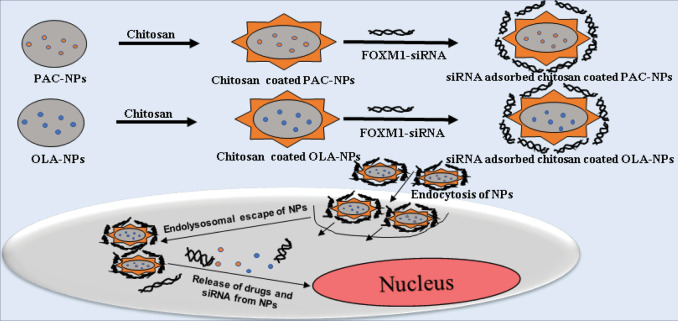
Abstract
Introduction: Triple-negative breast cancer (TNBC) is a lethal tumor with an advanced degree of metastasis and poor survivability as compared to other subtypes of breast cancer. TNBC which consists of 15 % of all types of breast cancer is categorized by the absence of expression of estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth factor receptor-2 (HER2). This is the main reason for the failure of current hormonal receptor-based therapies against TNBCs, thus leading to poor patient outcomes. Therefore, there is a necessity to develop novel therapies targeting this devastating disease.
Methods: In this study, we have targeted TNBC by simultaneous activation of apoptosis through DNA damage via cytotoxic agent such as paclitaxel (PAC), inhibition of PARP activity via PARP inhibitor, olaparib (OLA) and inhibiting the activity of FOXM1 proto-oncogenic transcription factor by using RNA interference technology (FOXM1-siRNA) in nanoformulations. Experiments conducted in this investigation include cellular uptake, cytotoxicity and apoptosis study using MDA-MB-231 cells.
Results: The present study validates that co-delivery of two drugs (PAC and OLA) along with FOXM1-siRNA by cationic NPs, enhances the therapeutic outcome leading to greater cytotoxicity in TNBC cells.
Conclusion: The current investigation focuses on designing a multifunctional drug delivery platform for concurrent delivery of either PAC or PARP inhibitor (olaparib) and FOXM1 siRNA in chitosan-coated poly(D, L-lactide-co-glycolide) (PLGA) nanoparticles (NPs) with the ability to emerge as a front runner therapeutic for TNBC therapy.
Keywords: TNBC, Nanoparticles, Paclitaxel, Olaparib, FOXM1-siRNA
Introduction
Triple-negative breast cancer (TNBC) has become the major reason for death due to cancer in women worldwide.1 It is characterized with a greater degree of distant metastasis and lesser survivability as compared to other types of breast cancer2 and categorized by the absence of progesterone receptors (PR), estrogen receptors (ER) and HER2 expression.3 The present chemotherapeutic agents targeting these hormonal receptors are ineffective against TNBCs. Thus, there is a necessity to develop novel amalgamated therapies targeting this devastating disease.
Paclitaxel (PAC) is an effective chemotherapeutic drug considered as a gold-standard against breast cancer.4 It mainly stabilizes microtubules and induces cell cycle blockade in G2/M stage subsequently leading to cell death.5 The major limitation for PAC is its water insolubility and non-specific toxicity to normal cells. However, it is a well-known fact that improved solubility and increased efficacy of PAC can be achieved by delivering it in different formulations.
Generally, TNBC patients are found with BRCA gene mutation.6 Thus, investigators have exploited the defective DNA repair pathways as a capable therapeutic approach for TNBC. In this context, PARP inhibitors have emerged as a potential anticancer therapy regimen for TNBC treatment.7 Olaparib (OLA), a PARP inhibitor has shown a profound effect on TNBC therapy.8 However, the incidence of severe side effects has also been reported with the treatment of OLA in different cancers.9
Forkhead Box M1 (FOXM1) is a proto-oncogenic transcription factor, which is related to the most clinical drug-resistant, aggressive cancer phenotype that leads to poor patient survival.10 It is reported to be highly expressed in TNBC.11 Thus, owing to its higher expression, the FOXM1 gene is emerging as an effective target in TNBC owing to its higher overexpression rate.
Considering the above limitations of the current drugs, now investigators have moved forward to find improved therapeutic tactics for TNBC treatment. Thus, targeting FOXM1 gene in TNBC may become a powerful technology for inhibiting tumor growth. In this context, the use of RNA interference (RNAi) technology has garnered impetus for silencing the different genes responsible for tumorigenesis.12 However, there is a major concern in its clinical use relatively owing to its short half-life and poor membrane permeability thereby leading to less cellular uptake.13 Thus, there is an urgent need for strategies for siRNA delivery for uplifting their therapeutic effect for cancer management.
In this context, the use of nanotechnology has emerged as a weapon to co-deliver the drugs and siRNA in single nanoformulation thereby surpassing the drawbacks of conventional drug delivery technologies.14 These nanosystems deliver the drugs and siRNA into cancer cells by enhanced permeability and retention (EPR) effect thus enhancing the therapeutic effects of the drug.15 Polymeric nanoparticles (NPs) have shown more efficiency as a drug delivery platform. For instance, poly(lactic-co-glycolide) (PLGA) has been mostly used for various biomedical use due to its biocompatible and biodegradable nature.16 However, for the delivery of siRNA, the surface of PLGA NPs needs to be modified with some cationic polymer such as chitosan in order to increase the transfection efficiency.17-19 Thus, the purpose of the current study was to improve the efficacy of drugs and also to reduce the therapeutic dose of PAC by combined administration with OLA and FOXM1-siRNA in nanoformulations. This system may increase the efficiency of PAC by attaining the combinatorial effect of PAC, OLA and siRNA in NP system that can be used for TNBC treatment in near future.
Materials and Methods
Materials
PLGA (50:50), PVA (30,000–70,000 kDa), Chitosan (low molecular weight), MTT reagent, Human duplex siRNA targeting FOXM1 (sequence: GCACUAUCAACAAUAGCCU), and FAM-labeled negative siRNA were obtained from Sigma-Aldrich (St. Louis, MO). Acetonitrile was bought from Merck. Primary antibody: FOXM1 (NBP1-30961) was obtained from Novus Biologicals, BI Biotech India Pvt. Ltd. Secondary anti-rabbit IgG, HRP-linked antibody was purchased from Cell Signalling, Technology, (CST, MA, US).
Cell lines and culture conditions
MDA-MB-231 was provided by Dr. Avinash Bajaj, Associate professor at Regional Centre for Biotechnology, Faridabad, Haryana, India. MDA-MB-231 cells were maintained in DMEM- High Glucose media containing 10% FBS, 1% glutamine, 1% Penicillin-Streptomycin solution and maintained at 37°C, 5% CO2 incubator (FormaTM Steri-Cycle CO2 incubator, Thermo Scientific, USA). All chemicals for cell culture were procured from Invitrogen.
Preparation of Paclitaxel/Olaparib loaded PLGA NPs
PAC and OLA encapsulated PLGA NPs were prepared by a single oil-in-water emulsion solvent-evaporation method using previously reported protocol.20 Briefly, 50 mg of PLGA and particular concentrations of drugs (either 5 mg of PAC or 5 mg of OLA) in 3 ml of ethanol:acetonitrile (1:1) mixture solution was mixed with 12 ml of PVA (2 % w/v) solution in water. The emulsion was sonicated for 2 minutes (with 55 W energy) in an ice bath by probe sonicator (Misonix S-4000 ultrasonic liquid processor (Misonix Sonicators, CT, USA)). Then, the organic solvent was evaporated by overnight stirring on a magnetic stirrer. The next day, then it was centrifuged at 151,263 × g for 20 minutes at 4°C. The pellet was suspended in 2 ml of deionized water and then lyophilized for 48 hours to get the powder form of drug encapsulated NPs.
Preparation of chitosan-coated PLGA NPs
Chitosan-coated NPs were prepared by incubating a particular concentration of chitosan solution with drug-loaded NPs.21 Briefly, 10 % w/w of chitosan was dissolved in 1% glacial acetic acid. 10 mg of PAC or OLA-NPs was added into the above chitosan solution and kept for overnight stirring at 4°C. The next day, the mixture was centrifuged at 10,600 × g for 10 minutes at 4°C. The supernatant was discarded to remove an excess of unbound chitosan. The collected pellet was dispersed in PBS followed by lyophilization for 24 hours to get chitosan-coated PAC or OLA loaded PLGA NPs (CS-PAC-NPs or CS-OLA-NPs).
Size and zeta potential measurement
The hydrodynamic diameter and surface charge of NPs were measured by dynamic laser spectroscopy using Nano Zetasizer ZS (Malvern, UK). 100 µg/mL of NPs suspension was prepared in distilled water followed by sonication in an ice bath for 30 seconds. Then the suspension was subjected to measure the size and zeta potential. The experiments were performed in triplicate.
Atomic force microscope (AFM)
Drug-loaded PLGA NPs (with or without chitosan coating) were subjected to AFM study (JPK nanowizard II, Germany) to evaluate the surface morphology of drug-loaded NPs. The protocol followed for the study has already been described in a previously published paper.22
Estimation of encapsulation efficiency
The amount of PAC or OLA encapsulated in NPs was estimated by UV/visible spectrophotometer (Perkin Elmer Inc, MA, USA). One milligram of NPs was added to 1 mL of ethanol:acetonitrile (1:1) mixture followed by sonication and centrifugation at 4°C for 10 minutes at 10 600 × g. Then, the supernatant was used for estimating the amount of PAC or OLA present in NPs using spectrophotometer at λmax = 227 nm and λmax = 221 nm for PAC and OLA respectively. The amount of drug present in NPs was calculated by using the standard plot of the drugs prepared in 1 mL of ethanol:acetonitrile (1:1) mixture using similar conditions as mentioned above. Then the entrapment efficiency of the drug was estimated by using a formula such as Encapsulation efficiency (%) = (Quantity of drug present in the NPs/Total drug used while preparing the formulation) X 100.
In vitro release kinetics study of PAC or OLA from NPs
The in vitro drug release pattern from the NPs was studied by following our previously published protocol with slight modification.23 10 mg of PAC or OLA loaded NPs were dispersed in PBS buffer (0.01 M, pH 7.4, comprising Tween 80 of 0.1%). The NPs dispersion was distributed equally into three Eppendorf tubes and then the tubes were placed in a shaker maintained at 37°C and 150 rpm in a shaker. The released drug quantity from the NPs was estimated by centrifuging the tubes for 10 minutes at 13 800 × g and 4°C for particular time intervals. Then, the supernatants were used for estimating the number of released drugs from the NPs. The collected supernatants were lyophilized and then the recovered powder was dissolved in ethanol:acetonitrile (1:1) solvent mixture to measure the amount of PAC or OLA present in NPs using UV/visible spectrophotometer at λmax = 227 nm and λmax = 221 nm respectively. The pellet was redispersed with 1 ml of fresh PBS followed by keeping it in a shaker for further study for 7 days. The in vitro release experiment was performed in triplicate.
Surface adsorption of FOXM1-siRNA on CS coated NPs by Gel retardation assay
FOXM1-siRNA was loaded into the NPs by the surface adsorption method. The adsorption of FOXM1-siRNA on the surface of chitosan-coated PLGA NPs was analyzed by agarose gel electrophoresis following our previous protocol.24 A range of concentration (0 to 50 μg) of chitosan-coated PLGA NPs were prepared in distilled water. 100 pmol concentration of siRNA was added dropwise to the NPs suspension and mixed gently. Then the mixture was incubated and lyophilized to get the powder form. The recovered powder was dispersed in 20 μL of sterile water followed by analysis in 1% agarose gel electrophoresis and the complexes were viewed under the gel-doc system.
Uptake of FAM-labeled siRNA in MDA-MB-231 cells by confocal microscope
siRNA uptake by cells was studied by using FLOWVIEW F3000 confocal microscope (Olympus, USA). The fluorescence property of FAM-labeled siRNA was used in order to track the siRNA in cells. Briefly, 50,000 cells per well were plated in 6-well plates (BD Biosciences, US) and kept in an incubator for 24 hours. Then, cells were incubated with 200 nM of only FAM-siRNA and siRNA-NPs for 4 hours. The media was taken out after 4 hours followed by washing with PBS and then viewed under the microscope. The experiment was carried out at least three times and the image from one experiment has been represented.
Quantitative analysis of FAM uptake in MDA-MB-231 cells by flow cytometry
Cellular uptake of FAM siRNA was done by BD FACS CantoTM II flow cytometer (BD Biosciences, USA). Briefly, 50,000 cells per ml of media were seeded in 6-well plates (BD Biosciences, US) for 24 hours. The next day, the old media was taken out and then the cells were incubated with media containing FAM-siRNA and FAM-siRNA-NPs for 4 hours in an incubator. Following washing with PBS FAM uptake was analyzed in the form of mean fluorescence intensity values as measured by flow cytometry. For all the flow cytometry experiments, a gate was set on the side scattered vs forward-scattered light plot for excluding the cell debris and free particles. Moreover, 10,000 total number ungated cells were analyzed in all the experiments.
Effect of siRNA on FOXM1 expression
The effect of siRNA on FOXM1 protein expression was studied by western blot assay.22 Briefly, 5 lakhs cells were plated in flasks. After overnight incubation, cells were exposed with FOXM1-siRNA both in native and in nanoformulation for 48 hours. After incubation, cells were collected by scraping and lysed by using NP-40 based lysis buffer (50 mM Tris-Cl buffer (pH 7.5),150 mM NaCl, 2 mM, EDTA and 1% NP-40) comprising cocktails of phosphatase and protease inhibitor (Sigma-Aldrich). The concentration of protein was estimated by the BCA protein estimation kit (Thermo Scientific, USA). The proteins (25 μg) were separated in SDS-polyacrylamide (10%) gel and then the bands were transferred into PVDF membrane. After transferring the protein, the membrane was incubated in 5% non-fat dry milk (w/v) blocking solution, followed by overnight incubation with FOXM1 primary antibody (1:1000 dilution). Next, the membrane was incubated with a secondary antibody (1:5000 dilution) for 1 hour at 25°C. Protein band signals were detected by using a chemiluminescence reagent. β-actin was used as control. The experiment was repeated two times and a representative picture of a single experiment is provided.
Evaluation of toxic effects of PAC, OLA and FOXM1 in MDA-MB-231 cells using MTT assay
The cytotoxic effect of PLGA-NPs without containing any drug (void-NPs), PAC and OLA in combination with FOXM1 (both in solution and NPs) in MDA-MB-231 cells were evaluated by MTT assay.25 Approximately 5000 cells/well were seeded into 96-well plates for 24 hours. Then, the cells were exposed to varying concentrations of only PAC/OLA (from 0 to 50 μg/mL) and 100 pmol of FOXM1 siRNA both in solution and in NPs to evaluate the toxic effect of both the drugs and siRNA in MDA-MB-231 cells. Controls included cells grown in the growth media. After 3 days, the old medium was aspirated followed by adding 100 µL of fresh media. Then, 10 µL of 5 mg/mL MTT stock solution was added and kept at 37°C for 3 hours. Then, the developed formazan crystals were dissolved by using 100 µL of dimethylsulfoxide (DMSO) as a stop solution. The optical density was estimated at 560 nm in a 96-well format microplate reader (Berthold Technologies, Germany). Percentage of cell viability was calculated and graphs were plotted using origin software.
Apoptosis study by flow cytometry
The effect of different treatments on cell death was assessed by flow cytometry.26 Briefly, 2 lakhs cells per well were plated in a 6-well plate and kept for 24 hours. Then the cells were exposed to PAC and/or OLA (1 μg/mL) either alone or in combination with FOXM1 siRNA (100 pmol) both in native or in formulation for 48 hours. After incubation, the cells were collected by trypsinization followed by washing with PBS. Then the cells were incubated in 500 μl of propidium iodide (PI) solution (10 μg/μL) for 1 hr in dark at room temperature. Then, the apoptotic cells were detected by analyzing the percentage of cells present in the sub G0/G1 phase by using a flow cytometer. The experiment was done in triplicate and a representative image of a single experiment was provided.
Statistical analysis
All experiments were analyzed by using a t-test and one-way analysis of variance (ANOVA) test. P values of less than 0.05 were considered as significant differences.
Results
Physiochemical characterization of drug-loaded PLGA and chitosan-coated PLGA NPs
In an attempt to deliver drug and siRNA in nanoformulation simultaneously, drug encapsulated polymeric (PLGA) NPs were synthesized by solvent evaporation method and then coated with chitosan to form cationic nanoformulation to be used against TNBC cells. The size of all the formulated PLGA NPs were in the nanometer range with poly dispersity index less than 1 and negative zeta potential. However, after coating with chitosan there is no such impact on size but zeta potential changed to positive as shown in Table 1.
Table 1. Physico-chemical characterization results of different nanoparticle formulation.
| Formulations | Size (nm) a | Zeta potential (mV) b | PDI c |
| PLGA-NPs | 144 ± 3.1 | -7.8 ± 3.9 | 0.74 ± 2.7 |
| PAC-NPs | 141 ± 1.3 | -13.8 ± 3.3 | 0.86 ± 3.1 |
| OLA-NPs | 170 ± 2.1 | -13.3 ± 2.7 | 0.66 ± 3.3 |
| CS-PAC-NPs | 220 ± 2.9 | 13.3 ± 1.9 | 0.77 ± 2.6 |
| CS-OLA-NPs | 221 ± 2.3 | 12 ± 3.1 | 0.71 ± 3.7 |
a Size in nm as measured by dynamic laser spectroscopy.
b Zeta potential in mV measured by zetasizer.
c Polydispersity index measured by dynamic laser spectroscopy.
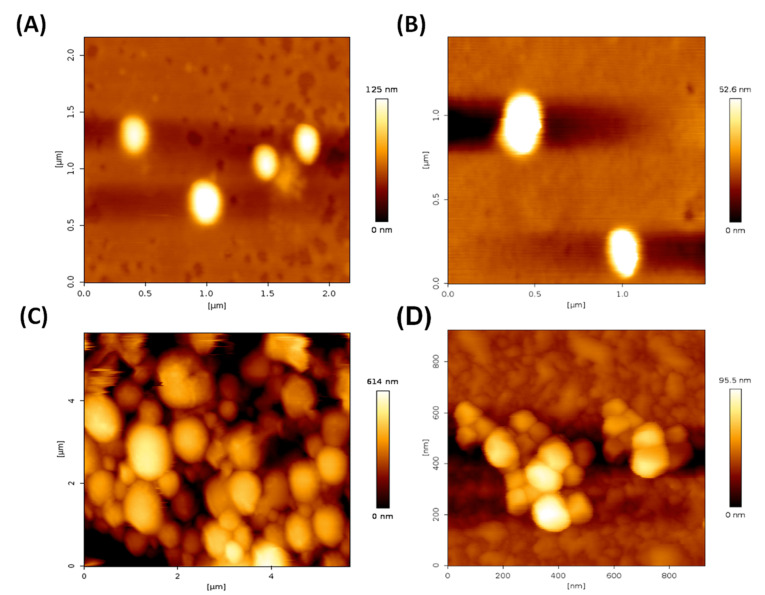
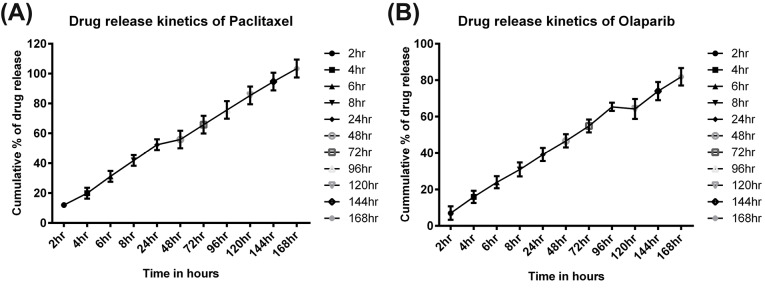
An effective nanoparticle system should have a high drug holding capacity in order to minimize the amount of carrier or NPs necessary for the administration to the disease site for cancer therapy. In this investigation, we attained an optimum amount of encapsulation efficiency for both the formulations PAC-NPs (~80%) and OLA-NPs (~60%). AFM images demonstrated the spherical and smooth surface of the nanoformulations both before and after coating of chitosan on drug-loaded PLGA NPs (Fig. 1A-D). The smooth and spherical shape of PLGA NPs have already been reported by many groups thus supporting our results.22 For achieving a complete remission of a disease like cancer, the constant presence of drugs at the tumor site is highly essential. Sustained-release formulations such as NPs helps in maintaining a constant drug concentration at the tumor site for an extended period. Thus, we have formulated a controlled and sustained-release formulations of PAC and OLA loaded PLGA NPs as seen from in vitro release kinetics study. These formulations have shown the sustained release of the drugs for a long time of over 7 days (Fig. 2A and B). The graphs showed a biphasic release of the drugs from the NPs. Initially burst release of surface adsorbed drugs occurred following a slow release from the inner core of the polymeric matrix.
Fig. 1.
Atomic force microscopy study of NPs. (A) PAC loaded PLGA NPs, (B) OLA loaded PLGA NPs, (C) Chitosan coated PAC loaded PLGA NPs and (D) Chitosan coated OLA loaded PLGA NPs.
Fig. 2.
Release kinetics study of drugs from the NPs. (A) In vitro release kinetics of PAC from PAC loaded PLGA NPs and (B) In vitro release kinetics of OLA from OLA loaded NPs. Data represented as mean ± SEM (n = 3).
PLGA NPs/siRNA complexes detection by gel retardation assay
The formation of the complex between positively charged chitosan-coated NPs and negatively charged siRNA was assessed by an agarose gel retardation experiment (Fig. 3). A particular concentration of siRNA was mixed with a range of concentrations of NPs (from 0 to 50 μg). In lane 1, 100 pmol of the only siRNA gave an intense band, while with increasing concentration of NPs, the siRNA gets condensed with positively charged NPs thereby showing no bands or decrease in band intensity.
Fig. 3.
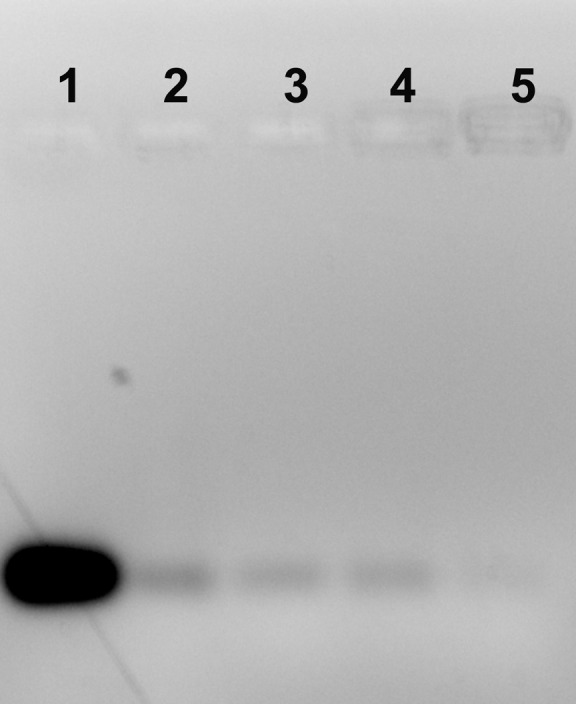
Gel retardation assay of a fixed concentration of siRNA with varying concentrations of chitosan-coated NPs analyzed by agarose gel electrophoresis. Lane 1: Native siRNA (100 pmol), lane 2: NPs (5 µg/mL), lane 3: NPs (10 µg/mL), lane 4: NPs (25 µg/mL) and lane 5: NPs (50 µg/mL).
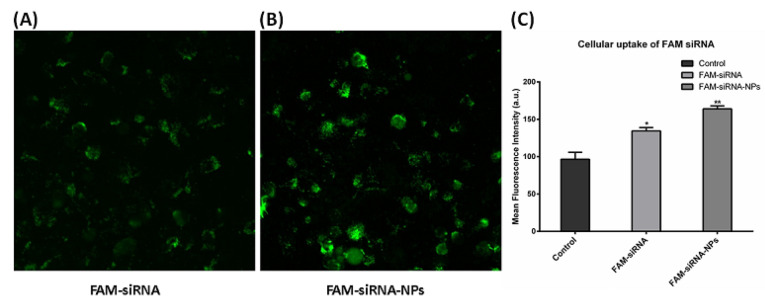
Cellular uptake of FAM-labeled siRNA in MDA-MB-231 cells by confocal microscopy and Flow cytometry
The cellular uptake of siRNA adsorbed chitosan-coated NPs was observed by a confocal microscope. FAM is a fluorescein synthetic dye generally used in oligonucleotide synthesis research. Thus, FAM-labeled siRNA was used to check the siRNA-NPs complex internalization into the cells. Briefly, MDA-MB-231 cells were incubated with FAM-labeled siRNA both in solution and nanoformulations for 4 hours (Fig. 4A). Results demonstrated higher uptake of siRNA, in cells incubated with FAM-siRNA-NPs in comparison to the native siRNA. This may be attributed due to the mucoadhesive nature of chitosan-coated NPs facilitating better uptake thereby increasing the fluorescence intensity. Lower uptake of siRNA may be due to negatively charge repulsion between siRNA and the cell membrane or degradation of siRNA in the cytosol.
Fig. 4.
Intracellular uptake of FAM conjugated siRNA both in native and in NPs in MDA-MB-231 cells. (A) 4 hours uptake of FAM-siRNA (native and NPs) into MDA-MB-231 cells by confocal microscope. (B) Quantitative estimation of FAM-siRNA (native and NPs) in MDA-MB-231 cells by flow cytometry. Data represented as mean ± SEM. (n = 3). **P < 0.01 for FAM-siRNA-NPs versus control.
Besides our flow cytometry uptake result also depicts higher uptake of FAM-siRNA in NPs as compared to the native one (Fig. 4B). This may be due to the endocytic mode of uptake of NPs into the cells while native siRNA, only diffuses through the membrane thus there are chances that the latter can be easily effluxed out of the cell thus resulting in lower uptake.
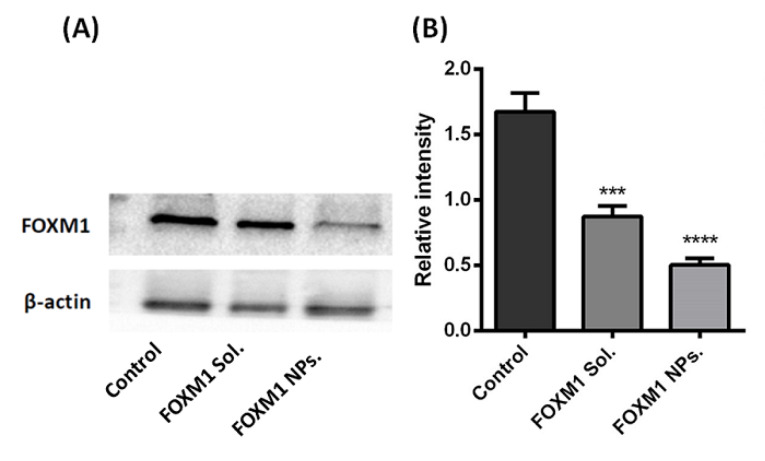
Effect of FOXM1-siRNA on protein expression
The effect of FOXM1-siRNA and FOXM1-NPs on the expression of FOXM1 protein was evaluated by western blotting (Fig. 5A). Our result depicts that siRNA inhibits the expression of FOXM1 protein in MDA-MB-231 cells. However, it can be noticed that siRNA in complex with NPs exhibited enhanced protein downregulation as compared to the native siRNA. This means that greater penetration of siRNA in NPs complex results in increased transfection efficiency subsequently demonstrating effective gene silencing. The significant difference in the effect of FOXM1-siRNA in solution and NPs can be observed in the densiometric graph (Fig. 5B).
Fig. 5.
Effect of FOXM1-siRNA on protein expression by western blot assay. (A) FOXM1 protein expression by western blot study and (B) Densiometric analysis of relative expression of FOXM1 protein expression treated with siRNA either in native or in NPs.
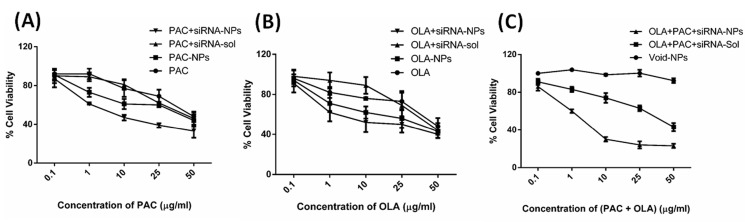
Cytotoxic effects of PAC, OLA and FOXM1 siRNA either in single or in combination in MDA-MB-231 cells
The cytotoxic effect of a range of concentration (from 0 to 50 μg/mL) of drugs (either PAC or OLA and PAC+OLA) along with a fixed concentration of FOXM1 siRNA (100 pmol) in both native and NPs formulations was assessed in MDA-MB-231 cells using MTT colorimetric assay (Fig. 6). The results demonstrate that both the drugs (either PAC or OLA) in native/NPs induce cytotoxicity in a concentration-dependent manner. Further, it is notable to state that both the drugs (either PAC or OLA) in NPs are inducing enhanced cytotoxicity than the drugs in native form. Additionally, combinational delivery of siRNA with any single drug (either PAC or OLA) in nanoformulation leads to greater cytotoxicity as compared to the single treatment. In Fig. 6A, a higher toxic effect of PAC with FOXM1 siRNA in nanoformulation can be observed. Similarly, in fig. 6B, OLA with siRNA in NPs induced more toxicity. Fig. 6C depicts that void-NPs are non-toxic to the cells. While maximum cytotoxicity was observed in cells treated with the combination of all the therapeutic components in nanoformulations as compared to all other treatment groups in MDA-MB-231 cells. The improved cytotoxicity by (PAC+OLA+siRNA)-NPs may be due to a lowering in the expression of FOXM1 mRNA/protein by FOXM1-siRNA and also the combinatorial effect of all the therapeutic compounds that lead to maximum cell death.
Fig. 6.
Cytotoxic effect study of different treatments MDA-MB-231 cells using MTT assay. (A) Cytotoxicity induced by different concentrations of only PAC and PAC+ FOXM1-siRNA (100pmol) both in solution and in nanoformulations, (B) Cytotoxicity induced by different concentrations of only OLA and OLA+FOXM1-siRNA (100 pmol) both in solution and in nanoformulations and (C) Cytotoxicity induced by different concentrations of (PAC+OLA) with FOXM1-siRNA (100pmol) both in solution and in nanoformulations. Data represented as mean ± SD (n = 3).
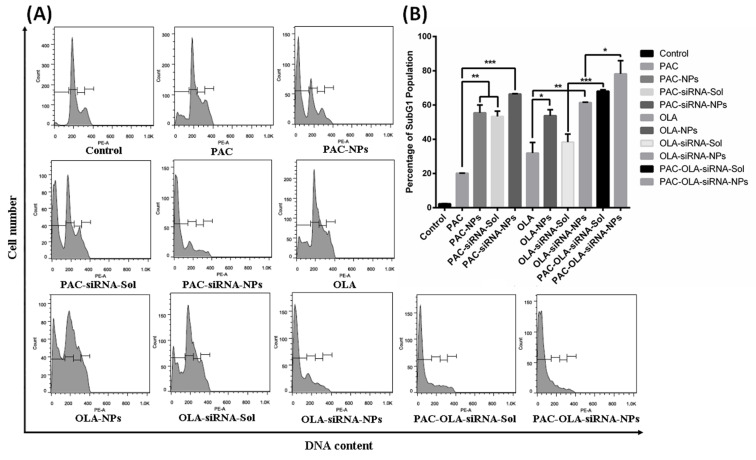
Cell Cycle Analysis by Flow Cytometry
The mode of induction of cell death by PAC, OLA and FOXM1 was investigated by cell cycle analysis using flow cytometry. The above therapeutic moieties mostly induce cell death primarily through the process of cell cycle arrest and apoptosis in cells. Fig. 7 represents the Sub G1 population of cells exposed to different treatment groups. Results depict that combination of PAC, OLA and siRNA can induce more apoptosis when compared to other treatments. Moreover, the NPs treatments have shown better apoptosis induction than their native counterparts.
Fig. 7.
Apoptosis analysis by cell cycle study using flow cytometry. (A) Histograms representing different cell cycle phases and (B) SubG0/G1 phase cell population indicating the apoptotic cells after treatment of MDA-MB-231 cells with different treatment groups. Data represented as mean ± S.E.M. (n = 3).
Discussion
PAC is a potent chemotherapeutic drug used for TNBC. However, it has some limitations such as poor solubility, toxicity to normal cells and development of multidrug resistance. Thus, to further enhance the efficacy of the drug, many combinational approaches are being explored. Independently, PARP inhibitor, OLA is also being used in combination with other drugs for TNBC treatment. Furthermore, FOXM1, a transcription factor is associated with the survivability of patients with TNBC. Thus, downregulation of FOXM1 expression may increase the sensitivity of the breast cancer cells towards many anticancer drugs. In this study, we found that PAC inhibited the growth of TNBC cells in a concentration-dependent manner. Additionally, a combination of PAC with OLA and FOXM1 siRNA inhibits cell growth and induces apoptosis significantly as compared to the monotherapy. Further to enhance the combinational effect of the drugs and siRNA by overcoming the limitations associated with both, nanoformulations were prepared and their efficacy was investigated in MDA-MB-231 cell line. Mostly, anticancer drugs are not only water-insoluble but also toxic to normal cells. Moreover, for a disease like cancer, we need a sustained and controlled drug release pattern at the tumor site in order to maintain an optimum therapeutic drug concentration.27 Thus, PAC and OLA loaded PLGA NPs were prepared to suffice the above objectives. Moreover, in this study siRNA has also been used to target the transcription factor FOXM1 in order to control cell proliferation. Besides, we have overcome the limitations associated with siRNA delivery by using cationic nanoformulations. Thus, in the present investigation, we are using chitosan-coated PLGA NPs for the delivery of FOXM1-siRNA and PAC or OLA to circumvent the drug resistance in TNBC.
Our study focuses on the preparation of different drug-loaded PLGA NPs were formulated for controlled release of PAC and OLA and then, these NPs were coated with chitosan to form cationic NPs. Physicochemical characterization results depict that PAC and/or OLA loaded NPs were in the size range below 200 nm with a negative charge of around -15 mV. AFM results demonstrated the smooth and spherical and morphology of the NPs. To further load siRNA in these NPs, the formulations were coated with chitosan to form cationic NPs. The physicochemical properties of chitosan-coated NPs confirm their positive charge thus confirming the successful coating of chitosan. The small particle size of the NPs will aid in easy penetration into the cells. Moreover, the cationic nature of the NPs will form a complex with the siRNA and the mucoadhesive nature of the chitosan will help the NPs to adhere easily to the cell surface. Furthermore, the release kinetics experiment was performed to study the release of drugs (PAC or OLA) in the buffer. The nanoformulations followed a sustained drug release pattern for a longer period owing to the release of incorporated or embedded drugs from the core of the NPs.
Effective gene silencing by siRNA can be achieved with increased transfection efficiency. Our gel retardation study confirms that greater transfection efficiency of siRNA is achieved by adsorbing it on coated NPs. Previously, Misra et al have also documented similar kind of results thus corroborating our study.24 In order to get efficient gene silencing, the siRNA has to be localized in the cells efficiently. For the same, a qualitative cellular uptake study was done using the confocal microscope. Our results depict higher uptake of siRNA-NPs complex as compared to the native siRNA. Similarly, enhanced uptake of FAM-siRNA-NPs was observed in MDA-MB-231 cells by FACS. It may be due to better interaction of positively charged coated NPs with the negatively charged cell surface thereby leading to increased transfection efficiency.28 Increased cellular intake of siRNA in complex with NPs may lead to enhanced gene silencing leading to better downregulation of FOXM1 protein expression. In this regard, Susa et al have demonstrated higher downregulation of protein expression in cells following treatment with siRNA-NPs complex, thus verifying our results.29 Followed by higher cellular uptake, the effect of various formulations on cell death was evaluated by MTT assay. Cytotoxicity assay results demonstrated that NPs are inducing more cell death than their native counterparts. Moreover, the combination of all the entities such as PAC, OLA and FOXM1 siRNA in the formulation are exhibiting the highest cytotoxic effect than all other treatments. This may be attributed to their higher cellular uptake and the combined effect of all the components present in the multifactorial approach. Cell cycle analysis results prove that both the drugs and siRNA-loaded NPs were able to arrest the cells in G0/G1 phase than other treatments due to the combinational effect of all the agents. Thus, the present investigation demonstrates that concurrent delivery of both the drugs (PAC and OLA) and FOXM1-siRNA by cationic NPs, augment the therapeutic outcome leading to improved cytotoxicity in MDA-MB-231 cells.
Conclusion
In summary, the current investigation validates the prospect of using PAC, OLA and FOXM1-siRNA adsorbed chitosan-coated PLGA nanosystems to precisely and proficiently control the resistance mechanism and target TNBC by inducing increased cytotoxicity. These chitosan-coated NPs may be an efficacious podium to conquer the boundaries of existing drug/gene delivery platforms for better management of TNBC in the future.
Funding sources
This work is supported by DST-SERB-NPDF funding project designated by a number PDF/2016/002494.
Ethical statement
Not applicable.
Competing interests
None to declare.
Authors’ contribution
RM and RSV designed the study and experiments. RM wrote the draft and performed all the experiments under the supervision of RSV. BP and SV helped RM in FACS and Western bolt experiments respectively.
Acknowledgments
RM would like to thank the SERB-Department of Science and Technology (DST), Government of India for grant assistance (PDF/2016/002494).
Research Highlights
What is the current knowledge?
√ The absence of three important surface markers such as ER, PR and HER2 have made TNBC treatment is a challenging mission.
√ Integration of nanotechnology with traditional chemotherapeutic strategy has emerged as a therapeutic boon to meet the lacunae of current treatment methodology.
What is new here?
√ A novel combination of PAC, OLA and a siRNA targeting to FOXM1 gene have been applied for killing TNBC cells efficiently.
√ Results depict the increased cytotoxicity and apoptosis in TNBC cells.
√ Thus, this tactic may be used as a potent therapeutic modality for TNBC treatment in near future.
References
- 1.Wu H, Wang Q, Zhong H, Li L, Zhang Q, Huang Q. et al. Differentially expressed microRNAs in exosomes of patients with breast cancer revealed by next-generation sequencing. Oncol Rep. 2019;43:240–250. doi: 10.3892/or.2019.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapadia CH, Ioele SA, Day ES. Layer-by-layer assembled PLGA nanoparticles carrying miR-34a cargo inhibit the proliferation and cell cycle progression of triple-negative breast cancer cells. J Biomed Mater Res A. 2020;108:601–613. doi: 10.1002/jbm.a.36840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu K, Wang D, Li Z, Du G, Guo Q, Wu T. et al. Heterogeneity of Triple-Negative Breast Cancer: Differences in Clinicopathologic and Ultrasound Features Between Premenopausal and Postmenopausal Patients. J Ultrasound Med. 2019;9999:1–9. doi: 10.1002/jum.15174. [DOI] [PubMed] [Google Scholar]
- 4.Bomane A, Goncalves A, Ballester PJ. Paclitaxel Response Can Be Predicted With Interpretable Multi-Variate Classifiers Exploiting DNA-Methylation and miRNA Data. Front Genet. 2019;10:1041. doi: 10.3389/fgene.2019.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Sayed AD, Elshenawy MA, Tulbah A, Al-Tweigeri T, Ghebeh H. Complete Response of Chemo-Refractory Metastatic Metaplastic Breast Cancer to Paclitaxel-Immunotherapy Combination. Am J Case Rep. 2019;20:1630–1635. doi: 10.12659/AJCR.918770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy P, Muggia F. Women's cancers: how the discovery of BRCA genes is driving current concepts of cancer biology and therapeutics. Ecancermedicalscience. 2019;13:904. doi: 10.3332/ecancer.2019.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantelidou C, Sonzogni O, De Oliveria Taveira M, Mehta AK, Kothari A, Wang D. et al. PARP Inhibitor Efficacy Depends on CD8(+) T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019;9:722–737. doi: 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews Hew T, Zuberi L. PARP Inhibitor Olaparib Use in a BRCA1-Positive Patient With Metastatic Triple-Negative Breast Cancer, Without the Initial Use of Platinum-Based Chemotherapy, Showing Significant Rapid Near Resolution of Large Liver Metastasis While Patient Experienced Gout-Like Symptoms. J Investig Med High Impact Case Rep. 2019;7:2324709619864989. doi: 10.1177/2324709619864989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo XX, Wu HL, Shi HY, Su L, Zhang X. The efficacy and safety of olaparib in the treatment of cancers: a meta-analysis of randomized controlled trials. Cancer Manag Res. 2018;10:2553–2562. doi: 10.2147/CMAR.S169558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Y, Wang W, Wang X, Xie H, Ye X, Shu P. Integrated network analysis identifies hsa-miR-4756-3p as a regulator of FOXM1 in Triple Negative Breast Cancer. Sci Rep. 2019;9:13830. doi: 10.1038/s41598-019-50248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saba R, Alsayed A, Zacny JP, Dudek AZ. The Role of Forkhead Box Protein M1 in Breast Cancer Progression and Resistance to Therapy. Int J Breast Cancer. 2016;2016:9768183. doi: 10.1155/2016/9768183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titze-de-Almeida SS, Brandao PRP, Faber I, Titze-de-Almeida R. Leading RNA Interference Therapeutics Part 1: Silencing Hereditary Transthyretin Amyloidosis, with a Focus on Patisiran. Mol Diagn Ther. 2020;24:49–59. doi: 10.1007/s40291-019-00434-w. [DOI] [PubMed] [Google Scholar]
- 13.Dua K, Wadhwa R, Singhvi G, Rapalli V, Shukla SD, Shastri MD. et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev Res. 2019;80:714–730. doi: 10.1002/ddr.21571. [DOI] [PubMed] [Google Scholar]
- 14.Wang BK, Yu XF, Wang JH, Li ZB, Li PH, Wang H. et al. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials. 2016;78:27–39. doi: 10.1016/j.biomaterials.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 15. Misra R, Kandoi S, Varadaraj, S. Vijayalakshmi, S. Nanda, A. Verma, R. S.∗. Nanotheranostics: A tactic for cancer stem cells prognosis and management. J Drug Deliv Sci Technol 2020; 55 101457. 10.1016/j.jddst.2019.101457 [DOI]
- 16.Chen C, Mei H, Shi W, Deng J, Zhang B, Guo T. et al. EGFP-EGF1-conjugated PLGA nanoparticles for targeted delivery of siRNA into injured brain microvascular endothelial cells for efficient RNA interference. PLoS One. 2013;8:e60860. doi: 10.1371/journal.pone.0060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagani HV, Josyula VR, Palanimuthu VR, Hariharapura RC, Gang SS. Improvement of therapeutic efficacy of PLGA nanoformulation of siRNA targeting anti-apoptotic Bcl-2 through chitosan coating. Eur J Pharm Sci. 2013;48:611–618. doi: 10.1016/j.ejps.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr CM. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine. 2007;3:173–183. doi: 10.1016/j.nano.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Li L, Rathinavelu A, Hao J, Narasimhan M, He M. et al. SiRNA drug delivery by biodegradable polymeric nanoparticles. J Nanosci Nanotechnol. 2006;6:2821–2828. doi: 10.1166/jnn.2006.436. [DOI] [PubMed] [Google Scholar]
- 20.Misra R, Sahoo SK. Intracellular trafficking of nuclear localization signal conjugated nanoparticles for cancer therapy. Eur J Pharm Sci. 2010;39:152–163. doi: 10.1016/j.ejps.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Alibolandi M, Amel Farzad S, Mohammadi M, Abnous K, Taghdisi SM, Kalalinia F. et al. Tetrac-decorated chitosan-coated PLGA nanoparticles as a new platform for targeted delivery of SN38. Artif Cells Nanomed Biotechnol. 2018;46:1003–1014. doi: 10.1080/21691401.2018.1477789. [DOI] [PubMed] [Google Scholar]
- 22.Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly(D,L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharm. 2011;8:852–866. doi: 10.1021/mp100455h. [DOI] [PubMed] [Google Scholar]
- 23.Misra R, Sahoo SK. Antibacterial activity of doxycycline-loaded nanoparticles. Methods Enzymol. 2012;509:61–85. doi: 10.1016/B978-0-12-391858-1.00004-6. [DOI] [PubMed] [Google Scholar]
- 24.Misra R, Das M, Sahoo BS, Sahoo SK. Reversal of multidrug resistance in vitro by co-delivery of MDR1 targeting siRNA and doxorubicin using a novel cationic poly(lactide-co-glycolide) nanoformulation. Int J Pharm. 2014;475:372–384. doi: 10.1016/j.ijpharm.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 25.Das M, Duan W, Sahoo SK. Multifunctional nanoparticle-EpCAM aptamer bioconjugates: a paradigm for targeted drug delivery and imaging in cancer therapy. Nanomedicine. 2015;11:379–389. doi: 10.1016/j.nano.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Xu K, Pian G, Sun S. Artesunate and sorafenib: Combinatorial inhibition of liver cancer cell growth. Oncol Lett. 2019;18:4735–4743. doi: 10.3892/ol.2019.10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30:5737–5750. doi: 10.1016/j.biomaterials.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Okabe N, Note Y, Hashiba K, Maeki M, Tokeshi M. et al. Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomater. 2020;102:341–350. doi: 10.1016/j.actbio.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Susa M, Iyer AK, Ryu K, Choy E, Hornicek FJ, Mankin H. et al. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010;5:e10764. doi: 10.1371/journal.pone.0010764. [DOI] [PMC free article] [PubMed] [Google Scholar]