Abstract
Molecular knowledge regarding the primary esophageal achalasia is essential for the early diagnosis and treatment of this neurodegenerative motility disorder. Therefore, there is a need to find the main microRNAs (miRNAs) contributing to the mechanisms of achalasia. This study was conducted to determine some patterns of deregulated miRNAs in achalasia. This case-control study was performed on 52 patients with achalasia and 50 nonachalasia controls. The miRNA expression profiling was conducted on the esophageal tissue samples using the next-generation sequencing (NGS). Differential expression of miRNAs was analyzed by the edgeR software. The selected dysregulated miRNAs were additionally confirmed using the quantitative reverse transcription polymerase chain reaction (qRT-PCR). Fifteen miRNAs were identified that were significantly altered in the tissues of the patients with achalasia. Among them, three miRNAs including miR-133a-5p, miR-143-3p, and miR-6507-5p were upregulated. Also, six miRNAs including miR-215-5p, miR-216a-5p, miR-216b-5p, miR-217, miR-7641 and miR-194-5p were downregulated significantly. The predicted targets for the dysregulated miRNAs showed significant disease-associated pathways like neuronal cell apoptosis, neuromuscular balance, nerve growth factor signaling, and immune response regulation. Further analysis using qRT-PCR showed significant down-regulation of hsa-miR-217 (p-value = 0.004) in achalasia tissue. Our results may serve as a basis for more future functional studies to investigate the role of candidate miRNAs in the etiology of achalasia and their application in the diagnosis and probably treatment of the disease.
Keywords: Achalasia, microRNA, next-generation sequencing, expression profiling, bioinformatics
1. Introduction
Achalasia is a chronic neurogenic esophageal motility disorder featured by impaired lower esophageal sphincter (LES) laxity and disturbed peristalsis (Triadafilopoulos et al., 2012). Its symptoms include progressive swallowing disorder, regurgitation, esophageal chest pain, aspiration, and eventually malnutrition (Sadowski et al., 2010). According to a population-based study, achalasia prevalence is more than 10/100,000, with a steady increasing trend from 2.5/100,000 in 1996 to 10.8/100,000 in 2007 (Sadowski et al., 2010). Survival of the patients with achalasia is significantly less than age-sex matched healthy people (Sadowski et al., 2010). Most patients underwent late diagnosis and ineffective treatment due to nonspecific symptoms of the disease and the absence of noninvasive diagnostic tests (Farrokhi and Vaezi, 2007).
The pathophysiology of achalasia is based on selective loss of inhibitory neurons in the myenteric network, which can interfere with the coordination of esophageal peristalsis and LES relaxation during swallowing (Ghoshal et al., 2012). Decreased levels of the nitric oxide synthase (NOS) and vasoactive intestinal polypeptide (VIP) as inhibitory neurotransmitters in the myenteric plexus disrupt esophageal neuromuscular function in the patients with achalasia (Ates and Vaezi, 2015). Although the exact mechanism of the disease is not fully understood, some studies have shown evidence regarding the association of the viral, autoimmune, and neurodegenerative factors (Furuzawa-Carballeda et al., 2016; Park and Vaezi, 2005).
MicroRNAs (miRNA) are a group of small noncoding RNAs which act as gene expression regulators in different disease-related pathways (Bartel, 2004). The miRNA system involves in various physiological and pathophysiological processes and behaves as potential prognostic biomarkers (Furer et al., 2010). Several studies showed the altered miRNAs expression in various disorders, including cancers (Fang et al., 2012), immune-mediated inflammatory diseases (Singh et al., 2013; Tahamtan et al., 2016), and nervous disturbances (Wang et al., 2014a).
Although few studies argue the association of the pathogenesis of achalasia with neurological communication, cholinergic signaling, and inflammation, studying miRNAs expression helps us to understand better the pathophysiology of achalasia. While investigating the effects of miRNAs on the pathogenesis of the abovementioned diseases has received considerable attention, their effects on the development of achalasia are still unclear. The present study performed the next-generation sequencing (NGS) with an analytical approach to identify reliable candidate miRNAs associated with the development of the disease.
2. Materials and methods
2.1. Participants and sampling
This matched case-control study was performed on 102 participants referred to the Digestive Diseases Research Center (DDRC) in the Shariati Hospital in Tehran-Iran between August 2015 and April 2016. All the patients with primary esophageal achalasia referring to the clinic for regular follow-up were recruited consecutively (N = 52). These patients aged ≥18 years old were diagnosed based on the clinical, radiological, endoscopic findings and high-resolution manometry. All the patients received the same pneumatic dilatation treatment and were classified into excellent, good, moderate, and poor categories according to the outcome. Patients in the excellent and good categories were considered as good responses to the treatment, and those in the moderate and poor categories were considered as poor responses to the treatment (Hasanzadeh et al., 2010). Controls were selected randomly from the individuals without dysphagia or esophageal lesions who visited the same clinic (N = 50). All the cases and controls were matched by age (±5 years) and sex. Participants underwent the endoscopic biopsy from the LES by an expert clinician. The samples were stored at –80 ºC for the subsequent experiments. Patients with other associated motility or nonmotility disorders, malignancy, or coagulopathy were excluded from the study.
This study was approved by the Ethics Committee of Golestan University of Medical Sciences (Ethics Code = 31078693122415). Participation in this study was optional. Informed written consent was obtained from all of the participants and their anonymity was preserved. The test results were considered confidential and only available to the physician and the moderator of the project.
2.2. RNA isolation and deep sequencing
Total RNA was extracted from all the samples (52 cases and 50 controls) using the Trizol reagent according to the manufacturers’ instructions (Invitrogen, Sweden). To increase the experiment power, total RNAs were obtained from each group of patients were pooled together (mixed equally) and sent for miRNA sequencing (pooled sample 1, 2, 3, 4). For the controls, total RNAs were pooled equally, and then two pooled samples were sent for miRNA profiling (pooled sample 5, 6). In brief, each pooled sample contained 15 and 25 extracted total RNAs of the patient and the control groups respectively.
The samples were sent to BGI (Beijing Genomics Institute) for miRNA sequencing. Bioanalyzer 2100 (Agilent, Santa Clara, CA) was employed to measure the RNA Integrity Number (RIN) for each sample. The samples with RIN greater than seven were considered for sequencing. The RNA purification, library construction, and sequencing procedures were conducted by the BGI Company. Each library was single-end sequenced on an Illumina HiSeq 4000 platform. The raw miRNA-Seq data were deposited and released in the Sequence Read Archive (SRA) database, with the BioProject accession number of PRJNA616451.
2.3. Analysis of small RNA sequencing data (NGS data)
The FASTQC was used to perform primary quality control of the miRNA-Seq data (version 0.11.5Babraham Bioinformatics (2021). FastQC [online]. Website http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [accessed 08- 03-2016]. ). Afterward, low-quality reads and adapter sequences of raw data were trimmed by the Trimmomatic software version 0.35 (Bolger et al., 2014) (parameters of trailing 20, max info 18:0.90, and minimum length 18). Reads with the length shorter than 18 bases were discarded after quality trimming, and the remaining reads were mapped against the Rfam database (Nawrocki et al., 2014) to eliminate unwanted noncoding. RNAs (rRNAs, tRNAs, snRNAs, and snoRNA). Subsequently, the remaining reads were analyzed using the miRDeep2 software version 0.0.8 to quantify known miRNAs and predict novel miRNAs (Friedländer et al., 2011). For efficient read mapping, clean reads in each sample were collapsed into a set of unique sequences with read numbers counted. Then, the unique sequences were aligned to the Ensembl GRCH37 human genome (Ensembl Release 68) and miRNAs sequences (miRBase database, version 21) (Kozomara and Griffiths-Jones, 2013). The aligned reads were quantified using the default settings of the miRDeep2 software with only one allowed mismatch within the read. On the other hand, putative novel miRNAs were predicted using the default settings in the miRDeep2 software. The predictions by the miRDeep2 software were filtered, with a miRDeep2 score >1, the length of nucleotides ≥50, and the predicted probability of being a miRNA > 60%. The difference in miRNAs expression (fold change) was analyzed by the edgeR package (version 1.4.5) in the R software. A fold change with an adjusted p-value or false discovery rate (FDR) less than 0.05 was considered statistically significant.
2.4. MiRNAs target prediction and gene enrichment analyses
Potential target genes of the differentially expressed miRNAs were predicted using the three target prediction programs, including PITA (Kertesz et al., 2007), TargetSpy (Sturm et al., 2010), and RNAhybrid (Rehmsmeier et al., 2004). If a gene was predicted by at least two programs, it was considered as a putative target. Since each program uses various algorithms to predict miRNA targets and has different levels of sensitivity and specificity, using the combination of them reduced the false positive. We applied the default parameters of each program for target prediction. The 3’UTR sequences were recovered by the Ensembl BioMart2Ensembl BioMart database [online]. Website http://rfam.xfam.org/ [accessed 10-04-16]. and then, were used for prediction. Finally, Gene Ontology (GO) and Kyoto Encyclopedia Genes and Genomes (Qiu, 2013) were applied to analyze the potential function and pathway of target genes.
2.5. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
For more confirmation, the expression levels of the dysregulated candidate miRNAs were measured in the esophageal tissues of the patients with achalasia (N = 28) and control individuals (N = 32) by the ABI 7300 real-time PCR machine (Applied Biosystems, USA). The process of cDNA synthesis of miRNAs was performed by the Reverse Transcription System Kit (ZistRoyesh, Iran) with a miR-specific stem-loop primer (Mohammadi-Yeganeh et al., 2013). The SNORD47 was measured as an internal normalization control using the 2-dct method. For qRT-PCR statistical analysis, differences between the two groups were tested by Student’s t-test and the Mann–Whitney U test (based on normality of data distribution) in the SPSS statistical software version 16.0. The difference with a probability value less than 0.05 was considered statistically significant.
3. Results
3.1. Esophageal tissues of the patients with achalasia expressed MiRNA profile different from the controls
Table 1 summarizes the clinical information of the patients. As demonstrated in Table 1, there is no significant difference in age (p-value = 0.48) and sex (p-value = 0.43) between the cases and controls. The miRNA sequencing results were compared between three groups: good response group including pooled samples 1 and 2 (those with good and excellent response to the dilatation treatment), poor response group consisting of pooled samples 3 and 4 (those with moderate and poor response to the dilatation treatment), and pooled samples 5 and 6 that were merged into control group to perform the transcriptome analysis based on the clinicians’ recommendation (Table 2). It was attempted to increase the statistical power through post-processing replication for each group.
Table 1.
Clinical data for 52 achalasia patients and 50 controls.
| Characteristic | Patients † | Controls † | p-value |
|---|---|---|---|
| Mean age (SD‡ ), year | 43.5 (1.6) | 45.8 (1.6) | 0.48 |
| Male/female no. (% male) | 31/21 (59.6) | 26/24 (52) | 0.43 |
| Vantrappen classification§ExcellentGoodModeratePoor | n (%)15 (28.8)15 (28.8)12 (23.1)10 (19.2) | ||
| Achalasia subtype¶Type 1Type 2Type 3 | n (%)9 (17.3)42 (80.8)1 (1.9 ) | ||
| Mean duration (months) of symptoms (SD) | 32.34 (2.06) | ||
| Baseline symptomsDysphagiaChest painRegurgitation | n (%)43 (82.7)7 (13.5)2 (3.8) |
†Unless otherwise indicated data are expressed as number (percentage) of patients. Percentages have been rounded and might not total 100. ‡SD: Standard deviation. §Vantrappen classification: Excellent, indicates no symptoms; Good, symptoms occurring less than once a week; Moderate, symptoms occurring more than once weekly; and Poor, persistent symptoms (Vantrappen and Hellemans, 1980).¶Achalasia subtype: Type 1 (classic) with minimal contractility in the esophageal body, type 2 with intermittent periodsof panesophageal pressurization, and type 3 (spastic) with premature or spastic distal esophageal contractions (Kahrilas et al., 2015).
Table 2.
Next-generation sequencing read counts and mapping result for individual samples.
| Post processing grouping | Clinical outcome after dilatation treatment | Sample ID | Total Reads | Mapped Reads | Mapped (%) | |
|---|---|---|---|---|---|---|
| Treat 1 | Good response | Excellent | Pooled sample1 | 30378288 | 15879232 | 0.523 |
| Good | Pooled sample2 | 26809904 | 13712314 | 0.511 | ||
| Treat 2 | Poor response | Fair | Pooled sample3 | 29249887 | 14814455 | 0.506 |
| Poor | Pooled sample4 | 30445887 | 16872768 | 0.554 | ||
| Treat 3 | Without treatment / Control | Control 1 | Pooled sample5 | 24835372 | 10948105 | 0.441 |
| Control 2 | Pooled sample6 | 29473046 | 13101058 | 0.445 | ||
The miRNA expression profiling analysis showed that 15 miRNAs were significantly differentially expressed in the tissues of the patients with achalasia (good or poor response groups) compared to the controls. Besides, our findings indicated that most of the dysregulated miRNAs (11 miRNAs) were downregulated and only four miRNAs were upregulated in the tissues of the patients with achalasia. Three miRNAs were significantly upregulated in both good and poor response group compared to the controls; miR-133a-5p (adjusted p-value < 0.001 for good response group and adjusted p-value = 0.005 for poor response group), miR-143-3p (adjusted p-value = 0.001 for good response group and adjusted p-value = 0.011 for poor response group) and miR-6507-5p (adjusted p-value = 0.001for good response group and adjusted p-value = 0.016 for poor response group). Besides, the NGS data showed hsa-miR-3609 was significantly upregulated only in a good response group compared to the controls (adjusted p-value = 0.021). Furthermore, we found six miRNAs that were downregulated significantly in both good and poor response groups (Figure1). These were miR-215-5p, miR-216a-5p, miR-216b-5p, miR-217 and miR-7641 with adjusted p-value < 0.001 and miR-194-5p (adjusted p-value = 0.01 for good response group and adjusted p-value = 0.005 for poor response group). Moreover, the good response group showed significant downregulation in the expression of four miRNAs, including hsa-miR-135a-5p, hsa-miR-4488, hsa-miR-122-5p, and hsa-miR-4449. On the other hand, the significant downregulation of hsa-miR-383-5p (adjusted p-value =0.001) was seen in the poor response achalasia group compared to the controls (Table 3). This study did not detect significant differential expression in any of the miRNAs between two groups of patients with achalasia (good and poor response groups) regarding the treatment outcome.
Table 3.
Fifteen significant upregulated and downregulated miRNAs in the achalasia tissues (Good response group and Poor response group) versus control tissues.
| MicroRNA | Treat 1 | Treat 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| FC† | log2FC† | p-value | Adjusted p-value | FC† | log2 FC† | p- value | A p-value* | |
| hsa-miR-217 | ↓0.020 | –5.644 | 3.98E-10 | 2.46E-07 | ↓ 0.31 | –1.69 | 9.68E-09 | 1.5E-06 |
| hsa-miR-216a-5p | ↓ 0.062 | –4.011 | 1.05E-09 | 2.65E-07 | ↓ 0.047 | –4.411 | 1.02E-10 | 3.14E-08 |
| hsa-miR-7641 | ↓ 0.155 | –2.689 | 1.28E-09 | 2.65E-07 | ↓ 0.160 | –2.644 | 2.28E-09 | 4.71E-07 |
| hsa-miR-216b-5p | ↓ 0.08 | –3.644 | 2.06E-09 | 3.18E-07 | ↓ 0.04 | –4.644 | 7.52E-13 | 4.65E-10 |
| hsa-miR-215-5p | ↓ 0.240 | –2.059 | 8.98E-07 | 0.000111 | ↓ 0.193 | –2.373 | 2.39E-08 | 2.95E-06 |
| hsa-miR-135a-5p | ↓ 0.173 | –2.531 | 2.14E-06 | 0.00022 | - | - | - | - |
| hsa-miR-194-5p | ↓ 0.368 | –1.442 | 0.000174 | 0.010725 | ↓ 0.353 | –1.502 | 7.62E-05 | 0.005888 |
| hsa-miR-4488 | ↓ 0.323 | –1.630 | 0.000571 | 0.029432 | - | - | - | - |
| hsa-miR-122-5p | ↓ 0.231 | –2.114 | 0.000723 | 0.03438 | - | - | - | - |
| hsa-miR-4449 | ↓ 0.302 | –1.727 | 0.000835 | 0.036864 | - | - | - | - |
| hsa-miR-133a-5p | ↑ 35 | 5.129 | 2.89E-06 | 0.000255 | ↑ 19 | 4.248 | 7.62E-05 | 0.005888 |
| hsa-miR-143-3p | ↑ 6.702 | 2.744 | 1.74E-05 | 0.001345 | ↑ 5.173 | 2.371 | 0.000166 | 0.011374 |
| hsa-miR-6507-5p | ↑ 44 | 5.459 | 2.36E-05 | 0.00162 | ↑ 24 | 4.585 | 0.000261 | 0.016122 |
| hsa-miR-3609 | ↑ 4.6 | 2.202 | 0.00038 | 0.021343 | - | - | - | - |
| hsa-miR-383-5p | - | - | - | - | ↓ 0.133 | –2.910 | 1.28E-05 | 0.001317 |
†FC, Fold change;*A p-value§, Adjusted p-value.
Figure 1.
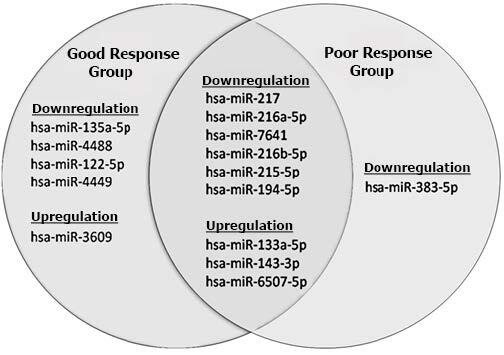
Candidate tissue miRNAs significantly differentially expressed in the patients with achalasia compared to the controls. Nine candidate miRNAs are common in samples of two achalasia groups. Good response group: achalasia patients with a good response to the pneumatic dilatation treatment. Poor response group: achalasia patients with poor response to the pneumatic dilation treatment.
3.2. Functional annotation of the candidate miRNAs
The biological process of GO and KEGG pathways of the 15 candidate miRNAs were analyzed based on the biological process. As detailed in Table 4, we introduced a list of the most significantly enriched terms and pathways of the target genes of candidate miRNAs involved in achalasia. Interestingly, GO analysis showed that the differentially expressed genes associated with the neuron apoptotic process (adjusted p-value = 0.004), neuronal death (adjusted p-value = 0.006), and immune response regulation (adjusted p-value = 0.008) were targeted by hsa-miR-143-3p. We found that the genes related to the cellular response to oxidative stress (adjusted p-value = 0.011), cellular aging (adjusted p-value = 0.011), axon regeneration and development (adjusted p-value = 0.031) and myelination (adjusted p-value = 0.031) were significantly enriched by the hsa-miR-217. Moreover, KEGG analysis showed that the genes involved in Glioma (adjusted p-value = 0.0001) and the Sphingolipid signaling pathway (adjusted p-value = 0.0006) were the most highly represented pathways enriched by the hsa-miR-143-3p. The genes associated with cancers, including non-small cell lung cancer (adjusted p-value = 0.001 for hsa-miR-143-3p & adjusted p-value = 0.004 for hsa-miR-217), prostate (adjusted p-value = 0.004 for hsa-miR-217), colorectal (adjusted p-value = 0.001 for hsa-miR-143-3p), bladder (adjusted p-value = 0.001 for hsa-miR-143-3p) and endometrial cancers (adjusted p-value = 0.004 for hsa-miR-217) were significantly enriched by the predicted target genes (Table 4).
Table 4.
The most significant enriched terms (potential function and pathway of target genes) based on biological process GO† enrichment (white rows) and KEGG‡ pathway (gray rows) of the miRNAs associated with achalasia.
| miRNA | Enriched Term | Target genes | A p-value* |
|---|---|---|---|
| hsa-miR-217 | Nonsmall cell lung cancer- Homo sapiens- hsa05223 | E2F3;KRAS;FOX3;FHIT | 0.004 |
| Endometrial cancer- Homo sapiens- hsa05213 | TCF7L2;PTEN;KRAS;FOXO3 | 0.004 | |
| Negative regulation of cell aging (GO:0090344) | PTEN;SIRT1;MARCH5 | 0.011 | |
| Cellular response to oxidative stress (GO:0034599) | NR4A2;TP53INP1;FOXO3;SIRT1;HIF1A;EZH2 | 0.011 | |
| Prostate cancer- Homo sapiens-hsa05215 | TCF7L2;PTEN;E2F3;KRAS | 0.016 | |
| Regulation of myelination (GO:0031641) | TCF7L2;PTEN;TNFRSF21 | 0.041 | |
| hsa-miR-216b-5p | Melanoma- Homo sapiens- hsa05218 | CDK6;CDK4;MAPK1;KRAS;FGF10 | 0.034 |
| Pathways in cancer-Homo sapiens-hsa05200 | CDK6;FZD5;TPM3;CDK4;COL4A4;FZD9;TCEB2;MAPK1;KRAS;FGF10 | 0.036 | |
| Signaling pathways regulating pluripotency of stem cells-Homo sapiens_hsa04550 | SMAD1;FZD5;FZD9;MAPK1;LHX5;KRAS | 0.036 | |
| Nonsmall cell lung cancer-Homo sapiens-hsa05223 | CDK6;CDK4;MAPK1;KRAS | 0.037 | |
| hsa-miR-215-5p | Cell cycle-Homo sapiens-hsa04110 | RB1;CDKN2D;CDKN2A;BUB1B;CDC7;TTK;CDC14A;ANAPC10;CDC20;ORC4;ORC1;CCNE1;RAD21;MCM3;MCM6;MAD2L1 | 0.012 |
| hsa-miR-143-3p | Glioma-Homo sapiens- hsa05214 | PDGFRA;MDM2;AKT1;MAPK1;BRAF;CALM3;KRAS;HRAS;IGF1R | -1.84E-05 |
| Sphingolipid signaling pathway- Homo sapiens- hsa04071 | CERS4;SGPL1;SPTLC2;PPP2R5E;BCL2;AKT1;MAPK1;KRAS;TNF;HRAS | -6.69E-05 | |
| MicroRNAs in cancer- Homo sapiens-has 05206 | TRIM71;PDGFRA;DNMT3A;PTGS2;MAPK7;ERBB3;FSCN1;MDM2;BCL2;MAPK1;KRAS;HRAS;CD44 | 0.0007 | |
| Nonsmall cell lung Cancer-Homo sapiens-hsa05223 | AKT1; MAPK1; BRAF; KRAS; FHIT; HRAS | 0.0012 | |
| Colorectal Cancer-Homo sapiens-hsa05210 | SMAD3; BCL2; AKT1; MAPK1; BRAF; KRAS | 0.0015 | |
| Bladder Cancer-Homo sapiens-hsa05219 | MDM2; MAPK1; BRAF; KRAS; HRAS | 0.0019 | |
| Regulation of neuron death (GO:1901214) | ERBB3;UBE2V2;BCL2;AKT1;XIAP;KRAS;BRAF;HRAS;TNF;BB | 0.004 | |
| Regulation of neuron apoptotic process (GO:0043523) | ERBB3;UBE2V2;BCL2;XIAP;KRAS;BRAF;HRAS;TNF;BBC3 | 0.004 | |
| Negative regulation of neuron death (GO:1901215) | ERBB3;UBE2V2;BCL2;AKT1;XIAP;KRAS;BRAF;HRAS | 0.006 | |
| Immune response regulating cell surface receptor signaling pathway GO:0002768) | PDGFRA;NCKAP1;PLEKHA1;YWHAB;LIMK1;ERBB | 0.0080 | |
| hsa-miR-6507-5p | cytokinesis_(GO:0000910) | RACGAP1;PRC1;NEK7;MYH9;CEP55;RHOB | 0.033 |
| hsa-miR-135a-5p | Jak STAT signaling pathway-Homo sapiens-hsa04630 | PIAS4;MYC;MPL;BCL2;STAT6;JAK2 | 0.002 |
| Signaling pathways regulating pluripotency of stem cells-Homo sapiens-hsa04550 | BMPR2;APC;MYC;JAK2;SMAD5;SKIL | 0.002 | |
| Colorectal cancer-Homo sapiens-hsa05210 | APC;MYC;BCL2;BIRC5 | 0.004 | |
| MicroRNAs in cancer-Homo sapiens-hsa05206 | MARCKS;BMPR2;APC;ROCK1;MYC;BCL2;IRS2 | 0.004 | |
| TGF beta signaling pathway-Homo sapiens-hsa04350 | BMPR2;ROCK1;MYC;SMAD5 | 0.008 | |
| Cellular response to BMP stimulus (GO:0071773) | HEYL;GATA6;SMAD5 | 0.031 | |
| Response to BMP (GO:0071772) | HEYL;GATA6;SMAD5 | 0.031 | |
| neuron_projection_regeneration_(GO:0031102) | BCL2;APOA1;JAK2 | 0.031 | |
| Axon development (GO:0061564) | BCL2;APOA1;JAK2 | 0.031 | |
| Axon regeneration (GO:0031103) | BCL2;APOA1;JAK2 | 0.031 | |
| Positive regulation of ntrinsic apoptotic signaling pathway (GO:2001244 | PIAS4;SIAH1;BCL2;SKIL | 0.031 | |
| hsa-miR-3609 | Pathways in cancer-Homo sapiens-hsa05200 | ITGB1;EGLN3;PRKCB;F2R;FZD9;XIAP;HIF1A;IGF1R;TGFBR2;BMP2;CCND1;MDM2;MAPK1;CRK;APPL1;F2RL3 | 0.020 |
| Proteoglycans in cancer Homo sapiens hsa05205 | ITGB1;CCND1;PRKCB;CAV1;FZD9;MDM2;RRAS2;MAPK1;HIF1A;THBS1;IGF1R | 0.020 | |
| Endocytosis-Homo sapiens hsa04144 | SH3GLB1;HSPA8;RAB5B;RAB4A;ZFYVE9;SH3KBP1;CAV1;F2R;EPS15L1;IGF1R;TGFBR2;RAB11FIP1;MDM2 | 0.020 | |
| Focal adhesion- Homo sapiens-hsa04510 | RAP1B;ITGB1;CCND1;PRKCB;CAV1;XIAP;PAK6;MAPK1;CRK;THBS1;IGF1R | 0.020 | |
| hsa-miR-194-5p | Adherens junction- Homo sapiens-hsa04520 | TJP1;EP300;RAC1;IGF1R | 0.048 |
| Proteoglycans in cancer-Homo sapiens-hsa05205 | CAV1;FZD6;RAC1;HBEGF;IGF1R | 0.049 | |
| Focal adhesion- Homo sapiens-hsa04510 | CAV1;TLN2;RAC1;IGF1R;ITGA9 | 0.049 | |
| HIF-1 signaling pathway-Homo sapiens-hsa04066 | CDKN1B;EP300;RBX1;IGF1R | 0.049 |
†GO, Gene Ontology; ‡KEGG, Kyoto Encyclopedia of Genes and Genomes; *A p-value, Adjusted p-value.
3.3. Novel predicted miRNAs in the esophageal tissue
Interestingly, the data analysis showed novel potential miRNA transcripts in the esophageal tissues were expressed in at least two different pooled samples with mean read counts greater than five in each group. All the rRNAs and tRNAs were excluded by Rfam database(Nawrocki, et al., 2014) and the identified novel miRNAs possessed the criteria of secondary structure in the RNA fold change. Thirty-six novel candidate miRNAs were identified with mammalian homologues using this approach (Table S1), but none of them was significantly changed in the achalasia. GO analysis showed that eight novel miRNAs are significantly related to the neurotransmission process (adjusted p-value = 0.03), axon development and regeneration (adjusted p-value = 0.02), cellular response to nerve growth factor (adjusted p-value = 0.03), and inflammation process (Table 5).
Table 5.
The most significant enriched terms (potential function and pathway of target genes) based on biological process GO† enrichment of the novel candidate miRNAs in the esophageal tissues.
| miRNA | Enriched Term | Target genes | A p-value* |
|---|---|---|---|
| 2:46348793..46348872 | Positive_regulation_of_neurotransmitter_transport_(GO:0051590) | DTNBP1 | 0.024 |
| Positive_regulation_of_neurotransmitter_secretion_(GO:0001956) | 0.024 | ||
| Anterograde_axon_cargo_transport_(GO:0008089) | 0.024 | ||
| Axon_cargo_transport_(GO:0008088) | 0.03 | ||
| Regulation_of_neurotransmitter_secretion_(GO:0046928) | 0.03 | ||
| Regulation_of_neurotransmitter_transport_(GO:0051588) | 0.03 | ||
| 3:186787298..186787358 | Neuroepithelial_cell_differentiation_(GO:0060563) | MITF | 0.046 |
| 6:104646203...104646269 | Cellular_response_to_interleukin-6_(GO:0071354) | GALT | 0.039 |
| Interleukin-6-mediated_signaling_pathway_(GO:0070102) | 0.027 | ||
| Response_to_interleukin-6_(GO:0070741) | 0.04 | ||
| 7:53776229...53776317 | Positive_regulation_of_interleukin8_biosynthetic_process_(GO:0045416) | PRG3 | 0.031 |
| Regulation_of_interleukin-8_production_(GO:0032677) | 0.031 | ||
| 15:60128283...60128360 | Neuron_projection_regeneration_(GO:0031102) | NEFL | 0.02 |
| Axon_development_(GO:0061564) | 0.02 | ||
| Axon_regeneration_(GO:0031103) | 0.02 | ||
| Anterograde_axon_cargo_transport_(GO:0008089) | 0.02 | ||
| Neurofilament_cytoskeleton_organization_(GO:0060052) | 0.02 | ||
| Axon_cargo_transport_(GO:0008088) | 0.02 | ||
| Response_to_axon_injury_(GO:0048678) | 0.02 | ||
| Positive_regulation_of_axonogenesis_(GO:0050772) | 0.024 | ||
| Negative_regulation_of_neuron_apoptotic_process_(GO:0043524) | 0.036 | ||
| Regulation_of_axonogenesis_(GO:0050770) | 0.036 | ||
| Negative_regulation_of_neuron_death_(GO:1901215) | 0.037 | ||
| Regulation_of_neuron_apoptotic_process_(GO:0043523) | 0.04 | ||
| Positive_regulation_of_neuron_differentiation_(GO:0045666) | 0.044 | ||
| Regulation_of_neuron_death_(GO:1901214) | 0.044 | ||
| Regulation_of_neuron_projection_development_(GO:0010975) | 0.046 | ||
| Positive_regulation_of_neurogenesis_(GO:0050769) | 0.047 | ||
| 20:38425194..38425268 | Cellular_response_to_nerve_growth_factor_stimulus_(GO:1990090) | RAP1A | 0.032 |
| Response_to_nerve_growth_factor_(GO:1990089) | RAP1A | 0.032 | |
| Positive_regulation_of_calcium_ion_transmembrane_transporter_activity_(GO:1901021) | ANK2 | 0.032 | |
| Negative_regulation_of_neurotransmitter_transport_(GO:0051589) | RAP1A | 0.032 | |
| Nerve_growth_factor_signaling_pathway_(GO:0038180) | RAP1A | 0.032 | |
| Negative_regulation_of_neurotransmitter_secretion_(GO:0046929) | RAP1A | 0.032 | |
| Regulation_of_neurotransmitter_secretion_(GO:0046928) | RAP1A | 0.047 | |
| 6:77781479...77781527 | Regulation_of_intrinsic_apoptotic_signaling_pathway_by_p53_class_mediator_(GO:1902253) | RRM2B | 0.004 |
| Negative_regulation_of_signal_transduction_by_p53_class_mediator_(GO:1901797) | 0.005 | ||
| Regulation_of_signal_transduction_by_p53_class_mediator_(GO:1901796) | 0.006 | ||
| Regulation_of_intrinsic_apoptotic_signaling_pathway_(GO:2001242) | 0.014 | ||
| Negative_regulation_of_apoptotic_signaling_pathway_(GO:2001234) | 0.018 | ||
| Regulation_of_apoptotic_signaling_pathway_(GO:2001233) | 0.028 | ||
| 12:29562570..29562639 | Calcium-mediated_signaling_using_intracellular_calcium_source_(GO:0035584) | HOMER2 | 0.039 |
| Regulation_of_interleukin-8_biosynthetic_process_(GO:0045414) | PRG3 | 0.039 | |
| Mast_cell_activation_involved_in_immune_response_(GO:0002279) | PLA2G3 | 0.039 | |
| Positive_regulation_of_interleukin-8_biosynthetic_process_(GO:0045416) | PRG3 | 0.039 | |
| Axoneme_assembly_(GO:0035082) | PLA2G3 | 0.042 |
3.4. Validation of the NGS results by the qRT-PCR analysis
Three candidate miRNAs (hsa-miR-217, hsa-miR-143-3p, and hsa-miR-133a-5p), with the highest expression changes, were selected from the NGS data to confirm the gene expression changes. The qRT-PCR was used to validate the results of NGS. The qRT-PCR findings revealed a significant decline of hsa-miR-217 expression in the achalasia tissues compared to the controls (p-value = 0.004). These findings validated the results of the same comparison conducted by the NGS method. The qRT-PCR findings of hsa-miR-143-3p and hsa-miR-133a-5p, similar to the NGS results, showed upregulated expression in the tissues of the patients with achalasia but, contrary to NGS, these findings were not significant (p-value = 0.457 and p-value = 0.840 respectively) (Figure 2).
4. Discussion
To the best of our knowledge, this study is the first study in which the miRNA expression in the tissues of the patients with achalasia was compared to the controls using the NGS approach. The complete pattern of the miRNAs associated with the achalasia was obtained using the NGS approach. Fifteen miRNAs had significant differential expression in the esophageal tissues of the patients with achalasia compared to the controls. It was confirmed that miR-217 was downregulated significantly, and miR-143-3p and hsa-miR-133a-5p were upregulated (p-value > 0.05) in the achalasia tissues using the stem-loop qPCR as similarly observed in the NGS results. In a recent study using the microarray method, Shoji et al. showed that only two miRNAs (miR‑361‑5p and miR‑130a) were upregulated in patients with achalasia, which is contrary to the present study. This difference may be attributed to the different methods used in each study for miRNA expression analysis. Moreover, they used middle esophageal mucosa for sampling, which could potentially have different gene expression from the LES (Shoji et al., 2017). Another study that evaluated the miRNA expression profiling by the microarray demonstrated upregulated expression of hsa-miR-133a-5p in achalasia tissue in line with our study (Palmieri et al., 2019). Both previous studies used the microarray method for sequencing. The NGS platforms have higher sensitivity and dynamic amplitude than microarrays with higher sequencing depth (Motameny et al., 2010). Furthermore, the NGS produces a more accurate and reliable sequence, even if the individual reads are less accurate (Kulski, 2016).
Functional annotation revealed that many miRNAs determined in our study are involved in neuronal cell apoptosis (hsa-miR-143-3p), myelination process (hsa-miR-217), and neuronal regeneration (hsa-miR-135a-5p). In accordance with our findings, a previous study showed the mechanism of esophageal dysfunction in response to neuronal destruction in patients with Parkinson’s disease (Qualman et al., 1984). Moreover, the current study found that hsa-miR-143-3p targeted the immune system which was shown to be dysregulated in achalasia patients. Although the etiology of primary esophageal achalasia remains unknown, several hypotheses suggest that inflammation and autoimmunity are associated with its pathogenesis (Hirano, 2006). The histopathology analysis of the esophageal tissues, indicated lymphocytic infiltration, myenteric inflammation, and aganglionosis during the achalasia (Sodikoff et al., 2016). The cytotoxic autoimmune responses can potentially trigger progressive neuronal apoptosis in the achalasia tissues (Kahrilas and Boeckxstaens, 2013). The evidence suggests that miRNAs play an important role in the development of neurodegenerative diseases (Kye and Inês do Carmo, 2014).
Some of the miRNAs that were significantly differentially expressed in the current study were previously reported as cancer-related miRNAs. For example, miR-217, assuming to have tumor suppressor function; has been reported downregulated in several cancers such as gastric cancer (Chen et al., 2015), pancreatic ductal adenocarcinoma (Vychytilova-Faltejskova et al., 2015), Esophageal Squamous Cell Carcinoma (ESCC) (Wang et al., 2015b), and colorectal cancer (Wang et al., 2015a). Moreover, similar to this study, reduced miR-216 expression was reported in other diseases, such as nonsmall cell lung cancer (Wang et al., 2014b), ESCC (Dong et al., 2016), nasopharyngeal carcinoma (Deng et al., 2011), and hepatocellular carcinoma (Liu et al., 2015). The tumor suppressor role of miR-217 and miR-216 may justify the high prevalence of esophageal cancer in patients with achalasia. Despite the pathological differences between neurodegenerative diseases (such as achalasia) and cancers, new evidence suggests that they have similar regulatory mechanisms (Grasso et al., 2014).
The present study indicated the upregulation of hsa-miR-143-3p in the achalasia tissues of the patients. The upregulation of miR-143 in the CD4+T cells, highlights the importance of this miRNA in autoimmune diseases (Martínez-Ramos et al., 2014). This finding is in agreement with the role of autoimmunity in the formation of achalasia.
The biological process of GO and KEGG assessments in this study demonstrated that phosphatase and tensin homolog (PTEN) and Sirtuin 1 (SIRT1) could be significant targets of miR-217 in the achalasia (Table 4). Some studies showed that PTEN has a direct role in neurodegeneration under oxidative stress conditions (Li et al., 2013b; Morris et al., 2010). SIRT1 levels are associated with neurodegenerative diseases which have a progressive and severe reduction in neuronal cells (Kim et al., 2007). These findings could be in line with the role of neurodegeneration in the development of achalasia.
Interestingly, our findings identified some genetic factors related to the candidate miRNAs similar to other studies which were associated with achalasia. For instance, the HLA genes which showed to be targeted by miR-122-5p in this study related to achalasia in another study (Ruiz-de-León et al., 2002). In the current study, some immune modulator genes, including Interleukin 10(IL-10) and Interleukin 23 Receptor (IL-23R), were predicted to be targeted by hsa-miR-143-3p and hsa-miR-216a-5 respectively (De León et al., 2010; Palmieri et al., 2016). Accordingly, these findings highlight the role of immunity and inflammation in the initiation and progression of achalasia (Table S2).
This research showed that miR-383-5p was downregulated in the patient with achalasia who had a poor response to the dilatation treatment. This miRNA might play a potential prognostic role in the prediction of the response to the treatment in patients with achalasia. However, further studies could confirm this finding. Other studies introduced the hsa-miR-383 as a tumor suppressor with a decreased level in the glioma, medulloblastoma, and testicular embryonal carcinoma cells (Li et al., 2013a; Lian et al., 2010; Xu et al., 2015; Xu et al., 2014). Our results demonstrated that dysregulated miR-216b could target the tropomyosin (TPM), the gene which encodes the beta-tropomyosin with an important role in the regulation of the calcium-dependent muscle contraction. A study showed the changes in the TPM expression on the achalasia tissues (Palmieri et al., 2016). These findings may emphasize the neuromuscular process in the pathogenesis and development of the achalasia (Table 4).
Other findings indicated that has-miR-135 was downregulated only in the patients with a good response to the treatment. Some studies showed that the induction of miR-135a expression in different types of cancers could suppress cell proliferation through target genes (c-MYC, STAT6, SMAD5, and BMPR2). Some research introduced miR-135a as a potential predictor of treatment outcome in some cancers (Yamada et al., 2013; Ahmad et al., 2018). The current study confirmed that these target genes could be considered as significant targets of has-miR-135 in achalasia (Table 4).
This investigation found that Caveolin1 (CAV1) involving in the calcium signaling pathway could be a significant target of hsa-miR-3609 and hsa-miR-194-5p which are differentially expressed in the achalasia tissues of the patients. This finding is in line with a study that showed the CAV1 target gene was differentially expressed in the achalasia tissues with a possible function related to the achalasia pathogenesis (Palmieri et al., 2016). It is generally accepted that calcium channel blockers can support LES relaxation and esophageal peristalsis in patients with achalasia (Dughera et al., 2011). This provides further support for the role of candidate miRNAs in the etiology of achalasia.
5. Conclusion
In conclusion, the results of the current study provide a comprehensive analysis of miRNA expression in the achalasia and may be used as a basis for future studies to investigate the role of candidate miRNAs in the etiology of achalasia. A significant downregulation was observed in the hsa-miR-217 in the LES samples of the achalasia patients with significant enrichment in myelination process ontology. Furthermore, the NGS miRNA expression profiling might be a suitable platform to classify the achalasia into different response groups concerning the outcome of dilatation treatment.
Acknowledgements
The authors would like to thank all the participants who have donated their samples to the study. The authors also thank the staff of the DDRC in the Shariati Hospital affiliated with Tehran University of Medical Sciences for their collaboration. This work was supported by the Golestan University of Medical Sciences (grant no: 940208018).
References
- Ahmad A Zhang W Wu M Tan S Zhu T Tumor-suppressive miRNA-135a inhibits breast cancer cell proliferation by targeting ELK1 and ELK3 oncogenes. Genes & Genomics . 2018;40:243–251. doi: 10.1007/s13258-017-0624-6. [DOI] [PubMed] [Google Scholar]
- Ates F Vaezi MF The pathogenesis and management of achalasia: current status and future directions. Gut Liver . 2015;9:449–449. doi: 10.5009/gnl14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP MicroRNAs: genomics, biogenesis, mechanism, and function. Cell . 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bolger A Lohse M Usadel B Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics . 2014;30 doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D Zhang D Lu Y Chen L Zeng Z microRNA-217 inhibits tumor progression and metastasis by downregulating EZH2 and predicts favorable prognosis in gastric cancer. Oncotarget . 2015;6:10868–10868. doi: 10.18632/oncotarget.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De León A De La Serna J Santiago J Sevilla C Fernández‐arquero M Association between idiopathic achalasia and IL23R gene. J Neurogastroenterol Motil . 2010;22:734–e218. doi: 10.1111/j.1365-2982.2010.01497.x. [DOI] [PubMed] [Google Scholar]
- Deng M Tang H Zhou Y Zhou M Xiong W miR-216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. Journalof Cell Science . 2011;124:2997–3005. doi: 10.1242/jcs.085050. [DOI] [PubMed] [Google Scholar]
- Dong S Yin H Dong C Sun K Lv P Predictive value of plasma microRNA-216a/b in the diagnosis of esophageal squamous cell carcinoma. Disease Markers . 2016;10 doi: 10.1155/2016/1857067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dughera L Chiaverina M Cacciotella L Cisarò F Management of achalasia. Clinical and Experimental Gastroenterology . 2011;4:33–33. doi: 10.2147/CEG.S11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y Fang D Hu J MicroRNA and its roles in esophageal cancer. Medical Science Monitor . 2012;18:RA22–RA30. doi: 10.12659/MSM.882509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhi F Vaezi MF Idiopathic (primary) achalasia. Orphanet Journal of Rare Diseases . 2007;2:38–38. doi: 10.1186/1750-1172-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedländer MR Mackowiak SD Li N Chen W Rajewsky N miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Research . 2011;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furer V Greenberg JD Attur M Abramson SB Pillinger MH The role of microRNA in rheumatoid arthritis and other autoimmune diseases. Clinical Immunology . 2010;136:1–15. doi: 10.1016/j.clim.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Furuzawa-Carballeda J Torres-Landa S Valdovinos MÁ Coss-Adame E Del Campo LAM New insights into the pathophysiology of achalasia and implications for future treatment. World Journal of Gastroenterology . 2016;22:7892–7892. doi: 10.3748/wjg.v22.i35.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal UC Daschakraborty SB Singh R Pathogenesis of achalasia cardia. World Journal of Gastroenterology . 2012;18:3050–3057. doi: 10.3748/wjg.v18.i24.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso M Piscopo P Confaloni A Denti MA Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules . 2014;19:c6891–6910. doi: 10.3390/molecules19056891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanzadeh A Mikaeli J Elahi E Mehrabi N Etemadi A Demographic, clinical features and treatment outcomes in 700 achalasia patients in Iran. Middle East Journal of Digestive Diseases . 2010;2:c91–c91. [PMC free article] [PubMed] [Google Scholar]
- Pathophysiology of achalasia and diffuse esophageal spasm. GI Motility online. doi: 10.1038/gimo22 . 2006.
- Kahrilas PJ Boeckxstaens G The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology . 2013;145:954–965. doi: 10.1053/j.gastro.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrilas PJ Bredenoord A Fox M Gyawali C Roman S The Chicago Classification of esophageal motility disorders. v3. 0. Neurogastroenterology & Motility . 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz M Iovino N Unnerstall U Gaul U Segal E The role of site accessibility in microRNA target recognition. Nature Genetics . 2007;39:1278–1278. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- Kim D Nguyen MD Dobbin MM Fischer A Sananbenesi F SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. The EMBO Journal . 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A Griffiths-Jones S miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research . 2013;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Next-generation sequencing—an overview of the history, tools, and “omic” applications. Next Generation Sequencing-Advances, Applications and Challenges . 2016. pp. 3–60.
- Kye MJ Inês do Carmo GG ( Frontiers in Cellular Neuroscience . 2014;8 doi: 10.3389/fncel.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KKW Pang JCS Lau KM Zhou L Mao Y MiR‐383 is downregulated in medulloblastoma and targets peroxiredoxin 3 (PRDX3) Brain Pathology . 2013a;23:413–425. doi: 10.1111/bpa.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P Mao WM Zheng ZG Dong ZM Ling ZQ Down-regulation of PTEN expression modulated by dysregulated miR-21 contributes to the progression of esophageal cancer. Digestive Diseases and Sciences . 2013b;58:3483–3493. doi: 10.1007/s10620-013-2854-z. [DOI] [PubMed] [Google Scholar]
- Lian J Tian H Liu L Zhang X Li W Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell Death &Disease . 2010;1 doi: 10.1038/cddis.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F Zhou S Deng Y Zhang Z Zhang E MiR-216b is involved in pathogenesis and progression of hepatocellular carcinoma through HBx-miR-216b-IGF2BP2 signaling pathway. Cell Death & Disease . 2015;6 doi: 10.1038/cddis.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Ramos R García-Lozano J Lucena J Castillo-Palma M García-Hernández F Differential expression pattern of microRNAs in CD4+ and CD19+ cells from asymptomatic patients with systemic lupus erythematosus. Lupus . 2014;23:353–359. doi: 10.1177/0961203314522335. [DOI] [PubMed] [Google Scholar]
- Mohammadi-Yeganeh S Paryan M Samiee SM Soleimani M Arefian E Development of a robust, low cost stem-loop real-time quantification PCR technique for miRNA expression analysis. Molecular Biology Reports . 2013;40:3665–3674. doi: 10.1007/s11033-012-2442-x. [DOI] [PubMed] [Google Scholar]
- Morris L Veeriah S Chan T Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene . 2010;29:3453–3464. doi: 10.1038/onc.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motameny S Wolters S Nürnberg P Schumacher B Next generation sequencing of miRNAs–strategies, resources and methods. Genes . 2010;1:70–84. doi: 10.3390/genes1010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki EP Burge SW Bateman A Daub J Eberhardt RY 0: updates to the RNA families database. Rfam 12 . 2014;43:130–137. doi: 10.1093/nar/gku1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri O Mazza T Bassotti G Merla A Tolone S microRNA‐mRNA network model in patients with achalasia. Neurogastroenterology & Motility . 2019;32 doi: 10.1111/nmo.13764. [DOI] [PubMed] [Google Scholar]
- Palmieri O Mazza T Merla A Fusilli C Cuttitta A Gene expression of muscular and neuronal pathways is cooperatively dysregulated in patients with idiopathic achalasia. Scientific Reports . 2016;6:31549–31549. doi: 10.1038/srep31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W Vaezi MF Etiology and pathogenesis of achalasia: the current understanding. The American Journal of Gastroenterology . 2005;100:1404–1414. doi: 10.1111/j.1572-0241.2005.41775.x. [DOI] [PubMed] [Google Scholar]
- Qiu YQ Wolkenhauer O. Cho KH. Yokota H KEGG Pathway Database. 2013;10:4419–4419. [Google Scholar]
- Qualman SJ Haupt HM Yang P Hamilton SR Esophageal Lewy bodies associated with ganglion cell loss in achalasia: similarity to Parkinson’s disease. Gastroenterology . 1984;87:848–856. [PubMed] [Google Scholar]
- Rehmsmeier M Steffen P Höchsmann M Giegerich R Fast and effective prediction of microRNA/target duplexes. RNA . 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-de-León A Mendoza J Sevilla-Mantilla C Arquero MF Pérez-de-la-Serna J Myenteric antiplexus antibodies and class II HLA in achalasia. Digestive Diseases and Sciences . 2002;47:15–19. doi: 10.1023/a:1013242831900. [DOI] [PubMed] [Google Scholar]
- Sadowski D Ackah F Jiang B Svenson L Achalasia: incidence, prevalence and survival. A population‐based study. Neurogastroenterology & Motility . 2010;22:e256–e261. doi: 10.1111/j.1365-2982.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- Shoji H Isomoto H Yoshida A Ikeda H Minami H MicroRNA-130a is highly expressed in the esophageal mucosa of achalasia patients. Experimental and Therapeutic Medicine . 2017;14:898–904. doi: 10.3892/etm.2017.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP Massachi I Manickavel S Singh S Rao NP The role of miRNA in inflammation and autoimmunity. Autoimmunity Reviews . 2013;12:1160–1165. doi: 10.1016/j.autrev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Sodikoff JB Lo AA Shetuni BB Kahrilas PJ Yang GY Histopathologic patterns among achalasia subtypes. Neurogastroenterology & Motility . 2016;28:139–145. doi: 10.1111/nmo.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm M Hackenberg M Langenberger D Frishman D TargetSpy: a supervised machine learning approach for microRNA target prediction. BMC Bioinformatics . 2010;11:1471–1471. doi: 10.1186/1471-2105-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtan A Inchley CS Marzban M Tavakoli‐Yaraki M Teymoori‐Rad M The role of microRNAs in respiratory viral infection: friend or foe? Reviews in Medical Virology . 2016;26:389–407. doi: 10.1002/rmv.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G Patti MG Gullo R Pandolfino JE Kahrilas PJ The Kagoshima consensus on esophageal achalasia. Diseases of the Esophagus . 2012;25:337–348. doi: 10.1111/j.1442-2050.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- Vantrappen G Hellemans J Treatment of achalasia and related motor disorders. Gastroenterology . 1980;79:144–154. [PubMed] [Google Scholar]
- Vychytilova-Faltejskova P Kiss I Klusova S Hlavsa J Prochazka V -198 and miR-217 as diagnostic and prognostic biomarkers for chronic pancreatitis and pancreatic ductal adenocarcinoma. Diagnostic Pathology . 2015;21:34a–34a. doi: 10.1186/s13000-015-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B Shen Z-l Zhao G Wang C Jiang K-w. BMC Cancer . 2015a;15:1–1. doi: 10.1186/s12885-015-1438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C Ji B Cheng B Chen J Bai B Neuroprotection of microRNA in neurological disorders (Review) Biomedical Reports . 2014a;2:611–619. doi: 10.3892/br.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R-T Xu Xu C-X Song Jin H Decreased expression of miR216a contributes to non–small-cell lung cancer progression. Clinical Cancer Research . 2014b;20:4705–4716. doi: 10.1158/1078-0432.CCR-14-0517. [DOI] [PubMed] [Google Scholar]
- Wang X Li M Wang Z Han S Tang X Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. Journal of Biological Chemistry . 2015b;290:3925–3935. doi: 10.1074/jbc.M114.596866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D Ma P Gao G Gui Y Niu X MicroRNA-383 expression regulates proliferation, migration, invasion, and apoptosis in human glioma cells. Tumor Biology . 2015;36:7743–7753. doi: 10.1007/s13277-015-3378-2. [DOI] [PubMed] [Google Scholar]
- Xu Z Zeng X Tian D Xu H Cai Q MicroRNA-383 inhibits anchorage-independent growth and induces cell cycle arrest of glioma cells by targeting CCND1. Biochemical and Biophysical Research Communications . 2014;453:833–838. doi: 10.1016/j.bbrc.2014.10.047. [DOI] [PubMed] [Google Scholar]
- Yamada Y Hidaka H Seki N Yoshino H Yamasaki T Tumor‐suppressive microRNA‐135a inhibits cancer cell proliferation by targeting the c‐MYC oncogene in renal cell carcinoma. Cancer Science . 2013;104:304–312. doi: 10.1111/cas.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]


