Abstract
Objective
Bronchial thermoplasty (BT) is a bronchoscopic procedure for patients with severe asthma. Although it has been suggested that BT works by reducing airway smooth muscle, the detailed mechanism underlying its effects is still unknown.
Methods
We performed xenon ventilation computed tomography (Xe-CT) before each BT procedure and six weeks after the third treatment to assess the improvement in lung ventilation at each separate lung region. The air trapping index in each lobe was defined as the mean trapping value (0: none, 1: mild, 2: moderate, and 3: severe) of the included segments.
Patients and Materials
Four patients were included.
Results
Asthma symptoms were improved after BT. The comparison of the scores at baseline with those after the third treatment showed that the air trapping index was improved in both the treated and untreated regions. However, neither the pulmonary function nor the exhaled nitric oxide was improved.
Conclusion
Using Xe-CT, we successfully evaluated the air trapping in patients who underwent BT. The improvement in asthma symptoms by BT may be related to the amelioration of peripheral lung ventilation in both the treated and untreated regions.
Keywords: xenon ventilation computed tomography, severe asthma, bronchial thermoplasty, air trapping
Introduction
The majority of asthma patients have their condition well-controlled by general treatment, such as inhalation drugs. However, some severe asthma patients have symptoms that are not sufficiently improved with these standard treatments. Bronchial thermoplasty (BT) is a bronchoscopic procedure that reduces bronchial smooth muscle using controlled thermal energy. Randomized controlled trials have shown that BT improves the daily asthma symptoms and frequency of severe asthma attacks among severe asthma patients, although the forced expiratory volumes in 1 second were not improved (1).
Pretolani et al. reported that the decrease in airway smooth muscle, which was first observed at three months after BT, was highly correlated with the improvement in symptoms (2), and that a decrease in airway smooth muscle was also observed in untreated middle lobes (3). In addition, the reduction of nerve fibers by BT was shown to be related to bronchospasm and improved asthma symptoms (4).
The local ventilatory condition can be clinically evaluated by lung ventilation scintigraphy; however, this approach is associated with several issues, such as exposure to radioactive isotopes and a low spatial resolution. Recently, xenon ventilation computed tomography (Xe-CT) was reported to be feasible for the assessment of lung ventilation without radio isotopes (5), and it can be used in asthma patients as well (6).
We herein report the changes in the ventilatory conditions, particularly in untreated middle lobes, before and after BT using Xe-CT.
Materials and methods
Study design
Severe asthma patients who received BT from January 2017 to April 2018 in our hospital were included in the study. Eligibility criteria included an age over 18 years old, uncontrolled asthma with high-dose inhaled corticosteroids (ICSs) and other controllers, including long-acting beta agonists (LABAs). All participants provided their written informed consent to a protocol approved by the ethics boards of Kobe University.
Patients were treated with BT according to the current standard method with conscious sedation (7). Participants underwent Xe-CT, pulmonary function tests, exhaled nitric oxide tests, and filled out an asthma control test (ACT) questionnaires in the previous week of each BT (Visit 1-3) and six weeks after the third BT (Visit 4). At visits 1 and 4, patients underwent forced oscillometry. At visit 1-3, patients had not started taking high-dose oral corticosteroids for the subsequent BT procedure.
Xe-CT
The mouth and nose of the patients were covered with oxygen masks using elastic straps. Patients inhaled 30% xenon and 70% oxygen mixed gas for 1 minute in the wash-in period and 100% oxygen for 2 minutes in the wash-out period using a xenon gas control system.
The end-tidal xenon concentration was monitored by the gas control system. When the concentration reached 30% in the wash-in period and 0% in the wash-out period, dual-energy CT was performed.
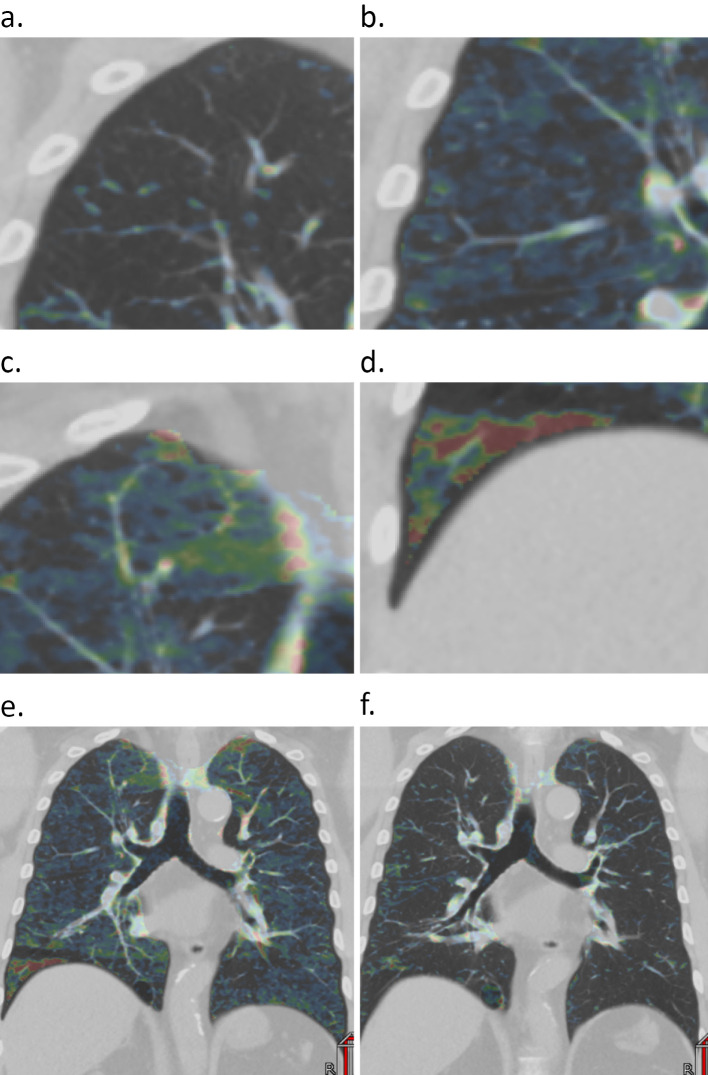
Using the three material decomposition method, the subtracted Hounsfield units (HU) for xenon was calculated for each voxel, as previously described (8). We evaluated air trapping based on a four-point rating scale consisting of none (0), mild (1), moderate (2), and severe (3) according to the maximal subtracted HU in each pulmonary segment on wash-out phase CT: 0-5, 5-10, 10-20, and >20 HU, respectively. Regions with a high HU of <50 mm2 were measured as regions where the xenon accumulation was one step weaker (Fig. 1a-d). The evaluation was performed by two radiologists independently, and the average was used as the score. The trapping index was defined as the average score of air trapping in the segments of each lobe.
Figure 1.
The assessment of the air trapping scores in each pulmonary segment with wash-out phase Xe-CT. Depending on the maximum xenon intensity in each segment, the scores were defined as none (a), mild (b), moderate (c), and severe (d). Representative Xe-CT images at baseline (e) and after the third BT procedure (f).
Forced oscillation technique (FOT)
Resistances at 5 Hz (R5) and 20 Hz (R20), R5-R20, reactance at 5 Hz (X5), the resonant frequency (Fres), and low-frequency reactance area (ALX) were measured using a MostGraph-01 (Chest M.I., Tokyo, Japan). During the measurements, the patients were kept in the sitting position, supporting their cheeks with their hands while wearing nose clips.
Statistical analyses
The statistical software program R, version 3.5.0, was used for analyses, and the results were expressed as the mean ± standard deviation (SD). A paired t-test was used to compare the exhaled nitric oxide and ACT score between groups. Wilcoxon's rank sum test was used to compare the air trapping score between groups, and Wilcoxon's signed rank test was used to compare changes in air trapping between groups.
Results
Patient characteristics
Three men and one woman were included this study, and eventually all patients who underwent BT participated (Table 1). All patients needed the maximal dose of inhaled corticosteroids, long-acting beta-agonists, long-acting muscarinic antagonists, and leukotriene receptor antagonists. Only one patient used biologics. No patients used oral corticosteroids as their daily controller. The ratios of forced expiratory volumes in 1 second to the predicted value were more than 60% in all participants. At visits 1 and 4, all participants had been free from oral corticosteroids (OCS) for at least 30 days (Supplementary material 1).
Table 1.
Patients Characteristics.
| case1 | case2 | case3 | case4 | |||||
|---|---|---|---|---|---|---|---|---|
| Age | 57 | 53 | 55 | 59 | ||||
| Sex | male | female | male | male | ||||
| BMI(kg/m2) | 31.3 | 26.3 | 28.2 | 20.3 | ||||
| Smoking(pack-year) | 45 | never | never | never | ||||
| Age of asthma onset | 55 | 28 | 50 | 49 | ||||
| Exacerbations in last year | 5 | 4 | 7 | 3 | ||||
| ACT score | 8 | 11 | 8 | 22 | ||||
| ICS dose(μg/day) | BUD1,600 | FP1,000 | FF200 | FF200 | ||||
| LABA | yes | yes | yes | yes | ||||
| LAMA | yes | yes | yes | yes | ||||
| LTRA | yes | yes | yes | yes | ||||
| Antihistamine | no | no | yes | no | ||||
| Omalizumab | no | no | yes | no | ||||
| Mepolizumab | no | no | no | no | ||||
| Daily OCS | no | no | no | no | ||||
| FVC(L) | 3.29 | 2.84 | 3.39 | 3.28 | ||||
| FEV1(L) | 2.28 | 2.36 | 2.54 | 2.02 | ||||
| %FEV1 | 70.6% | 97.7% | 74.5% | 67.1% | ||||
| Total IgE(IU/L) | 17.0 | 1,131.7 | 371.5 | 577.0 | ||||
| Eosinophils(/μl) | 261 | 248 | 360 | 43 |
BT
All patients completed the BT procedure three times. The mean numbers of activations were 49.2±18.5, 36.7±7.46, 35.5±13.2, and 37.7±8.87 in the right lower lobes, left lower lobes, right upper lobes, and left upper lobes, respectively.
ACT score
The ACT scores improved from visit 1 (12.2±5.76) to visit 4 (20.7±1.92) (p<0.05). Due to exacerbation after the BT procedure, the ACT score of patient 4 temporarily declined at visit 3 (Fig. 2).
Figure 2.
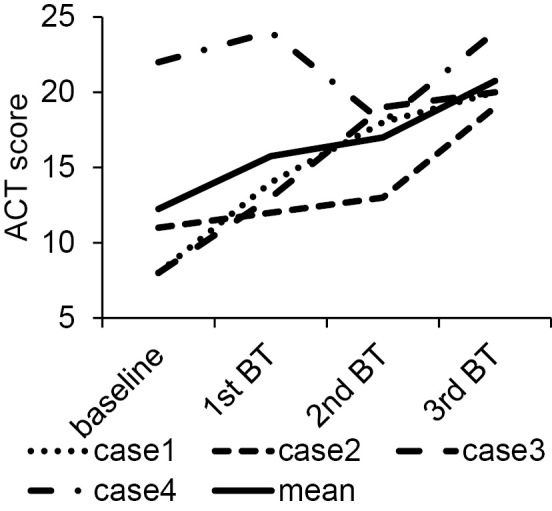
The changes in the ACT scores in each case and the mean at each timepoint.
Air trapping score on Xe-CT
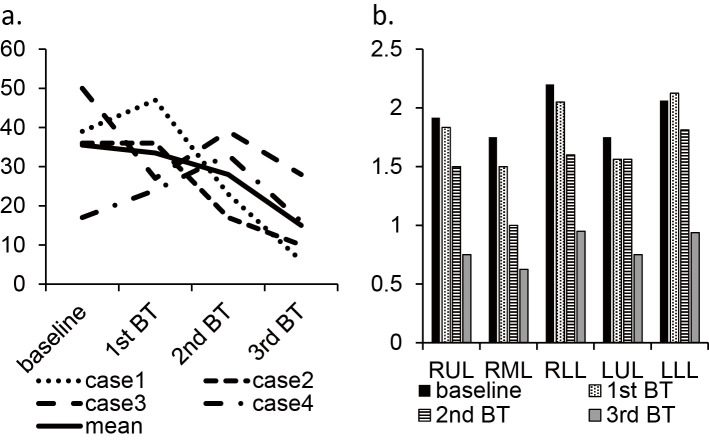
Except for Case 4, a decrease in air trapping was observed by BT (Fig. 3a). The mean trapping index of each lobe decreased after BT, as in the untreated right middle lobe (Fig. 3b). There were no adverse effects, including headache, nausea, somnolence, seizure, and hypopnea, related to xenon inhalation.
Figure 3.
The change in the total trapping score (sum of scores in all segment) in each case (a) and the trapping index (mean scores of each segment consisting the lobe) (b).
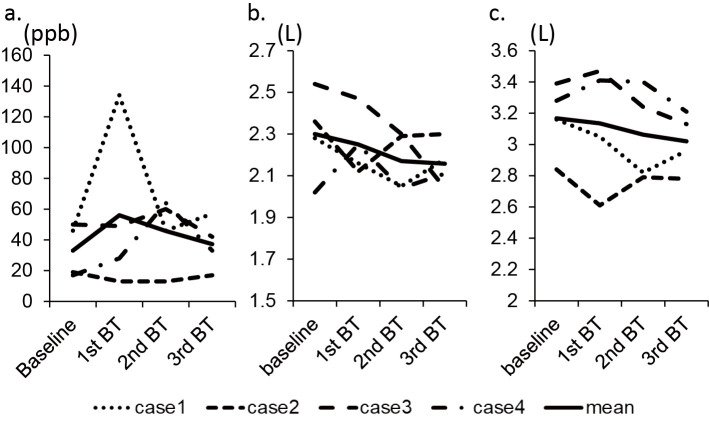
The pulmonary function test and exhaled nitric oxide
The forced expiratory volume in one second and exhaled nitric oxide value were not significantly changed after the BT procedure (Fig. 4).
Figure 4.
The changes in FeNO (a), FEV1 (b), and FVC (c) in each case are shown.
FOT parameters
In case 2, the values of airway resistance (R5, R20 and R5-R20) were decreased, and reactance (X5) was less negative than 1st visit. These changes suggest that airway obstruction had improved. However, in case 1, the results were completely the opposite, and in cases 3 and 4, the values had not changed (Table 2).
Table 2.
Changes of FOT Parameters
| case1 | case2 | case3 | case4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | pre | Post | |||||||||
| R5 (cmH2O/L/s) | 0.46 | 3.46 | 5.73 | 1.1 | 0.41 | 0.36 | 0.27 | 0.27 | ||||||||
| R20 (cmH2O/L/s) | 2.02 | 2.44 | 4.5 | 1.98 | 0.7 | 0.71 | 0.84 | 0.78 | ||||||||
| R5-R20 (cmH2O/L/s) | -1.58 | 1.02 | 1.23 | -0.88 | -0.29 | -0.33 | -0.57 | -0.51 | ||||||||
| X5 (cmH2O/L/s) | -1.27 | -1.85 | -7.58 | -0.71 | -0.03 | -0.11 | -0.1 | -0.1 | ||||||||
| Fres (Hz) | 14.1 | 15.29 | 21.53 | 15.96 | 5.83 | 8.62 | 6.33 | 7.85 | ||||||||
| ALX (cmH2O/L/s x Hz) | 8.07 | 10.45 | 58.66 | 4.89 | 0.11 | 0.41 | 0.36 | 0.43 | ||||||||
Discussion
In this study, using Xe-CT, we found that the air trapping of lungs improved after BT alongside the improvement in asthma symptoms. Furthermore, improvement of air trapping was not limited to the treatment site but was also observed in the untreated right middle lobe. However, there was no improvement in the forced expiratory volume in one second or fractional exhaled nitric oxide.
Langton et al. reported the improvement in the residual volume at 6 months after BT in 32 patients but no changes in other spirometric parameters (9). The improvement of air trapping on Xe-CT may be the result of ventilatory changes in small airways, thus not affecting the forced expiratory volumes in one second. In addition, these changes in the ventilatory condition in small airways may be caused by the suppression of bronchospasm and a reduction in airway smooth muscle.
To assess the ventilatory mechanics at the resting respiratory level, we also used a FOT examination. FOT is often used as an auxiliary examination to evaluate airway obstruction and elasticity in asthma patients. Commonly, R5 and R20 are the indicators of total and proximal airway resistance, respectively, and R5-R20 (the difference of R5 and R20) is interpreted as the index of distal or small airway resistance. However, there are no official statements or physiological basis to support this interpretation at present. In adult asthmatics, the R5-R20 is higher and the X5 more negative than in healthy subjects (10), and R5-R20 has been reported to be modestly correlated with the alveolar concentration of nitric oxide, a marker of peripheral airway inflammation (11).
However, very few studies have focused on FOT parameters and BT. Miki et al reported a case in which the airway resistance and reactance were improved by BT (12). In contrast, Langton et al. reported that neither the airway resistance nor reactance was changed despite significant clinical improvements (13). In our study, the FOT parameters, including R5-R20 and X5, were improved in one case, declined in another case, and were unchanged in the other two cases, despite all cases showing clinical improvement.
Of note, in contrast to pulmonary function tests, both Xe-CT and FOT are examined in the resting respiratory state. Therefore, these tests more closely reflect the daily respiratory state. However, an index that can be objectively determined, as in the pulmonary function test, has not been established. These methods were assumed to be effective for complementarily estimating the changes in the ventilatory condition in the patients who underwent BT.
Several limitations associated with the present study warrant mention. First, participants had to take high-dose OCS for the subsequent BT procedure, which may have affected the ACT and air trapping scores. The results at visits 2 and 3 may have been affected by systemic steroids, as some patients had only finished OCS a short while before the evaluation. However, at visits 1 and 4, the patients were examined over one month after finishing OCS, so the effect is considered to have been very small. Second, the number of patients was very small, and consequently, we were unable to fully assess the effect of BT in other types of asthma patients, such as patients with a low forced expiratory volumes in one second and daily OCS users. We need to perform Xe-CT in such patients undergoing BT. Finally, there is no established method for analyzing air trapping on Xe-CT, similar to FOT. We should evaluate more patients, including neutrophilic asthmatics and nonresponders to BT. Studying cases in which the symptoms have improved despite no improvement in Xe-CT findings may help clarify the mechanism of BT from lung functional imaging.
Conclusion
In the present study, we successfully evaluated the changes in air trapping in patients who underwent BT using Xe-CT. The improvement in air trapping is correlated with the symptoms. Further analyses focused on the lung ventilator conditions are needed to elucidate the involved mechanism and clinical effectiveness of BT.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by JSPS KAKENHI 17K09615 to Kazuyuki Kobayashi.
Supplementary Material
Days after last OCS use at each visit
References
- 1. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 181: 116-124, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pretolani M, Bergqvist A, Thabut G, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathologic correlations. J Allergy Clin Immunol 139: 1176-1185, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Pretolani M, Dombret MC, Thabut G, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med 190: 1452-1454, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Facciolongo N, Di Stefano A, Pietrini V, et al. Nerve ablation after bronchial thermoplasty and sustained improvement in severe asthma. BMC Pulm Med 18: 29, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu GM, Zhao Y, Zhang LJ, Schoepf UJ. Dual-energy CT of the lung. Am J Roentgenol 199 (Suppl): S40-53, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Kim WW, Lee CH, Goo JM, Park SJ, Kim JH, Park EA, Cho SH. Xenon-enhanced dual-energy CT of patients with asthma: dynamic ventilation changes after methacholine and salbutamol inhalation. Am J Roentgenol 199: 975-981, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 173: 965-969, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Ohno Y, Yoshikawa T, Takenaka D, et al. Xenon-enhanced CT using subtraction CT: basic and preliminary clinical studies for comparison of its efficacy with that of dual-energy CT and ventilation SPECT/CT to assess regional ventilation and pulmonary functional loss in smokers. Eur J Radiol 86: 41-51, 2017. [DOI] [PubMed] [Google Scholar]
- 9. Langton D, Ing A, Bennetts K, et al. Bronchial thermoplasty reduces gas trapping in severe asthma. BMC Pulm Med 18: 155, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boudewijn IM, Telenga ED, van der Wiel E, et al. Less small airway dysfunction in asymptomatic bronchial hyperresponsiveness than in asthma. Allergy 68: 1419-1426, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto H, Niimi A, Jinnai M, et al. Association of alveolar nitric oxide levels with pulmonary function and its reversibility in stable asthma. Respiration 81: 311-317, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Miki K, Miki M, Yoshimura K, et al. Improvement of exertional dyspnea and breathing pattern of inspiration to expiration after bronchial thermoplasty. Allergy Asthma Clin Immunol 14: 74, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langton D, Ing A, Sha J, et al. Measuring the effects of bronchial thermoplasty using oscillometry. Respirology 24: 431-436, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Days after last OCS use at each visit