Abstract
We herein report a rare case of a 66-year-old man with refractory chylothorax. Although he had been treated with moderate doses of prednisolone (PSL) on suspicion of pleuritis with Sjögren syndrome, the pleural effusion expanded after the reduction of PSL. Further workup including histopathological examinations of pleura led to the diagnosis of IgG4-RD with bilateral chylothorax without any leakage from the thoracic duct. Combination therapy with high-dose PSL plus rituximab successfully decreased the pleural effusion. This is a very rare case of IgG4-related pleuritis with chylothorax and the first report of its successful treatment with rituximab.
Keywords: IgG4-related disease, pleuritis, chylothorax, rituximab
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a systemic fibro-inflammatory disease characterized by serum IgG4 elevation and distinctive histopathological findings, such as lymphoplasmacytic infiltrate with abundant IgG4-positive plasma cells, storiform fibrosis, and obliterative phlebitis (1,2). Almost all organs in the body, such as the central nervous system (CNS), lacrimal glands, salivary glands, thyroid, lungs, pancreas, biliary duct, liver, gastrointestinal tract, kidneys, prostate, retroperitoneum and lymph nodes, can be affected by IgG4-RD (3).
While the lungs are involved in 9-18% of IgG4-RD patients (4-7), pleural involvement is observed in only 4% (4,5). Pleural effusion is uncommon, but previous reports have shown that it is usually exudative (8).
Chylothorax, which is characterized by milky-appearing pleural fluid with elevated triglyceride levels or the presence of chylomicrons, is caused by the extravasation of chyle into the pleural space due to obstruction or damage of a thoracic duct or its tributaries or transdiaphragmatic flow from the peritoneal cavity (9). The etiologies of chylothorax include several causes, such as trauma (surgical or non-surgical), malignancy, lymphatic disorders, infection, chylous ascites, and other miscellaneous causes (10); however, chylothorax due to IgG4-RD has almost never been reported.
We experienced a rare case of IgG4-RD with refractory chylothorax that was successfully treated with high-dose prednisolone (PSL) and rituximab (RTX). We report this case with a review of previous case reports of IgG4-related pleuritis.
Case Report
A 66-year-old Japanese man with a history of pollen allergy and thyroidectomy for Graves-Basedow disease was admitted to another hospital with a 2-month history of leg edema, eyelid edema, and dyspnea on exertion. Computed tomography (CT) demonstrated pleural and pericardial effusions, and a pericardiocentesis revealed the fluid as a nonspecific inflammatory effusion with increased numbers of lymphocytes without any infection. Increasing the levothyroxine dose for latent hypothyroidism and initiation of furosemide therapy did not decrease the effusion. He was transferred to our department.
At his first admission to our hospital, whole-body CT demonstrated pericardial effusion, bilateral pleural effusion, and testicular hydrocele. No swelling of the lacrimal or salivary glands nor pancreatic enlargement was observed. The right pleural effusion was exudative with a total cell count of 2,410/mm3 (lymphocytes, 75%) and neither malignant cells nor bacteria. Serum anti-SS-A/Ro antibody was slightly positive (15.4 U/mL; normal range, <10.0 U/mL) on an enzyme-linked immunosorbent assay but negative with the double immunodiffusion method. Other autoantibodies, including anti-SS-B/La, anti-CCP, anti-dsDNA, anti-RNP, anti-Scl70, and anti-neutrophil cytoplasmic antibodies, were all negative. Sialometry showed a rate of 1.008 mL/minute (within normal range), while salivary gland scintigraphy showed a slightly decreased uptake and secretory function. A lip biopsy demonstrated grade 2 lymphocytic infiltration according to Greenspan's classification (11), with only a few IgG4-positive plasma cells. The Schirmer test and rose bengal dye staining test were positive only in the left eye. Although he did not meet the ACR 2012 classification criteria (12), we suspected Sjögren syndrome with serositis.
PSL 40 mg/day (0.5 mg/kg) was initiated. Both the pleural and pericardial effusion amount decreased; however, tapering of the PSL led to exacerbation of the pleural effusion.
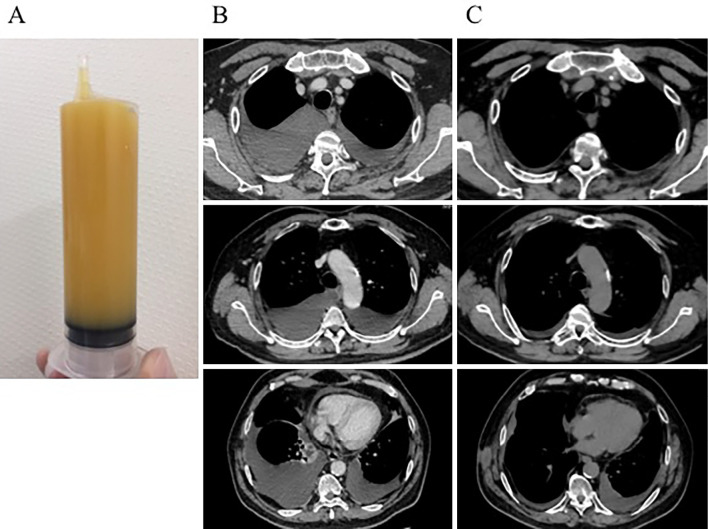
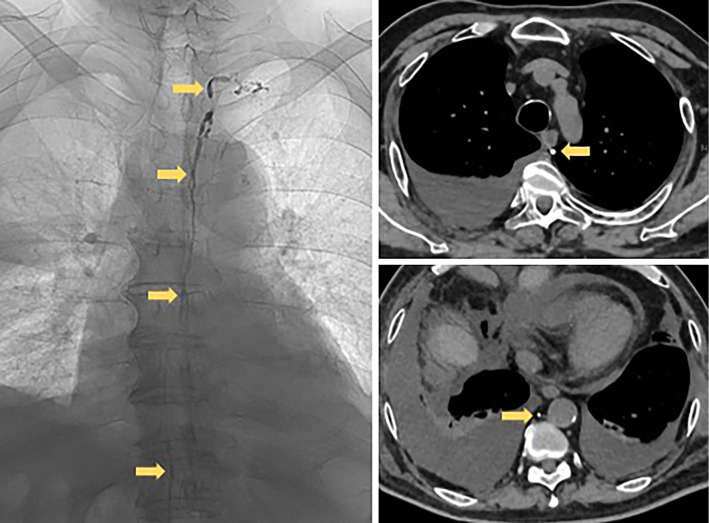
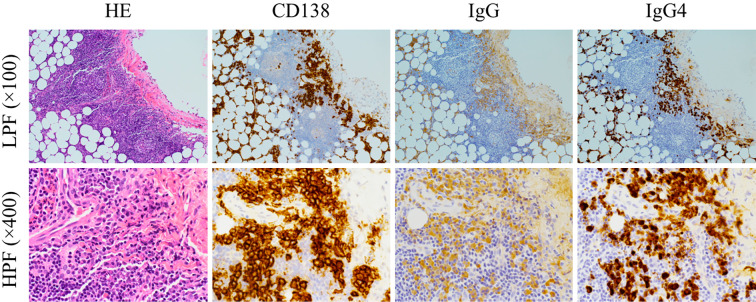
At his second admission, he presented with swelling of the lacrimal glands. Laboratory data are shown in Table 1. The white blood cell count in the peripheral blood was 8,200/μL (neutrophil 87.7%, lymphocyte 8.7%). Serum IgG and IgG4 levels were 1,500 mg/dL and 264 mg/dL, respectively. Autoantibodies were all negative. Serum levels of C-reactive protein (CRP), soluble interleukin-2 receptor (IL-2R), free T3, free T4, and thyroid-stimulating hormone (TSH) were also within the normal ranges (0.04 mg/dL, 394 U/mL, 2.9 pg/mL, 1.7 ng/dL and 1.67 μIU/mL, respectively). Chest CT showed marked bilateral pleural effusion with passive atelectasis and slight pericardial effusion (Fig. 1B). Thoracentesis for the right pleural effusion (Table 1) revealed turbid yellow fluid (Fig. 1A) with a total cell count of 810/mm3 (lymphocytes 84%), total protein 5.7 g/dL, adenosine deaminase (ADA) 25.1 U/L, total cholesterol 84 mg/dL, triglyceride 300 mg/dL, and the presence of chylomicrons, compatible with chylothorax. A cytologic examination was negative for malignancy. General bacterial and mycobacterial cultures of the pleural fluid were negative. He had no history of trauma or thoracic surgery. Lymphangiography did not show any leakage or obstruction of the thoracic duct (Fig. 2). While thoracoscopy did not reveal any tumor, amyloid deposits, or leakage from the thoracic duct, a thoracoscopic surgical pleural biopsy demonstrated infiltration by lymphocytes and plasma cells with ectopic germinal centers under the pleural mesothelium. Approximately 50% of IgG-positive plasma cells were IgG4-positive (Fig. 3). Storiform fibrosis and obstructive phlebitis were not found in this small specimen.
Table 1.
Laboratory Data on 2nd Admission.
| Peripheral blood | CRP | 0.04 | mg/dL | Pleural effusion | ||||||||||
| WBC | 8,200 | /μL | ESR | 15 | mm/hr | Cell count | 810 | /μL | ||||||
| Neut | 87.7 | % | Ferritin | 85 | ng/mL | poly | 0 | /μL | ||||||
| Lymp | 8.7 | % | IgG | 1,500 | mg/dL | mono | 680 | /μL | ||||||
| Mono | 3.2 | % | IgG4 | 264 | mg/dL | others | 130 | /μL | ||||||
| Eosino | 0.2 | % | IgA | 226 | mg/dL | pH | 7.5 | |||||||
| Baso | 0.2 | % | IgM | 70 | mg/dL | specific gravity | 1.016 | |||||||
| RBC | 5.05 | ×106/μL | C3 | 109 | mg/dL | TP | 5.7 | g/dL | ||||||
| Hb | 15.5 | g/dL | C4 | 24 | mg/dL | Alb | 3.3 | g/dL | ||||||
| Plt | 288 | ×103/μL | CH50 | 59.3 | U/mL | Amy | 45 | U/L | ||||||
| Cryoglobulin | negative | Glu | 131 | mg/dL | ||||||||||
| Biochemistry/Serology | LDH | 88 | U/L | |||||||||||
| TP | 7.4 | g/dL | Antibody | T-cho | 84 | mg/dL | ||||||||
| Alb | 4.2 | g/dL | ANA | negative | TG | 300 | mg/dL | |||||||
| T-Bil | 0.5 | mg/dL | SS-A | 3.3 | U/mL | ADA | 25.1 | U/L | ||||||
| BUN | 24.2 | mg/dL | SS-B | <1.0 | U/mL | Hyaluronic Acid | 6,670 | ng/mL | ||||||
| Cre | 1.25 | mg/dL | ds-DNA | 3.8 | U/mL | CEA | 1.8 | ng/mL | ||||||
| UA | 9.2 | mg/dL | RNP | <2.0 | U/mL | |||||||||
| Na | 139.5 | mEq/L | MPO-ANCA | <1.0 | U/mL | |||||||||
| K | 3.8 | mEq/L | PR3-ANCA | <1.0 | U/mL | |||||||||
| Cl | 100 | mEq/L | ||||||||||||
| AST | 13 | U/L | Tumor marker | |||||||||||
| ALT | 10 | U/L | sIL-2R | 394 | U/mL | |||||||||
| LDH | 163 | U/L | CEA | 1.6 | ng/mL | |||||||||
| ALP | 235 | U/L | CYFRA | 1.7 | ng/mL | |||||||||
| γ-GTP | 55 | U/L | SCC | 0.7 | ng/mL | |||||||||
| Amy | 62 | U/L | proGRP | 41.5 | ng/mL | |||||||||
| CK | 70 | U/L | ||||||||||||
| T-cho | 192 | mg/dL | Infection marker | |||||||||||
| HbA1c | 7.0 | % | procalcitonin | <0.03 | ng/mL | |||||||||
| KL-6 | 193 | U/mL | T-SPOT | negative | ||||||||||
| BNP | 8.0 | pg/mL | GPLcore-IgA | negative | ||||||||||
| fT3 | 2.9 | ng/dL | CMV-Ag | negative | ||||||||||
| fT4 | 1.7 | ng/dL | β-D | <6.0 | pg/mL | |||||||||
| TSH | 1.67 | μIU/mL | ||||||||||||
TP: total protein, Alb: albumin, T-Bil: total bilirubin, BUN: blood urea nitrogen, Cre: creatinine, UA: uric acid, Amy: amylase, T-cho: total cholesterol, KL-6: Krebs von den Lungen-6, BNP: brain natriuretic peptide, fT3: free triiodothyronine, fT4: free thyroxine, TSH: thyroid-stimulating hormone, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, ANA: antinuclear antibody, SS-A: anti-SS-A/Ro antibody, SS-B: anti-SS-B/La antibody, ds-DNA: anti-double-stranded DNA antibody, RNP: anti-RNP antibody, MPO-ANCA: myeloperoxidase-anti-neutrophil cytoplasmic antibody, PR3-ANCA: proteinase3-anti-neutrophil antibody, sIL-2R: soluble interleukin-2 receptor, GPLcore-IgA: glycopeptidolipid core IgA antibody, CMV-Ag: cytomegalovirus antigenemia, β-D: (1-3-)β-D-glucan, ADA: adenosine deaminase
Figure 1.
Pleural effusion and CT images before and after treatment. The right pleural effusion appeared as turbid yellow fluid (A). Bilateral pleural effusion and pericardial effusion were seen at the second admission (B). After treatment with a combination of high-dose corticosteroids and rituximab, the pleural and pericardial effusion was significantly decreased (C).
Figure 2.
Lymphangiography images. Lymphangiography showed the intact structure of the thoracic duct (yellow arrows) and revealed no leakage or obstruction of the duct.
Figure 3.
Histopathological images from the pleural biopsy. A pleural biopsy showed the infiltration of lymphocytes and plasma cells with ectopic germinal centers under the pleural mesothelium, and more than 50% of IgG-positive plasma cells were IgG4-positive. HE: Hematoxylin and Eosin staining, LPF: low-power field, HPF: high-power field
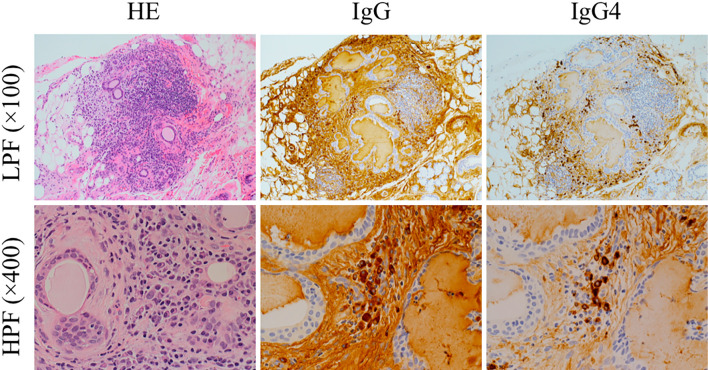
We performed another lip biopsy, and the specimen showed excessive IgG4-positive plasma cell infiltration [48 cells/high-power field (HPF)] with >50% IgG4/IgG (Fig. 4). According to the diagnostic criteria of IgG4-related respiratory disease (13), the patient met the following: i) pleural involvement with CT, ii) elevated serum IgG4 level, iii) pleural biopsy findings (lymphoplasmacytic infiltration and increased IgG4 positive cells), and iv) IgG4-related sialadenitis confirmed with a lip biopsy. Thus, we diagnosed him with “definite” IgG4-related disease.
Figure 4.
Histopathological images from the lip biopsy. A lip biopsy revealed focal IgG4-positive plasma cell infiltration, with up to 48 cells/HPF and an IgG4/IgG ratio exceeding 50%. HE: Hematoxylin and Eosin staining, LPF: low-power field, HPF: high-power field
Regarding mimickers of IgG4-RD, sarcoidosis was considered unlikely because of the lack of any elevation in the level of angiotensin-converting enzyme (ACE) or hypercalcemia on blood tests and no findings of granulomas in the biopsy specimens. The pleural specimens did not show angiocentricity or granuloma formation, so lymphomatoid granulomatosis was also deemed unlikely in this case. No fever or high CRP levels were observed in this patient, which made Multicentric Castleman disease unlikely.
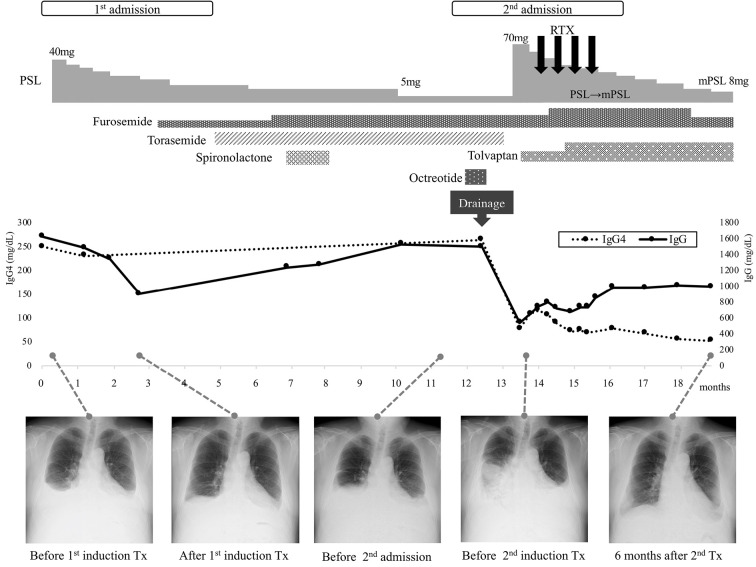
The treatment and clinical course of this patient are shown in Fig. 5. We first performed continuous drainage of the pleural fluid with fasting, intravenous hyperalimentation, and octreotide for two weeks. The octreotide was used in an off-label manner with the patient's consent to reduce lymphatic flow in the thoracic duct (14). The pleural drainage decreased the effusion temporarily, but the production of new fluid did not cease, resulting in the marked loss of serum levels of albumin, IgG, and IgG4. We started high-dose PSL 70 mg (1 mg/kg/day) with RTX (375 mg/m2, weekly, 4 times). The off-label use of RTX for IgG4-RD was approved by the authorized committee in our hospital [approval number: 2020-012] with informed consent from the patient. The pleural effusion gradually decreased. Later, we switched from PSL to methylprednisolone (mPSL) due to the latter's lower mineralocorticoid activity and better transferability to the lung (15,16). Six months from induction therapy, the pleural effusion had significantly improved (Fig. 1C).
Figure 5.
Summary of clinical course of this patient. The pleuritis showed an insufficient response to the first induction therapy with moderate-dose PSL and diuretics. However, the second induction therapy with high-dose PSL and RTX resulted in the significant improvement of pleuritis and a reduction in the serum IgG4 levels. PSL: prednisolone, mPSL: methylprednisolone, RTX: rituximab, Tx: treatment
Discussion
The present patient developed refractory chylothorax due to IgG4-RD diagnosed histopathologically with a pleural specimen. The response to a moderate dose of PSL was poor. High-dose PSL and additional RTX resulted in marked improvement. Chylothorax presenting as IgG4-related pleuritis is quite rare, and to our knowledge, this is the first report of the successful treatment of IgG4-related pleuritis with RTX.
Pleural involvement is reportedly rare; indeed, Fei et al. found that 87 of 248 patients with IgG4-RD in a prospective cohort (35.1%) had intrathoracic involvement (17), although the involvement was mainly in the lungs and lymph nodes, including hilar and mediastinal lymphadenopathy, in 52.9%, solid nodules in the lungs in 25.3%, alveolointerstitial opacities in 20.7%, round ground-glass opacities in 9.2%, and bronchovascular opacities in 20.7%. Pleural nodules and thickening were observed in 16.1%, but pleural effusion was seen in only 4.6%.
We summarized 37 previous case reports with IgG4-RD related pleuritis in Table 2 (18-48). Patients with IgG4-related pleuritis were predominantly men (78%), and the mean age was 63.5±14.7 years old. Bilateral pleural effusion was seen in 21 cases, while 11 cases (right, n=7 cases; left, n=4) had unilateral effusion. In the 24 cases with pleural effusion findings available, 22 showed an exudative pattern, while bloody effusion was seen in 2 cases and transudative effusion in 1 case. Pleural effusion cytometry revealed predominantly lymphocytes among total cases, with a high concentration of IgG4 revealed in 10 cases. Our present findings were consistent with those of previous cases with regard to the age, percentage of men, and rate of bilateral exudative pleural effusion.
Table 2.
A Summary of 37 Previous Case Reports with IgG4-RD Related Pleuritis.
| Case No. | Reference | Age | Gender | Side | Pleural effusion test | Pleural biopsy | Serum | Associated diseases | Initial PSL dose (/day) |
Immunosuppressant | PSL effect for pleuritis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell count (/μL) | Lymph (%) |
IgG (mg/dL) | IgG4 (mg/dL) | ADA (IU/L) | IgG4-positive plasma cells (/HPF) | IgG4/IgG | IgG (mg/dL) |
IgG4 (mg/dL) |
||||||||||||
| Non-chylothorax cases | ||||||||||||||||||||
| 1 | 18 | 74 | M | Right | N/D | N/D | N/D | N/D | N/D | N/D | N/D | 46 | N/D | N/D | none | none | - | Good response | ||
| 2 | 19 | 65 | M | Left | Exudative | N/D | lymp 32%, Plasma 32% | 3,005 | 1,510 | N/D | N/D | N/D | 3,142 | 1194% | Mikulicz’s disease | 30 mg | - | Good response | ||
| 3-7 (5 cases) | 20 | 62 (49-76) | M (all) | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | high in 3/3 cases (100%) | high in 2/4 cases (50%) | 3/5 cases | N/D | N/D | N/D | ||
| 8 | 21 | 63 | F | Bilateral | bloody | N/D | lymphocyte and plasma cells dominant | N/D | N/D | N/D | N/D | N/D | 2,450 | 420 | history of autoimmune pancreatitis | dose unknown | - | Good response | ||
| 9 | 22 | 78 | M | Bilateral | Exudative | N/D | mononuclear cell dominant | N/D | 590 | 34.1-46.7 | 17.6 | 85.4 | 1,604 | 483 | - | none | - | Partial response | ||
| 10 | 23 | 85 | M | Bilateral (Left dominant) | Exudative | 2,600 | 87% | 3,403 | 2,090 | 122 | N/D | N/D | 4,121 | 2,740 | salivary glands, lymph nodes, orbital lesion, bile duct, gastric glands | 30 mg | - | Good response | ||
| 11 | 24 | 73 | M | Right | Exudative (bloody) | 741 | 69% | 3,358 | 907 | 59.8 | N/D | N/D | 4,219 | 1,500 | pericarditis, retroperitoneal fibrosis | 30 mg | - | Good response | ||
| 12 | 68 | M | Left | Exudative | 4,800 | 92% | 2,809 | 571 | 104.4 | N/D | N/D | 1,471 | 372 | Mikulicz’s disease | 30 mg | - | Good response | |||
| 13 | 25 | 29 | F | Bilateral | Exudative | N/D | 93% | N/D | N/D | N/D | >30 | 92% | N/D | 136 | pericardium | 40 mg | - | Good response | ||
| 14 | 26 | 57 | M | Bilateral | N/D | N/D | N/D | N/D | N/D | N/D | N/D | >40% | N/D | 970 | none | N/D | - | Good response | ||
| 15 | 27 | 69 | M | Right | Exudative | N/D | dominant | 4,276 | N/D | 70.6 | N/D | 50% | 3,570 | 2,380 | lymph node | 0.5 mg/kg | - | Good response | ||
| 16 | 28 | 71 | F | Right | N/D | N/D | N/D | N/D | N/D | N/D | 27.3 | 84% | 1,756 | 684 | periaortitis | 40 mg | - | Good response | ||
| 17 | 29 | 74 | F | Bilateral | Exudative | N/D | N/D | N/D | N/D | normal | 91 | 91% | N/D | 740 | interstitital pneumonia | 25 mg (0.5mg/kg) | - | Good response | ||
| 18 | 30 | 48 | M | Bilateral | Exudative | N/D | lymphocyte dominant | N/D | N/D | N/D | N/D | 24% | N/D | 248 | lymph node | 0.6 mg/kg | - | Good response | ||
| 19 | 31 | 63 | M | Right | Exudative | N/D | N/D | N/D | N/D | N/D | 73 | >40% | N/D | 284 | none | 40 mg | MTX (for PsA) |
Good response | ||
| 20 | 32 | 74 | M | Left | Transudative | N/D | N/D | N/D | N/D | N/D | N/D | 30% | 1,300 | 217 | lymph node, neuromyopathy | 40 mg | - | Good response | ||
| 21 | 33 | 68 | F | Bilateral | Exudative | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | 307 | uterine enlargement | 0.6 mg/kg | - | Good response | ||
| 22 | 34 | 58 | M | Bilateral | N/D | N/D | N/D | N/D | N/D | N/D | 52 | 50% | 1,200 | 141 | none | 37.5 mg | AZP→ MTX |
Good response | ||
| 23 | 35 | 32 | M | Biateral | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | 550 | pericarditis | 30 mg | - | Good response | ||
| 24 | 36 | 78 | M | Bilateral | N/D | N/D | N/D | N/D | N/D | N/D | 100 | 70% | N/D | 760 | Sclerosing cholangitis, constrictive pericarditis | N/D | - | Good response | ||
| 25 | 37 | 70 | M | Bilateral | N/D | N/D | N/D | N/D | N/D | N/D | none (after treatment) | none (after treatment) | normal | 437 | pericarditis, Aortitis | high dose mPSL 3days→PSL 1mg/kg | CYC | Good response | ||
| 26 | 70 | M | Right | Exudative | N/D | dominant | N/D | N/D | N/D | N/D | >50% | N/D | 224 | mediastinitis | 0.6 mg/kg | - | Good response | |||
| 27 | 38 | 70 | M | Bilateral (Right dominant) | Exudative | 5,400 | 93.80% | 4,409 | 1,070 | 75.6 | >10 | >40% | 2,518 | 1,030 | none | 40 mg | - | Good response | ||
| 28 | 39 | 84 | M | Bilateral | N/D | 448 | Plasma 53% | N/D | N/D | N/D | 45 | N/D | N/D | 306 | none | 40 mg | - | Good response | ||
| 29 | 40 | 43 | F | Right | Exudative | N/D | 80-97% | N/D | N/D | 4.6-7.0 | 80 | >40% | normal | 125 | Pericardial effusion, abdominal effusion | 30 mg | - | Good response | ||
| 30 | 41 | 55 | M | Bilateral | Exudative | 1,952 | 52% | N/D | N/D | N/D | N/D | N/D | 3,260 | 534 | pericarditis, lacrimal gland, | 80 mg | MMF | N/D | ||
| 31 | 42 | 65 | M | Bilateral | Exudative | 2,700/7,160 | 90%/97% | N/D | 124/125 | 23.0/20.5 | 50 | 40% | 1,490 | 164 | none | 30 mg | AZP | Partial response | ||
| 32 | 43 | 81 | M | Bilateral | Exudative | 3,450 | 69% | N/D | N/D | 85.0 | >50 | <40% | 2,807 | 233 | none | 30 mg | - | Partial response | ||
| 33 | 44 | 70 | F | Bilateral | Exudative | N/D | N/D | 3,269 | 1,280 | 75.4 | N/D | N/D | 3,877 | >1,500 | pericarditis | N/D | N/D | Good response | ||
| 34 | 45 | 72 | F | Left | Exudative | 5,483 | 99% | N/D | N/D | 80.2 | >50 | >40% | 5,310 | >1,500 | lymph node | none | - | (naturally dissappered) | ||
| 35 | 46 | 46 | M | Bilateral | Exudative | N/D | 68% | N/D | 256 | 36.4 (normal) | 22 | 42% | N/D | 142 | N/D | 30 mg | - | Good response | ||
| Cylorhorax cases | ||||||||||||||||||||
| 36 | 47 | 69 | M | Bilateral | Exudative (Chylothorax) | 3980/5,870 | 92%/88.5% | 2,696/2,647 | 571/653 | 40.8/39.9 | N/D | 90% | 1,539 | 277 | none | 30 mg | - | Partial response | ||
| 37 | 48 | 16 | M | Bilateral | Exudative (Chylothorax) | N/D | N/D | N/D | N/D | 15 | 62 | 40% (mediastinal biopsy) | N/D | 1,650 | none | 1 mg/kg | AZP | Poor response (surgical obliteration) | ||
| present case | Sakata et al. | 66 | M | Bilateral | Exudative (Chylothorax) | 810 | 84% | N/D | N/D | 25.1 | 50% | 1500 | 264 | lacrimal and salivery gland, pericarditis | 70mg | RTX | Poor response | |||
M: male, F: female, N/D: not determined, MTX: methotrexate, AZP: azathioprine, CYC: 381 cyclophosphamide, MMF: mycophenolate mofetil, RTX: rituximab
Interestingly, in previously reported cases of IgG4-related pleuritis, 10 out of 15 cases showed high levels of ADA in the pleural fluid (>40 U/L), which is usually measured as an auxiliary tool for the diagnosis of tuberculous pleuritis (49). Although careful ruling out of tuberculous pleuritis is necessary using other examinations, such as Ziehl-Neelsen staining (50), elevated ADA levels in pleural fluid may be useful for identifying IgG4-RD pleuritis because such a condition reflects the strong activation of lymphocytes. The levels of ADA in the pleural fluid of the present case were within normal limits.
Only two previous cases of IgG4-related pleuritis presenting as chylothorax have been reported (47,48). Kato et al. reported a 69-year-old man with IgG4-related pleuritis, demonstrating right-sided chylothorax and left-sided non-chylothorax pleuritis (47). The right-side chylothorax persisted while the left-side pleuritis improved with corticosteroids. Another case, reported by Goag et al., was a young man with bilateral chylothorax (48) unresponsive to high-dose PSL with azathioprine or octreotide and a limited low-fat diet with medium-chain triglyceride supplementation. He ultimately had to undergo exploratory thoracotomy and surgical obliteration. In contrast to most non-chylothorax IgG4-pleuritis patients, who tend to show a good response to treatment, IgG4-related pleuritis with chylothorax is likely to have a poor response to PSL. The pathogenesis of chylothorax in IgG4-RD is unclear. Lymphangiography in our case did not reveal any leakage from the thoracic duct, suggesting potential micro-damage to the lymphatic channels hampering centripetal lymph propulsion from the periphery of the pleural surface. However, we lacked histological evidence of this, so more cases need to be accumulated to clarify the mechanism involved.
Many kinds of immunosuppressant drugs, such as azathioprine, methotrexate, cyclophosphamide, and mycophenolate mofetil, have been used to try to treat refractory IgG4-RD, but the optimal drug in combination with PSL is still unclear (51). Reports of the effectiveness of RTX in IgG4-RD have been increasing (51-54). Regarding the mechanisms underlying IgG4-RD, RTX, which depletes peripheral B cells, is a reasonable addition to therapy not only to prevent the repletion of short-lived plasmablasts and plasma cells but also to interfere with the maintenance of CD4+ T cell memory (55). Furthermore, a French nationwide study demonstrated the efficacy of RTX for both induction therapy and the maintenance of remission (56). Therefore, we selected RTX in our refractory case, and his pleural effusion gradually disappeared with a steroid-sparing effect. To our knowledge, this is the first case report suggesting the effectiveness of RTX in IgG4-related pleuritis.
In conclusion, we experienced a case of refractory IgG4-related pleuritis with chylothorax that was improved with high-dose PSL and RTX. More cases need to be accumulated in order to clarify the clinical manifestations of IgG4-RD pleuritis and its appropriate treatment.
We declare that we have obtained written informed consent from this patient to publish this case report.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 366: 539-551, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet 385: 1460-1471, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Umehara H, Okazaki K, Masaki Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol 22: 1-14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 34: 1812-1819, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Fernández-Codina A, Martinez-Valle F, Pinilla B, et al. IgG4-related disease: results from a multicenter Spanish registry. Medicine (Baltimore) 94: e1275, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol 67: 2466-2475, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 94: e680, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryu JH, Sekiguchi H, Yi ES. Pulmonary manifestations of immunoglobulin G4-related sclerosing disease. Eur Respir J 39: 180-186, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg 84: 15-20, 1997. [PubMed] [Google Scholar]
- 10. Riley LE, Ataya A. Clinical approach and review of causes of a chylothorax. Respir Med 157: 7-13, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Greenspan JS, Daniels TE, Talan N, Sylvester RA. The histopathology of Sjögren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol 37: 217-229, 1974. [DOI] [PubMed] [Google Scholar]
- 12. Shiboski SC, Shiboski CH, Creswell L, et al. American College of Rheumatology classification criteria for Sjögren syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 64: 475-487, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsui S, Yamamoto H, Minamoto S, Waseda Y, Mishima M, Kubo K. Proposed diagnostic criteria for IgG4-related respiratory disease. Respir Investig 54: 130-132, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med 12: 264-267, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Heming N, Sivanandamoorthy S, Meng P, Bounab R, Annane D. Immune effects of cotricosteroids in sepsis. Front Immunol 9: 1736, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vichyanond P, Irvin CG, Larsen GL, Szefler SJ, Hill MR. Penetration of corticosteroid into the lung: evidence for a difference between methylprednisolone and prednisolone. J Allergy Clin Immunol 84: 867-873, 1989. [DOI] [PubMed] [Google Scholar]
- 17. Fei Y, Shi J, Lin W, et al. Intrathoracic involvements of immunoglobulin G4-related sclerosing disease. Medicine (Baltimore) 94: e2150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamashita K, Haga H, Kobashi Y, Miyagawa-Hayashino A, Yoshizawa A, Manabe T. Lung involvement in IgG4-related lymphoplasmacytic vasculitis and interstitial fibrosis: report of 3 case and review of the literature. Am J Surg Pathol 32: 1620-1626, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Miyake K, Moriyama M, Aizawa K, et al. Peripheral CD4+ T cells showing a Th2 phenotype in a patient with Mikulicz's disease associated with lymphadenopathy and pleural effusion. Mod Rheumatol 18: 86-90, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Zen Y, Inoue D, Kitao A, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 33: 1886-1893, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Rossi G, Marchioni A, Guicciardi N, Cadioli A, Cavazza A. Recurrent pleural and pericardium effusions in a white women with IgG4-related syndrome. Am J Surg Pathol 33: 802-803, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto H, Suzuki T, Yasuo M, et al. IgG4-related pleural disease diagnosed by a re-evaluation of chronic bilateral pleuritis in a patient who experienced occasional acute left bacterial pleuritis. Intern Med 50: 893-897, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Tanaka H, Ichiyasu H, Notsute D, Yamashita A, Hamamoto J, Kohrogi H. A case of systemic IgG4-related disease with bilateral pleural effusions. Nihon Kokyuki Gakkai Zasshi 49: 214-220, 2011(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 24. Suzuki N, Saeki T, Shimaoka Y, et al. Two cases of IgG4-related disease with pleural effusion. Nihon Kokyuki Gakkai Zasshi 49: 97-102, 2011(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 25. Sekiguchi H, Horie R, Utz JP, Ryu JH. IgG4-related systemic disease presenting with lung entrapment and constrictive pericarditis. Chest 142: 781-783, 2012. [DOI] [PubMed] [Google Scholar]
- 26. Kojima M, Nakazato Y, Kaneko Y, Sugihara S, Masawa N, Nakamura N. Cytological findings of IgG4-related pleural effusion: a case report. Cytopathology 24: 338-340, 2013. [DOI] [PubMed] [Google Scholar]
- 27. Hara Y, Shinkai M, Yamaguchi N, et al. A case of steroid effective pleuritis and lymphadenopathy needed to distinguish IgG4-related disease from multicentric Castleman's disease. Nihon Kokyuki Gakkai Zasshi 2: 544-549, 2013(in Japanese, Abstract in English). [Google Scholar]
- 28. Ishida M, Hodohara K, Furuya A, et al. Concomitant occurrence of IgG4-related pleuritis and periaortitis: a case report with review of the literature. Int J Clin Exp Pathol 7: 808-814, 2014. [PMC free article] [PubMed] [Google Scholar]
- 29. Ishida A, Furuya N, Nishisaka T, Mineshita M, Miyazawa T. IgG4-related pleural disease presenting as a massive bilateral effusion. J Bronchology Interv Pulmonol 21: 237-241, 2014. [DOI] [PubMed] [Google Scholar]
- 30. Choi JH, Sim JK, Oh JY, et al. A case of IgG4-related disease presenting as massive pleural effusion and thrombophlebitis. Tuberc Respir Dis (Seoul) 76: 179-183, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corcoran JP, Culver EL, Psallidas I, et al. A 63-year-old man with a recurrent right-sided pleural effusion. Thorax 70: 504-507, 2015. [DOI] [PubMed] [Google Scholar]
- 32. Waheed W, Nickerson J, Ambaye AB, Babi MA, Tandan R. IgG4-related neuromyopathy associated with recurrent pleural effusion. J Clin Neuromuscul Dis 16: 210-219, 2015. [DOI] [PubMed] [Google Scholar]
- 33. Ohkubo H, Miyazaki M, Oguri T, Arakawa A, Kobashi Y, Niimi A. A rare case of IgG4-related disease involving the uterus. Rheumatology (Oxford) 54: 1124-1125, 2015. [DOI] [PubMed] [Google Scholar]
- 34. Gajewska ME, Rychwicka-Kielek BA, Sørensen K, Kubik M, Hilberg O, Bendstrup E. Immunoglobulin G4-related pleuritis - A case report. Respir Med Case Rep 19: 18-20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karim AF, Verdijk RM, Guenoun J, van Hagen PM, van Laar JA. An inflammatory condition with different faces: immunoglobulin G4-related disease. Neth J Med 74: 110-115, 2016. [PubMed] [Google Scholar]
- 36. Kondo T, Uehara T. Immunoglobulin G4-related disease with fibroinflammatory lesions in the pleura, bile ducts and pericardium. CMAJ 188: 972, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. González-Moreno J, Losada-López I, Gállego-Lezaun C, García-Gasalla M, Gómez Bellvert C, Ortego Centeno N. Serosal involvement in IgG4-related disease: report of two cases and review of the literature. Rheumatol Int 36: 1033-1041, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Ikuyama Y, Hachiya T, Komatsu M, Nakamura T, Yamamoto H, Hanaoka M. A case of pleuritis diagnosed with IgG4-related disease. Nihon Kokyuki Gakkai Zasshi 6: 78-83, 2017(in Japanese, Abstract in English). [Google Scholar]
- 39. Krause ML, Yi ES, Warrington KJ. Pulmonary IgG4-related disease and colon adenocarcinoma: possible paraneoplastic syndrome. Int J Rheum Dis 20: 654-656, 2017. [DOI] [PubMed] [Google Scholar]
- 40. Tong X, Bal M, Wang W, Han Q, Tian P, Fan H. IgG4-related disease involving polyserous effusions with elevated serum interleukin-6 levels: a case report and literature review. Immunol Res 65: 944-950, 2017. [DOI] [PubMed] [Google Scholar]
- 41. Vu K, Gupta R, Frater J, Atkinson J, Ranganathan P. A 55-year-old man with periorbital and inguinal masses, pericarditis, and pleuritis. Arthritis Care Res (Hoboken) 69: 730-736, 2017. [DOI] [PubMed] [Google Scholar]
- 42. Kita T, Araya T, Ichikawa Y, et al. IgG4-related pleuritis with no other organ involvement. Am J Med Sci 356: 487-491, 2018. [DOI] [PubMed] [Google Scholar]
- 43. Nagayasu A, Kubo S, Nakano K, et al. IgG4-related pleuritis with elevated adenosine deaminase in pleural effusion. Intern Med 57: 2251-2257, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fukiya M, Marukawa K, Shimizu T, et al. Cytologically proven IgG4-positive plasma cells in pleural effusion of a patient clinically compatible with IgG4-related disease. J Jpn Soc Clin Cytol 57: 50-55, 2018(in Japanese). [Google Scholar]
- 45. Makimoto G, Ohashi K, Taniguchi K, et al. Long-term spontaneous remission with active surveillance in IgG4-related pleuritis: a case report and literature review. Respir Med Case Rep 28: 100938, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yosokawa N, Shirai R, Tanaka H, Kurose K, Oga T, Oka M. Thoracoscopic findings in IgG4-related pleuritis. Intern Med 59: 257-260, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kato E, Takayanagi N, Ishiguro T, Kagiyama N, Shimizu Y, Sugita Y. IgG4-related pleuritis with chylothorax. Intern Med 53: 1545-1548, 2014. [DOI] [PubMed] [Google Scholar]
- 48. Goug EK, Park JE, Lee EH, et al. A case of extensive IgG4-related disease presenting as massive pleural effusion, mediastinal mass, and mesenteric lymphadenopathy in a 16-year-old male. Tuberc Respir Dis (Seoul) 78: 396-400, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: a systematic review and meta-analysis. PLoS One 14: e0213728, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology 24: 962-971, 2019. [DOI] [PubMed] [Google Scholar]
- 51. Akiyama M, Takeuchi T. IgG4-related disease: beyond glucocorticoids. Drugs Aging 35: 275-287, 2018. [DOI] [PubMed] [Google Scholar]
- 52. Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 74: 1171-1177, 2015. [DOI] [PubMed] [Google Scholar]
- 53. Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DM, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 91: 57-66, 2012. [DOI] [PubMed] [Google Scholar]
- 54. Hart PA, Topazian MD, Witzig TE, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut 62: 1607-1615, 2013. [DOI] [PubMed] [Google Scholar]
- 55. Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol 9: 315-347, 2014. [DOI] [PubMed] [Google Scholar]
- 56. Ebbo M, Grados A, Samson M, et al. Long-term efficacy and safety of rituximab in IgG4-related disease: data from a French nationwide study of thirty-three patients. PLoS One 12: e0183844, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]