Abstract
Background
A variety of skin manifestations have been associated with COVID-19 infection. Acral lesions on hands and feet, closely resembling chilblains, have been reported in association with COVID-19, which are nonspecific. These acro-ischemic painful lesions have been described mainly in asymptomatic and mildly symptomatic pediatric COVID-19 positive patients, without a precise pathogenetic mechanism. COVID-19-induced chilblains may portend an indolent course and a good outcome. In young patients, the IFN-1 response induces microangiopathic changes and produces a chilblain lupus erythematosus-like eruption with vasculitic neuropathic pain features.
Objectives
This paper presented a case series of pediatric patients with COVID-19-related skin lesions and neuropathic-like pain.
Methods
Clinical outcomes were collected from 11 patients diagnosed with painful erythematous skin lesions with neuropathic-like pain and positive IgG for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Results
It is a mildly symptomatic condition not related to severe pain rates, and it is treated with paracetamol due to the transitory nature of the problem, which provides good results.
Conclusions
A particular point of interest is skin lesion manifestation as a further indirect sign of SARS-CoV-2 infection. Due to the initial manifestation of chilblains in pauci-symptomatic pediatric patients, they need to be immediately tested and isolated. Chilblains can be considered a clinical clue to suspect SARS-CoV-2 infection and help in early diagnosis, patient triage, and infection control.
Keywords: COVID-19; Child, Vasculitis; Neuropathic-like Pain; Coronavirus
1. Background
On Dec 31, 2019, the World Health Organization reported an unexplained lower respiratory tract infection in Wuhan, China (1). A new virus belonging to the coronavirus (CoV) family has been attributed as the etiological agent for the disease, and it has been named "severe acute respiratory syndrome coronavirus 2" (SARS-CoV-2) (2).
The virus originates from a reservoir of bats and an unknown intermediate host, infecting humans through an interspecies transmission (3-5). The novel coronavirus has an extraordinary capacity to spread due to prolonged contagiousness and infectivity among asymptomatic hosts. The rapid spread was declared a pandemic due to SARS-CoV-2 on Mar 11, 2020 (6).
Interestingly, various skin manifestations have been associated with COVID-19 infection, including urticaria, erythematous and exanthematic rash. However, skin lesions reported in association with COVID-19 are nonspecific and lack prognostic significance (7, 8).
There is an increasing concern about the clinical implications of clotting disorders underlying acute acro-ischemic lesions in asymptomatic or mildly symptomatic COVID-19 patients (9). These acro-ischemic painful lesions have been described mainly in asymptomatic and mildly symptomatic pediatric COVID-19 positive patients, without a precise pathogenetic mechanism.
2. Objectives
This paper presented a case series of pediatric patients with COVID-19 concomitant skin lesions and neuropathic-like pain. A particular point of interest is skin lesion manifestation as a further indirect sign of SARS-CoV-2 infection.
3. Methods
3.1. Study Cohort
Clinical outcomes were collected from 11 COVID-19 pediatric patients diagnosed with painful erythematous skin lesions with neuropathic-like pain. Data from the patients’ acro-ischemic skin lesions were gathered in April 2020 at Ospedale dei Colli, Naples. Informed consent was obtained from the patients’ parents to present this case series.
3.2. Clinical Work-up
Multiple diagnostic procedures like nasopharyngeal swabs and laboratory tests were conducted as part of the standard infection work-up in this series of patients. Moreover, the serological tests for SARS-CoV-2 virus-antibodies were assessed.
3.3. Pain Assessment and Treatment
Patients suffering from neuropathic-like pain without a history of the Raynaud phenomenon or acrocyanosis were treated in a multidisciplinary pain management center. During the physical examination, pain characteristics, including intensity and neuropathy, were analyzed using the numeric rating scale (NRS) and DN4 questionnaire (DN4), respectively (10). All the patients were treated with age- and weight-adjusted paracetamol 15 mg/kg per dose, to a maximum of 750 mg per dose, every 6 - 8 hours, with a maximum of 3,000 mgs daily for ten days. The treatment's primary goal was to achieve pain relief over the skin lesions.
3.4. Statistical Analysis
Descriptive statistics were used for socio-demographic data, diagnoses, and pain characteristics. Data summarized as continuous variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as numbers and percentages.
4. Results
4.1. Study Cohort
This case series presented 11 pediatric patients treated in April 2020 (mean age, 11 ± 2,19 SD, years old; range, 8 - 15 years old), including seven males and four females. The diagnosis made by a pediatrician and a first aid physician was established through the presence of "acro-ischemic skin lesion," with positive IgG antibodies for SARS-CoV-2 laboratory test results.
4.2. Clinical Work-up
At the first clinical evaluation, two of the patients had presented fever and cough in the previous month, and four of the patients had bilateral conjunctivitis. None of the patients had a past medical history of the Raynaud phenomenon or acrocyanosis. All the patients admitted to our pain center were diagnosed with painful or itchy erythematous skin lesions affecting distal extremities (especially the digits), with a self-limiting course associated with neuropathic-like pain features. The serological tests for SARS-CoV-2 virus-antibodies revealed IgG ( > 5 AU / mL) in all the case series patients. Moreover, all the patients had a positive PCR test. They were admitted to the Pain Clinic between 3 and 13 days after the serological test resulted positive.
4.3. Pain Assessment and Treatment
At the time of the physical examination performed in our pain center, nine out of the 11 (81.8%) patients complained of mild-moderate pain, and the mean pain score among all the patients was 4/10 on the NRS and 7/10 on the DN4 questionnaire (Figure. 1).
Figure 1. Values of NRS (gray) and DN4 (black) for each of the 11 patients.
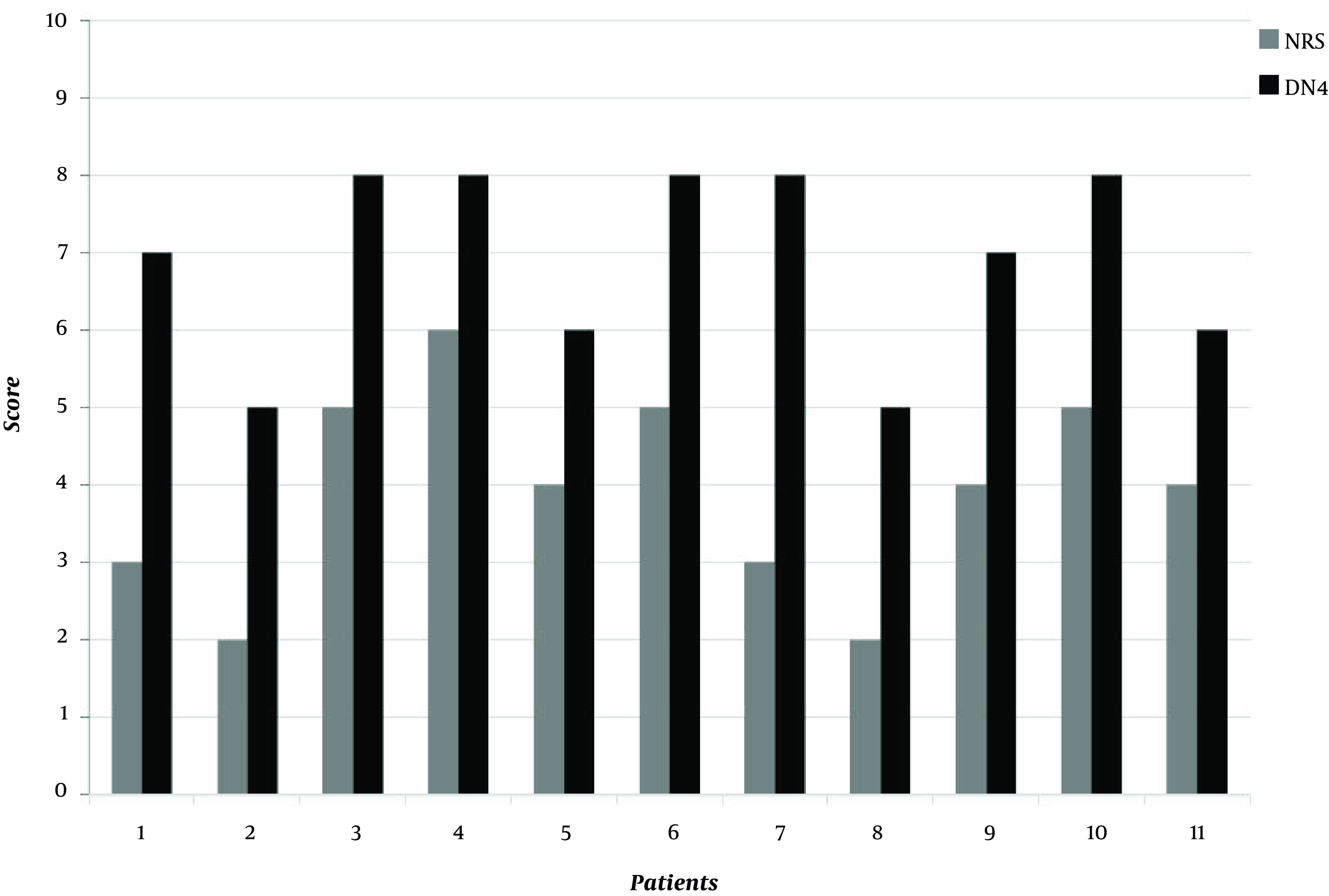
Table 1 summarizes information on neuropathic pain characteristics, as defined by the DN4 questionnaire in our series. All the patients experienced three or more neuropathic pain characteristics (100%). Typical pain features experienced by all the patients over the cutaneous lesions, were an aching pain with itching and burning of mild intensity.
Table 1. Symptoms and Personal History of the Patients.
| Symptom and Personal History | No. (%) |
|---|---|
| Allodynia | 10 (90.91) |
| Itching | 8 (72.73) |
| Burning | 10 (90.91) |
| Pain | 11 (100) |
| Acrocyanosis | 11 (100) |
| Family history | 0 (0) |
| Infection | 4 (36.36) |
| Neuropathic features | 11 (100) |
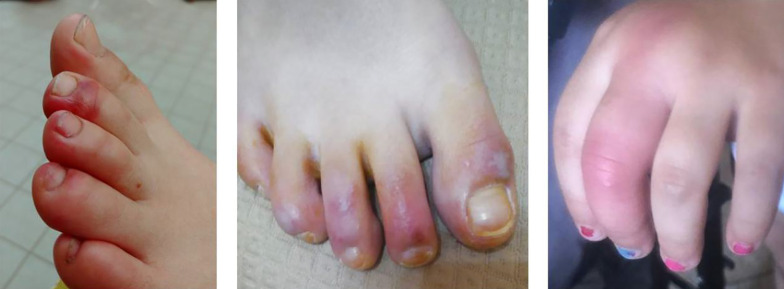
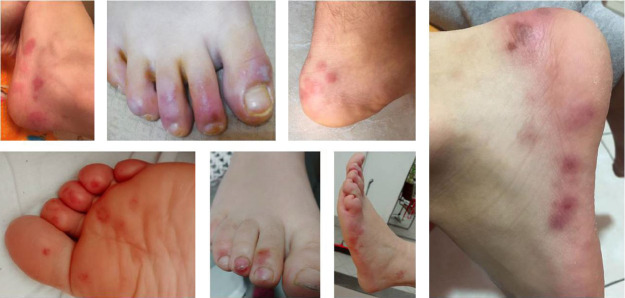
The lesions showed a chilblain-like pattern, with red to purple macules, plaques, and nodules, as well as an erythema multiforme-like pattern (i.e., rounded erythematous macules and vesicles that tend to coalesce). The lesions were localized in the upper and lower extremities in two (18.1%) and nine (81.8%) out of the 11 cases, respectively (Figures 2 - 4).
Figure 2. Chilblain-like pattern.
Figure 4. Skin ulcers.
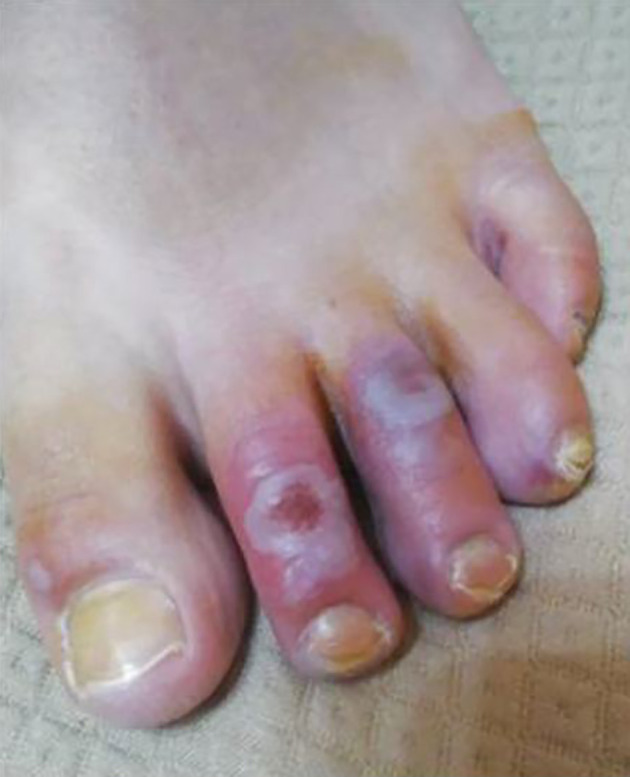
Figure 3. Red to purple macules, plaques, and nodules, also with erythema multiform-like.
The natural history of these skin lesions showed an evolution from an edematous to an erythematous state. Three (27.3%) of the cases evolved into ulcerative lesions, and one (9.1%) of the cases developed an infection and was treated with Mupirocin 2% ointment three times per day. Twelve to 15 days after the clinical evaluation of the skin lesions, complete healing was obtained in all the patients with the zeroing of the NRS.
5. Discussion
Chilblain is a localized disease that manifests acral inflammatory lesions (11). The lesions can be erythematous or purple and are intensely pruritic or painful. Furthermore, the lesions are more likely to appear during the winter, affecting the proximal phalanges' toes and dorsum, and frequently affect female teenagers (12, 13). Chilblains can present as a secondary feature of diseases, such as systemic lupus erythematosus (LE), Behçet disease, chronic myelomonocytic leukemia, metastatic breast carcinoma, cryoglobulinemia, cold agglutinin disease, macroglobulinemia, Aicardi-Goutieres syndrome, and anorexia nervosa (14, 15). In our case series, pediatric patients presenting chilblains did not show any history of skin manifestations, such as Raynaud syndrome, vascular diseases, or cutaneous LE. Coronavirus has been suspected as the etiology of the recent increase of acro-ischemic lesions, specifically chilblains, in pediatric patients, during the COVID-19 outbreak (16).
Skin lesions have been reported in different disease stages, presenting an indolent course, either as the first sign or as a late manifestation in positive COVID-19 patients (16). The lesions are located mainly on distal limbs, and the feet are more affected than the hands.
The monogenic autoinflammatory interferonopathies provoke microangiopathy, which manifests clinically as chilblains. The clinical similarities with LE chilblains are not surprising due to the type 1 interferons (IFN-1) etiopathological mechanism. The antiviral and immunostimulatory properties of IFN-1 have been confirmed in acute viral infections, like Epstein-Barr, or IFN-1 mediated diseases, like LE, whose pathogenesis simulates a viral-induced immune response (17, 18).
INF-1 is critical in the immune response to SARS-COV-2, triggering the expressions of INF-1 inducible genes. Elderly patients mount an inadequate or postponed IFN-1 response, developing hypercytokinemia and increasing morbidity and mortality due to the typical damage pattern in COVID-19 patients with high interleukin levels in the second phase of the disease.
Like in this case series, pediatric patients develop an INF-1 response, and they are affected by skin lesions (19), indicating the benignity of COVID-19 without developing the cytokine storm. The reason is the surge in IFN-1 that causes downregulation of other cytokines.
Hence, microangiopathic-associated chilblain lesions in pediatric patients have a different pathological mechanism from thrombotic-related acral-ischemia lesions observed in severely ill COVID-19 patients. Thrombotic-related acral-ischemia lesions show an hypercoagulopathy state and elevated D-dimer levels with a subsequent higher likelihood of thrombi formation and consequent thromboembolic events, increasing tissue susceptibility to ischemia (20).
COVID-19-induced chilblains may portend an indolent course and a good outcome. In young patients, the IFN-1 response induces microangiopathic changes and produces a chilblain LE-like eruption with vasculitic neuropathic pain features (21, 22).
Vasculitis neuropathy usually has patchy and asymmetrical distribution, affecting mainly distal lower limbs (23, 24). Pain is a critical clinical feature of neuropathy in more than 80% of patients. It is often severe, and it may be aching or throbbing rather than burning and characteristically more neuropathic in quality (22, 24). It may also be present in patients in intensive care (25).
Neuropathic pain has been defined as "pain initiated or caused by a primary lesion or dysfunction in the nervous system," which can affect both the peripheral and central nervous systems, causing various pathophysiological mechanisms, such as inflammatory reactions and neuroplastic changes (26, 27). It is characterized by sensory abnormalities ranging from numbness to hypersensitivity (hyperalgesia/allodynia) and can be objectively assessed by quantitative sensory testing considering perception thresholds for a light touch, vibration, and thermal and pain sensation (28, 29). It affects the quality of life (30) and may be treated both pharmacologically or non-pharmacologically (31-33). The same neurological illnesses causing neuropathic pain also, or instead, have been suggested to cause itching. Thus, neuropathic pain or itching indicates pathophysiological abnormalities in the peripheral or central nervous system (34). In our case series, the skin lesions were defined by a neuropathic-like pain pattern assessed with the DN4 questionnaire.
5.1. Conclusions
Acro-ischemic lesions, described in our case series, with neuropathic-like features of pain are present in asymptomatic and mildly symptomatic pediatric COVID-19 IgG positive patients, without a precise pathogenetic mechanism.
Coronavirus has been suspected as the etiology of the recent increase of acro-ischemic lesions, and further studies are needed to confirm this correlation.
SARS-CoV-2 infection-induced chilblains may portend in pediatric patients an indolent course and a good outcome of the illness. The mechanism in young patients is related to the rise of INF-1 levels, downregulating other cytokines, and preventing the cytokine storm.
Paracetamol, in young patients, appears to be effective in treating moderate and transitory pain associated with these lesions.
It is a mildly symptomatic condition not related to severe pain rates due to the transitory nature of the problem. Our patients were treated only with paracetamol, which provided good results.
To conclude, we aim to alert clinicians to the initial manifestation of chilblains in pauci-symptomatic pediatric patients, who need to be immediately tested and isolated. The reason is that chilblains can be considered a clinical clue to suspect SARS-CoV-2 infection and help in early diagnosis, patient triage, and infection control.
Footnotes
Authors' Contribution: AMS, AS, GLB, MT, and MTDD initially screened the cases. AP cared for the final diagnosis and therapy. GV, FI, and GAA made most of the literature search. All the authors participated in the elaboration of the data and contributed to preparing the first draft of the manuscript.
Conflict of Interests: The authors declare that they have no conflicts of financial and other interests.
Ethical Approval: This case series was based on reports of cases treated in the regular everyday clinical practice and thus did not need any previous approval by the Institutional Ethics Committee.
Funding/Support: The authors received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: The consent to report the cases as a group was obtained from the studied children's parents by preserving their privacy.
Contributor Information
Alfonso Papa, Email: alfonsopapa2@gmail.com.
Anna Maria Salzano, Email: annasalzano@hotmail.com.
Maria Teresa Di Dato, Email: marisita@libero.it.
Giuliano Lo Bianco, Email: giulianolobianco@gmail.com.
Mariangela Tedesco, Email: tedescomariangela@alice.it.
Antonio Salzano, Email: antonio.salzano2@aslnapoli2nord.it.
Dariusz Myrcik, Email: dariuszmyrcik@me.com.
Farnad Imani, Email: farnadimani@yahoo.com.
Giustino Varrassi, Email: giuvarr@gmail.com.
Ghodrat Akhavan Akbari, Email: dr.akhavanakbari@gmail.com.
Antonella Paladini, Email: antopaladini@gmail.com.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–9. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization [cited February 27, 2021];Naming the coronavirus disease (COVID-19) and the virus that causes it. . 2021 Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 3.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–8. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92(6):595–601. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau SKP, Luk HKH, Wong ACP, Li KSM, Zhu L, He Z, et al. Possible bat origin of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis. 2020;26(7):1542–7. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–7. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212–3. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 8.Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–5. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1-2):29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Prakash S, Weisman MH. Idiopathic chilblains. Am J Med. 2009;122(12):1152–5. doi: 10.1016/j.amjmed.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Raza N, Habib A, Razvi SK, Dar NR. Constitutional and behavioral risk factors for chilblains: A case-control study from Pakistan. Wilderness Environ Med. 2010;21(1):17–21 e1. doi: 10.1016/j.wem.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Tonoli RE, Souza PR. Case for diagnosis. Chilblains. An Bras Dermatol. 2012;87(4):649–50. doi: 10.1590/s0365-05962012000400027. [DOI] [PubMed] [Google Scholar]
- 14.Lutz V, Cribier B, Lipsker D. Chilblains and antiphospholipid antibodies: report of four cases and review of the literature. Br J Dermatol. 2010;163(3):645–6. doi: 10.1111/j.1365-2133.2010.09829.x. [DOI] [PubMed] [Google Scholar]
- 15.White KP, Rothe MJ, Milanese A, Grant-Kels JM. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11(1):1–5. doi: 10.1111/j.1525-1470.1994.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 16.Andina D, Noguera-Morel L, Bascuas-Arribas M, Gaitero-Tristan J, Alonso-Cadenas JA, Escalada-Pellitero S, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37(3):406–11. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeed M. Lupus pathobiology based on genomics. Immunogenetics. 2017;69(1):1–12. doi: 10.1007/s00251-016-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh H, Kaur H, Singh K, Sen CK. Cutaneous manifestations of COVID-19: A systematic review. Adv Wound Care (New Rochelle). 2021;10(2):51–80. doi: 10.1089/wound.2020.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J, et al. [Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia]. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 21.Kolivras A, Dehavay F, Delplace D, Feoli F, Meiers I, Milone L, et al. Coronavirus (COVID-19) infection-induced chilblains: A case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489–92. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graf J, Imboden J. Vasculitis and peripheral neuropathy. Curr Opin Rheumatol. 2019;31(1):40–5. doi: 10.1097/BOR.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 23.Collins MP, Hadden RD. The nonsystemic vasculitic neuropathies. Nat Rev Neurol. 2017;13(5):302–16. doi: 10.1038/nrneurol.2017.42. [DOI] [PubMed] [Google Scholar]
- 24.Pergolizzi JJ, Magnusson P, LeQuang JA, Breve F, Paladini A, Rekatsina M, et al. The current clinically relevant findings on COVID-19 pandemic. Anesth Pain Med. 2020;10(2):e103819. doi: 10.5812/aapm.103819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoodpoor A, Shadvar K, Ghamari AA, Mohammadzadeh Lameh M, Asghari Ardebili R, Hamidi M, et al. Management of critically Ill patients with COVID-19: What we learned and what we do. Anesth Pain Med. 2020;10(3) doi: 10.5812/aapm.104900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–5. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 27.Baron R. Mechanisms of disease: neuropathic pain--a clinical perspective. Nat Clin Pract Neurol. 2006;2(2):95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 28.Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care. 2012;39(3):561–71. doi: 10.1016/j.pop.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am. 2003;14(2):261–86. doi: 10.1016/s1047-9651(02)00122-5. [DOI] [PubMed] [Google Scholar]
- 30.Girach A, Julian TH, Varrassi G, Paladini A, Vadalouka A, Zis P. Quality of life in painful peripheral neuropathies: A systematic review. Pain Res Manag. 2019;2019:2091960. doi: 10.1155/2019/2091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Pharmacological management of painful peripheral neuropathies: A systematic review. Pain Ther. 2020 doi: 10.1007/s40122-020-00210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Non-pharmacological management of painful peripheral neuropathies: A systematic review. Adv Ther. 2020;37(10):4096–106. doi: 10.1007/s12325-020-01462-3. [DOI] [PubMed] [Google Scholar]
- 33.Amorizzo E, Colini-Baldeschi G. Peripheral nerve stimulation: Two cases of intractable neuropathic pain. Anesth Pain Med. 2021;11(2):e113162. doi: 10.5812/aapm.113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33(12):550–8. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]