Abstract
Hospital healthcare workers (HCWs) are at increased risk of contracting COVID-19 infection. We aimed to determine the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in HCWs in Ireland. Two tertiary referral hospitals in Irish cities with diverging community incidence and seroprevalence were identified; COVID-19 had been diagnosed in 10.2% and 1.8% of staff respectively by the time of the study (October 2020). All staff of both hospitals (N = 9038) were invited to participate in an online questionnaire and blood sampling for SARS-CoV-2 antibody testing. Frequencies and percentages for positive SARS-CoV-2 antibody were calculated and adjusted relative risks (aRR) for participant characteristics were calculated using multivariable regression analysis. In total, 5788 HCWs participated (64% response rate). Seroprevalence of antibodies to SARS-CoV-2 was 15% and 4.1% in hospitals 1 and 2, respectively. Thirty-nine percent of infections were previously undiagnosed. Risk for seropositivity was higher for healthcare assistants (aRR 2.0, 95% confidence interval (CI) 1.4–3.0), nurses (aRR: 1.6, 95% CI 1.1–2.2), daily exposure to patients with COVID-19 (aRR: 1.6, 95% CI 1.2–2.1), age 18–29 years (aRR: 1.4, 95% CI 1.1–1.9), living with other HCWs (aRR: 1.3, 95% CI 1.1–1.5), Asian background (aRR: 1.3, 95% CI 1.0–1.6) and male sex (aRR: 1.2, 95% CI 1.0–1.4). The HCW seroprevalence was six times higher than community seroprevalence. Risk was higher for those with close patient contact. The proportion of undiagnosed infections call for robust infection control guidance, easy access to testing and consideration of screening in asymptomatic HCWs. With emerging evidence of reduction in transmission from vaccinated individuals, the authors strongly endorse rapid vaccination of all HCWs.
Key words: Coronavirus, COVID-19, healthcare workers, SARS-CoV-2 antibodies, seroprevalence
Background
Healthcare workers (HCWs), and those they live with, are at increased risk of contracting COVID-19 viral infection [1–3]. Raised antibody levels to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are an excellent indicator of COVID-19 infection [4]. To date there are no published literature on the seroprevalence of antibodies to SARS-CoV-2 infection in Irish HCWs, but it is known from surveillance data that a high proportion of the COVID-19 cases notified were HCWs [5]. Understanding the transmission and potential immunity dynamics of SARS-CoV-2 in hospitals in Ireland is key to controlling this pandemic at national and hospital levels and adds valuable information to the growing evidence based on the transmission patterns of COVID-19 among HCWs.
Hospital 1 is a tertiary referral hospital in the south inner city of Dublin, the capital city of Ireland (population 1.2 million) and has almost 4700 employees and just over 1000 beds. From March to May 2020 (first wave of the pandemic in Ireland [6]) 9.6% of the staff of Hospital 1 tested positive for SARS-CoV-2 infection via polymerase chain reaction (PCR), and by the start of October (the start of the second wave of the pandemic in Ireland [6]) 10.2% of staff had tested positive by PCR. Hospital 2 is a comparable tertiary referral hospital with almost 4400 employees and over 500 beds, located in Galway, in the West of Ireland (population 80 000); 1.8% of its HCWs had a PCR-confirmed infection at some stage during the time period from March to May 2020 and this remained at 1.8% until the start of October 2020. Hospital 1 is one of the largest acute hospitals in Dublin city; hospital 2 is the main acute hospital serving the city of Galway. Both hospitals received patients with COVID-19 infection throughout the first wave of the pandemic in Ireland, and breakdown by ward and specialty is similar. The community incidence of COVID-19 infection in County Galway was significantly lower than that in County Dublin during this time period, which covered the first wave of the pandemic in Ireland and the start of the second wave [6]. The community seroprevalence was also significantly lower in the West of Ireland as compared with the greater Dublin area; community seroprevalence was 3.1% for hospital 1 and 0.6% for hospital 2 in June 2020 [7, 8].
The purpose of the study was to determine the prevalence of anti-SARS-CoV-2 antibodies in HCWs in these two hospitals with diverging community and healthcare rates of infection to improve the understanding of HCWs’ risk factors (demographic, living arrangements and work-related risks) for SARS-CoV-2 infection and to inform risk reduction activities and help health services to prepare for further waves of the pandemic. The study will be repeated in April 2021 to assess changes in overall seroprevalence, changes in individual serostatus over time and antibody response to COVID-19 vaccination.
Methods
Study design
This is a cross-sectional study of the seroprevalence of circulating antibodies to SARS-CoV-2, carried out from 14th to 23rd October 2020. All staff members of both hospitals were invited to participate in an online self-administered consent process and online questionnaire, followed by blood sampling for SARS-CoV-2 antibody testing. Electronic consent and patient reported outcomes were captured using Castor; an eClinical platform that enables decentralised clinical trials [9]. Technical support and walk-in phlebotomy clinics were provided for participants who had difficulty with the online consent process. Information collected in the questionnaire included demographic information, contact details, place and type of work, level of contact with patients, previous COVID-19 symptoms and testing, history of close contact with a confirmed case of COVID-19 and living arrangements. Blood samples were processed anonymously. All samples were tested on two testing platforms: the Abbott Architect SARS-CoV-2 immunoglobulin (Ig)-G assay and the Roche Elecsys anti-SARS-CoV-2 immunoassay [10–12]. Samples with an index result in the Abbott manufacturer's suggested positive and greyzone underwent additional testing in the National Virus Reference Laboratory (NVRL) using the Wantai SARS-CoV-2 AB ELISA distributed by Fortress Diagnostics [13]. A positive result on any of the three assays was considered a positive result. Results were discussed in person with any participant who requested this.
Statistical analysis
Frequencies and percentages were calculated for socio-demographic, epidemiological and clinical characteristics, including antibody results. Characteristics of those with a positive SARS-CoV-2 antibody result were compared to those with undetectable antibody, using the chi-square test. Univariate logistic regression was used to calculate relative risks along with their 95% confidence intervals (CIs) to assess the association between SARS-CoV-2 antibody result and characteristics of the study participants. Multivariable regression analysis was conducted to control for negative and positive confounding and to calculate adjusted relative risks (aRR). No explicit finite population correction or reweighting was carried out. All analyses were conducted in Stata 16 (StataCorp, 2019; College Station, TX, LLC) and R 4.0.3 (R Core Team, 2020, www.R-project.org/).
Results
Participation rates and demographics
All staff working in both hospitals (9038 people) were invited to participate in the study. In hospitals 1 and 2, 65% (3042/4692) and 63% (2745/4395) of staff participated in both questionnaire and blood sample, respectively.
The socio-demographic characteristics of participants were similar in both hospitals. Seventy-seven percent were female, with a median age of 39.5 years (IQR 30.4–48.9); 5.1% of participants were >60 years of age. Regarding ethnicity, 77% of participants were white Irish, 10% Asian (13% in hospital 1 and 7% in hospital 2), 9.5% other white background (majority born in Poland, USA and UK) and 2% African or any other black background. Ninety-one percent of participants live with others, and 31% live with other HCWs. The majority (36%) of participants were nursing staff, 19% were allied health care staff, 17% medical/dental staff, 13% administration staff, 7.5% general support staff, 5% healthcare assistants (HCAs) and 2% other HCWs, broadly reflecting the HCW breakdown of the hospital staff (Table 1). Participation rates among staff groupings were also similar in both hospitals; nurses and HCA were slightly under-represented at 59% and 39% uptake respectively. In all other groups participation rates were above 60%.
Table 1.
Participant characteristics by hospital and total number of participants
| Hospital 1 (N = 3042) | Hospital 2 (N = 2745) | P valuea | Total (n = 5788) | ||||
|---|---|---|---|---|---|---|---|
| Participant characteristics | n | % | n | % | N | % | |
| Age groups | |||||||
| 18–29 | 728 | 24 | 632 | 23 | 0.717 | 1350 | 23 |
| 30–39 | 831 | 27 | 785 | 29 | 1617 | 28 | |
| 40–49 | 793 | 26 | 722 | 26 | 1515 | 26 | |
| 50–59 | 532 | 18 | 468 | 17 | 1001 | 17 | |
| Over 60 | 158 | 5.2 | 146 | 5.3 | 304 | 5.3 | |
| Sex | |||||||
| Female | 2326 | 77 | 2152 | 78 | 0.117 | 4478 | 77 |
| Male | 716 | 24 | 592 | 22 | 1308 | 23 | |
| Missing | – | – | 1 | 0.04 | 1 | 0.02 | |
| Ethnicity | |||||||
| Irish | 2262 | 74 | 2182 | 80 | 4444 | 77 | |
| Any other white background | 267 | 8.8 | 284 | 10 | <0.001 <0.001 |
551 | 10 |
| Any Asian background | 393 | 13 | 184 | 7 | 577 | 10 | |
| Any African or black background | 65 | 2.1 | 48 | 1.8 | 113 | 2.0 | |
| Other | 55 | 1.8 | 46 | 1.7 | 101 | 1.8 | |
| Missing | – | – | 1 | 0.04 | 1 | 0.02 | |
| Country of birtha | |||||||
| Ireland | 2182 | 72 | 2091 | 76 | 4273 | 74 | |
| Country of birtha | |||||||
| UK | 152 | 5.0 | 192 | 7.0 | 344 | 5.9 | |
| India | 201 | 6.6 | 98 | 3.6 | 299 | 5.2 | |
| Philippines | 166 | 5.5 | 25 | 0.9 | 191 | 3.3 | |
| Poland | 24 | 0.8 | 48 | 1.8 | 72 | 1.2 | |
| USA | 22 | 0.7 | 38 | 1.4 | 60 | 1.0 | |
| Other | 295 | 9.7 | 253 | 9.2 | 548 | 9.5 | |
| Education | |||||||
| Primary | 27 | 0.9 | 2 | 0.1 | <0.001 | 29 | 0.5 |
| Secondary | 420 | 14 | 264 | 10 | 684 | 12 | |
| Third level | 1300 | 43 | 1245 | 45 | 2545 | 44 | |
| Post-graduate | 1295 | 43 | 1232 | 45 | 2527 | 44 | |
| Missing | – | – | 2 | 0.1 | 2 | 0.03 | |
| Role | |||||||
| Admin | 454 | 15 | 349 | 13 | <0.001 | 803 | 14 |
| Medical/dental | 460 | 15 | 522 | 19 | 982 | 17 | |
| Nursing/midwifery | 1045 | 34 | 1019 | 37 | 2064 | 36 | |
| Allied health | 616 | 20 | 475 | 17 | 1012 | 19 | |
| General support | 255 | 8.4 | 179 | 6.5 | 434 | 7.5 | |
| Health care assistant | 157 | 5.2 | 129 | 4.7 | 286 | 4.9 | |
| Other | 55 | 1.8 | 72 | 2.6 | 127 | 2.2 | |
| Lives with | |||||||
| Alone | 256 | 8.4 | 223 | 8.1 | 0.020 | 479 | 8.3 |
| Lives with | |||||||
| With others | 2768 | 91.0 | 2518 | 91.7 | 5286 | 91.3 | |
| Missing | 18 | 0.6 | 4 | 0.2 | 22 | 0.4 | |
| Lives with HCW | |||||||
| Yes | 928 | 31 | 839 | 31 | 0.983 | 1767 | 31 |
| Lives with HCW | |||||||
| No | 2060 | 68 | 1859 | 68 | 3919 | 68 | |
| Missing | 54 | 1.8 | 47 | 1.7 | 101 | 1.8 | |
Calculated using the χ2 test.
Previous testing and COVID-19-related characteristics of the participants
Hospital 1 staff had a higher percentage of previously confirmed COVID-19 infection; 9.6% of participants reported having tested positive at some stage by PCR compared to 2.7% of participants in hospital 2 (Table 2). Table 2 highlights the COVID-19-related characteristics of the participants by hospital.
Table 2.
COVID-19-related characteristics by hospital and total number of participants
| Hospital 1 (N = 3042) | Hospital 2 (N = 2745) | Total (N = 5788) | |||||
|---|---|---|---|---|---|---|---|
| Participant characteristics | n | % | N | % | P valuea | n | % |
| Contact of a COVID-19 case | |||||||
| Yes | 1185 | 39 | 519 | 19 | <0.001 | 1704 | 30 |
| No | 1847 | 61 | 2224 | 81 | 4071 | 70 | |
| Missing | 10 | 0.3 | 2 | 0.1 | 12 | 0.2 | |
| Setting of close contact | |||||||
| Contact at work | 1039 | 88 | 456 | 88 | 0.916 | 1495 | 88 |
| Contact outside of work | 146 | 12 | 63 | 12 | 209 | 12 | |
| Daily contact with COVID-19 patients | |||||||
| Contact with COVID-19 patients | 510 | 17 | 392 | 14 | <0.001 | 902 | 16 |
| Contact with patients without COVID-19 | 1611 | 53 | 1634 | 60 | 3245 | 56 | |
| No patient contact | 918 | 30 | 717 | 26 | 1635 | 28 | |
| Missing | 3 | 0.1 | 2 | 0.1 | 5 | 0.1 | |
| Previous COVID-19 symptoms | |||||||
| No symptoms | 1359 | 45 | 1517 | 55 | <0.001 | 2876 | 50 |
| Had symptoms | 1683 | 55 | 1228 | 45 | 2911 | 50 | |
| Severity | |||||||
| No symptoms | 1359 | 45 | 1517 | 55 | <0.001 | 2876 | 50 |
| Only minor symptoms | 1214 | 40 | 945 | 34 | 2159 | 37 | |
| Significant symptoms | 442 | 15 | 259 | 9.4 | 701 | 12 | |
| Hospitalised | 27 | 0.9 | 24 | 0.9 | 51 | 0.9 | |
| Previous COVID-19 PCR test | |||||||
| Yes | 1685 | 55 | 1093 | 40 | <0.001 | 2778 | 48 |
| No | 1353 | 45 | 1650 | 60 | 3003 | 52 | |
| Missing | 4 | 0.1 | 2 | 0.1 | 6 | 0.1 | |
| Previous positive COVID-19 PCR test | |||||||
| Yes | 292 | 9.6 | 75 | 2.7 | <0.001 | 367 | 6.3 |
| No | 2746 | 90.3 | 2668 | 97.2 | 5414 | 93.6 | |
| Missing | 4 | 0.1 | 2 | 0.1 | 6 | 0.1 | |
Calculated using the χ2 test.
Seroprevalence of antibodies to SARS-CoV-2
In hospital 1, the overall seroprevalence of antibodies to SARS-COV-2 was 15% (464/3042). Regarding the level of patient contact, the seroprevalence was 21% (108/510) in participants reporting daily contact with patients with known or suspected COVID-19 infection (we defined this as the high-risk group), 17% (269/1611) in those who reported daily contact with patients without known or suspected COVID-19 infection (intermediate-risk group) and 9.5% (87/918) in those who reported little or no patient contact (low-risk group). In hospital 2, the overall seroprevalence of antibodies to SARS-COV-2 was 4.1% (112/2745); 7.1% (28/392) in the high-risk group; 4.6% (75/1634) in the intermediate-risk group and 1.3% (9/717) in the low-risk group.
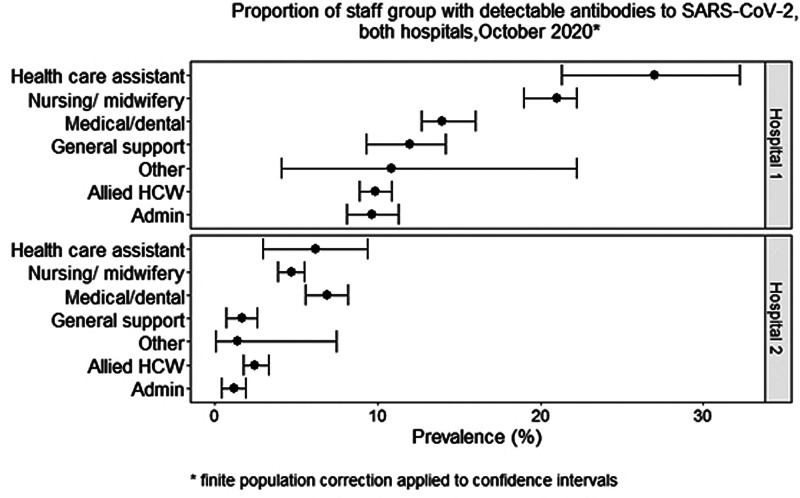
When looking at seroprevalence by role, the combined data for both hospitals showed the highest seroprevalence in HCAs, with 18% of those participating in the study having detectable antibodies. This was followed by nurses at 13% and medical/dental staff at 10%. The group with the lowest seroprevalence was the administration staff at 6%. Figure 1 shows the proportion of each staff group with detectable antibodies by hospital.
Fig. 1.
Proportion of staff group with detectable antibodies to SARS-CoV-2, both hospitals, October 2020.
SARS-CoV-2 antibody and previous diagnosis and symptoms
Ninety-five percent (350/367) of those who had previously confirmed infection by PCR had detectable antibodies. In total, 227/576 (39%) of those with positive antibodies had not previously been diagnosed with COVID-19 infection; this represented 3.9% of all participants. Although 63% (142/227) of these participants with a previously undiagnosed infection reported having had symptoms at some stage, it is impossible to know if these symptoms coincided with the time of undiagnosed infection. Sixteen percent (90/576) of those with detectable antibodies had experienced no symptoms consistent with COVID-19.
Risk factors for antibody positivity to SARS-CoV-2
Tables 3 and 4 show the prevalence of SARS-CoV-2 IgG antibodies by participant characteristics for both hospitals combined. (For the prevalence of SARS-CoV-2 IgG antibodies by participant characteristics for the individual hospitals see Tables A–D, Supplementary annex).
Table 3.
Prevalence of SARS-CoV-2 antibodies by participant characteristics, both hospitals
| Participant characteristics | Total | SARS-CoV-2 antibody detected | P valuea | |
|---|---|---|---|---|
| N | n | % (95% CI) | ||
| Age groups (years) | ||||
| 18–29 | 1350 | 177 | 13 (11–15) | <0.001 |
| 30–39 | 1617 | 168 | 10 (8.9–12) | |
| 40–49 | 1515 | 124 | 8.2 (6.9–9.7) | |
| 50–59 | 1001 | 77 | 7.7 (6.1–9.5) | |
| Over 60 | 304 | 30 | 9.9 (6.8–14) | |
| Sex | ||||
| Female | 4478 | 422 | 9.4 (8.6–10) | 0.013 |
| Male | 1308 | 154 | 12 (10–14) | |
| Ethnicity | ||||
| Irish | 4444 | 384 | 8.6 (7.8–9.5) | <0.001 |
| Any other white background | 551 | 62 | 11 (8.7–14.2) | |
| African and any other black background | 113 | 16 | 14 (8.3–22) | |
| Asian background | 577 | 107 | 19 (16–22) | |
| Other | 101 | 7 | 6.9 (2.6–15) | |
| Country of birtha | ||||
| Ireland | 4273 | 373 | 8.7 (7.9–9.6) | <0.001 |
| UK | 344 | 32 | 9.3 (6.5–13) | |
| India | 299 | 54 | 18 (14–31) | |
| Philippines | 191 | 47 | 25 (19–31) | |
| Poland | 72 | 10 | 14 (6.9–24) | |
| USA | 60 | 3 | 5.0 (1.0–14) | |
| Other | 548 | 57 | 10 (8.0–13) | |
| Education | ||||
| Primary | 29 | 4 | 14 (3.9–32) | 0.055 |
| Secondary | 684 | 61 | 8.9 (6.9–11) | |
| Third level | 2545 | 283 | 11 (9.9–12) | |
| Post-graduate | 2527 | 228 | 9.0 (7.9–10) | |
| Role | ||||
| Admin | 803 | 48 | 6.0 (4.4–7.9) | <0.001 |
| Medical/dental | 982 | 102 | 10 (8.6–13) | |
| Nursing/midwifery | 2064 | 263 | 13 (11−14) | |
| Allied health | 1091 | 73 | 6.7 (5.3–8.3) | |
| General support | 434 | 33 | 7.6 (5.3–11) | |
| Health care assistant | 286 | 50 | 18 (13–22) | |
| Other | 127 | 7 | 5.5 (2.2–11) | |
| Lives with | ||||
| Alone | 479 | 28 | 5.9 (3.9–8.3) | 0.007 |
| With others | 5286 | 546 | 10 (9.5–11) | |
| Missing | 22 | 2 | 9.1 (1.1–29) | |
| Lives with HCW | ||||
| Yes | 1767 | 234 | 13 (12–15) | <0.001 |
| No | 3919 | 332 | 8.5 (7.6–9.4) | |
| Missing | 101 | 10 | 9.9 (4.9–18) | |
Calculated using the χ2 test.
Table 4.
Prevalence of SARS-CoV-2 antibodies by COVID-19-related characteristics, both hospitals
| Participant characteristics | Total | SARS-CoV-2 IgG detected | P valuea | |
|---|---|---|---|---|
| N | n | % (95% CI) | ||
| Contact of a COVID-19 case | ||||
| Yes | 1704 | 325 | 19 (17–21) | <0.001 |
| No | 249 | 249 | 6.1 (5.4–6.9) | |
| Missing | 10 | 2 | 167 (2.1–48) | |
| Setting of close contact | ||||
| Contact at work | 1495 | 269 | 18 (16–20) | 0.002 |
| Contact outside of work | 209 | 56 | 27 (21–33) | |
| Workplace exposure | ||||
| Daily contact with COVID-19 patients | 902 | 136 | 15 (13–18) | <0.001 |
| Daily contact with patients without COVID | 3245 | 344 | 11 (9.6–12) | |
| No patients contact | 1635 | 96 | 5.9 (4.9–7.1) | |
| Previous COVID-19-like symptoms | ||||
| No symptoms | 2876 | 92 | 3.2 (2.6–3.9) | <0.001 |
| Had symptoms | 2911 | 484 | 17 (15–18) | |
| Previous COVID-19 PCR test | ||||
| Yes | 2778 | 474 | 17 (16–19) | <0.001 |
| No | 3003 | 102 | 3.4 (2.8–4.1) | |
| Previous positive COVID-19 PCR test | ||||
| Yes | 367 | 350 | 95.4 (92.7–97.3) | <0.001 |
| No | 5414 | 226 | 4.2 (3.7–4.7) | |
Calculated using the χ2 test.
On multivariable analysis of the combined hospital data the aRR of detectable antibody was higher for the following characteristics: working in hospital 1 (aRR 3.7, 95% CI 3.0–4.5, P < 0.001), working as an HCA (aRR 2.0, 95% CI 1.4–3.0, P = 0.001), working as a nurse (aRR 1.6, 95% CI 1.1–2.2, P = 0.007), daily exposure to patients with confirmed or suspected COVID-19 infection (aRR 1.6, 95% CI 1.2–2.1, P = 0.002), daily contact with patients not known or suspected to have COVID-19 infection (aRR 1.4, 95% CI 1.1–1.8, P = 0.008), age 18–29 years (aRR 1.4, 95% CI 1.1–1.9, P = 0.006), living with others (aRR 1.5, 95% CI 1.0–2.1, P = 0.048), living with other HCW (aRR 1.3, 95% CI 1.1–1.5, P = 0.007), being of Asian background (aRR 1.3, 95% CI 1.0–1.6, P = 0.028) and male sex (aRR 1.2, 95% CI 1.0–1.4, P = 0.046) (Table 5). (For multivariable analysis by hospital see Tables A and F, Supplementary annex.)
Table 5.
Association between risk factors and the presence of SARS-CoV-2 antibodies, both hospitals
| Participant characteristics | Unadjusted relative risk (95% CI) | P value | aRR (95% CI) | P value |
|---|---|---|---|---|
| Hospital | ||||
| Hospital 2 | Ref. | |||
| Hospital 1 | 3.7 (3.1–4.6) | <0.001 | 3.7 (3.0–4.5) | <0.001 |
| Age groups (years) | ||||
| 18–29 | 1.7 (1.3–2.2) | <0.001 | 1.4 (1.1–1.9) | 0.006 |
| 30–39 | 1.4 (1.1–1.8) | 0.022 | 1.2 (0.9–1.5) | 0.217 |
| 40–49 | 1.1 (0.8–1.4) | 0.656 | 1.0 (0.8–1.3) | 0.978 |
| 50–59 | Ref. | |||
| Over 60 | 1.3 (0.9–1.9) | 0.224 | 1.4 (0.9–2.0) | 0.112 |
| Sex | ||||
| Female | Ref. | |||
| Male | 1.3 (1.1–1.5) | 0.012 | 1.2 (1.0–1.4) | 0.046 |
| Ethnicity | ||||
| Irish | Ref. | |||
| Any other white background | 1.3 (1.0–1.7) | 0.041 | 1.3 (1.0–1.6) | 0.068 |
| African and other black background | 1.6 (1.0–2.7) | 0.037 | 1.3 (0.8–2.0) | 0.299 |
| Asian background | 2.2 (1.8–2.6) | <0.001 | 1.3 (1.0–1.6) | 0.028 |
| Other | 0.8 (0.4–1.7) | 0.549 | 0.6 (0.2–1.3) | 0.177 |
| Country of birth | ||||
| Ireland | Ref. | Did not enter | ||
| India | 2.1 (1.6–2.7) | <0.001 | ||
| Philippines | 2.8 (2.2–3.7) | <0.001 | ||
| UK | 1.1 (0.8–1.5) | 0.717 | ||
| Poland | 1.6 (0.9–2.9) | 0.119 | ||
| USA | 0.6 (0.2–1.7) | 0.324 | ||
| Other | 1.2 (0.9–1. 6) | 0.193 | ||
| Education | ||||
| Primary | 1.5 (0.6–3.8) | 0.365 | Did not enter | |
| Secondary | 1.0 (0.8–1.3) | 0.933 | ||
| Third level | 1.2 (1.0–1.5) | 0.013 | ||
| Post-graduate | Ref. | |||
| Role | ||||
| Admin | Ref. | |||
| Doctor/Dental | 1.7 (1.3–2.4) | 0.001 | 1.2 (0.8–1.7) | 0.327 |
| Nursing | 2.1 (1.6–2.9) | <0.001 | 1.6 (1.1–2.2) | 0.007 |
| HCA | 2.9 (2.0–4.2) | <0.001 | 2.0 (1.4–3.0) | 0.001 |
| General support | 1.3 (0.8–2.0) | 0.270 | 0.9 (0.6–1.4) | 0.687 |
| Allied HCW | 1.1 (0.8–1.6) | 0.531 | 0.9 (0.6–1.3) | 0.635 |
| Other | 0.9 (0.4–2.0) | 0.837 | 1.0 (0.5–2.1) | 0.941 |
| Lives with | ||||
| Alone | Ref. | |||
| With others | 1.8 (1.2–2.6) | 0.002 | 1.5 (1.0–2.1) | 0.048 |
| Lives with HCW | ||||
| No | Ref. | |||
| Yes | 1.6 (1.4–1.8) | <0.001 | 1.3 (1.1–1.5) | 0.007 |
| Contact of a COVID-19 case | ||||
| No | Ref. | Did not enter | ||
| Yes | 3.1 (2.7–3.6) | <0.001 | ||
| Close contact at worka | ||||
| No | 1.5 (1.2–1.9) | 0.002 | Did not enter | |
| Yes | Ref. | |||
| Workplace exposure to COVID-19 patients | ||||
| No patient contact | Ref. | |||
| Daily contact with patients without COVID-19 | 1.8 (1.5–2.3) | <0.001 | 1.4 (1.1–1.8) | 0.008 |
| Daily contact with COVID-19 patients | 2.6 (2.0–3.3) | <0.001 | 1.6 (1.2–2.1) | 0.002 |
| Previous COVID-19-like symptoms | ||||
| No | Ref. | Did not enter | ||
| Yes | 5.2 (4.2–6.5) | <0.001 | ||
Calculated for close contacts of COVID-19 cases only (n = 1704).
Discussion
Overall seroprevalence
The seroprevalence between hospitals 1 and 2 differed by fourfold, reflecting the difference in incidence and seroprevalence in the community in the two locations; the seroprevalence in both locations was six times the community seroprevalence [7, 8]. A Swedish study found HCW seroprevalence to be three times higher than the community seroprevalence during the first wave of the pandemic [14]. A Greek study found HCW seroprevalence to be between 10 and 22 times higher than the general population [15]; this was attributed in part to insufficient use/availability of personal protective equipment (PPE) in the hospital setting. Infection prevention and control (IPC) measures were the same in hospitals 1 and 2 (based on national guidelines) and there have been no issues with PPE availability in either of the hospitals involved in our study at any stage thus far during the pandemic. In both hospitals staff were re-deployed to improve the hospital's capacity to deal with the outbreak, however staff were not deployed to areas that would have been outside of their scope of practice, and all staff had training on the correct use of PPE. The seroprevalence in hospital 1 was similar to that found in a recent unpublished study in another hospital in the same city [16], suggesting that one of the main risks for infection in HCWs is the community incidence; a higher community incidence means that HCWs are more likely to be exposed by the nature of their work which involves direct contact with other people, both patients and other HCWs. Although this risk disproportionately affected those with closer patient contact, the risk to HCWs was higher than in the community, even for those who reported little or no patient contact.
The seroprevalence in both hospitals fell within the wide range (1–45%) previously described in other studies [15–21], and fell either side of the European estimate of 8.5% from the meta-analysis published in November 2020 [22].
Previous symptoms and testing
Five percent of participants with a previous PCR-confirmed COVID-19 infection did not have detectable antibodies. The manufacturers' reported test sensitivity is >95% for each assay used [11–13], so while some of these may be false negative results, it is also possible that these participants did not mount an antibody response following infection with COVID-19, or that they had antibody levels below the limits of detection of the test. We feel that a false-positive PCR result is less likely, but also possible.
In both hospitals, the seroprevalence was higher than the known PCR-confirmed diagnoses of COVID-19 infection of the same timeframe (15% vs. 10% in hospital 1, and 4.1% vs. 1.8% in hospital 2), and was also higher than the self-reported previously confirmed diagnoses (15% vs. 9.6% in hospital 1, 4.1% vs. 2.7% in hospital 2). Sixteen percent of participants with positive antibodies reported never having experienced symptoms that were consistent with infection with COVID-19. Thirty-nine percent of infections in our study were undiagnosed, even though both hospitals had onsite PCR testing available to HCWs with symptoms or close contact with a confirmed case of COVID-19 from mid-March 2020. It is likely that these undiagnosed HCWs were working during the infectious period, with potential for onwards transmission to patients and other staff members if proper use of PPE and other IPC measures were not strictly adhered to. This highlights the importance of early detection and reinforces the importance of clear messaging to HCWs about not working when symptomatic. It also highlights the necessity for universal adherence to standard infection control precautions at all times, compliance with transmission-based precautions and appropriate use of PPE including face masks in the hospital setting, irrespective of symptoms [23]. This finding also supports the recommendation for screening of asymptomatic staff when a patient case of hospital-acquired infection, or hospital outbreak of infection with COVID-19 occurs [24]. Mass serial screening of asymptomatic HCWs should be considered. This intervention has been shown to be effective in certain settings [25, 26]. However, the frequency of testing that would be required to have a significant impact on transmission of infection from HCWs has not been established, and other studies have found the impact of this intervention to be uncertain and the logistical challenges it poses to the health service are significant [27–29].
Risk factors for antibody positivity
The main risk factors identified to be significantly associated with SARS-CoV-2 antibody positivity were working in hospital 1, being an HCA, being a nurse, performing roles associated with close patient contact (especially those working directly with COVID-19 patients), living with others, living with other HCWs, being of Asian ethnicity, being aged 18–29 years and being male. Similar risk factors have also been identified in other studies, including the meta-analysis of European studies [22, 30, 31]. Those of Asian background had a higher risk than those of white Irish background. This was a significant finding on multivariable analysis (MVA), with other factors including exposure accounted for. It is possible that there are other social factors relating to ethnicity that were not evaluated in our study and that are contributing to this risk. Studies conducted in other countries have also found Black individuals to be at a higher risk, and many studies have found higher risk in combined BAME (Black, Asian and Minority Ethnic) groups. Black individuals in our study did have a higher overall seroprevalence at 19%, and a higher relative risk of antibody positivity, however this finding was not statistically significant on multivariate analysis, possible due to smaller number of Black participants compared to Asian participants. Other studies have highlighted close patient contact as a risk factor for disease acquisition [32], including specifically the role of nurse or HCAs [15, 31]. We found daily contact with patients with COVID-19 infection to carry a higher relative risk of antibody positivity with comparison to daily contact with patients without COVID-19 infection. Studies have differed on this result; a German study showed a higher seroprevalence among the intermediate risk group with comparison to the high-risk group, potentially due to less scrupulous adherence to infection control precautions including the use of PPE on non-COVID wards [17]. A Spanish study found no significant correlation between role or direct patient contact and antibody positivity, although community incidence was higher in their setting [18].
Having a household contact is known to be a significant risk factor for disease acquisition [33]. In our study, living with others (and especially living with other HCWs) was significantly associated with antibody positivity, which supports the theory that a proportion of the HCW contracting COVID-19 are doing so in their home environment. Other studies have found some correlation between size of household and antibody positivity [18] but to the best of our knowledge this is the first study to find a statistically significant correlation between living with other HCWs and being antibody positive.
Limitations
Our study has several limitations. First, information on COVID-19 symptoms and test results were self-reported and thus could be biased. Second, although the uptake rate of 64% overall is good for an opt-in study, there may be a selection bias; it is possible that those who chose not to take part did so due to busier workload, for example, those working on a COVID-19 ward, and therefore with a higher risk of COVID-19 infection. One of the main reasons for overall good recruitment was the incentive of each participant receiving their individual result. This too may introduce a selection bias; those who already know that they have had COVID-19 infection may have been less interested in participating, as well as those who may have already had private antibody testing done elsewhere, which could lead to an under-estimate in the true seroprevalence. Conversely, those who had a previously confirmed infection by PCR may have had more interest in participating to see if they had gained antibodies (and potential immunity). Third, although the communication strategy was an important part of the recruitment process, the study took part during our second wave of the pandemic, and therefore also relied heavily on engagement with information technology (IT) platforms (email, messenger groups and hospital intranet) and less on face-to-face announcements. The online consent process, questionnaire and blood test booking system risks exclusion of those who are less literate in IT. This was identified as a potential limitation from the start, and attempts were made to mitigate this selection bias. Multilanguage information and plain English were used, and groups identified as potentially at risk of exclusion on this basis were targeted directly for inclusion in the study, with small-group sessions to aid consent and questionnaire completion and walk-in clinics for phlebotomy. In the fourth instance, in testing on two different platforms, we chose to prioritise sensitivity over specificity. However, the rate of discordant results was low, and unlikely to have had a significant effect on the results; there were only 21 samples with a positive result on the Abbott Architect assay that did not have a positive result on either the Roche Elecsys assay or the Wantai ELISA. In the fifth instance, this population was surveyed in October, at the start of the second wave of the pandemic in Ireland. A proportion of the HCW workforce in Ireland is transient, and the staff included in the study may not have worked in the same hospital during the first wave of the pandemic. However, over 90% of participants in each hospital reported that had been working in the same hospital during the first wave of the pandemic. And finally, even though the sample size was large, some covariate partners were small and thus rendered further analysis lacking in statistical power and precision (e.g. interactions terms or further stratified analysis).
Conclusion and recommendations
The overall seroprevalence of antibodies to SARS-CoV-2 was 15% in hospital 1 and 4.1% in hospital 2, reflecting the difference in community incidence and seroprevalence in each area, and suggesting that the main risk factor for acquisition of COVID-19 infection in HCWs is the local community incidence. The HCW seroprevalence was six times the community seroprevalence in each area. Specific risk factors for antibody positivity included being an HCA or nurse, daily contact with patients (especially those known or suspected to have COVID-19 infection), age 18–29 years, living with others, in particular living with other HCWs, being of Asian background and being male. The degree of previously undiagnosed and asymptomatic infections highlights the need for ongoing universal adherence to infection control guidance including the use of appropriate PPE in the hospital setting, as well as the importance of early case detection. It is essential that HCWs have easy access to testing, even with mild or no symptoms. As the national COVID-19 vaccination programme is rolled out, we expect that access to testing for HCWs will still be critical. Screening of asymptomatic HCWs in the setting of hospital-acquired patient infection or outbreaks is important and regular screening of asymptomatic HCWs needs to be considered depending on local epidemiology.
This study is a unique comparison between two hospitals in the areas of differing community incidence, in which IPC measures were the same. It is the first study, to the best of our knowledge, to specifically delineate the relationship between living with other HCWs and risk of antibody positivity. This study is paramount in improving understanding of transmission dynamics and HCW risk factors (demographic, workplace- and household-related) in hospitals in Ireland. The high proportion of undiagnosed infections underscores the importance of all interventions to reduce infection in the hospital setting. This bundle should include robust infection control guidance and adherence to that guidance, with scrupulous attention to standard and transmission-based precautions including the use of appropriate PPE in the hospital setting, easy access to testing for HCWs and prompt outbreak investigation. This study will be crucial in informing the vaccination strategy and roll-out of HCWs in Ireland. Emerging evidence of reduced transmission of infection by vaccine recipients [34] endorses prompt vaccination of all HCWs.
Acknowledgements
We acknowledge the National Public Health Emergency Team (NPHET) who recommended that this study take place, the study steering group who planned the study and critically evaluated this manuscript. The study team who coordinated the running of the study in each hospital, the hospital management at both sites for their support for the study and the staff of both hospitals who participated. We would especially like to acknowledge the phlebotomy departments in each hospital for facilitating the sampling of almost 6000 participants, the microbiology, virology and biochemistry laboratories in each hospital for processing the samples on two different assays, the National Virus Reference Laboratory of Ireland for additional testing and the human resources department for their help with denominator data.
Financial support
This work was supported by the Irish Health Service Executive COVID-19 budget.
Ethical standards
Ethical approval was obtained from the National Research Ethics Committee for COVID-19 in Ireland.
Data availability statement
This dataset is not available to the public for ethical reasons of data protection as certain individuals may be identifiable from the data.
Contributor Information
PRECISE Study Steering Group:
Lorraine Doherty, Niamh Allen, Colm Bergin, Niall Conlon, Lisa Domegan, Catherine Fleming, Margaret Fitzgerald, Cillian de Gascun, Joan Gallagher, Derval Igoe, Mary Keogan, Noirin Noonan, Cliona O'Farrelly, Una Ni Rian, and Breda Smyth
Collaborators: PRECISE Study Steering Group
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268821000984.
click here to view supplementary material
Conflict of interest
None of the authors have any conflicts of interest to declare.
References
- 1.Shah ASV, et al. (2020) Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. British Medical Journal 371, m3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson U and Fraenkel C-J (2020) COVID-19: risks to healthcare workers and their families. British Medical Journal 371, m3944. [DOI] [PubMed] [Google Scholar]
- 3.Richterman A, Meyerowitz EA and Cevik M (2020) Hospital-acquired SARS-CoV-2 infection: lessons for public health. Journal of the American Medical Association 324, 2155. [DOI] [PubMed] [Google Scholar]
- 4.Watson J, Richter A and Deeks J (2020) Testing for SARS-CoV-2 antibodies. British Medical Journal 370, m3325. [DOI] [PubMed] [Google Scholar]
- 5.HPSC COVID-19 in Healthcare Workers [Internet] (2021) HPSC COVID-19 in Healthcare Workers. Available at https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/surveillance/covid-19casesinhealthcareworkers/.
- 6.HPSC Covid Cases in Ireland [Internet]. [cited 2021 Jan 13]. Available at https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/casesinireland/epidemiologyofcovid-19inireland/COVID19%20Daily%20infographic.pdf.
- 7.Preliminary report of the results of the Study to Investigate COVID-19 Infection in People Living in Ireland (SCOPI): A national seroprevalence study, June-July 2020 [Internet]. Available at https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/scopi/SCOPI%20report%20preliminary%20results%20final%20version.pdf.
- 8.HPSC Surveillance for COVID-19 [Internet] (2021) HPSC. [cited 2021 Jan 6]. Available at https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/surveillance/.
- 9.Castor EDC (2021) Castor Electronic Data Capture. [online]. Available at https://castoredc.com. [Internet]. 2021. Available at https://www.castoredc.com/.
- 10.SARS-CoV-2 IgG Architect - Instructions for Use, FDA [Internet]. [cited 2020 Dec 14]. Available at https://www.fda.gov/media/137383/download.
- 11.ARCHITECT System SARS-COV-2IgG/lgM e- Assay CD-ROM - WW (excluding US) List number 6514-03. Information update 07th October 2021.
- 12.Elecsys® Anti-SARS-CoV-2 Immunoassay for the qualitative detection of antibodies (incl. IgG) against SARS-CoV-2 [Internet]. [cited 2020 Dec 14]. Available at https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html.
- 13.WANTAI SARS-CoV-2 Ab ELISA - FDA [Internet]. Available from https://www.fda.gov/media/140929/download
- 14.Rudberg A-S et al. (2020) SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nature Communications 11, 5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psichogiou M et al. (2020) Antibodies against SARS-CoV-2 among health care workers in a country with low burden of COVID-19. PLoS One 15, e0243025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nearly one-fifth of Tallaght hospital staff found to have coronavirus antibodies. [cited 2020 Dec 12]; Available at https://www.thejournal.ie/tallaght-hospital-staff-antibody-study-5245379-Oct2020/.
- 17.Korth J et al. (2020) SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. Journal of Clinical Virology 128, 104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Basteiro AL et al. (2020) Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nature Communications 11, 3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jespersen S et al. (2020) SARS-CoV-2 seroprevalence survey among 17,971 healthcare and administrative personnel at hospitals, pre-hospital services, and specialist practitioners in the central Denmark region. Clinical Infectious Diseases, ciaa1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steensels D et al. (2020) Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. Journal of the American Medical Association 324(2), 195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houlihan C et al. (2020) SARS-CoV-2 virus and antibodies in front-line health care workers in an acute hospital in London: preliminary results from a longitudinal study. Infectious Diseases (except HIV/AIDS). [cited 2020 Jul 24]. medRxiv 2020.06.08.20120584. Available at http://medrxiv.org/lookup/doi/10.1101/2020.06.08.20120584. [Google Scholar]
- 22.Galanis P et al. (2020) Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. Journal of Hospital Infection 108, 120–134, February 01, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HPSC Infection Prevention and Control Precautions for COVID-19 [Internet]. [cited 2021 Jan 12]. Available at https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/guidance/infectionpreventionandcontrolguidance/.
- 24.Brown CS et al. (2020) Snapshot PCR surveillance for SARS-CoV-2 in hospital staff in England. Journal of Infection 81, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivett L et al. (2020) Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife 9, e58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moynan D et al. (2020) The role of healthcare staff COVID-19 screening in infection prevention & control. Journal of Infection 81, e53–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treibel TA et al. (2020) Asymptomatic health-care worker screening during the COVID-19 pandemic – authors’ reply. The Lancet 396, 1394–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow A et al. (2020) Asymptomatic health-care worker screening during the COVID-19 pandemic. The Lancet 396, 1393–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.HPSC General guide on management of COVID-19 outbreaks in the workplace [Internet]. [cited 2021 Jan 12]. Available at https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/guidance/outbreakmanagementguidance/Guidance%20of%20work%20place%20outbreaks.pdf.
- 30.Aldridge RW et al. (2020) Black, Asian and minority ethnic groups in England are at increased risk of death from COVID-19: indirect standardisation of NHS mortality data. Wellcome Open Research 5, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin CA et al. (2020) Demographic and occupational determinants of anti-SARS-CoV-2 IgG seropositivity in hospital staff. Journal of Public Health (Oxford), fdaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai X et al. (2020) Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. Journal of the American Medical Association Network Open 3, e209666–e209666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar N et al. (2021) Seroprevalence of antibodies against SARS-CoV-2 among health care workers in Mumbai, India. Asia Pacific Journal of Public Health 33(1), 126–128. [DOI] [PubMed] [Google Scholar]
- 34.Voysey M, et al. Single Dose Administration, and the Influence of the Timing of the Booster Dose On Immunogenicity and Efficacy of ChAdOx1 nCoV-19 (AZD1222) Vaccine. Available at https://ssrn.com/abstract=3777268. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268821000984.
click here to view supplementary material
Data Availability Statement
This dataset is not available to the public for ethical reasons of data protection as certain individuals may be identifiable from the data.