent longer intervals between rises in dKO eggs were due to reduced Ca2+ influx and not to the timing of injection or inability of dKO eggs to efficiently translate the Plcz cRNA, we injected another cRNA encoding for a fluorescently tagged calcium-calmodulin kinase (CaMKII) reporter, Camui, and quantified the fluorescent induced signal in WT and dKO eggs. We selected this cRNA because it is expressed quickly and its accumulation does not cause cell cycle progression. Both WT and dKO eggs displayed similar fluorescence intensities at each time point (Fig. S6C,D) indicating that dKO eggs are capable of efficiently translating injected cRNAs, and that the differences in Ca2+ parameters observed between WT and dKO eggs cannot be attributed to delayed PLCz expression.
We next examined the impact of dKO eggs on Ca2+ responses induced by the sperm during in vitro fertilization (IVF). As noted, fertilization-induced Ca2+ oscillations persisted in eggs of both TRPV3 and tKO mice (Carvacho et al., 2013; Bernhardt et al., 2015). Consistent with previous results, WT eggs displayed normal Ca2+ oscillations in response to IVF (Fig. 5A), whereas the frequency of oscillations in dKO eggs was substantially lower (Fig. 5B), with mean frequencies of 2.55±0.69 oscillations/h for WT eggs versus 1.59±0.74 for dKO eggs, and 1.4±0.51 for tKO eggs (not graphed, but included for analysis; P<0.001 for both KO groups). We also quantified the stark difference in AUC for the first transient, and observed that this parameter is significantly reduced in dKO eggs compared to WT eggs (3.73±2.2 versus 1.90±1.03 AU, P<0.001 in WT and dKO eggs, respectively; Fig. 5C). Additionally, just as after injection of Plcz cRNA, the first-rise AUC was not different between WT and tKO fertilized eggs (Fig. 5C), confirming the cumulative impact of the loss of these channels on the internal Ca2+ stores. dKO fertilized eggs also displayed a reduced total number of Ca2+ transients per egg (1.97±0.64 transients versus 5.4±2.8 transients in WT eggs; P<0.001; Fig. S6E), as well as increased lag time until the first Ca2+ transient (22.6±9.0 min for WT eggs versus 33.4±17.9 min for dKO eggs; P<0.05; Fig. S6F).
Fig. 5.
Absence of TRPV3 and CaV3.2 channels significantly affects the pattern of Ca2+ oscillations post-fertilization or following ICSI. (A,B) Oscillations induced by IVF in WT eggs (n=20) (A) versus dKO eggs (n=34) (B). (C) Quantification of parameters. Left y-axis: number of oscillations in 60 min. tKO eggs (n=15) inseminated concurrently were included in the analysis. WT:dKO, ***P<0.001; WT:tKO, ***P<0.001. Right y-axis: AUC of first rise. WT:tKO, P>0.05; WT:dKO, ***P<0.001. (D,E) Oscillations induced by ICSI in WT eggs (n=11; D) vs dKO eggs (n=11; E). (F) Quantification of ICSI parameters with the time intervals between the first and second peaks plotted on the left y-axis; WT:dKO, ***P<0.001. The numbers of oscillations within 200 min are plotted on the right y-axis; WT:dKO, ***P<0.001. ns, not significant (Student's t-test).
Lastly, we tested the ability of dKO eggs to oscillate after intracytoplasmic sperm injection (ICSI) (Fig. 5D,E). dKO eggs mounted fewer oscillations (Fig. 5F), and with irregular patterns after ICSI, compared to WT eggs. The mean time interval between the first and second rises was also much greater in dKO eggs (48.2±15.1 min versus 25.2±9.79 min for WT eggs; P<0.001; Fig. 5F). These data suggest that TRPV3 and CaV3.2 channels are not required for the initiation of fertilization, but remarkably affect the periodicity of such oscillations, and are therefore physiological contributors to the [Ca2+]i responses during mouse fertilization.
Functional TRPM7-like channels are present in eggs of dKO mice
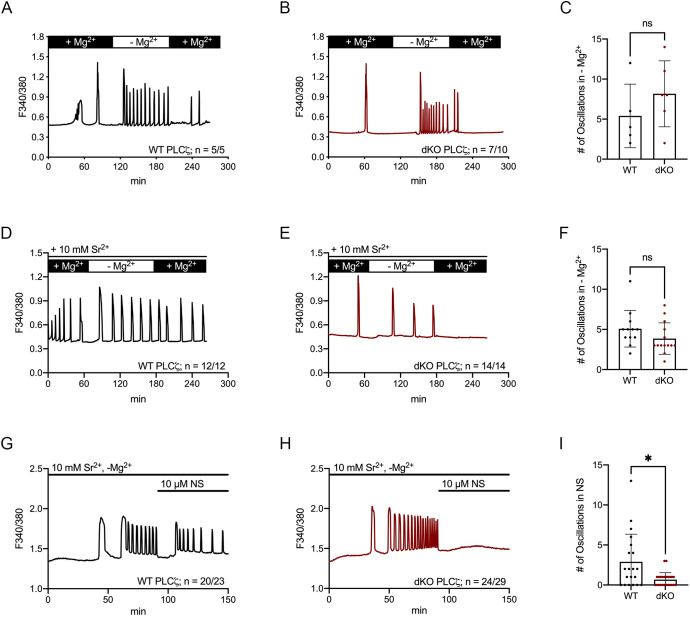
The fact that oocytes/eggs from mouse lines with separate or combined deletions of Trpv3 or Cacna1h showed partly filled [Ca2+]ER, and that fertilization-induced oscillations were slowed but not prevented, suggests the presence of Ca2+ influx by another channel(s). TRPM7 presence and functional expression have been identified in oocytes, eggs and embryos using electrophysiology (Bernhardt et al., 2018; Carvacho et al., 2016). This unique chanzyme is modulated at different levels by intracellular and extracellular concentrations of the divalent cation Mg2+ (Bates-Withers et al., 2011). Remarkably, the concentrations of Mg2+ in some commonly used culture media, including holding media such as HEPES-buffered Tyrode's lactate solution (TL-HEPES), are quite different and possibly high enough to partially obstruct channels such as the TRPM7 channel, which might compromise embryo development in several species (Herrick et al., 2015). This possible scenario was evidenced in a study in which fertilization-induced embryo development was modified by culture in media with lower concentrations of [Mg2+]o (Ozil et al., 2017), and the same study showed that the frequency of sperm-initiated oscillations was greater in the presence of low levels of [Mg2+]o (Ozil et al., 2017).
To determine whether indeed [Mg2+]o was modifying Ca2+ oscillations in our system, we monitored oscillations after expression of Plcz cRNA under conditions in which Mg2+ concentrations were sequentially altered. Consistent with the results of others, we observed that removing [Mg2+]o from the bathing medium caused a sharp increase in the frequency of oscillations in both WT and dKO eggs (Fig. 6A,B), although the mean frequency of the oscillations in Mg2+-free conditions was not significantly different between WT eggs (5.4±3.9 rises) and dKO eggs (8.17±4.1 rises; P>0.05). Bringing back Mg2+ to the original concentrations slowed the oscillations in WT eggs, although largely terminated them in dKO eggs (Fig. 6A–C). We also observed an increase in the frequency of oscillations under Mg2+-free conditions when Sr2+ was the present divalent cation (Fig. 6D–F) (P>0.05), and re-addition of Mg2+ terminated oscillations in dKO eggs but not in WT eggs (Fig. 6D,E). The presence of SrCl2-induced oscillations in Plcz cRNA-injected dKO eggs is a remarkable observation because it is the first time we are able to initiate Sr2+ oscillations in eggs devoid of TRPV3 channels. These results suggest that PLCz expression/fertilization stimulates divalent influx in MII eggs through a channel that is not TRPV3, and the fact that Sr2+ influx was susceptible to external [Mg2+]o implies that TRPM7 may be underpinning this influx in dKO eggs. We examined this possibility by adding the TRPM7 inhibitor NS8593, which acutely terminated oscillations in dKO eggs but was without effect on, or transiently slowed the responses of, WT eggs (Fig. 6G–I; WT: 2.9±3.4 rises after NS8593 versus 0.667±0.87 rises in dKO eggs; P=0.01), suggesting that TRPM7 underlies the Sr2+ oscillations in Plcz cRNA-injected dKO eggs. Similarly, in GV dKO oocytes, SrCl2 largely fails to induce oscillations, but removal of [Mg2+]o promoted them; these characteristics are also likely to be mediated by TRPM7 (Fig. S7A,B).
Fig. 6.
External Mg2+ modifies Plcz cRNA-induced oscillations in eggs. Ca2+ oscillations induced after Plcz cRNA injection in MII eggs. (A,B) Oscillations in the presence or absence of MgCl2 in WT (A) and dKO (B). (C) Number of oscillations under Mg2+-free conditions; P>0.05 (Student's t-test). (D,E) Oscillations in the presence of 10 mM SrCl2, and in the presence or absence of MgCl2, in WT (D) and dKO (E). (F) Number of oscillations under Mg2+-free conditions; P>0.05 (Student's t-test). (G,H) Oscillations in the presence of 10 mM SrCl2, Mg2+- and Ca2+-free media, and after addition of 10 µM NS8593 (NS), in WT (G) and dKO (H). (I) Number of oscillations after addition of NS8593; *P=0.01 (unpaired Student's t-test with Welch's correction). Monitoring was continuous throughout changes in MgCl2 concentrations. ns, not significant.
Finally, we hypothesized that to compensate for the lack of two crucial Ca2+ channels, the PM expression of TRPM7 would be higher in dKO eggs. We also speculated that this current would be more sensitive to the inhibitory effects of NS8593. Indeed, in response to a ramp protocol, we observed higher TRPM7-like current density values at +90 mV in dKO eggs (6.09±1.29 pA/pF) compared to WT eggs (4.31±1.25 pA/pF) (P>0.05) (Fig. 7A), and inhibition with NS8593 induced a greater decrease in outward currents in dKO eggs (1.27±0.93 pA/pF, versus dKO basal; P<0.001) than in WT eggs (1.50±0.935 pA/pF, versus WT basal; P<0.01) (Fig. 7B,C). Together, these results suggest that TRPM7 is upregulated and accounts for a greater portion of the NS8593-sensitive current in WT eggs than in dKO eggs.
Fig. 7.
dKO eggs show increased NS8593-sensitive currents. (A,B) Ramp protocol from −110 mV to +90 mV to measure TRPM7 current (HP: 0 mV). WT (black trace) versus dKO (red trace). Traces display a mean of n=4 recordings. (A) TRPM7 basal response. (B) TRPM7 response in the presence of 30 µM NS8593. (C) Mean response across ten sweeps per condition. WT basal versus WT blocked, *P=0.01; dKO basal versus dKO blocked, ***P<0.001. (D,E) Ni2+ influx was measured by the quenching of Mag-Fura2-AM. 500 µM Ni2+ was added after 5 min of recording of baseline values. (D) Representative WT trace (black, n=10). (E) Representative dKO trace (red, n=12). (F) Quantification of the quench rate; **P=0.007 (Student's t-test).
We also examined Ni2+ influx in WT and dKO eggs as TRPM7 displays high permeability to Ni2+ (Fleig and Penner, 2004; Li et al., 2006). As shown in Fig. 7D–F, Ni2+ influx, which was assessed by the rate of Mag-Fura2-AM quenching, was enhanced to a greater extent in dKO eggs than in WT eggs (WT: −28.9±6.67 versus dKO: −36.0±4.14; P=0.007). The increase in TRPM7-like currents combined with the increased Ni2+ permeability in dKO eggs suggests an increased function of TRPM7 channels in eggs lacking TRPV3 and CaV3.2 channels.
DISCUSSION
The extent to which CaV3.2 and TRPV3 channels are responsible for maintaining and increasing Ca2+ store content during maturation, as well as their combined impact on fertilization, is not fully known. Our results show that their simultaneous absence greatly impacts Ca2+ homeostasis in oocytes and eggs, and compromises the ability to initiate regularly spaced, frequent Ca2+ transients after fertilization. Yet, while diminished in frequency, [Ca2+]i oscillations are sustained, and when induced in the absence of [Mg2+]o, the frequency is enhanced. Remarkably, dKO eggs fail to mount oscillations when exposed to Sr2+, although PLCz expression restores the ability of these eggs to support Sr2+ oscillations, which are potentiated by the removal of [Mg2+]o. These results, together with the termination of oscillations caused by NS8593, suggest the participation of TRPM7 channels in sustaining the residual oscillations in dKO eggs. Collectively, our data show that CaV3.2–TRPV3 dKO eggs are a perfect platform to gain insights into the contribution of these channels to the regulation of Ca2+ homeostasis during maturation and fertilization, and assess the presence of other channels responsible for the totality of Ca2+ in oocytes and eggs.
dKO fertility
The function of CaV3.2 and TRPV3 channels, single or combined, does not appear necessary for oocyte maturation or ovulation, as a normal number of oocytes complete maturation and reach the MII stage in single-KO females (Bernhardt et al., 2015; Cheng et al., 2010; as well as our own results with dKO females in this study). Remarkably, we found a decline in the fertility of dKO females, especially after the third litter, which also coincides with parturitions occurring at greater, although inconsistent, intervals. It is presently unclear what the underlying cellular or molecular reasons that progressively compromise fertility are, as these defects are not observed in single-KO lines. Future studies should examine histological sections of the ovaries at different ages, as well as the collection of embryos following timed mating to elucidate the factor(s) compromising fertility in this model.
Ca2+ store content in eggs of dKO mice
Using Ca2+-imaging measurements after the addition of Ca2+ ionophore and/or TG, we found that dKO eggs showed vastly reduced total Ca2+ store content compared to WT eggs and even compared to eggs from single-channel KO lines. Remarkably, the levels of [Ca2+]ER appeared undisturbed in the eggs of these genetic lines except in TRPV3 KO eggs, which suggests that the remaining channel(s) and/or transporter can effectively compensate in the absence of one or two of these channels. Interestingly, using the FRET probe, D1ER, we could not observe significant differences in YFP/CFP ratios before IO addition, an estimate of basal [Ca2+]ER levels. Nevertheless, the addition of IO caused a greater and faster efflux of [Ca2+]ER in dKO eggs than in WT eggs, suggesting that the content and/or refilling of the ER is altered in dKO eggs. The lower levels of [Ca2+]ER in dKO eggs after IO addition could be explained by a bigger fractional efflux of Ca2+ in these eggs, or by a rapid and slight refilling of the stores in WT eggs from the Ca2+ present in nominal Ca2+-free medium. Regardless, the [Ca2+]ER dynamics are altered in dKO eggs. Lastly and importantly, the stores of dKO eggs were not empty, which suggests that these oocytes are still capable of Ca2+ influx. Given that mouse eggs express TRPM7, it is possible that TRPM7 mediates the partial filling of the Ca2+ stores.
Sr2+ responses in dKO oocytes and eggs
We previously reported that, unlike what occurs in WT eggs, Sr2+ is unable to induce oscillations in V3KO MII eggs, which implicates this channel in Sr2+ influx (Carvacho et al., 2013). In direct contrast to these data in MII eggs, V3KO GV oocytes display normal oscillations (Ardestani et al., 2020; Carvacho et al., 2016), suggesting that other channel(s) underlie Sr2+ influx at this stage. In cardiac Purkinje cells, research showed that CaV channels mediate divalent cation influx, including that of Sr2+ (Hirano et al., 1989a,b), and our results confirm these findings because Sr2+ oscillations were nearly absent in dKO GV oocytes. The observation that some dKO GV oocytes still displayed a few rises and that oscillations were rescued by reduction or removal of [Mg2+]o suggests TRPM7 as a candidate channel. Altogether, our results demonstrate an important switch in the regulation of divalent cation homeostasis during oocyte maturation, as whereas CaV3.2 mediates the majority of Sr2+ influx in GV oocytes (Ardestani et al., 2020), this role is taken over by TRPV3 in unfertilized MII eggs (this paper; Carvacho et al., 2013). Given the importance of divalent cation homeostasis during maturation and fertilization, the cellular and molecular mechanisms underlying this change in the channel(s) through which Sr2+, and possibly other divalent cations, gain access into the cell during oocyte maturation should be elucidated.
Ca2+ oscillations post-activation and -fertilization
Fewer dKO eggs than WT eggs initiated Ca2+ oscillations in response to a variety of stimuli including fertilization, and those that displayed oscillations showed decreased frequency, while the persistence of oscillations also seemed shortened. It is unclear how the absence of these channels undermines the mounting of robust Ca2+ responses, but a logical interpretation is that reduced Ca2+ influx causes slower refilling of the internal stores, impairing the periodicity of the oscillations (Wakai et al., 2013). There are several ways that reduced store refilling might undermine the frequency of the oscillations. One possibility is that an intra-store threshold Ca2+ level is needed to sensitize the ER IP3R1s to the prevalent cytoplasmic inositol 1,4,5-triphosphate (IP3) levels {[IP3]} (Taylor and Tovey, 2010; Vais et al., 2020). This sensitization will induce IP3R1-mediated Ca2+ release and a global Ca2+ rise. Therefore, any process that slows the accumulation of luminal Ca2+ would delay IP3R1 sensitization, increasing the intervals between rises. Alternatively, a slower Ca2+ influx could delay the steady, but subtle, Ca2+ ER leak that progressively increases basal cytosolic Ca2+, leading to the sharp upstroke of each global Ca2+ rise, slowing, therefore, the frequency of oscillations. The Ca2+ ER leak stimulates IP3R1 by a mechanism known as Ca2+-induced Ca2+ release (CICR) (Lock and Parker, 2020; Rahman and Taylor, 2009). Our D1ER sensor results suggest a pivotal role for [Ca2+]ER levels in determining the frequency of oscillations, as the lower [Ca2+]ER levels prevalent in stimulated dKO eggs would demand longer times to refill and delay IP3R1 sensitization, consequently slowing down the next [Ca2+]i rise and the overall frequency of oscillations. Lastly, it is also possible that the ER Ca2+ leak stimulates the catalytic activity of PLCz, leading to acute IP3 production and Ca2+ release, or Ca2+-induced IP3 production, which could trigger the next Ca2+ rise (Sanders et al., 2018). This possibility is, however, less likely to dictate the frequency of oscillations because the [IP3] rise is not present in the initial [Ca2+]i rises, rather it is of small magnitude even after the initial Ca2+ rises, and the [IP3] peaks occur after each Ca2+ rise (Matsu-ura et al., 2019). Nevertheless, the Ca2+-induced IP3 production that follows each [Ca2+]i rise is likely to assist in maintaining [IP3] above basal levels. Therefore, our results suggest that diminished Ca2+ influx in dKO eggs delays either the intraluminal and/or cytoplasmic sensitization of IP3R1s by Ca2+, effectively reducing the frequency of oscillations. Regardless of the specific mechanism(s), our results here are the first to show – without pharmacological manipulation, molecular overexpression or abnormal concentrations of extracellular Ca2+ – that Ca2+ influx following sperm entry is a pivotal contributor to setting the frequency of fertilization-induced Ca2+ oscillations in mammals.
The rescue of Sr2+ oscillations in dKO eggs by PLCz deserves special mention. As noted, V3KO eggs fail to mount oscillations in response to Sr2+ (Carvacho et al., 2013), a finding we extend here by showing that dKO eggs are also unable to initiate oscillations. Remarkably, injection of Plcz cRNA rescued the ability of Sr2+ to induce oscillations in dKO eggs, although how PLCz and fertilization may promote Sr2+ influx is unknown. It is possible nonetheless that activation of the phosphoinositide pathway and its downstream products, Ca2+, IP3, diacylglycerol (DAG) and PKC (also known as PRKC), singly and/or in conjunction, are able to stimulate Sr2+ influx while sensitizing the IP3R1, and thereby promote the oscillations. Consistent with this, a previous report suggested that a PKC might stimulate Ca2+ influx during fertilization (Halet, 2004), although the channel(s) targeted by the kinase and its role during fertilization are unknown. It is worth noting, however, that the PKC isoform(s) examined in the study were not the predominant isoforms expressed in mouse eggs, and only high amplitude [Ca2+]i rises stimulated the translocation of the kinase. Therefore, future studies should ascertain the precise PM channel target(s) modified by PLCz, as it may provide insights into the mechanisms of fertilization.
Functional role of TRPV3 and CaV3.2 in mouse oocytes and eggs
Given that fertilization-initiated oscillations are still present in dKO eggs, it is worth asking what the function of these channels is in oocytes and eggs. This question is especially relevant in the case of CaV3.2 channels, which are voltage-gated channels, because, in contrast to invertebrate species, mouse eggs during fertilization reportedly experience negligible changes in membrane potential (Igusa et al., 1983; Jaffe and Cross, 1984). However, those experiments were performed using conventional electrophysiological techniques that likely modified membrane integrity, thereby impeding accurate measurements of subtle membrane potential changes (Carvacho et al., 2018). Importantly, eggs display CaV3.2-like currents, although at the eggs' reigning resting membrane potential of ∼−30 mV to −40 mV (Bernhardt et al., 2015; Day et al., 1998; Peres, 1986), they are expected to remain largely inactive. Nevertheless, a portion of CaV3.2 channels may display persistent inward currents at low voltages, referred to as ‘window currents’ (Bernhardt et al., 2015; Williams et al., 1997). These currents have been detected in several cell types at or near the resting potential of unfertilized mouse oocytes and eggs (Carbone et al., 2014). Additionally, the negative reversal potential we observed in the CaV recordings of dKO eggs (Fig. 2C) might reflect the very low permeability of the membrane to ions in the absence of these two main Ca2+ influx channels, and the inhibition of TRPM7 by the high Ca2+ concentrations (Li et al., 2006) in which CaV3.2 recordings are performed. It is, therefore, possible that the small-scale ionic changes mediated by the T-type channel currents potentiate the function of another channel(s) present in eggs, as its elimination worsened the fertilization-induced oscillations in V3KO eggs (Carvacho et al., 2013) and in TRPM7 conditional knockout (cKO) eggs (Bernhardt et al., 2018). Future studies should determine whether CaV3.2 channels act in concert with other channels in oocytes and eggs to maintain internal Ca2+ stores and support oscillations.
The role of TRPV3 also needs some re-examination because Trpv3−/− eggs did not show changes in oscillation frequency post-fertilization (Carvacho et al., 2013). However, we show here that the elimination of both channels has severe effects on the eggs' Ca2+ store content and on oscillations after fertilization and/or Plcz cRNA injection, including specific reduction of the AUC of the first Ca2+ rise. Moreover, a recent study (Ardestani et al., 2020) and our results here show that elimination of TRPV3 caused a reduction in the total Ca2+ store content in GV oocytes, and a reduced [Ca2+]ER content in eggs. It is therefore plausible that these channels in mouse eggs compensate for each other's loss of function, and can sustain Ca2+ store content and oscillations in the event of the loss of a single channel. It is also possible that there are undetermined endogenous modulators of TRPV3 and CaV3.2 that are present in oocytes and eggs, and how their activity is regulated will be the subject of future studies. Finally, although the exact function(s) of these channels remain(s) unknown, we provide evidence that they contribute to the maintenance of Ca2+ homeostasis pre- and post-fertilization. Future studies should examine how the absence of one channel impacts the function of the other.
TRPM7 and residual Ca2+ influx in dKO eggs
The findings that the internal Ca2+ stores are not empty in dKO oocytes/eggs and that residual oscillations occur in dKO eggs after fertilization suggest the presence of other channels in these cells. TRPM7 has been reported to be essential for embryonic development (Jin et al., 2008), and we demonstrated its functional expression in oocytes and eggs (Bernhardt et al., 2018; Carvacho et al., 2016), which places it as a primary candidate to underpin the oscillations in dKO eggs. Consistent with this view, a recent study showed that the combined elimination of Cacna1h and Trpm7 compromised the accumulation of Ca2+ during oocyte maturation and the periodicity of sperm-induced oscillations (Bernhardt et al., 2018). Our results here show that the reduced frequency of Plcz cRNA-initiated Ca2+ oscillations and the number of oscillating dKO eggs lacking Trpv3 and Cacna1h are stimulated by lowering [Mg2+]o, a change that stimulates the function of TRPM7. Lowering [Mg2+]o also enhanced Sr2+-induced oscillations in dKO eggs, and these Plcz cRNA-induced Sr2+ oscillations were terminated in these eggs by addition of the TRPM7 inhibitor NS8395. Additionally, we found that TRPM7-like currents are higher in dKO eggs than in WT eggs, and Ni2+ influx is also enhanced in dKO eggs versus WT eggs. Therefore, it is possible that the change in expression and/or function of TRPM7 in dKO eggs is sufficient for the initiation and persistence of the low-frequency oscillations induced by fertilization. It is worth noting that the role of TRPM7 in pre-implantation embryo development is likely significant besides its role in Ca2+ influx and egg activation, given that it is also permeable to other divalent cations such as Zn2+ and Mg2+ (Bates-Withers et al., 2011), and that its functional and molecular expression increase in two-cell embryos and thereafter (Carvacho et al., 2016; Zeng et al., 2004).
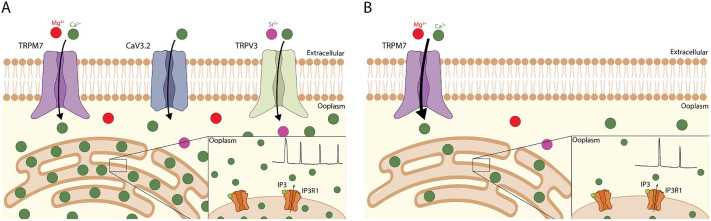
In conclusion, the initiation and persistence of Ca2+ oscillations that induce embryo development in the mouse, and possibly in other mammals, rely on the concerted action of at least three PM divalent-permeable ion channels expressed in eggs (Fig. 8). Gaining insight into the mechanism of Ca2+ influx in oocytes and eggs will aid in the design of conditions that improve developmental competence. Moreover, the identification of the complete set of channels that underlie Ca2+ influx in all mammals will make possible the development of specific channel blockers that could be turned into novel, non-hormonal methods of contraception to be used in humans or in other species to control population growth.
Fig. 8.
Ca2+ homeostasis is affected in dKO eggs. (A) Model WT MII egg expressing TRPV3 (green channel), CaV3.2 (blue channel), TRPM7 (purple channel) and ER (beige shape). Ca2+ ions (green circles), Mg2+ ions (red circles) and Sr2+ ions (magenta circles) are also shown. A WT egg is able to accumulate adequate [Ca2+]i to support the normal pattern of PLCz-induced oscillations (black Ca2+-imaging profile in inset). (B) Model dKO MII egg expressing only TRPM7 (purple channel). A dKO egg exhibits less Ca2+ influx, diminished pattern of PLCz-induced oscillations (black profile in inset) and thus decreased [Ca2+]ER. Insets: beige shape, ER lumen; orange channels, IP3R1; light-green shapes, IP3; green circles, Ca2+ ions.
MATERIALS AND METHODS
Animal husbandry
WT and dKO mice were generated by breeding a female Trpv3−/− mouse (Carvacho et al., 2013) (a generous gift from Dr H. Xu, University of Michigan, Ann Arbor, MI) with a mixed C57BL/6J and 129/SvEvTac background to a male Cacna1h−/− mouse (The Jackson Laboratory, Bar Harbor, ME) with a B6;129-Cacna1htm1Kcam/J background to generate F1 offspring heterozygous for Trpv3 and Cacna1h (dHET; +/−). Initial dKO and WT mice were obtained by intercross of dHETs and maintained on a mixed C57BL/6 and 129/SvEvTac background. Ear clips from offspring were collected prior to weaning, and confirmation of genotype was performed after most experiments.
Oocyte collection
Fully mature GV oocytes were collected from the ovaries of 6- to 10-week-old females that were superovulated by intraperitoneal (i.p.) injection of 5 IU pregnant mare serum gonadotropin (PMSG; Calbiochem, EMD Biosciences). GVs were collected and recovered into a TL-HEPES solution supplemented with 5% heat-treated fetal calf serum (FCS; Gibco) and 100 µM 3-isobutyl-1-methylxanthine (IBMX) to block spontaneous progression of meiosis. In vivo matured MII eggs were collected by i.p. injection of 5 IU human chorionic gonadotropin (hCG; Calbiochem, EMD Biosciences) 46–48 h post-PMSG stimulation. Ovulated, MII-arrested eggs were obtained by rupturing the oviducts with fine forceps in TL-HEPES solution supplemented with 5% FCS 12–14 h post-hCG stimulation. Cumulus cells were removed using 0.1% bovine testes hyaluronidase (Sigma-Aldrich, St Louis, MO) and gentle aspiration through a pipette. All procedures were performed according to research animal protocols approved by the University of Massachusetts Institutional Animal Care and Use Committee. For Fig. S2C, images were taken on a Nikon (Melville, NY) dissection microscope outfitted with a 1X shutter. For Fig. S2D, images were taken on a Nikon Diaphot microscope outfitted with an 8 megapixel camera.
Genotyping/PCR analysis
Mice were identified and genotyped using tissue from an ear clip, which was collected and lysed using tail lysis buffer (50 mM Tris-HCl pH 8.8, 1 mM EDTA pH 8, 0.5% Tween 20, 0.3 mg/ml proteinase K). Genomic DNA was then stored at −20°C for later use in PCR analysis. Mouse genotyping was routinely performed using PCR analysis followed by fractionation on a 1.2% agarose gel. For Trpv3, F7622, 5′-GACATGCCATGCAAAAAACTACCA-3′ and R28432, 5′-GTCTGTTATATGTACAGGCATGG-3′ were used. The Trpv3 WT and mutant alleles yielded products of 800 bp and 300 bp, respectively. For Cacna1h, 11395, 5′-ATTCAAGGGCTTCCACAGGGTA-3′, 11396, 5′-CATCTCAGGGCCTCTGGACCAC-3′ and oIMR2063, 5′-GCTAAAGCGCATGCTCCAGACTG-3′ were used. All primers were purchased from IDT Technologies (Coralville, IA). The Cacna1h WT and mutant alleles yielded products of 480 bp and 330 bp, respectively.
[Ca2+]i imaging and reagents
[Ca2+]i monitoring was performed as previously reported by our laboratory (Kurokawa et al., 2007). Briefly, eggs were loaded with the Ca2+ sensitive dye Fura-2-acetoxymethyl ester (Fura-2AM; Molecular Probes, Invitrogen, Carlsbad, CA). Oocytes/eggs were loaded with 1.25 µM Fura-2AM supplemented with 0.02% pluronic acid (Molecular Probes) for 20 min at room temperature. To estimate [Ca2+]i, oocytes/eggs were thoroughly washed and immobilized on a glass-bottom monitoring dish (MatTek, Ashland, MA) submersed in FCS-free TL-HEPES under mineral oil. Oocytes/eggs were monitored under a Nikon Diaphot microscope outfitted for fluorescence measurements. The objective used was a 20× Nikon Fluor. The excitation lamp was a 75 W Xenon lamp, and emitted light >510 nm was collected by a cooled Photometrics SenSys CCD camera (Roper Scientific, Tucson, AZ) using NIS-Elements software (Nikon). Oocytes/eggs were alternatively illuminated with 340 nm and 380 nm light by a MAC5000 filter wheel/shutter control box (Ludl Electronic Productions), and fluorescence was captured every 20 s.
For experiments in which the concentration of Mg2+ was changed, two blunt capillaries were secured to micromanipulators on either side of the glass-bottom dish and inserted into the monitoring drop. One capillary was attached to a perfusion manifold via polyethylene tubing leading to open syringes filled with either normal, Mg2+ containing medium or Mg2+-free medium. The other capillary was connected via polyethylene tubing to a closed syringe that acted as a manual vacuum when suction was applied with the plunger. Medium was flowed in a slow, laminar fashion over the eggs during fluorescence intervals, while suction was simultaneously applied to change the concentration of Mg2+. Complete perfusion lasted about 2 min, and monitoring was continuous throughout the process.
To examine the role of Ca2+ influx in refilling [Ca2+]ER, we monitored eggs in nominal Ca2+-free, FCS-free TL HEPES. After a 5–8-min baseline recording, [Ca2+]ER levels were assessed by the addition of 10 µM TG (Calbiochem, San Diego, CA), an inhibitor of the SERCA pump, which induced a Ca2+ leak via an unknown mechanism. TG-induced Ca2+ rises were regarded as [Ca2+]ER content that could be estimated from the AUC of the [Ca2+]i rise using Prism (GraphPad Software, La Jolla, CA). In other experiments, the addition of 2.5 µM IO, a Ca2+ ionophore, was used to assess the total store content of the egg. IO-induced Ca2+ rises were regarded as the total [Ca2+]i that could be estimated from the AUC of the [Ca2+]i rise using Prism. Data were first normalized to individual mean baseline values as minimum, and mean WT peak values as maximum. Data are displayed in Fig. 3C and F as a transform using y=k*y; where k represents the reciprocal of the respective mean WT values for AUC and relative maximum. Baseline was calculated from the mean of the y values from x=0 to x=5 min.
To detect Ni2+ influx, oocytes were loaded with 0.5 μM Mag-Fura-2AM (Invitrogen) supplemented with 0.02% Pluronic acid for 10 min at room temperature (RT). Monitoring of Ni2+ influx, which caused a decrease in the intensity of Mag-Fura-2 at both wavelengths (340 nm and 380 nm), was performed in nominal Mg2+-free medium. Mag-Fura-2 was excited as for Fura-2 and fluorescence captured every 20 s. Quench rate was measured by the change in F380 values over time point immediately after the addition of Ni2+ until the end of recording (∼30–35 min);
FRET and Ca2+ imaging
To estimate the relative concentrations of Camui, the emissions of CFP, YFP and ratio imaging of the Camui (YFP/CFP) were monitored using a CFP excitation filter, dichroic beam splitter, and CFP and YFP emission filters (Chroma Technology, Rockingham, VT; ET436/20X, 89007bs, ET480/40m and ET535/30m). Eggs were then attached on glass-bottom dishes and placed on the stage of an inverted microscope. CFP and YFP intensities were collected every 20 s by a cooled Photometrics SenSys CCD camera, and intensities were compared between groups under examination. The rotation of excitation and emission filter wheels was controlled using the MAC5000 filter wheel/shutter control box (Ludl) and NIS-Elements software. Imaging was performed on an inverted epifluorescence microscope using a 20× objective.
To estimate relative changes in [Ca2+]ER, emission ratio imaging of D1ER (YFP/CFP) was performed as for Camui, and as performed by us previously (Wakai et al., 2013). To measure [Ca2+]ER and [Ca2+]i simultaneously, eggs that had been injected with Cameleon D1ER cRNA were loaded with 1 µM Rhod-2AM supplemented with 0.02% pluronic acid for 40 minutes at RT. Eggs then were attached on glass-bottom dishes (MatTek) and placed on the stage of an inverted microscope. CFP, YFP and Rhod-2AM intensities were collected every 20 s by a cooled Photometrics SenSys CCD camera (Roper Scientific, Tucson, AZ). The rotation of excitation and emission filter wheels was controlled using the MAC5000 filter wheel/shutter control box (Ludl) and NIS-Elements software.
Confocal microscopy
Live-cell imaging of oocytes/eggs expressing fluorescent-tagged proteins was performed using a laser-scanning confocal microscope (Nikon A1 Resonant Confocal with six-color TIRF) fitted with a 63×, 1.4 NA oil-immersion objective lens. Images were acquired with NIS-Elements software and pseudocolored in Photoshop CS (Adobe).
Electrophysiology
Whole-cell currents were measured at 22–24°C using an Axopatch 200B amplifier digitized at 10 kHz (Digidata 1440A) and filtered at 5 kHz. Electrophysiology recordings were performed on the same day of egg isolation up to 8 h post-collection. Cumulus-free superovulated eggs were maintained in K+-supplemented simplex optimized medium with amino acids (KSOMAA) at 37°C and 5% CO2. Shortly before measurement, eggs were aspirated briefly in acid Tyrode's solution (pH 2.5) to remove the zona pellucidae. Data were analyzed using Clampfit (Molecular Devices) and GraphPad Prism. Pipettes of 1–3 MΩ resistance were made from glass capillaries (593600, A-M Systems, Sequim, WA), and typical seals of 1–3 GΩ were achieved before breaking into eggs. Series resistance was compensated by 40–60%. The intracellular solution contained the following: 152 mM Cs-methanesulfonate, 1 mM Cs-BAPTA, 10 mM HEPES, 2 mM MgATP, 0.3 mM NaGTP, 8 mM NaCl, pH 7.3 adjusted with CsOH. The external solution contained the following: 125 mM NaCl, 6 mM KCl, 20 mM CaCl2, 1.2 mM MgCl2, 20 mM HEPES-NaOH, pH: 7.4. The response to 2-APB was measured in external solution containing the following: 140 mM NaCl, 10 mM HEPES, 10 mM glucose, 4 mM KCl, 1 mM MgCl2, 2 mM CaCl2. All voltages were corrected for calculated junction potentials present between the intracellular and external solutions before seal formation. TRPV3 currents were elicited by voltage ramps from −100 mV to 100 mV (600 ms, every 2 s), in the presence of 2-APB. The holding potential was 0 mV. CaV3.2 currents were elicited by 50 ms duration depolarization steps from −100 mV to 50 mV in 10 mV increments. The holding potential was −80 mV. For experiments using inhibitors, seals were obtained in external solution containing 20 mM CaCl2 followed by equilibration in 2 mM CaCl2. All currents are displayed as current density (pA/pF). Statistical analyses (paired two-tailed Student's t-tests) were performed using GraphPad Prism.
Parthenogenetic activation
For TRPV3-mediated egg activation, oocytes/eggs were collected as described above in TL-HEPES supplemented with 5% FCS (and 100 µM IBMX for GV experiments). For Ca2+ monitoring, oocytes/eggs were loaded with Fura-2AM, then immobilized to a glass-bottom monitoring dish (MatTek) under nominal Ca2+- and FCS-free TL-HEPES supplemented with 10 mM SrCl2 (and 100 µM IBMX for GV experiments) submersed in mineral oil. For activation, eggs were incubated in 5% CO2 at 37°C for 2 h in Ca2+-free Chatot, Ziomek or Bavister (Chatot et al., 1989) medium supplemented with either 3 mg/ml bovine serum albumin (BSA; Sigma-Aldrich) or 0.01% polyvinyl alcohol and 10 mM SrCl2. Eggs were then washed and transferred to KSOMAA, and cultured to the two-cell stage. Eggs were evaluated at 5–6 h and 22–24 h post-treatment under phase-contrast microscopy. Activated eggs were classified according to the following criteria: (1) pronucleus (PN) group, zygotes forming a single PN with first and second polar bodies (5 h post-treatment); (2) cleaved group, eggs undergoing immediate cleavage after 24 h. Eggs without second polar bodies or PN formation, or those failing to cleave, were considered as non-activated (MII egg). Fragmented eggs were excluded from analysis.
Preparation of cRNAs and microinjections
The sequences encoding for Camui (generously gifted by Dr Margaret Stratton, University of Massachusetts Amherst, Amherst, MA) and the full-length of mouse Plcz cDNA, a kind gift from Dr K. Fukami (Tokyo University of Pharmacy and Life Science, Tokyo, Japan) were subcloned into a pcDNA6 vector (pcDNA6/Myc-His B; Invitrogen). Plasmids were linearized with a restriction enzyme downstream of the insert, and cDNAs were in vitro transcribed using the T7 or SP6 mMESSAGE mMACHINE Kit (Ambion, Austin, TX) as previously described according to the promoter present in the construct. A Poly (A)-tail was added to the mRNAs using a Tailing Kit (Ambion), and poly(A)-tailed RNAs were eluted with RNAase-free water and stored in aliquots at −80°C. Microinjections were performed as described previously (Lee et al., 2016). cRNAs were centrifuged, and the top 1–2 ml was used to prepare micro drops from which glass micropipettes were loaded by aspiration. cRNAs were delivered into eggs by pneumatic pressure (PLI-100 picoinjector, Harvard Apparatus, Cambridge, MA). Each egg received 5–10 pl, which is ∼1–3% of the total volume of the egg. Injected MII eggs were allowed for translation up to 4 h in KSOMAA. AUC was calculated via the integral of the first transient from x1 (first point of inflection) to x2 (last point before return to baseline).
Sperm isolation
Spermatozoa for IVF procedures were obtained from 10- to 16-week-old male CD1 mice. The cauda epididymis of the sacrificed male was collected and sliced with scissors in 500 µl Toyoda, Yokoyama, Hosi (TYH) medium supplemented with 4 mg/ml BSA. The epididymis was incubated for 10–15 min at 37°C and 5% CO2 in TYH after which it was removed, whereas sperm were incubated for an additional 1 h under the same conditions.
IVF
For standard IVF, expanded cumulus–oocyte complexes were released from the oviduct and directly transferred to 90 µl drops of TYH medium supplemented with 4 mg/ml BSA that was equilibrated overnight in 5% CO2 at 37°C, and 0.1–0.3×106 sperm/ml were added. Complexes were incubated for 1 h, washed of excess sperm and loaded with Fura-2AM for Ca2+ monitoring as described above. We also performed IVF in zona-free oocytes to detect [Ca2+]i responses in WT and dKO mice. Procedures were performed as previously described (Bernhardt et al., 2015).
Statistical analysis
Values from three or more experiments performed on different batches of eggs were used for evaluation of statistical significance. Prism was used to perform the unpaired Student's t-tests, one-way ANOVA, chi-square calculations and graph productions. All data are presented as mean±s.d. Differences were considered significant at P<0.05.
Chemical reagents
IO, TG, PMSG and hCG were purchased from Calbiochem. Fura-2AM, Mag-Fura-2AM and pluronic acid were purchased from Invitrogen. All other chemicals were from Sigma-Aldrich, unless otherwise specified.
Supplementary Material
Acknowledgements
We thank Ms Changli He for technical support, Dr James Chambers for electrophysiology and microscopy support, and Ms Cristina Parrella for assistance with maintaining a breeding colony of mice and preliminary culture studies of pre-implantation embryos.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.M., G.A., I.C., R.F.; Methodology: A.M., G.A., I.C., R.F.; Software: A.M., I.C.; Validation: A.M., H.A., I.C., R.F.; Formal analysis: A.M., I.C.; Investigation: A.M., G.A., H.A., I.C., R.F.; Resources: R.F.; Data curation: A.M., H.A., I.C., R.F.; Writing - original draft: A.M., G.A., I.C., R.F.; Writing - review & editing: A.M., G.A., I.C., R.F.; Supervision: I.C., R.F.; Project administration: R.F.; Funding acquisition: R.F.
Funding
These studies were supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) [HD051872 and HD092499 to R.F.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Deposited in PMC for release after 12 months.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.257956
References
- Ardestani, G., Mehregan, A., Fleig, A., Horgen, F. D., Carvacho, I. and Fissore, R. A. (2020). Divalent cation influx and calcium homeostasis in germinal vesicle mouse oocytes. Cell Calcium 87, 102181. 10.1016/j.ceca.2020.102181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates-Withers, C., Sah, R. and Clapham, D. E. (2011). TRPM7, the Mg 2+ inhibited channel and kinase. Adv. Exp. Med. Biol. 704, 173-183. 10.1007/978-94-007-0265-3_9 [DOI] [PubMed] [Google Scholar]
- Bernhardt, M. L., Zhang, Y., Erxleben, C. F., Padilla-Banks, E., McDonough, C. E., Miao, Y. L., Armstrong, D. L. and Williams, C. J. E. (2015). CaV3.2 T-type channels mediate Ca2+ entry during oocyte maturation and following fertilization. J. Cell Sci. 93, 13004. 10.1242/jcs.180026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt, M. L., Stein, P., Carvacho, I., Krapp, C., Ardestani, G., Mehregan, A., Umbach, D. M., Bartolomei, M. S., Fissore, R. A. and Williams, C. J. (2018). TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization. Proc. Natl. Acad. Sci. USA 115, E10370-E10378. 10.1073/pnas.1810422115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, M. J. (2002). The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32, 235-249. 10.1016/S0143416002001823 [DOI] [PubMed] [Google Scholar]
- Berridge, M. J., Lipp, P. and Bootman, M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11-21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Berridge, M. J., Bootman, M. D. and Roderick, H. L. (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517-529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Carbone, E., Calorio, C. and Vandael, D. H. F. (2014). T-type channel-mediated neurotransmitter release. Pflugers Arch. Eur. J. Physiol. 466, 677-687. 10.1007/s00424-014-1489-z [DOI] [PubMed] [Google Scholar]
- Carvacho, I., Lee, H. C., Fissore, R. A. and Clapham, D. E. (2013). TRPV3 Channels mediate strontium-induced mouse-egg activation. Cell Rep. 5, 1375-1386. 10.1016/j.celrep.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho, I., Ardestani, G., Lee, H. C., McGarvey, K., Fissore, R. A. and Lykke-Hartmann, K. (2016). TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci. Rep. 6, 34236. 10.1038/srep34236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho, I., Piesche, M., Maier, T. J. and Machaca, K. (2018). Ion channel function during oocyte maturation and fertilization. Front. Cell Dev. Biol. 6, 63. 10.3389/fcell.2018.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatot, C. L., Ziomek, C. A., Bavister, B. D., Lewis, J. L. and Torres, I. (1989). An improved culture medium supports development of random-bred 1-cel mouse embryos in vitro. J. Reprod. Fertil. 86, 679-688. 10.1530/jrf.0.0860679 [DOI] [PubMed] [Google Scholar]
- Chen, C.-C., Lamping, K. G., Nuno, D. W., Barresi, R., Prouty, S. J., Lavoie, J. L., Cribbs, L. L., England, S. K., Sigmund, C. D., Weiss, R. M.et al. (2003). Abnormal coronary function in mice deficient in α1H T-type Ca2+ channels. Science (80-.) 302, 1416-1418. 10.1126/science.1089268 [DOI] [PubMed] [Google Scholar]
- Cheng, X., Jin, J., Hu, L., Shen, D., Dong, X.-P., Samie, M. A., Knoff, J., Eisinger, B., Liu, M.-L., Huang, S. M.et al. (2010). TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331-343. 10.1016/j.cell.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, M. K., Lee, H., Mizuno, A., Suzuki, M. and Caterina, M. J. (2004). 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 24, 5177-5182. 10.1523/JNEUROSCI.0934-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, M. L., Johnson, M. H. and Cook, D. I. (1998). Cell cycle regulation of a T-type calcium current in early mouse embryos. Pflugers Arch. Eur. J. Physiol. 436, 834-842. 10.1007/s004240050712 [DOI] [PubMed] [Google Scholar]
- Deguchi, R., Shirakawa, H., Oda, S., Mohri, T. and Miyazaki, S. (2000). Spatiotemporal analysis of Ca2+ waves in relation to the sperm entry site and animal-vegetal axis during Ca2+ oscillations in fertilized mouse eggs. Dev. Biol. 218, 299-313. 10.1006/dbio.1999.9573 [DOI] [PubMed] [Google Scholar]
- Ducibella, T., Huneau, D., Angelichio, E., Xu, Z., Schultz, R. M., Kopf, G. S., Fissore, R., Madoux, S. and Ozil, J.-P. (2002). Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev. Biol. 250, 280-291. 10.1006/dbio.2002.0788 [DOI] [PubMed] [Google Scholar]
- FitzHarris, G., Marangos, P. and Carroll, J. (2007). Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev. Biol. 305, 133-144. 10.1016/j.ydbio.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Fleig, A. and Penner, R. (2004). The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol. Sci. 25, 633-639. 10.1016/j.tips.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Florman, H. M. and Fissore, R. A. (2015). Fertilization in mammals. In Knobil and Neill's Physiology of Reproduction (ed. Plant T. M. and Zeleznik A. J.), Volume 1, pp. 149-196. New York, NY, USA: Academic Press. [Google Scholar]
- Gordo, A. C., Rodrigues, P., Kurokawa, M., Jellerette, T., Exley, G. E., Warner, C. and Fissore, R. (2002). Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol. Reprod. 66, 1828-1837. 10.1095/biolreprod66.6.1828 [DOI] [PubMed] [Google Scholar]
- Halet, G. (2004). PKC signaling at fertilization in mammalian eggs. Biochim. Biophys. Acta Mol. Cell Res. 1742, 185-189. 10.1016/j.bbamcr.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Herrick, J. R., Strauss, K. J., Schneiderman, A., Rawlins, M., Stevens, J., Schoolcraft, W. B. and Krisher, R. L. (2015). The beneficial effects of reduced magnesium during the oocyte-to-embryo transition are conserved in mice, domestic cats and humans. Reprod. Fertil. Dev. 27, 323-331. 10.1071/RD13268 [DOI] [PubMed] [Google Scholar]
- Hille, B. (2001). Ion Channels of Excitable Membranes, 3rd edn, pp. 112-113. Sunderland, MA, USA: Sinauer Associates, Inc. [Google Scholar]
- Hirano, Y., Fozzard, H. A. and January, C. T. (1989a). Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje fibers. Am. J. Physiol. Heart Circ. Physiol. 256, H1478-H1492. 10.1152/ajpheart.1989.256.5.H1478 [DOI] [PubMed] [Google Scholar]
- Hirano, Y., Fozzard, H. A. and January, C. T. (1989b). Inactivation properties of T-type calcium current in canine cardiac Purkinje cells. Biophys. J. 56, 1007-1016. 10.1016/S0006-3495(89)82745-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner, V. L. and Wolfner, M. F. (2008). Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev. Dyn. 237, 527-544. 10.1002/dvdy.21454 [DOI] [PubMed] [Google Scholar]
- Hu, H.-Z., Gu, Q., Wang, C., Colton, C. K., Tang, J., Kinoshita-Kawada, M., Lee, L. Y., Wood, J. D. and Zhu, M. X. (2004). 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 279, 35741-35748. 10.1074/jbc.M404164200 [DOI] [PubMed] [Google Scholar]
- Hu, H., Grandl, J., Bandell, M., Petrus, M. and Patapoutian, A. (2009). Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc. Natl. Acad. Sci. USA 106, 1626-1631. 10.1073/pnas.0812209106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa, Y., Miyazaki, S.-I. and Yamashita, N. (1983). Periodic hyperpolarizing responses in hamster and mouse eggs fertilized with mouse sperm. J. Physiol. 340, 633-647. 10.1113/jphysiol.1983.sp014784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, L. A. and Cross, N. L. (1984). Electrical properties of vertebrate oocyte membranes. Biol. Reprod. 30, 50-54. 10.1095/biolreprod30.1.50 [DOI] [PubMed] [Google Scholar]
- Jin, J., Desai, B. N., Navarro, B., Donovan, A., Andrews, N. C. and Clapham, D. E. (2008). Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science (80-.) 322, 756-760. 10.1126/science.1163493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. T., Carroll, J. and Whittingham, D. G. (1995). Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J. Biol. Chem. 270, 6671-6677. 10.1074/jbc.270.12.6671 [DOI] [PubMed] [Google Scholar]
- Kang, D., Hur, C.-G., Park, J.-Y., Han, J. and Hong, S.-G. (2007). Acetylcholine increases Ca2+ influx by activation of CaMKII in mouse oocytes. Biochem. Biophys. Res. Commun. 360, 476-482. 10.1016/j.bbrc.2007.06.083 [DOI] [PubMed] [Google Scholar]
- Kline, D. and Kline, J. T. (1992). Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol. 149, 80-89. 10.1016/0012-1606(92)90265-I [DOI] [PubMed] [Google Scholar]
- Kurokawa, M., Yoon, S. Y., Alfandari, D., Fukami, K., Sato, K. I. and Fissore, R. A. (2007). Proteolytic processing of phospholipase Cζ and [Ca2+]i oscillations during mammalian fertilization. Dev. Biol. 312, 407-418. 10.1016/j.ydbio.2007.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. C., Yoon, S.-Y., Lykke-Hartmann, K., Fissore, R. A. and Carvacho, I. (2016). TRPV3 channels mediate Ca2+ influx induced by 2-APB in mouse eggs. Cell Calcium 59, 21-31. 10.1016/j.ceca.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Li, M., Jiang, J. and Yue, L. (2006). Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 127, 525-537. 10.1085/jgp.200609502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock, J. T. and Parker, I. (2020). Ip3 mediated global Ca2+ signals arise through two temporally and spatially distinct modes of Ca2+ release. eLife 9, e55008. 10.7554/eLife.55008.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, T., Kanaji, T., Nakade, S., Kanno, T. and Mikoshiba, K. (1997). 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(l,4,5)P3-induced Ca2+ release. J. Biochem. 122, 498-505. 10.1093/oxfordjournals.jbchem.a021780 [DOI] [PubMed] [Google Scholar]
- Matsu-ura, T., Shirakawa, H., Suzuki, K. G. N., Miyamoto, A., Sugiura, K., Michikawa, T., Kusumi, A. and Mikoshiba, K. (2019). Dual-FRET imaging of IP 3 and Ca 2+ revealed Ca 2+ -induced IP 3 production maintains long lasting Ca 2+ oscillations in fertilized mouse eggs. Sci. Rep. 9, 4829. 10.1038/s41598-019-40931-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, S.-I. and Igusa, Y. (1981). Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature 290, 702-704. 10.1038/290702a0 [DOI] [PubMed] [Google Scholar]
- Ozil, J.-P., Markoulaki, S., Toth, S., Matson, S., Banrezes, B., Knott, J. G., Schultz, R. M., Huneau, D. and Ducibella, T. (2005). Egg activation events are regulated by the duration of a sustained [Ca 2+]cyt signal in the mouse. Dev. Biol. 282, 39-54. 10.1016/j.ydbio.2005.02.035 [DOI] [PubMed] [Google Scholar]
- Ozil, J.-P., Sainte-Beuve, T. and Banrezes, B. (2017). [Mg2+]o/[Ca2+]o determines Ca2+ response at fertilization: Tuning of adult phenotype? Reproduction 154, 675-693. 10.1530/REP-16-0057 [DOI] [PubMed] [Google Scholar]
- Palmer, A. E., Jin, C., Reed, J. C. and Tsien, R. Y. (2004). Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA 101, 17404-17409. 10.1073/pnas.0408030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington, J., Jones, K. T., Lai, F. A. and Swann, K. (1999). The soluble sperm factor that causes Ca2+ release from sea-urchin (Lytechinus pictus) egg homogenates also triggers Ca2+ oscillations after injection into mouse eggs. Biochem. J. 341, 1-4. 10.1042/bj3410001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington, J., Davis, L. C., Galione, A. and Wessel, G. (2007). Flipping the switch: How a sperm activates the egg at fertilization. Dev. Dyn. 236, 2027-2038. 10.1002/dvdy.21255 [DOI] [PubMed] [Google Scholar]
- Peres, A. (1986). Resting membrane potential and inward current properties of mouse ovarian oocytes and eggs. Pflügers Arch. Eur. J. Physiol. 407, 534-540. 10.1007/BF00657512 [DOI] [PubMed] [Google Scholar]
- Peres, A. (1987). The calcium current of mouse egg measured in physiological calcium and temperature conditions. J. Physiol. 391, 573-588. 10.1113/jphysiol.1987.sp016757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, T. and Taylor, C. W. (2009). Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels. 3, 226-232. 10.4161/chan.3.4.9247 [DOI] [PubMed] [Google Scholar]
- Sanders, J. R., Ashley, B., Moon, A., Woolley, T. E. and Swann, K. (2018). PLCζ induced Ca2+ oscillations in mouse eggs involve a positive feedback cycle of Ca2+ induced InsP3 formation from cytoplasmic PIP2. Front. Cell Dev. Biol. 6, 780. 10.3389/fcell.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, C. M., Larman, M. G., Parrington, J., Cox, L. J., Royse, J., Blayney, L. M., Swann, K. and Lai, F. A. (2002). PLCζ: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129, 3533-3544. 10.1242/dev.129.15.3533 [DOI] [PubMed] [Google Scholar]
- Swann, K., Saunders, C. M., Rogers, N. T. and Lai, F. A. (2006). PLCζ(zeta): A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin. Cell Dev. Biol. 17, 264-273. 10.1016/j.semcdb.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Taylor, C. W. and Tovey, S. C. (2010). IP(3) receptors: toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2, a004010. 10.1101/cshperspect.a004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais, H., Wang, M., Mallilankaraman, K., Payne, R., McKennan, C., Lock, J. T., Spruce, L. A., Fiest, C., Chan, M. Y.-L., Parker, I.et al. (2020). Er-luminal [ca2+] regulation of insp3 receptor gating mediated by an er-luminal peripheral ca2+-binding protein. eLife 9, e53531. 10.7554/eLife.53531.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai, T. and Fissore, R. A. (2013). Ca2+ homeostasis and regulation of ER Ca2+ in mammalian oocytes/eggs. Cell Calcium 53, 63-67. 10.1016/j.ceca.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai, T., Vanderheyden, V. and Fissore, R. A. (2011). Ca2+ signaling during mammalian fertilization: requirements, players, and adaptations. Cold Spring Harb. Perspect. Biol. 3, a006767. 10.1101/cshperspect.a006767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai, T., Zhang, N., Vangheluwe, P. and Fissore, R. A. (2013). Regulation of endoplasmic reticulum Ca2+ oscillations in mammalian eggs. J. Cell Sci. 126, 5714-5724. 10.1242/jcs.136549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker, M. (2006). Calcium at fertilization and in early development. Physiol. Rev. 86, 25-88. 10.1152/physrev.00023.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S. R., Tóth, T. I., Turner, J. P., Hughes, S. W. and Crunelli, V. (1997). The “window” component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J. Physiol. 505, 689-705. 10.1111/j.1469-7793.1997.689ba.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L.-J., Sweet, T.-B. and Clapham, D. E. (2010). International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the Mammalian TRP ion channel family. Pharmacol. Rev. 62, 381-404. 10.1124/pr.110.002725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, F., Baldwin, D. A. and Schultz, R. M. (2004). Transcript profiling during preimplantation mouse development. Dev. Biol. 272, 483-496. 10.1016/j.ydbio.2004.05.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.