Abstract
Background
Caudal anesthesia is an effective method of pain management, which can be successfully employed to minimize post-thoracotomy pain in pediatric patients. However, its main disadvantage is the short postoperative analgesic period, which can be prolonged by the concurrent administration of one of many adjuvants.
Objectives
This prospective randomized, blinded study aimed to compare the efficacy of dexmedetomidine versus morphine as adjuvants to bupivacaine in caudal anesthesia for thoracic surgeries in pediatric patients.
Methods
Fifty patients were randomly allocated into two equal groups. To achieve caudal epidural block anesthesia, the patients in group M (n = 25) were administered morphine and bupivacaine, while group D (n = 25) received a mixture of dexmedetomidine and bupivacaine. The primary outcome of this study was the postoperative analgesic duration achieved. The secondary outcomes included morphine administration in the first 24 hours following caudal block anesthesia, the face, legs, activity, cry, consolability (FLACC) scale scores, and adverse effects, including vomiting, itching, bradycardia, hypotension, and respiratory depression.
Results
The results showed that patients who had received dexmedetomidine achieved a longer postoperative analgesia as compared to those who had received morphine (P < 0.001). Postoperatively, the heart rate, blood pressure, pain score, and mean consumption of morphine were lower in group D as compared to the group M. There was no significant difference in the adverse effects between the two groups.
Conclusions
The use of dexmedetomidine as an adjuvant to bupivacaine for caudal anesthesia during pediatric thoracic surgeries induced better and prolonged postoperative analgesia as compared to morphine.
Keywords: Caudal Block, Pediatric, Dexmedetomidine, Thoracic Surgeries
1. Background
Anesthetic management of pediatric thoracic surgeries is challenging and requires meticulous control of acute post-thoracotomy pain, which is a common problem. Besides, post-thoracotomy pain may cause postoperative morbidities and lead to a longer intensive care unit (ICU) stay if not properly controlled (1). Several techniques have been used for the management of post-thoracotomy pain, including opioid infusion, epidural anesthesia, caudal anesthesia, paravertebral block, intercostal nerve block, and serratus anterior plane block (2).
Caudal anesthesia is a simple method of anesthesia, commonly used in pediatric surgeries. It is an effective method of postoperative pain management in children undergoing cardiac and thoracic surgeries (3). Its main disadvantage is the short postoperative analgesic period achieved, which can be prolonged by the concomitant administration of one of many adjuvants (4)) or insertion of a neuraxial catheter for continuous analgesic administration, which requires experience, skills, and financial resources (5).
Many adjuvants, such as morphine, fentanyl, and dexmedetomidine, have been administered as part of caudal anesthesia regimens with variable degrees of efficacy. Opioids are the most commonly used adjuvants. However, their use carries the risk of respiratory depression, constipation, pruritus, nausea, and vomiting (6). Dexmedetomidine shows a strong affinity to the α2 adrenergic receptors, enhancing the analgesic effects, without causing respiratory or cardiovascular adverse effects (7). The analgesic effects of dexmedetomidine, when used in neuraxial anesthesia, are well established (8).
2. Objectives
There is currently no consensus regarding the best adjuvant for caudal anesthesia. Besides, the use of caudal anesthesia during thoracic surgeries for the pediatric population has not been studied adequately. Therefore, this study aimed to compare the efficacy of dexmedetomidine versus morphine as adjuvants to bupivacaine in caudal anesthesia for pediatric patients undergoing thoracic surgeries.
3. Methods
This prospective randomized, blinded trial was conducted at a tertiary pediatric cardiac center after obtaining approval from the institutional ethics committee. Written informed consent forms were signed by the legal guardians of pediatric patients who were to participate in the study. The clinical trial registration number of this study is NCT04445636. Fifty patients aged 1 - 6 years, who were scheduled for thoracotomy surgery and were classified as class I to III based on the American Society of Anesthesiologists (ASA) physical status criteria, were enrolled in this study.
The exclusion criteria were as follows: the presence of an infection at the site of caudal injection; more than three hours of endotracheal intubation from the time of caudal injection; failed extubation at the end of surgery; failed caudal block (defined as a > 20% increase in the mean blood pressure and heart rate with skin incision); coagulopathy; mental retardation and congenital anomalies of the sacrum; the legal guardian's refusal to allow the child’s participation in the study; and a history of an allergic reaction to either bupivacaine, dexmedetomidine, or morphine.
The patients were randomly allocated to two equal groups with the help of a statistician, who used an online random number generator. To achieve caudal block anesthesia, patients in group M (n = 25) were administered morphine and bupivacaine, while group D (n = 25) received a mixture of dexmedetomidine and bupivacaine. Blindness was achieved by generating code numbers for each patient. These codes were placed into sequentially numbered sealed opaque envelopes by a research assistant, who was not involved in the study. The first anesthetist, who was not involved in patient management, was responsible for opening the envelopes and preparing the required drug combinations in accordance with the instructions in each envelope. The first anesthetist then delivered the prepared anesthetics to the second blinded and experienced anesthetist, who conducted caudal anesthesia for each patient. Also, an experienced nurse who recorded the data in the pediatric intensive care unit (PICU) was blinded to the study.
The primary outcome of this study was the postoperative analgesic duration achieved by the two anesthetics mixtures. This was specifically defined as the interval between caudal block anesthesia and achieving a FLACC score ≥ 4 (Table 1). The secondary outcomes included morphine administration at 24 hours after the caudal block anesthesia, procedure duration, postoperative FLACC scores, and postoperative adverse effects, including vomiting, itching, bradycardia (HR ≤ 60), hypotension (BP < 20% of the baseline measurement), and respiratory depression (SpO2 ≤ 92).
Table 1. The FLACC Score a.
| Categories | Scoring | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Face | No particular expression or smile | Occasional grimace or frown, withdrawn, disinterested | Frequent to constant quivering chin, clenching jaw |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn up |
| Activities | Lying quietly, normal position moves easily | Squirming, shifting back and forth, tense | Arched, rigid, or jerking |
| Cry | No cry (awake or asleep) | Moans or whimpers; occasional complaint | Crying steadily, screams or snobs, frequent complaints |
| Consolability | Content, relaxed | Reassured by occasional touching, hugging, or being talked to, distractable | Difficult to console or comfort |
aThe five categories of (F) face; (L) legs; (A) activity; (C) cry; and (C) consolability are scored from 0 to 2 (total score: 0 - 10).
A detailed review of each patient’s medical history and clinical examinations were carried out before the preoperative IV medications were administered, which included ketamine (0.5 mg.kg - 1) and atropine (0.01 mg.kg - 1); they were administered at 15 minutes before anesthesia induction. In the operating theater, each patient was connected to an electrocardiogram (ECG), pulse oximeter, and non-invasive blood pressure (NIBP) monitors. Anesthesia was induced with intravenous ketamine (1 mg.kg - 1), fentanyl (2 mcg.kg - 1), and atracurium (0.5 mg.kg - 1). Following anesthesia induction, the patients were intubated and connected to the anesthesia machine after complete muscle relaxation was confirmed. Anesthesia was maintained with isoflurane 1.2% and atracurium (0.1 mg.kg - 1), administered within 20-minute intervals. A nasopharyngeal temperature probe, central venous catheter, and arterial catheter were inserted. Each patient's heart rate, blood pressure, SpO2, ECG graphs, temperature, and EtCO2 were monitored continuously throughout the procedure, and the problems were managed accordingly.
Next, each patient was turned to the lateral position to administer a single-injection caudal block under complete aseptic precautions. The caudal space was localized anatomically and confirmed with a popping sensation after passing a 23-G needle through the sacrococcygeal ligament. The correct needle position was confirmed by the whoosh test and needle aspiration to make sure that the blood and the cerebrospinal fluid (CSF) were not aspirated (9) The patients in group M were administered 1.25 mL/kg of bupivacaine 0.25%, mixed with 30 mcg.kg-1 of preservative-free morphine sulfate, while patients in group D were administered 1.25 ml.kg-1 of bupivacaine 0.25%, mixed with 2 mcg.kg-1 of dexmedetomidine (10).
At the end of the surgery, the patients were extubated in the operative room after fulfilling the criteria for extubation. They were then transferred to the PICU for close monitoring according to the institutional guidelines. For pain management, all patients regularly received 10 mg.kg- of paracetamol within six-hour intervals. Also, the FLACC scores were assessed and recorded every four hours. If the FLACC score was ≥ 4, an additional morphine dose of 100 mcg.kg-1 was administered intravenously.
Each patient’s heart rate, arterial blood pressure (ABP), and SpO2 were continuously monitored. The vital signs were recorded every hour for the first postoperative 24 hours. The duration of postoperative analgesia was defined as the time interval between the injection of caudal drugs and a FLACC score ≥ 4 postoperatively. Besides, adverse effects, such as nausea, vomiting, bradycardia (HR < 60/min), hypotension (a 20% decrease from the baseline), and respiratory depression (oxygen saturation < 92%), were recorded. Pain assessment based on the FLACC score is described in the Table 1 (11).
3.1. Statistical Analysis
SPSS version 15 for Microsoft Windows (SPSS Inc., Chicago, IL, USA) was used for data analysis. Categorical data are presented as frequency (%) and analyzed using chi-square test. Continuous data were examined for normality using Shapiro-Wilk test and presented as mean (standard deviation [SD]) or median (interquartile range) as appropriate. Continuous data were analyzed using unpaired t-test or Mann-Whitney test as appropriate. Repeated measures analysis of variance was performed using the ANOVA test, with post-hoc pairwise comparisons using Bonferroni test. P-values less than 0.05 were considered statistically significant.
3.2. Estimation of Sample Size
The primary outcome of this study was the duration of postoperative analgesia. In a previous study (12), the duration of postoperative analgesia was reported to be 410 ± 32 minutes. The sample size was calculated using the Medcalc program to detect a mean difference of 10% in the duration of analgesia (410 minutes) between the two groups. A minimum of 50 patients (25 patients per group) was calculated to produce a study power of 80% and an alpha error of 0.05. The sample size was increased by 20% (60 patients) to compensate for the dropouts.
4. Results
Sixty pediatric patients, who were scheduled for thoracotomy surgeries, were assessed for the study eligibility. Ten patients were excluded from the study. Six were excluded due to the legal guardian’s refusal to allow the child’s participation. Also, four patients were excluded because of caudal failure. Finally, 50 patients were considered eligible for the study and enrolled. They were divided into two groups (25 patients per group) as previously stated and were included in the study through interventions, follow-up, and analysis (Figure 1).
Figure 1. The flow diagram of the study.
There were no significant differences in terms of the demographic data between the two groups. Also, no significant differences were found between group M and group D regarding the type of surgery, duration of procedures, or ASA classification, as shown in Table 2.
Table 2. The Patients’ Demographic Data, ASA Classification, and Procedure-Related Data. Values Are Presented as Number and Percentagea.
| Group M | Group D | |
|---|---|---|
| Age (y) | 3.2 ± 1.6 | 3.5 ± 1.2 |
| Male | 16 (64) | 18 (72) |
| Female | 9 (36) | 7 (28) |
| ASA I | 15 (60) | 12 (48) |
| ASA II & III | 10 (40) | 13 (52) |
| Procedure duration (min) | 118.05 ± 29.16 | 123.3 ± 30.48 |
| Procedure | ||
| PDA ligation | 9 (36) | 8 (32) |
| Lung lobectomy | 8 (32) | 7 (28) |
| Lung cyst excision | 8 (32) | 10 (40) |
a Values are expressed as Mean ± SD or No. (%).
A significantly longer postoperative duration of analgesia was reported in group D compared to group M (P < 0.001). In group M, the achieved duration of postoperative analgesia ranged from 360 to 540 minutes, with a mean of 414 minutes (SD: 54 min). In group D, the duration of postoperative analgesia ranged from 480 to 840 minutes, with a mean of 636 minutes (SD: 112.2 min). The results only showed a slight difference in the mean morphine use between group M and group D. The average morphine use was 1.4 mg in group M versus 1 mg in group D (P = 0.275), as shown in Table 3.
Table 3. Time Until the Administration of the First Rescue Analgesic Medication and the Total Dose of Morphine in the First 24 Hoursa.
| Morphine | Dexmedetomidine | P-Value | |
|---|---|---|---|
| Postoperative analgesic duration (min) | 414 ± 54 | 636 ± 112.2 | < 0.001 |
| Morphine consumption (mg) | 1.4 ± 1 | 1 ± 0.9 | 0.27 |
a Data are expressed as Mean ± SD.
The postoperative pain management was assessed using the FLACC score. Higher scores were recorded in group D, especially in the first 12 hours after caudal block anesthesia, as shown in Table 4.
Table 4. The Postoperative FLACC Scores in the Two Groups (Mann-Whitney U test).
| FLACC | Morphine | Dexmedetomidine | P-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | Min. | Max. | Median | Min. | Max. | ||
| After 4 h | 4 | 3 | 4 | 2 | 2 | 3 | < 0.001 |
| After 8 h | 4 | 3 | 4 | 2 | 2 | 3 | < 0.001 |
| After 12 h | 4 | 3 | 5 | 3 | 3 | 4 | 0.002 |
| After 16 h | 4 | 3 | 5 | 4 | 4 | 5 | 0.652 |
| After 20 h | 4 | 2 | 6 | 4 | 3 | 5 | 0.922 |
| After 24 h | 3 | 2 | 5 | 3 | 2 | 4 | 0.306 |
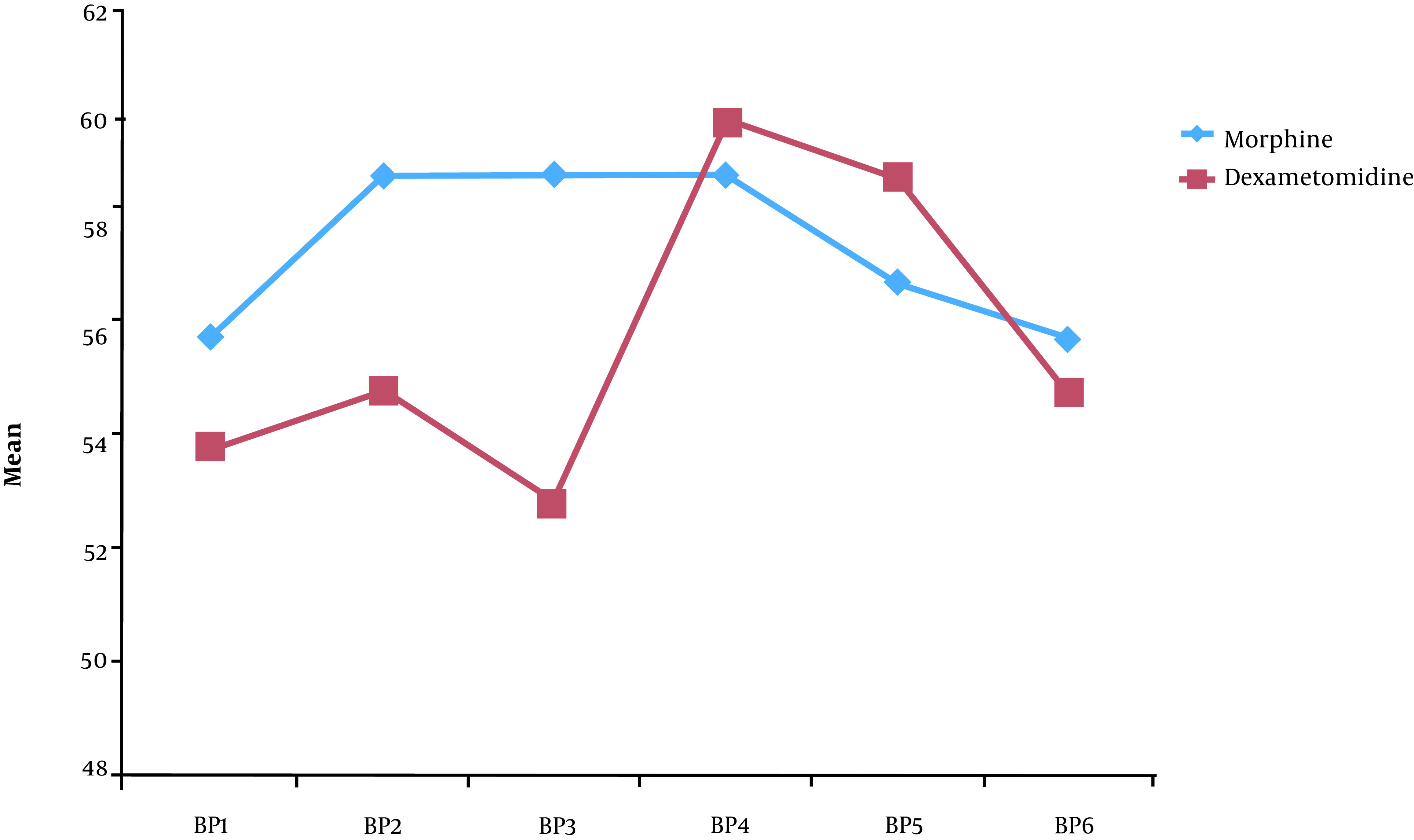
The patients’ vital signs were recorded every four hours after caudal injections and for a period of 24 hours. Lower heart rate and blood pressure were recorded in group D. The patients’ heart rate and blood pressure were especially lower in group D within the first 12 hours after caudal injections, as shown in Figures 2 and 3.
Figure 2. The heart rate of patients in the two groups postoperatively.
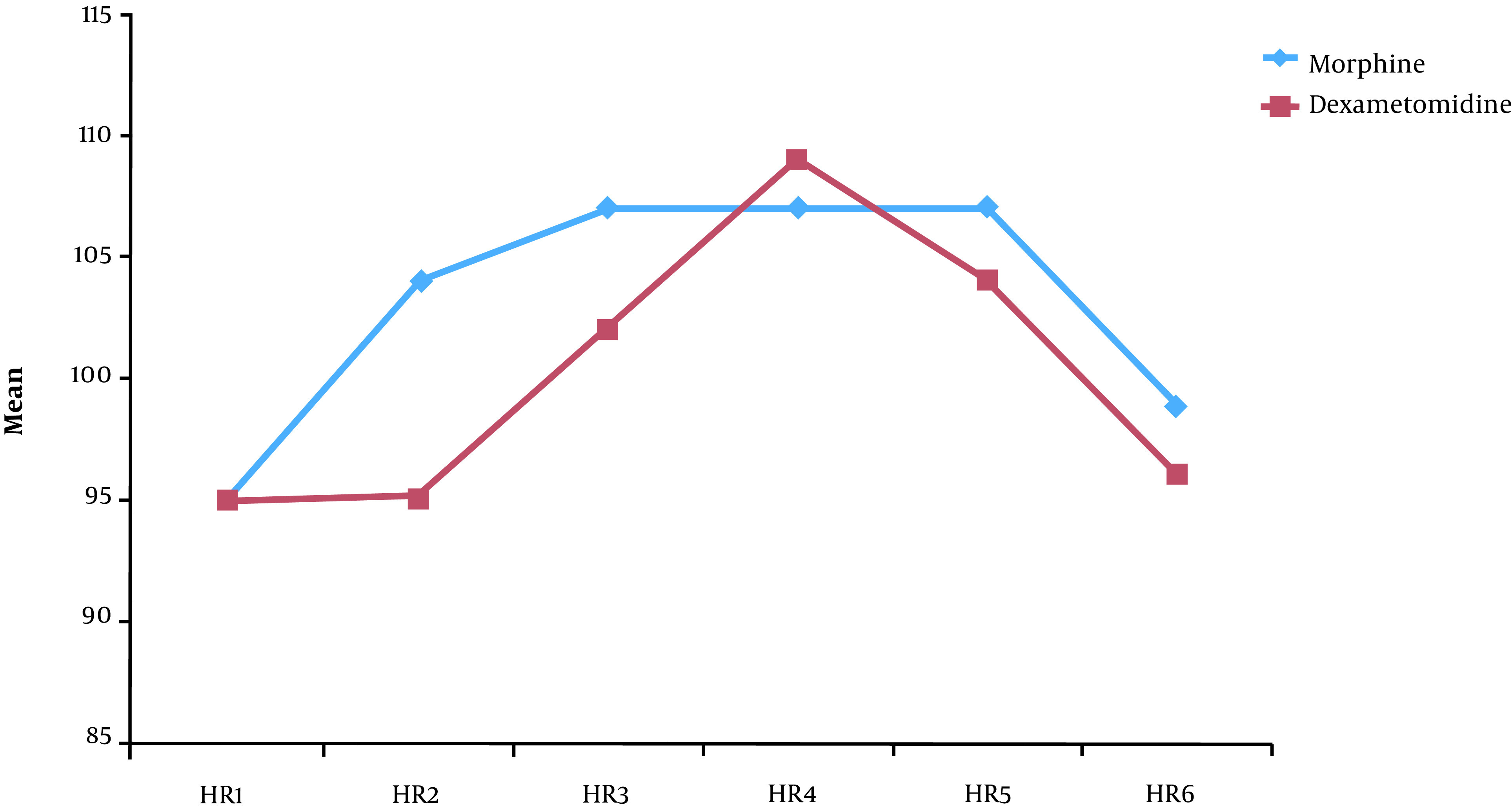
Figure 3. Blood pressure of patients in the two groups postoperatively.
4.1. Postoperative Side Effects
In the present study, four patients experienced vomiting during the postoperative period; three of them had received morphine, and one had received dexmedetomidine. Two patients from group M experienced itching, while no itching was recorded in Group D. There were no recorded incidences of respiratory depression in either of the groups (the lowest recorded oxygen saturation at 94%). All patients were discharged from the PICU to regular ward rooms within less than 24 hours, with the exception of two patients. These two patients remained in the PICU for more than 24 hours due to bed unavailability in the hospital wards (not due to medical necessity) (Table 5).
Table 5. The Postoperative Side Effects.
| Group M | Group D | |
|---|---|---|
| Vomiting | 3 (12%) | 1 (4%) |
| Itching | 2 (4%) | 0 |
| Delayed PICU stay ( > 24 h) | 1 | 1 |
| Hypotension | 0 | 0 |
| Bradycardia | 0 | 0 |
| Respiratory depression | 0 | 0 |
5. Discussion
In the current study, we found that the use of dexmedetomidine as an adjuvant to bupivacaine in caudal anesthesia prolonged the duration of postoperative analgesia following thoracic surgeries in pediatric patients, as compared to caudal morphine, without causing any side effects. Dexmedetomidine has been used as an adjuvant with caudal bupivacaine for a long time. Some studies have compared this drug with other adjuvants, such as morphine and fentanyl, but not in pediatric thoracic surgeries. Caudal anesthesia is commonly used to provide regional anesthesia in pediatric patients undergoing infraumbilical surgeries and is less commonly used to provide anesthesia for thoracic and upper abdominal surgeries.
The analgesic effect of dexmedetomidine may be attributed to either the effect of this drug on reducing the spinal sympathetic outflow at the presynaptic ganglionic sites or activation of the descending medullispinal noradrenergic pathway (13). Epidural opioids are believed to act as pre- and postsynaptic receptors in the spinal cord dorsal horn to achieve a selective block of nociceptive pathways (14). In this regard, Saadawy et al. assessed the efficacy of dexmedetomidine when administered as an adjuvant to caudal bupivacaine. He found that this mixture prolonged the duration of postoperative analgesia while reducing the need for additional analgesic medications, without any significant respiratory depression or changes in the hemodynamics (15).
A similar study by Imani et al. revealed that adding dexmedetomidine to ropivacaine in caudal block anesthesia potentiated the analgesic effect and decreased the analgesic requirements following infraumbilical surgeries in children without causing any side effects (16). Moreover, Al-Zaben et al. compared the efficacy of 2 mcg.kg-1 and 1 mcg.kg-1 of dexmedetomidine when used with bupivacaine for caudal block anesthesia. Their study reported that both doses led to the significant prolongation of postoperative analgesia, with minimal differences in the side effects when compared with bupivacaine alone (17).
Moreover, Gousheh et al. compared the effects of dexmedetomidine and morphine in epidural anesthesia during leg fracture surgeries. They concluded that dexmedetomidine was a better adjuvant than morphine, as it produced a longer analgesic effect and was more effective in pain control, without causing significant side effects or significant changes in the hemodynamics. Their results are consistent with the results of the present study; it should be noted that we used dexmedetomidine in supraumbilical surgeries. Also, unlike our study, Gousheh et al. focused on adult patients (18).
Three recent meta-analyses examined the efficacy and side effects of dexmedetomidine in caudal anesthesia for pediatric patients. These meta-analyses focused on infraumbilical procedures; their findings and conclusions are comparable to those of our study. Moreover, Tong et al. concluded that dexmedetomidine could delay the need for the first rescue analgesia. In our study, the side effects and hemodynamic changes due to dexmedetomidine addition were reportedly insignificant (19) Trifa et al. also recommended adding dexmedetomidine to the local anesthetic agent for pediatric patients undergoing infraumbilical surgeries (20). Moreover, a meta-analysis by Wang confirmed that dexmedetomidine increased the duration of post-caudal analgesia. However, dexmedetomidine increased the occurrence of bradycardia when used as part of caudal block (21).
In our study, although the heart rate and blood pressure were lower in the dexmedetomidine group, bradycardia and hypotension were insignificant findings. Another study by Nasr and Abdelhamid examined caudal anesthesia in supraumbilical surgeries. Their study compared the effects of dexmedetomidine versus fentanyl in cardiac surgeries, and their results are comparable to our findings. They reported that the addition of dexmedetomidine to caudal bupivacaine in cardiac surgeries improved the postoperative analgesia (22).
Moreover, Nguyen et al. reported that caudal anesthesia reduced the need for intraoperative opioid usage, but did not improve postoperative analgesia after pediatric cardiac surgeries. In this study, they focused on the effects of caudal anesthesia in supraumbilical procedures. Their study was, however, a retrospective study that analyzed the data of patients who had undergone cardiopulmonary bypass surgeries; it should be noted that these surgeries typically result in significant physiological changes (23). Besides, a similar retrospective study by Leyvi et al. concluded that post-induction administration of caudal anesthesia did not significantly change the outcomes of pediatric cardiac surgeries (24). We believe that further prospective studies are needed to assess the efficacy of caudal analgesia in supraumbilical, thoracic, and cardiac surgeries.
This study had some limitations. First, we did not use different concentrations of morphine or dexmedetomidine and did not include a placebo group. Second, we did not compare caudal versus intravenous opioid infusion. Third, we only focused on postoperative variables. Although we recorded the intraoperative variables, we did not include them in our statistical analysis, and we concentrated on the postoperative analgesic effects of caudal anesthesia.
5.1. Conclusions
Dexmedetomidine can produce prolonged and better postoperative analgesia as compared to morphine when used as an adjuvant to bupivacaine in caudal anesthesia for thoracic surgeries in pediatric patients.
Footnotes
Authors' Contribution: A.I.: Contribution to the study conception, design of the study, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript drafting, manuscript preparation, manuscript editing, and manuscript review; H.H.: Contribution to the study conception, study design, definition of intellectual content, literature search, clinical studies, experimental studies, data analysis, statistical analysis, manuscript drafting, manuscript preparation, manuscript editing, and manuscript review; A.G.: Contribution to the study conception, study design, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis, manuscript drafting, manuscript preparation, manuscript editing, and manuscript review. All authors read and approved the final manuscript.
Clinical Trial Registration Code: The clinical trial registration number for this study is NCT04445636.
Conflict of Interests: The authors declare no conflict of interest.
Ethical Approval: This prospective randomized, blinded trial was conducted in a tertiary pediatric cardiac center after obtaining approval from the relevant institutional ethics committee (N239-2020).
Funding/Support: This study was self-funded. There is no financial/material support for this research.
Informed Consent: Informed written consent forms were signed by the legal guardians of pediatric patients who participated in the study.
Contributor Information
Ahmed Abdelaziz Ismail, Email: ahmed.abdelaziz81@yahoo.com.
Hamza Mohamed Hamza, Email: hamza.kandel@gmail.com.
Ahmed Ali Gado, Email: gado_shahen@yahoo.com.
References
- 1.Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104(3):594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Agamohammdi D, Montazer M, Hoseini M, Haghdoost M, Farzin H. A comparison of continuous thoracic epidural analgesia with bupivacaine versus bupivacaine and dexmedetomidine for pain control in patients with multiple rib fractures. Anesth Pain Med. 2018;8(2):e60805. doi: 10.5812/aapm.60805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sendasgupta C, Makhija N, Kiran U, Choudhary SK, Lakshmy R, Das SN. Caudal epidural sufentanil and bupivacaine decreases stress response in paediatric cardiac surgery. Ann Card Anaesth. 2009;12(1):27–33. doi: 10.4103/0971-9784.45010. [DOI] [PubMed] [Google Scholar]
- 4.Tripi PA, Palmer JS, Thomas S, Elder JS. Clonidine increases duration of bupivacaine caudal analgesia for ureteroneocystostomy: a double-blind prospective trial. J Urol. 2005;174(3):1081–3. doi: 10.1097/01.ju.0000169138.90628.b9. [DOI] [PubMed] [Google Scholar]
- 5.Suksompong S, von Bormann S, von Bormann B. Regional catheters for postoperative pain control: Review and observational data. Anesth Pain Med. 2020;10(1):e99745. doi: 10.5812/aapm.99745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetta DF, Fares KM, Abedalmohsen AM, Abdel-Wahab AH, Elfadl GMA, Ali WN. Epidural dexmedetomidine infusion for perioperative analgesia in patients undergoing abdominal cancer surgery: randomized trial. J Pain Res. 2018;11:2675–85. doi: 10.2147/JPR.S163975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imani F, Rahimzadeh P, Faiz HR, Nowruzina S, Shakeri A, Ghahremani M. Comparison of the post-caesarean analgesic effect of adding dexmedetomidine to paracetamol and ketorolac: A randomized clinical trial. Anesth Pain Med. 2018;8(5):e85311. doi: 10.5812/aapm.85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imani F, Zaman B, De Negri P. Postoperative pain management: Role of dexmedetomidine as an adjuvant. Anesth Pain Med. 2021;10(6) doi: 10.5812/aapm.112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis MP, Thomas P, Wilson LF, Mulholland RC. The 'whoosh' test. A clinical test to confirm correct needle placement in caudal epidural injections. Anaesthesia. 1992;47(1):57–8. doi: 10.1111/j.1365-2044.1992.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 10.Wiegele M, Marhofer P, Lonnqvist PA. Caudal epidural blocks in paediatric patients: a review and practical considerations. Br J Anaesth. 2019;122(4):509–17. doi: 10.1016/j.bja.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–7. [PubMed] [Google Scholar]
- 12.Chertin B, Zeldin A, Kocherov S, Ioscovich A, Ostrovsky IA, Gozal Y. Use of caudal analgesia supplemented with low dose of morphine in children who undergo renal surgery. Curr Urol. 2016;9(3):132–7. doi: 10.1159/000442867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimzadeh P, Faiz SHR, Imani F, Derakhshan P, Amniati S. Comparative addition of dexmedetomidine and fentanyl to intrathecal bupivacaine in orthopedic procedure in lower limbs. BMC Anesthesiol. 2018;18(1):62. doi: 10.1186/s12871-018-0531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omar Mostafa M, Makram Botros J, Sayed Khaleel AM. Effect of dexmedetomidine versus nalbuphine as an adjuvant on paravertebral block to manage postoperative pain after mastectomies. Anesth Pain Med. 2018;8(2):e13308. doi: 10.5812/aapm.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53(2):251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 16.Imani F, Farahmand Rad R, Salehi R, Alimian M, Mirbolook Jalali Z, Mansouri A, et al. Evaluation of adding dexmedetomidine to ropivacaine in pediatric caudal epidural block: A randomized, double-blinded clinical trial. Anesth Pain Med. 2021;11(1) doi: 10.5812/aapm.112880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA, Al-Ghanem SM, Al-Mustafa MM, Alja'bari AN, et al. Comparison of caudal bupivacaine alone with bupivacaine plus two doses of dexmedetomidine for postoperative analgesia in pediatric patients undergoing infra-umbilical surgery: a randomized controlled double-blinded study. Paediatr Anaesth. 2015;25(9):883–90. doi: 10.1111/pan.12686. [DOI] [PubMed] [Google Scholar]
- 18.Gousheh M, Akhondzadeh R, Rashidi M, Olapour A, Moftakhar F. Comparison of dexmedetomidine and morphine as adjuvants to bupivacaine for epidural anesthesia in leg fracture surgery: A randomized clinical trial. Anesth Pain Med. 2019;9(4) doi: 10.5812/aapm.91480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong Y, Ren H, Ding X, Jin S, Chen Z, Li Q. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: a meta-analysis. Paediatr Anaesth. 2014;24(12):1224–30. doi: 10.1111/pan.12519. [DOI] [PubMed] [Google Scholar]
- 20.Trifa M, Tumin D, Tobias JD. Dexmedetomidine as an adjunct for caudal anesthesia and analgesia in children. Minerva Anestesiol. 2018;84(7):836–47. doi: 10.23736/S0375-9393.18.12523-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang XX, Dai J, Dai L, Guo HJ, Zhou AG, Pan DB. Caudal dexmedetomidine in pediatric caudal anesthesia: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99(31):e21397. doi: 10.1097/MD.0000000000021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasr DA, Abdelhamid HM. The efficacy of caudal dexmedetomidine on stress response and postoperative pain in pediatric cardiac surgery. Ann Card Anaesth. 2013;16(2):109–14. doi: 10.4103/0971-9784.109744. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen KN, Byrd HS, Tan JM. Caudal analgesia and cardiothoracic surgery: a look at postoperative pain scores in a pediatric population. Paediatr Anaesth. 2016;26(11):1060–3. doi: 10.1111/pan.12990. [DOI] [PubMed] [Google Scholar]
- 24.Leyvi G, Taylor DG, Reith E, Stock A, Crooke G, Wasnick JD. Caudal anesthesia in pediatric cardiac surgery: does it affect outcome? J Cardiothorac Vasc Anesth. 2005;19(6):734–8. doi: 10.1053/j.jvca.2005.01.041. [DOI] [PubMed] [Google Scholar]


