Abstract
Introduction
In this study, two cases that demonstrate the importance of bedside echocardiography and hands-off telemedicine technology for diagnosis and intervention in patients with coronavirus disease 2019 (COVID-19) are discussed.
Case Presentation
We report two cases of cardiac emergency associated with COVID-19. Case 1 is a 50-year-old female patient with chronic hypertension and chronic renal failure. Case 2 is a 64-year-old female with atrial fibrillation and recent stroke. Both were admitted to an isolation intensive care unit that was designated specifically to patients with COVID-19.
Conclusions
During admission, both patients had sudden deterioration characterized by oxygen desaturation and hypotension necessitating inotropic support. As a result, for both patients, bedside echocardiography was performed by the attending intensivist. Echocardiographic findings showed cardiac tamponade and acute pulmonary embolism, respectively, which were confirmed by a cardiologist through telemedicine technology. Proper emergency management was initiated, and both patients recovered well. Limited bedside transthoracic echocardiography had a front-line impact on the treatment and outcome of the two patients with COVID-19. By implementing telemedicine technology, the lives of two patients were saved, demonstrating the significance of telemedicine in isolation intensive care units in the developing countries during the COVID-19 pandemic.
Keywords: Cardiac Tamponade, COVID-19, Limited Echocardiography, Pulmonary Embolism, Telemedicine
1. Introduction
Coronavirus disease 2019 (COVID-19) represents an unprecedented medical challenge, exposing the problems of the health care and communication systems (1). Since the emergence of COVID-19 in China, the pandemic spread curve of COVID-19 has followed an exponential trend. Coronavirus virus disease 2019 affects the cardiovascular system and provokes myocardial dysfunction through various mechanisms, hence increasing morbidity and mortality. We describe the cases of two patients diagnosed with COVID-19 that required admission to the intensive care unit and subsequently experienced life-threatening cardiac complications.
2. Case Presentation
2.1. Case 1, Cardiac Tamponade
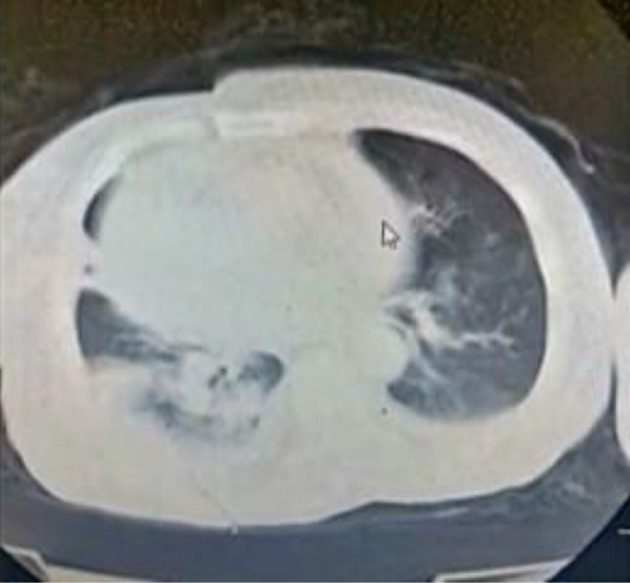
A 50-year-old female patient presented to our university hospital with fever and nonproductive cough. Her medical history included chronic hypertension (treated with bisoprolol) and chronic renal impairment, which was managed conservatively without hemodialysis. The patient tested positive for COVID-19 through reverse transcriptase polymerase chain reaction (RT-PCR) detection assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Laboratory tests showed hyperkalemia (potassium level was 6 mmol/L), metabolic acidosis, high serum urea (200 mg/dL), and serum creatinine level of 11 mg/dL. The patient underwent emergent hemodialysis, and on day two of hospitalization, she was transferred to our isolation hospital dedicated to patients with COVID-19 infection. On admission, a computed tomography (CT) scan of the chest was performed and showed bilateral ground-glass appearance, mild right-sided pleural effusion, and enlarged cardiac silhouette (most probably pericardial effusion, Figure 1).
Figure 1. Computed tomography of the chest showing bilateral ground-glass appearance, mild right-sided pleural effusion, and enlarged cardiac silhouette (likely pericardial effusion).
Upon admission to the isolation ICU, a physical examination of the patient revealed blood pressure of 150/90 mmHg, heart rate of 100 beats per minute, respiratory rate of 22 breaths per minute, and oxygen saturation (SpO2) of 88%. The patient was placed in the prone position with venturi oxygen mask delivering an FiO2 of 50%, resulting in a subsequent increase of the SpO2 to 92%. Arterial blood gases (ABG) analysis showed PaO2 to FiO2 ratio (P/F ratio) of 100. The laboratory results at that time showed ferritin 1200 ng/mL (normal 13 - 150), troponin 149 ng/L (normal 2 - 100), D-dimer 3.4 mg/mL (normal up to 0.5), CRP 159 mg/L (normal < 6), and total CK 300 U/L (normal 0 - 190) with normal value of CK-MB. We started methylprednisolone 2 mg/kg. On the following day, the patient was intubated and placed on mechanical ventilation secondary to progressively worsening respiratory distress. Norepinephrine infusion was started to stabilize blood pressure. The ECG showed sinus tachycardia and diffuse low QRS voltage. The patient suddenly developed pulmonary edema, and oxygen saturation decreased rapidly to the point of hypoxemic cardiac arrest. Immediately, 1 mg epinephrine was injected intravenously, FiO2 was placed at 100%, and chest compressions were started simultaneously. The patient responded with return of spontaneous circulation after 4 minutes of cardio-pulmonary resuscitation (CPR). The patient remained hypotensive (systolic blood pressure less than 90 mmHg) that required the addition of an inotropic infusion (epinephrine).
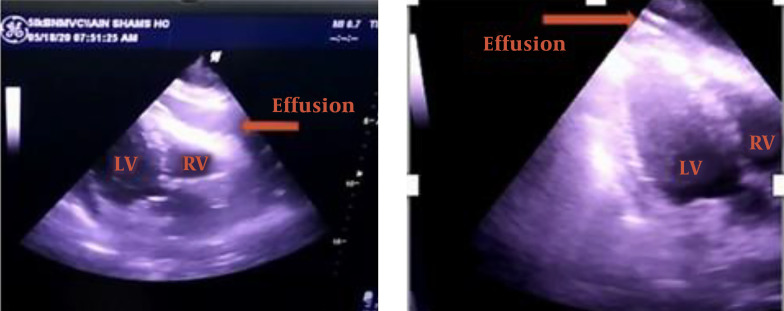
There were no cardiologists in the isolation hospital; thus, the ICU physician performed bedside transthoracic echocardiography (TTE, Figure 2), which revealed a large circumferential pericardial effusion around the entire heart with signs of early right ventricular diastolic collapse, a dilated but collapsing inferior vena cava, and lack of regional wall motion abnormalities. Using telemedicine technology, the intensivist sent the TTE video accompanied by the patient’s medical records to a cardiologist. The cardiologist confirmed the diagnosis and recommended urgent pericardiocentesis. The intensivist performed echo-guided pericardiocentesis using a central venous catheter, removing ~ 600 mL of clear serous fluid using the subcostal approach. A significant rise in blood pressure was noted along with improvement of the hemodynamic parameters. A sample from the pericardial fluid aspirate was sent to the laboratory for further analysis, and follow-up monitoring by TTE showed no pericardial effusion (Figure 2). The patient had a hemodialysis session with ultrafiltration of 1.5 liters. After dialysis, the hemodynamics continued to improve, and vasopressor drugs were decreased. Oxygen saturation increased, and FiO2 requirement subsequently decreased. The results of pericardial fluid aspirate supported serous contents, but the laboratory did not examine it for the presence of SARS-COV-2.
Figure 2. On the left is an echocardiograph of a subcostal coronal view of the heart showing pericardial effusion. On the right, we have the same view after pericardiocentesis with no visible effusion (LV, left ventricle; RV, right ventricle).
2.2. Case 2, Acute Pulmonary Embolism
A 64-year-old female patient presented to our university hospital with acute left-sided hemiplegia and dysarthria. The patient had a history of atrial fibrillation (AF) (on warfarin and digoxin). Magnetic resonance imaging (MRI) study of the brain showed bilateral acute midbrain and thalamic infarction. On the third day of hospital stay, the patient developed fever and dry cough, and RT-PCR of SARS-CoV-2 resulted positive. The blood tests showed international normalized ratio (INR) 1.03, ferritin 1200 ng/mL, troponin 10 ng/L, D-dimer 2.3 mg/mL, CRP 161 mg/L, and normal kidney and liver functions. Transthoracic echocardiogram showed good left ventricle ejection fraction (LVEF = 60%), moderate mitral regurgitation (MR), moderate tricuspid regurgitation, dilated right atrium, moderately dilated right ventricle (RV), good RV systolic function with pulmonary hypertension, and estimated right ventricle systolic pressure (RVSP) of 64 mmHg. The patient was transferred to our isolation hospital. On admission, the chest CT showed bilateral ground-glass appearance and sub-pleural consolidation mostly at the right lung field. The patient was admitted to the ICU, physical examination revealed a Glasgow coma scale of 13 (the patient had dysarthria), and bilateral lower limbs pitting edema was extended to the knee level. Blood pressure was 130/70 mmHg, heart rate was 90 beats per minute (atrial fibrillation with controlled ventricular response), respiratory rate was 18 breath per minute, and oxygen saturation was 96% (with nasal oxygen cannula 2 liters/minute). Of note, the patient was not cooperative for conscious prone positioning. An ABG showed P/F of 280. After the consensus of cardiologists, neurologists and intensivists, it was decided to keep the patient on low molecular weight heparin (enoxaparin 40 mg subcutaneous q12h) to avoid hemorrhagic transformation of the cerebrovascular stroke. Per protocol, the patient was placed on a combination of azithromycin, hydroxychloroquine, and methylprednisolone 2 mg/kg.
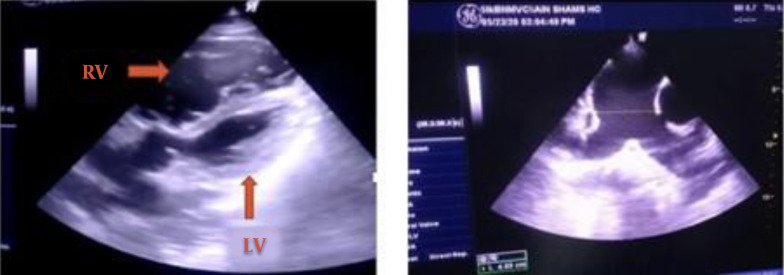
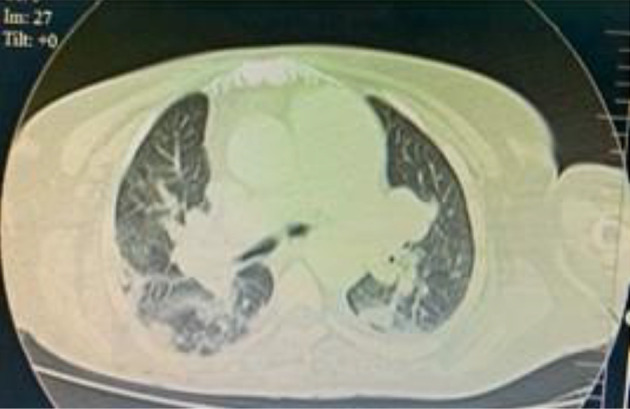
Systolic blood pressure started to decrease, and norepinephrine infusion was initiated. Blood pressure continued to decrease over days, resulting in a gradual escalation of the norepinephrine infusion until it was at 0.1 mcg/kg/minute on the sixth day of admission. The central venous pressure (CVP) was 15 - 18 mmHg, and urine output (UOP) was 0.5 mL/kg/hour. The ICU physician performed a TTE, showing dilated pulmonary arteries, dilated right atrium, hugely dilated right ventricle with mild RV free wall hypokinesia, intact interatrial septum (IAS), no visible thrombi in the main pulmonary artery (MPA), and peripheral proximal branches, dilated inferior vena cava with collapse < 50%, and normal LV dimensions and systolic function. These findings were confirmed by a cardiologist through telemedicine technology (Figure 3). CT pulmonary angiography was performed urgently, and it showed right middle lobar segmental branch occlusion consistent with thrombosis (Figure 4). There were no radiology physicians inside the isolation hospital. Therefore, deep veins duplex was not feasible. The diagnosis of acute pulmonary embolism was made, and we attributed it to the hypercoagulable state associated with COVID-19.
Figure 3. The left photo shows a modified apical 5 chamber view with a severely dilated RV compressing the LV (LV, left ventricle; RV, right ventricle). The right photo shows a short-axis view at the level of the great vessels showing a dilated pulmonary trunk: 4.9 cm and branches with no visible thrombi.
Figure 4. Computed tomography of the chest showing a right middle lobar segmental branch occlusion consistent with thrombosis.
The laboratory tests showed lactic metabolic acidosis and high fibrinogen degradation products (280 mg/mL), and the ABG revealed P/F of 180. In response, fibrinolysis was not indicated due to recent stoke and concern for hemorrhagic conversion. Consensus between pulmonologists, cardiologists, neurologists, and intensivists was that therapeutic doses of enoxaparin (80 mg subcutaneous q 12h) would be most effective. A dobutamine infusion was added, and blood pressure increased dramatically. The norepinephrine infusion dose was decreased, and two days later it was completely stopped. For right-sided heart failure, directed medical treatment was started with daily the administration of furosemide.
Two days later, the dobutamine infusion was weaned. Blood pressure was 130/80 mmHg, CVP 12 - 15 mmHg, and UOP increased to 1 mL/kg/hour. The patient was transferred to the medical floor with progressive clinical and hemodynamic improvement until discharged after one week.
3. Discussion
3.1. SARS CoV-2 and Cardiac System, Risk and Prognosis
Coronavirus disease 2019 is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2). Potential theories that explain the cardiovascular effects from COVID-19 include the cytokine storm triggered by the disease (3) and hypoxemia-induced myocardial damage (4). Moreover, a hypothesis is raised about binding of SARS CoV-2 to myocyte receptors, propagating the internalization and subsequent replication of the virus. However, there are no autopsy reports about viral inclusion bodies in the heart (5).
Cardiovascular disease (CVD) is a common comorbidity in patients with COVID-19 (6, 7). Data from the Center of Disease Control in China demonstrated that CVD was reported in 4.2% of the patients and represented 22.7% of the mortality cases (8).
Zheng et al. concluded that COVID-19 patients with underlying CVD have an adverse prognosis due to an aggravated course of pneumonia and acute myocardial injury, which necessitates ICU admission and increases mortality (9).
3.2. Cardiac Presentations Secondary to COVID-19
Coronavirus disease 2019 has been associated with various direct and indirect cardiovascular consequences, including acute coronary syndrome, myocarditis, arrhythmias, and heart failure (10).
3.2.1. Cardiac Tamponade
Viral infections come at the top of the list of causes of pericardial diseases (11). Coronavirus disease 2019 infection triggers a systemic inflammatory response. Also, renal failure patients requiring hemodialysis are at an increased risk of uremic pericarditis and pericardial effusion (12).
Two case reports have discussed cardiac tamponade secondary to COVID-19 in patients with a history of cardiac diseases (cardiomyopathy and previous myopericarditis) (13, 14). Herein, we presented a case of exacerbated pericardial effusion in a non-cardiac patient with a history of renal impairment. Our patient was diagnosed with moderate pericardial effusion, which worsened rapidly in two days till causing life-threatening cardiac tamponade. This supports the importance of baseline TTE in COVID-19 patients and regular monitoring by echocardiographic assessment in certain high-risk groups. In renal failure patients, daily assessment with bedside TTE may protect patients from life-threatening cardiac tamponade complicating the course of COVID-19.
3.2.2. Pulmonary Embolism
Pulmonary embolism (PE) is a common complication of COVID-19, impacting body hemostasis (15). The thromboembolic consequences of COVID-19 are explained by hypoxia, immobilization and cytokine storm in the form of an increase in procoagulant factors such as fibrinogen and D-dimer (16). The incidence of PE is remarkably high in critically ill patients, which favors the recommendation of high pharmacological thromboprophylaxis in patients with the severe form of COVID-19 (17).
In our second case, the patient was receiving quite high prophylactic doses of enoxaparin due to AF while trying to avoid the hemorrhagic transformation of the recent stroke (first week from the onset of the neurological insult) (18). However, the high prophylactic dose did not prevent the incidence of PE, which complicated the clinical course. This was described in a retrospective study in two French ICUs. The study showed a 69% incidence rate of thromboembolic events in severe ICU COVID-19 patients on prophylactic doses of anticoagulant therapy (19). It was recommended that early therapeutic doses of anticoagulant therapy should be administered in critically ill patients affected by COVID-19. We would also reinforce this recommendation and suggest using non-vitamin-K antagonists (direct oral anticoagulant DOAC), when possible and necessary (20).
Determining when to initiate anticoagulant therapy in patients with atrial fibrillation is still an unresolved clinical challenge. The risk-benefit ratio of this clinical dilemma, especially in COVID-19 patients, is equivocal because both PE and the hemorrhagic transformation of stroke have deleterious effects on patient outcomes.
We recommend risk stratification of hemorrhagic transformation considering the use of anticoagulant therapy, size of the infarcted area, and the patient’s comorbidities (21). Additionally, when making anticoagulation decisions, cerebral microbleeds should be considered, something that is independently associated with intracranial hemorrhage (22). Cerebral microbleeds are hypointense areas diagnosed with MRI. These hypointense areas are considered the neuroimaging biomarkers of small cerebral vessels with higher risk of bleeding (22). After risk assessment, we recommend starting direct oral anticoagulants (DOACs), which have been proven as effective as warfarin in the primary and secondary prevention of atrial fibrillation-related ischemic stroke. Direct oral anticoagulants also carry half the risk of intracranial bleeding and might help in preventing the development of PE in this high-risk group (23). Indeed, future studies are necessary to validate these recommendations.
We emphasize that follow-up TTE is mandatory in COVID-19 patients with any hemodynamic derangement to rule out any associated cardiac complications. Also, performing CT pulmonary angiography may prove beneficial in confirming the diagnosis of PE as it was with our experience.
Once PE is confirmed, the risk of hemorrhagic transformation of cerebral infarct due to full anticoagulation is smaller than untreated pulmonary embolism. Of course, this patient carried absolute contraindication to thrombolysis, and at same time, responded appropriately to managing hemodynamics with dobutamine infusion.
3.3. Role of the Bed-Side Echography in Isolation Hospital
The cardiovascular system appears to have complex interactions with COVID-19, which reflects the cardiac tropism of SARS CoV-2. This necessitates heightened cardiovascular care in isolation hospitals considering that delayed cardiology consultation may lead to deleterious complications.
There is a case report of a patient who was suffering from stable acute coronary syndrome, and the coronary angiography was subsequently postponed as a result of COVID-19 pandemic (24). One month later, the patient presented with left ventricle pseudoaneurysm, necessitating open heart surgery for the resection of the aneurysm and repair of the myocardium. The same was observed by our TTE assessment, as both patients suffered life-threatening cardiac complications.
Bedside echocardiography had a role in determining the cause of cardiac deterioration as pericardial, left, and right-sided lesions in COVID-19 patients. However, it is essential to know that echocardiography is not the primary diagnostic tool in pulmonary embolism but gives indirect data for diagnosis, unlike tamponade where echocardiography is the mainstay tool for diagnosis and treatment.
Intensivists who are well-trained on echocardiography and pericardiocentesis might diagnose and manage serious cardiac events until the arrival of the cardiologist. Therefore, the training of intensivists on basic echocardiography and emergency procedures is of utmost importance and would aid in saving patients’ lives, especially during such a pandemic (25). Also, limited rapid critical care bedside transthoracic echocardiography would decrease exposure of the echocardiographer to COVID-19 and protect healthcare professionals without affecting patient management and outcome.
3.4. Telemedicine
With the emergence of COVID-19, telemedicine has emerged as a critical hands-off technology providing rapid access to specialists who are not immediately available in the ICU24. Moreover, telemedicine can reduce the transmission of COVID-19 among health care providers without compromising the safety of patients, as it facilitates patient care without direct contact (26) and in different settings (27). In the cases mentioned above, the intensivists sent the echocardiography videos to cardiologists complete with medical history and hemodynamic data of the patients, and they provided patient care until the arrival of the specialist. Telemedicine was not established in our university institute; thus, the videos of the echocardiography findings were captured by the camera of smartphones, and they were sent through WhatsApp to the cardiologist.
Telemedicine technology is less time consuming and improves patient management and outcomes, especially in cardiac emergencies during the COVID-19 pandemic.
3.5. Limitations and Conclusion
This study has the limitations of any case report. It would be necessary to perform a case series study to draw better conclusions.
The cases reported in this study are indicative of two very important possible complications in COVID-19 patients. Their management represented a good example of how these patients should be treated. Moreover, the importance of basic knowledge on the use of echocardiography for ICU physicians was highlighted. The implementation of telemedicine in ICUs is also very important. In both cases, these two aspects have represented the most important support to save the lives of two patients.
Acknowledgments
The authors are grateful to the Paolo Procacci Foundation for the support in editing this manuscript.
Footnotes
Authors' Contribution: The initial draft of the manuscript was prepared by IMS and SST. All the other authors have contributed to the review and modification of the paper. All authors have reviewed and approved the final draft of the manuscript.
Conflict of Interests: None declared.
Ethical Approval: None declared.
Funding/Support: No funds were received for this research.
Informed Consent: Both patients were informed of the potentiality to publish their anonymized case stories and provided their informed consent.
Contributor Information
Islam Mohammed Sheata, Email: ur.islam87@gmail.com.
Scott Richard Smith, Email: ssmith@anest.ufl.edu.
Heba Kamel, Email: hebanossier@outlook.com.
Giustino Varrassi, Email: giuvarr@gamil.com.
Farnad Imani, Email: farnadimani@yahoo.com.
Abdolreza Dayani, Email: dr.a.dayani@gmail.com.
Dariusz Myrcik, Email: dariuszmyrcik@me.com.
Ivan Urits, Email: ivanurits@gmail.com.
Omar Viswanath, Email: viswanoy@gmail.com.
Sameh Salem Taha, Email: drsamehtaha@med.asu.edu.eg.
References
- 1.Pergolizzi JV, LeQuang JA, Taylor R, Wollmuth C, Nalamachu M, Varrassi G, et al. Four pandemics: Lessons learned, lessons lost. Signa Vitae. 2021;17(1):1–5. [Google Scholar]
- 2.Mallapaty S. Why does the coronavirus spread so easily between people? Nature. 2020;579(7798):183. doi: 10.1038/d41586-020-00660-x. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoodpoor A, Shadvar K, Ghamari AA, Mohammadzadeh Lameh M, Asghari Ardebili R, Hamidi M, et al. Management of critically Ill patients with COVID-19: What we learned and what we do. Anesth Pain Med. 2020;10(3):e104900. doi: 10.5812/aapm.104900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rekatsina M, Paladini A, Moka E, Yeam CT, Urits I, Viswanath O, et al. Healthcare at the time of COVID-19: A review of the current situation with emphasis on anesthesia providers. Best Pract Res Clin Anaesthesiol. 2020;34(3):539–51. doi: 10.1016/j.bpa.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pergolizzi JJ, Magnusson P, LeQuang JA, Breve F, Paladini A, Rekatsina M, et al. The current clinically relevant findings on COVID-19 pandemic. Anesth Pain Med. 2020;10(2):e103819. doi: 10.5812/aapm.103819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimzadeh P, Faiz HR, Farahmandrad R, Hassanlouei B, Habibi A, Hedayati Emami S, et al. Clinical Features and Prognosis of Invasive Ventilation in Hospitalized Patients with COVID-19: A Retrospective Study. Anesth Pain Med. 2020;10(6):e108773. doi: 10.5812/aapm.108773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epidemiology Working Group for Ncip Epidemic Response. Chinese Center for Disease Control Prevention [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–87. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, et al. [2015 ESC Guidelines for the diagnosis and management of pericardial diseases]. Kardiol Pol. 2015;73(11):1028–91. doi: 10.5603/KP.2015.0228. [DOI] [PubMed] [Google Scholar]
- 12.Gunukula SR, Spodick DH. Pericardial disease in renal patients. Semin Nephrol. 2001;21(1):52–6. doi: 10.1053/snep.2001.18378. [DOI] [PubMed] [Google Scholar]
- 13.Hua A, O'Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41(22):2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2(9):1326–30. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation. 2020;142(2):184–6. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 16.Debuc B, Smadja DM. Is COVID-19 a new hematologic disease? Stem Cell Rev Rep. 2021;17(1):4–8. doi: 10.1007/s12015-020-09987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altavilla R, Caso V, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, et al. Anticoagulation after stroke in patients with atrial fibrillation to bridge or not with low-molecular-weight heparin? Stroke. 2019;50(8):2093–100. doi: 10.1161/STROKEAHA.118.022856. [DOI] [PubMed] [Google Scholar]
- 19.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–6. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pergolizzi Jr JV, Magnusson P, Varrassi G, LeQuang JA, Mitchell K, Taylor R, et al. NOAC in patients with a single CHA2DS2-VASc risk factor. J Integr Cardiol. 2020;2(2) doi: 10.47275/2690-862x-113. [DOI] [Google Scholar]
- 21.Honig A, Percy J, Sepehry AA, Field TS, Gomez AG, Benavente OR. Abstract TP318: Hemorrhagic transformation in acute ischemic stroke: A quantitative systematic review. Stroke. 2018;49(Suppl 1) doi: 10.1161/str.49.suppl_1.TP318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): A multicentre observational cohort study. Lancet Neurol. 2018;17(6):539–47. doi: 10.1016/S1474-4422(18)30145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019;18(1):117–26. doi: 10.1016/S1474-4422(18)30356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimac G, Marzouk M, Dumont E, Paradis JM. When a delayed cardiology consultation leads to a massive left ventricle pseudoaneurysm: Collateral effects of the COVID-19 pandemic. Eur Heart J. 2020;41(32):3102. doi: 10.1093/eurheartj/ehaa480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moka E, Paladini A, Rekatsina M, Urits I, Viswanath O, Kaye AD, et al. Best practice in cardiac anesthesia during the COVID-19 pandemic: Practical recommendations. Best Pract Res Clin Anaesthesiol. 2020;34(3):569–82. doi: 10.1016/j.bpa.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679–81. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 27.Puntillo F, Giglio M, Brienza N, Viswanath O, Urits I, Kaye AD, et al. Impact of COVID-19 pandemic on chronic pain management: Looking for the best way to deliver care. Best Pract Res Clin Anaesthesiol. 2020;34(3):529–37. doi: 10.1016/j.bpa.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]