Abstract
Therapeutic drug monitoring (TDM) is the measurement of drug and antidrug antibody concentrations in individuals to guide treatment decisions. In patients with Crohn disease (CD), TDM, used either reactively or proactively, is emerging as a valuable tool for optimization of tumor necrosis factor (TNF) antagonist therapy. Reactive TDM is carried out in response to treatment failure, whereas proactive TDM involves the periodic monitoring of patients responding to TNF antagonist therapy to allow treatment optimization. In patients with CD, most of the available data for TDM relate to the first-to-market TNF antagonist infliximab and, to a lesser extent, to adalimumab and certolizumab pegol. Several gastroenterology associations, including the American Gastroenterology Association, have endorsed the use of reactive TDM in patients with active CD. However, fewer recommendations currently exist for the use of proactive TDM, although several new prospective randomized controlled trials evaluating proactive TDM strategies have been published. In this review, the current evidence for reactive and proactive TDM is discussed, and a proactive treatment algorithm for certolizumab pegol based on previously published threshold concentrations is proposed.
Keywords: antidrug antibody, certolizumab pegol, proactive TDM, reactive TDM, therapeutic drug monitoring
INTRODUCTION
Treating patients who have Crohn disease (CD) with tumor necrosis factor (TNF) antagonist monoclonal antibodies is often complicated by high interpatient variability in clinical response, in part because of differences in pharmacokinetics.1-3 Furthermore, because these monoclonal antibodies are large molecular-weight foreign proteins, they can elicit an immune response in a subgroup of patients with CD, resulting in the formation of antidrug antibodies (ADAbs). Negative consequences include reduced efficacy as a result of increased drug clearance, reduced binding to target, and increased likelihood of adverse reactions through several mechanisms.1
Therapeutic drug monitoring (TDM), in which serum drug and ADAb concentrations are measured in individual patients, is emerging as a valuable tool to optimize therapy with TNF antagonists. It can be performed reactively or proactively: Reactive TDM is carried out in response to treatment failure (secondary nonresponse), whereas proactive TDM is the periodic monitoring of patients responding to TNF antagonist therapy to allow optimization of treatment by dose adjustment to a target drug concentration. Because TNF antagonist treatment regimens consist of both an induction and a maintenance phase, there are a number of time points when proactive TDM is potentially useful. The aims of this review are to provide an overview of assays and guidelines on TDM and of clinical studies investigating TDM of TNF antagonists, in particular infliximab, adalimumab, and certolizumab pegol, for the treatment of CD, and to provide practical advice for clinicians on the use of both reactive and proactive TDM.
TDM OF TNF ANTAGONISTS IN CD
Most of the available data for TDM relate to the first-to-market TNF antagonist infliximab and, to a lesser extent, to adalimumab and certolizumab pegol.
Assays for TDM
Several assays and assay formats have been developed to measure TNF antagonist drug and ADAb concentrations in serum, some of which are commercially available, either as a kit or as a service provided by a specialized laboratory.4, 5 The most common assay formats are enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, and the homogeneous mobility shift assay. Some first-generation assays (irrespective of their format) are drug-sensitive—ie, they do not detect ADAbs in the presence of the drug (TNF antagonist) because free drug in the serum sample competes for ADAbs with the drug that is used to coat the assay plate. A second generation of ADAb assays is now available that is drug-resistant.6-9
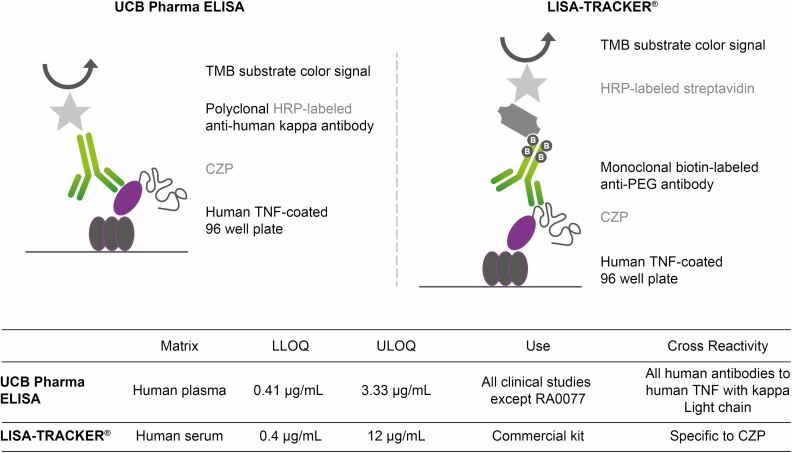
A comparative study indicated that 4 commercially available assays for infliximab were suitable for TDM, even in the presence of infliximab ADAbs or TNF in the serum sample.10 Similarly, a comparative study evaluating 2 commercially available assays for the assessment of adalimumab concentrations found that both methods were accurate and suitable for TDM.11 However, between assays, absolute concentrations of TNF antagonist drugs and ADAbs can differ: A comparison of the LISA-TRACKER (Theradiag) ELISA-based certolizumab pegol assay for the quantification of certolizumab pegol with a custom-made ELISA assay developed by UCB Pharma (validated in line with U.S. Food and Drug Administration/European Medicines Agency regulatory requirements for bioanalytical methods) showed that certolizumab pegol concentrations measured with the LISA-TRACKER certolizumab pegol assay were in good agreement with the ELISA assay developed by UCB Pharma, with a reported Bland-Altman mean ratio of 1.19 (95% confidence interval [CI], 1.13-1.25), suggesting a less than 20% variation in certolizumab pegol concentration between the 2 analytic methods.12 Overall, the good agreement between these two assays suggests that data measured with either assay can be extrapolated to clinical practice.12 Although the certolizumab pegol concentrations measured with the LISA-TRACKER certolizumab pegol assay were higher than those measured the UCB Pharma ELISA, the variability between these 2 assays is to be expected, given that the different format and reagents of these assays (see Fig. 1 for a comparison).12
FIGURE 1.
Comparison of 2 enzyme-linked immunosorbent (ELISA) assays for therapeutic drug monitoring in patients treated with certolizumab pegol: the LISA-TRACKER CZP assay* and a custom-made ELISA assay developed by UCB Pharma. Republished with permission of Future Science Ltd. on behalf of UCB Pharma, Copyright © 2017; permission conveyed through Copyright Clearance Center, Inc. Adapted with permission by Paul et al.12 *LISA-TRACKER is a product developed and manufactured by Theradiag, France. Miraca Life Sciences (now Inform Diagnostics) has a license to run these assays in the United States. CZP indicates certolizumab pegol; HRP, horseradish peroxidase; LLOQ, lower limit of quantification; PEG, polyethylene glycol; TMB, 3,3’,5,5’-tetramethylbenzidine; ULOQ, upper limit of quantification.
Furthermore, although a comparison of commercially available homogenous mobility shift assay and ELISA to assess infliximab and adalimumab concentrations found good correlation between the assays for each biologic (infliximab: r = 0.861; P < 0.001; adalimumab: r = 0.935; P < 0.001), agreement between the assays was weak (infliximab intraclass correlation coefficient = 0.356 [95% CI, −0.069 to 0.720]; P < 0.001; adalimumab intraclass correlation coefficient = 0.395 [95% CI, −0.073 to 0.759]; P < 0.001).13 Therefore, if the aim is to establish whether a particular cutoff concentration of a TNF antagonist has been reached as part of a TDM approach, it is always recommended to select the assay that was initially used to establish the respective threshold.
Often the slow turnaround time of the ELISA-based assays (up to 8 hours) has constrained the immediate implementation of treatment decisions based on test results. This situation has led to the development of more rapid assays with a time-to-result of 20 minutes,14, 15 which will in time allow clinicians to make appropriate treatment decisions based on rapidly available test results at the point of care.
Guidelines for Reactive and Proactive TDM in Inflammatory Bowel Disease
Several American, Australian, Canadian, and European gastroenterology associations have issued recommendations regarding the use of TDM of biologics in a reactive and/or proactive setting.16-21
Reactive TDM
Guidelines from the American Gastroenterological Association (AGA) conditionally recommend the use of reactive TDM in patients with active inflammatory bowel disease receiving TNF antagonists.16, 22 Trough concentrations (ie, the concentration of a drug in a patient just before the next dose) for reactive TDM in patients with active disease on maintenance TNF antagonist therapy, as suggested by the AGA, are ≥5 µg/mL, ≥7.5 µg/mL, and ≥20 µg/mL for infliximab, adalimumab, and certolizumab pegol, respectively. The AGA trough concentration thresholds have also been endorsed by the American College of Gastroenterology.18 A consensus statement funded by the Gastroenterological Society of Australia (GESA) endorsed the use of reactive TDM in patients with secondary nonresponse.19 Trough infliximab and adalimumab concentrations of 3.8 µg/mL and 5 to 12 µg/mL, respectively, were considered appropriate by the GESA panel to achieve clinical remission in luminal CD.19 Furthermore, the Toronto consensus statement by the Canadian Association of Gastroenterology recommended the use of TDM in patients who had lost response to anti-TNF therapy to help with clinical decision-making and dose optimization.20 A guideline/consensus paper by the European Crohn’s and Colitis Organization concurred that TDM can be beneficial in patients with difficult cases, notably in patients with primary or secondary treatment failure.17 An expert panel led by Melmed et al23, 24 recommended testing for TNF antagonist and ADAb concentrations in patients with secondary loss of response (LOR) and primary nonresponse.
Proactive TDM
Guidelines that endorse a proactive TDM approach have been less prevalent than those endorsing a reactive approach. The AGA guidelines, for example, do not make any recommendations regarding the use of routine proactive TDM in patients with quiescent IBD receiving TNF antagonists.16, 22 Those that do endorse a proactive approach do so very cautiously or only in certain clinical scenarios. For example, the GESA panel proposed that proactive TDM should be considered on a periodic basis only, when test results are likely to affect management decisions.19 Furthermore, an expert panel led by Melmed et al23, 24 endorsed testing for TNF antagonist and ADAb concentrations both during the first year of maintenance therapy and after the initial dose after a drug holiday.
Current guidelines do not address the use of TDM during induction, although several studies have shown the association of early adequate drug exposure with long-term clinically important outcomes.25 Furthermore, different phenotypes (eg, fistulizing CD) may require different therapeutic thresholds.26, 27
TDM for Infliximab
Factors affecting infliximab exposure
A subgroup of patients (6%–17%) treated with regularly scheduled infliximab (without dose interruption) develop ADAbs,28-31 resulting in drug–ADAb complexes that are cleared from the body much more quickly than the uncomplexed drug.18 This development negatively impacts overall exposure to the drug and consequently lowers infliximab trough concentrations. Low trough concentrations are associated with poor clinical outcome, such as LOR. A meta-analysis based on 10 studies and 668 patients found an association between infliximab ADAbs and LOR. The relative risk of LOR to therapy in patients with infliximab ADAbs was 3-fold higher than in patients who did not develop ADAbs (relative risk = 3.2; 95% CI, 1.9-5.5; P < 0.0001).32 In some patients, ADAb formation is “transient”—ie, ADAbs disappear over time and LOR is reversed, whereas in many other patients ADAb formation is persistent and LOR is sustained.33 Monitoring infliximab and ADAb concentrations in patients receiving infliximab therapy is important to help distinguish between these 2 patient subgroups based on ADAb status, thus aiding clinical decision-making. In patients who test positive for ADAbs, combination therapy with immunomodulators (IMMs; eg, azathioprine) in addition to TNF antagonist use is recommended to suppress ADAbs formation and is suggested to reverse the enhanced clearance of the TNF antagonist.34
Factors other than ADAbs have also been shown to correlate with increased infliximab drug clearance, including serum albumin and C-reactive protein (CRP) concentrations and body weight.35-38
Exposure–response relationship
Considerable data support an association between exposure, as defined by blood drug concentrations, and clinically important outcomes in patients treated with infliximab, adalimumab, or certolizumab pegol, indicating that this association is a class effect. This finding holds out the possibility that greater efficacy could be obtained by individualizing drug dosing to ascertain optimal exposure in individual patients, consistent with the principle of proactive TDM. Posthoc analyses that have evaluated the exposure–response relationship of infliximab identified a trough concentration >3 µg/mL during maintenance as being predictive of lower disease activity or sustained remission in patients with CD.39-41 A posthoc analysis from the Active Ulcerative Colitis Trials indicated that the proportion of patients achieving clinical remission increased with increasing quartiles of serum infliximab concentrations, with similar trends observed for clinical response and mucosal healing.42 Specifically, infliximab serum concentrations ≥18.6 µg/mL at week 2, ≥10.6 µg/mL at week 6, and ≥34.9 µg/mL at week 8 (induction time points) were associated with a week 8 Mayo Clinic endoscopic subscore ≤1.43 Infliximab serum concentrations ≥5.1 µg/mL at week 14 and ≥2.3 µg/mL at week 30 (maintenance time points) were associated with a week 30 Mayo Clinic endoscopic subscore ≤1, whereas higher concentrations of ≥6.7 µg/mL and ≥3.8 µg/mL, respectively, were associated with the more stringent outcome of a subscore of 0.43 A retrospective, single-center study identified an infliximab concentration ≥15 µg/mL at the end of the induction period (week 6; P = 0.025) as an independent factor of mucosal healing.44 Although these studies were conducted in patients with ulcerative colitis (UC), they are still relevant to this review because similar associations have also been observed in CD: in a posthoc analysis of ACCENT 1, a multicenter, randomized, placebo-controlled study of patients with CD, serum infliximab trough concentrations of >3.5 µg/mL at week 14 were predictors of durable sustained response during infliximab maintenance therapy at 5 mg/kg.40
Reactive vs proactive TDM
A number of studies have evaluated TDM of infliximab in a reactive setting. A prospective, 8-week cohort study evaluated TDM in patients with CD or UC who had received dose intensification after secondary failure to infliximab; an increase in infliximab trough concentration after dose intensification was associated with mucosal healing in both groups of patients (P = 0.001; Table 1).45 Furthermore, in a prospective, randomized, controlled cost-effectiveness study, patients with secondary infliximab treatment failure were randomized to either a conventional dose intensification (5 mg/kg every 4 weeks) or interventions using an algorithm based on combined infliximab and infliximab ADAb measurements. The algorithm-based treatment approach achieved similar clinical, biological, and quality-of-life outcomes to conventional dose intensification but at significantly lower costs (ie, 34% at week 12; P < 0.001); 46 this cost reduction was maintained for up to 1 year (Table 1).47
TABLE 1.
Overview of Trials Investigating TDM of TNF Antagonists for CD
| Study | Number of Patients | Study Design | Intervention | Primary Endpoint | Results |
|---|---|---|---|---|---|
| Efficacy studies | |||||
| Vande Casteele, Ferrante, et al (TAXIT trial)3 | N = 263 patients with CD or UC | 1-y prospective randomized controlled trial | IFX | Clinical and biochemical remission at 1 y after optimization phase | No differences in clinical, biological, and endoscopic remission between IFX TC-based dosing and clinically based dosing arms; however, TC-based dosing was associated with fewer flares requiring rescue therapy and more efficient use of drug. |
| D’Haens et al (TAILORIX trial)49 | N = 122 patients with CD randomized to 3 maintenance regimens: (1) dose intensification based on clinical symptoms, biomarker analysis, and serum TC IFX; (2) dose intensification of IFX to 5-10 mg/kg based on the same criteria; (3) IFX dose intensification to 10 mg/kg based on clinical symptoms alone | Prospective randomized double-blind controlled trial | IFX | Sustained, steroid-free clinical remission from weeks 22-54 and absence of ulceration at 1 y based on endoscopy | Proactive IFX TC-based dose intensification was not superior to clinically based dose intensification. |
| Paul, Del Tedesco, et al45 | N = 52 patients with CD or UC with secondary failure to IFX | Prospective 8-wk cohort study | IFX | n/a | An increase in IFX TC after dose optimization was associated with mucosal healing in patients with CD and with UC (P = 0.001). |
| Assa et al63 | N = 78 pediatric patients with CD naïve to treatment with TNF antagonists | Nonblinded randomized controlled trial | ADA | Sustained corticosteroid-free clinical remission at all visits (wks 8-72) | Proactive TDM of ADA TCs resulted in significantly higher rates of corticosteroid-free clinical remission than did reactive TDM. |
| Chiu et al55 CLASSIC subanalysis | N = 275 patients with CD receiving ADA as induction therapy in CLASSIC I and II studies | Prospective study | ADA | n/a | A positive correlation between serum ADA and remission was identified at several time points up to week 56. |
| Karmiris et al60 | N = 168 patients with CD after failure of IFX therapy | Prospective observational study | ADA | n/a | ADA TCs were lower in patients who discontinued; patients with ADA ADAbs had lower median ADA TC throughout entire follow-up period (P < 0.0001) |
| Bodini, Giannini, Savarino, et al54 | N = 23 patients with CD | Prospective 72-week study | ADA | n/a | ADA TCs were significantly higher (11.9 µg/mL) in patients with remission vs mild and moderate/severe disease (5.5 µg/mL; P = 0.0002). |
| Ward et al71 | N = 19 patients with CD on maintenance ADA regimen had ADA concentrations measured repeatedly to predefined schedule | Prospective observational study | ADA | n/a | ADA concentrations ≥4.9 µg/mL obtained during the first 9 days predicted therapeutic ADA TCs with reasonable confidence. |
| Vande Casteele, Feagan, Vermeire, et al70 | N = 2157 patients with CD | CZP simulation study based on data from 9 clinical trials | CZP | n/a | CZP concentrations of 36 µg/mL and 15 µg/mL at weeks 6 and 12 were associated with attaining a combined efficacy outcome of CDAI ≤150 and FCP ≤250 µg/g at week 26. |
| Cost-effectiveness studies | |||||
| Steenholdt et al46, 47 | N = 69 patients with secondary IFX failure were randomized to conventional dose intensification (5 mg/kg every 4 weeks) or interventions based on serum IFX and IFX ADAbs using an algorithm | Randomized controlled single-blind study | IFX | Accumulated mean cost per patient for CD at week 12 in the algorithm group vs the group receiving conventional dose intensification | Algorithm-based treatment achieved similar clinical, biological, and quality of life outcomes to conventional dose intensification but at significantly lower costs; this cost reduction was maintained for up to 1 year. |
ADA indicates adalimumab; CDAI, Crohn’s Disease Activity Index; CZP, certolizumab pegol; IFX, infliximab; n/a, not available; TC, trough concentration.
A number of studies have reported differences in clinical outcomes between patients who were proactively managed using TDM vs those who were conventionally managed. In the Trough Concentration Adapted Infliximab Treatment (TAXIT) randomized controlled trial, patients with CD (n = 178) or UC (n = 85) on maintenance infliximab therapy all underwent proactive TDM with dose optimization to achieve trough concentrations within the 3–7 μg/mL window.3 This intervention, in patients with subtherapeutic drug exposure, led to a significant increase in the proportion of patients in clinical and biochemical remission, whereas in patients with supratherapeutic drug exposure, significant cost reductions were achieved with attainment of response and remission status. After dose optimization, patients were randomized; those who were managed based on clinical factors had a greater need for rescue therapy compared with patients who were managed based on trough concentrations of infliximab. However, overall remission rates were similar between the 2 arms 1 year after proactive dose optimization in all participants (ie, the primary endpoint; Table 1). The limitations of the TAXIT trial are the design (ie, carryover effect of dose optimization in all patients before randomization) and the relatively short follow-up.3 A follow-up retrospective study of TAXIT evaluating longer-term outcomes showed that concentration-based dosing was associated with fewer infliximab discontinuations for all reasons than dosing based on clinical factors (P = 0.04).48
TAILORIX, a randomized controlled trial, evaluated 122 patients with CD who were randomized to 3 maintenance regimens: (1) infliximab dose intensification to 10 mg/kg based on clinical symptoms alone; (2) dose intensification in 2.5 mg/kg increments based on clinical symptoms, biomarker analysis, and serum trough concentrations of infliximab; and (3) dose intensification of infliximab to 5 to 10 mg/kg based on clinical symptoms, biomarker analysis, and serum trough concentrations of infliximab (Table 1).49 Infliximab trough concentration–based dose intensification was not superior to clinically based dose intensification with regard to achieving the primary endpoint of steroid-free clinical remission from weeks 22 to 54 and the absence of ulceration at 1 year based on endoscopy.49 However, note that only 14% of patients in TAILORIX actually underwent dose optimization based on infliximab trough concentrations in the combined intervention groups.
The concept that combination therapy with IMMs may not be necessary if adequate TNF antagonist concentrations are attained using proactive TDM is supported by a number of studies. In a pilot observational study, optimization of infliximab (“optimized monotherapy”) to a trough concentration of ≥5 µg/mL using TDM was reported as an alternative strategy to combination therapy with concomitant IMMs in a subset of patients.50 A retrospective study also showed that infliximab trough levels of >5 µg/mL had similar drug persistence after at least 6 months regardless of IMM therapy.51 Furthermore, in a posthoc analysis of the Study of Biologic and Immunomodulator-Naïve Patients in Crohn’s Disease trial, stratification of infliximab trough concentrations showed a similar outcome (corticosteroid-free remission at week 26) within each concentration quartile regardless of IMM therapy.52
In summary, considerable research supports an exposure–response relationship in patients treated with the approved dosing regimen of infliximab; however, the controlled studies performed to date do not provide strong evidence for proactive TDM. Conversely, reactive TDM has become well established in the management of patients with IBD and has some controlled data to support its use.
TDM for Adalimumab
Factors affecting adalimumab exposure
A comprehensive population pharmacokinetics (PK) analysis of adalimumab in patients with moderate-to-severe CD was recently published. Intense serum sampling enabled characterization of the absorption phase of this subcutaneously administered drug and revealed a 4-fold difference in the range of serum adalimumab concentrations 7 days after the first dose (160 mg). Substantial interindividual variability was also observed in clearance, and the presence of adalimumab ADAbs and higher lean body weight were found to be predictors of accelerated drug clearance.53
Exposure–response relationship
The relationship between trough adalimumab concentrations and clinical outcomes in the management of patients with CD is similar to that of infliximab, albeit less well described. Increased adalimumab trough serum concentrations have been reported as a key predictor of improved therapeutic outcome in CD.53-57 A prospective subanalysis study evaluating data from the Clinical Assessment of Adalimumab Safety and Efficacy Studied as Induction Therapy in Crohn’s Disease I and II trials identified a positive association between serum adalimumab concentration and remission at several time points up to week 56 (Table 1).55 However, in this study it was not possible to identify cutoff concentrations indicative of clinical remission.55 In a prospective 72-week study, trough adalimumab concentrations were significantly higher (11.9 µg/mL) in patients with remission vs mild and moderate/severe disease (5.5 µg/mL; P = 0.0002; Table 1).54 In addition, in a cross-sectional study of 40 patients, median trough concentrations of adalimumab were higher in patients in clinical remission (6.0 µg/mL) than in those with active disease (3.2 µg/mL; P < 0.012).57 Finally, in a cross-sectional study evaluating 118 trough serum samples from adalimumab-treated patients, higher adalimumab trough serum concentrations were associated with remission (P < 0.001), with an adalimumab serum cutoff value of >5.85 µg/mL being identified as a positive predictor for attaining remission. High adalimumab ADAb concentrations were positively associated with disease activity.56
Endpoints such as mucosal healing (ie, lack of endoscopic and histologic inflammation) may require even higher adalimumab concentrations (in the range of 13-14 µg/mL) than those required for clinical endpoints, as was reported in a cross-sectional study evaluating 66 patients with CD and UC.58 Furthermore, in a cross-sectional study of 40 patients, trough concentrations were higher in patients with mucosal healing (6.5 µg/mL) than in those without mucosal healing (4.2 µg/mL; P < 0.005). Trough concentrations and duration of adalimumab treatment were independently associated with mucosal healing. Absence of mucosal healing was associated with trough concentrations <4.9 µg/mL.57 Similar to what has been observed for infliximab, the presence of adalimumab ADAbs was associated with a higher LOR to adalimumab.59-61 The recent Study to Evaluate the Safety and Efficacy of Two Drug Regimens in Subjects With Moderate to Severe Ulcerative Colitis trial compared higher adalimumab induction doses with the standard induction dosing regimen. After 8 weeks of treatment, standard adalimumab induction doses resulted in similar clinical and endoscopic efficacy compared with the high-induction doses. The similar efficacy was observed despite adalimumab trough concentrations being higher after the higher induction dosing regimen vs the standard induction dosing regimen (mean [standard deviation] = 19.3 [9.5] µg/mL vs 8.0 [4.9] µg/mL, respectively).62 These results indicate that not all patients may benefit from higher doses, but rather a selection of patients who are at risk of accelerated drug clearance.
Reactive vs proactive TDM
Reactive vs proactive TDM of adalimumab was compared in the Pediatric Crohn’s Disease Adalimumab Level-Based Optimization Treatment trial, a nonblinded randomized controlled trial of 78 pediatric patients with CD.63 Proactive TDM of adalimumab trough concentrations resulted in significantly higher rates of corticosteroid-free clinical remission at all visits from weeks 8 to 72 (P = 0.002; primary endpoint; Table 1) compared with reactive TDM.63 Furthermore, a positive effect was noted for a composite endpoint including sustained corticosteroid-free clinical remission, CRP (≤0.5 mg/dL), and fecal calprotectin (FCP; ≤150 µg/g; P = 0.003), a surrogate marker for mucosal healing.63 However, although the effect of proactive TDM was significant for clinical remission, the benefit was only seen for mild exacerbations, not for moderate to severe exacerbations. Furthermore, drug-related discontinuations were similar between the proactive TDM and the reactive TDM treatment arms.63
In summary, an exposure–response relationship similar to that established for infliximab has been shown for adalimumab. Proactive vs reactive TDM were directly compared in a randomized controlled trial, with a significant effect on clinical remission being observed in patients with mild exacerbations.
TDM for Certolizumab Pegol
Factors affecting certolizumab pegol exposure
Similar to infliximab and adalimumab, a subgroup of patients receiving certolizumab pegol develop ADAbs that may result in increased drug clearance from the body.64-66 A population PK model was developed that made it possible to describe the time-varying nature of covariates and certolizumab pegol ADAb concentrations and how this affects certolizumab pegol PK parameters, such as clearance.67 This model predicted that high certolizumab pegol ADAb concentrations are more likely to lead to certolizumab pegol exposure below therapeutic concentrations compared with lower certolizumab pegol ADAb concentrations.67 Whereas this population PK model identified time-varying body weight and CRP as additional factors that increase certolizumab pegol clearance, it identified albumin as a factor that decreases clearance.67
Exposure–response relationship
An exposure–response relationship has been described for certolizumab pegol, similar to infliximab and adalimumab. Two studies have evaluated certolizumab pegol concentrations and certolizumab pegol ADAbs and correlated these with clinical outcomes in patients with CD.64, 68 A posthoc analysis by Colombel, Sandborn, Allez, et al68 reported that higher certolizumab pegol serum concentrations at week 8 (n = 80) were associated with endoscopic response (P = 0.0016; area under the receiver operating curve = 0.69) and clinical remission at week 10 (P = 0.0302, area under the receiver operating curve = 0.70) in patients with CD. At week 54 (n = 45), the rates of endoscopic remission correlated with certolizumab pegol plasma concentrations. Furthermore, there was an inverse relationship between certolizumab pegol plasma concentrations and body weight (P = 0.0373) and CRP concentrations (P = 0.0014) at baseline.68
In a retrospective study using endoscopic and radiologic data to investigate certolizumab pegol treatment in patients with IBD, certolizumab pegol trough concentrations >27.5 µg/mL were associated with radiological healing, radiological response, and symptomatic response. No association was found between median certolizumab pegol trough concentrations and mucosal healing. Lower trough concentrations (<27.5 µg/mL) were significantly associated with changes in clinical management (including a decrease in dose interval, addition of an IMM, or certolizumab pegol discontinuation; P = 0.007).64
A recent longitudinal report from the 7-year Pegylated Antibody Fragment Evaluation in Crohn’s Disease: Safety and Efficacy-3 study (N = 594) examined the impact of transient (defined as >2.4 U/mL with transient or no effect on certolizumab pegol plasma concentration [>5 μg/mL]) vs persistent (defined as >2.4 U/mL with continuous impact on certolizumab pegol plasma concentration) certolizumab pegol ADAbs on certolizumab pegol concentrations and markers of inflammation.69 There were 134 (22.6%) patients who were positive for ADAbs at least once during the study. Of those, 40 were categorized as having transient ADAbs and 94 were categorized as having persistent ADAbs.69 In patients with persistent certolizumab pegol ADAbs, median plasma concentrations of the inflammatory biomarkers CRP and FCP were significantly higher (P < 0.05 at some visits) and plasma certolizumab pegol concentrations were significantly lower (P < 0.0001) compared with patients who were certolizumab pegol ADAb-negative.69 However, in patients with transient certolizumab pegol ADAbs, plasma certolizumab pegol, CRP, and FCP concentrations were similar to those in patients who were certolizumab pegol ADAb-negative, which may suggest that transient ADAbs may not affect certolizumab pegol concentrations or the clinical response to certolizumab pegol.69
When the above-mentioned population PK model was applied to pooled data from 9 clinical trials investigating certolizumab pegol in adult patients with CD, approximate certolizumab pegol concentrations of 36 µg/mL at week 6 and 15 µg/mL at week 12 were associated with attainment of a robust combined efficacy outcome (Crohn’s Disease Activity Index ≤ 150 and FCP ≤ 250 µg/g, respectively) at week 26 (Table 1).70 Knowledge of specific drug concentration thresholds that corresponded with efficacy outcomes, such as the week 6 certolizumab pegol concentrations of 36 µg/mL and 15 µg/mL at week 12 identified in this analysis, may inform TDM and guide decisions in clinical practice.70
In summary, similar to infliximab and adalimumab, extensive research supports an exposure–response relationship in patients with CD receiving approved doses of certolizumab pegol. Concentration thresholds have been identified that correspond with efficacy outcomes.
A theoretical construct for proactive certolizumab pegol TDM
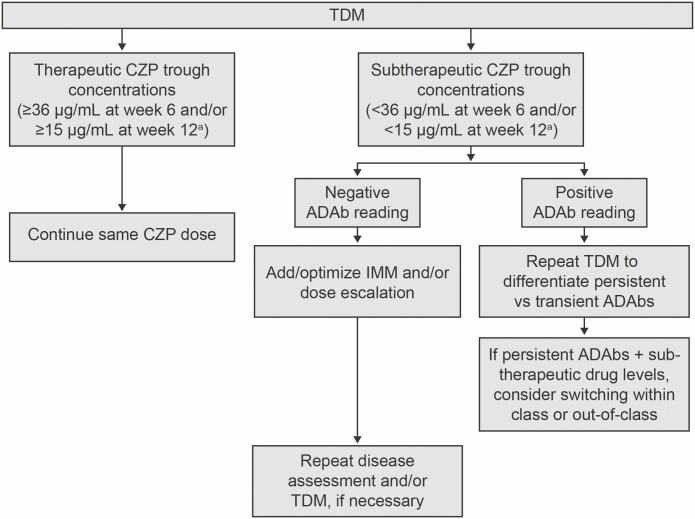
Although no controlled studies evaluating proactive TDM of certolizumab pegol have been conducted to date, we propose a theoretical construct for TDM of certolizumab pegol in patients in clinical remission based on previously published certolizumab pegol trough concentration thresholds, as illustrated in Fig. 2. In this treatment algorithm, certolizumab pegol trough concentrations are measured after induction and during maintenance therapy. Patients with subtherapeutic certolizumab pegol trough concentrations (<36 µg/mL at week 6 and/or <15 µg/mL at week 12, as described previously70) are identified and evaluated further for ADAb status. Patients with negative ADAbs are considered for the addition or optimization of IMM therapy and/or dose escalation. Patients with positive ADAbs may need repeat TDM (perhaps after an intervention such as dose escalation and/or addition or optimization of IMM therapy at provider discretion) to differentiate between persistent vs transient ADAbs. In patients with confirmed persistent ADAb status, switching to another TNF antagonist or to a different drug class should be considered.
FIGURE 2.
Proposed treatment approach for proactive TDM in clinical practice in patients in clinical remission receiving CZP maintenance therapy. aCZP cutoff concentrations based on Vande Casteele, Feagan, Vermeire, et al.70 CZP indicates certolizumab pegol.
LIMITATIONS OF CURRENT DATA AND KNOWLEDGE GAPS
Limitations of the data on which the proposed TDM treatment algorithm for certolizumab pegol is based include the absence of endoscopic outcomes, which were often not assessed in historic trials of CD. There is a need for studies evaluating clinical outcomes that support the long-term benefits of a proactive TDM strategy, with regard not only to steroid-free clinical remission but also to endoscopic and histologic healing. Furthermore, the causal relationship between certolizumab pegol exposure and clinical outcomes is not clearly established. A potential solution is a prospective, interventional clinical trial looking at early and optimized dosing of certolizumab pegol vs a reactive approach per standard of care.
CONCLUSIONS
There is substantial variability in pharmacokinetics and observed response rates between TNF antagonists in CD. We find that TDM offers the potential to optimize therapy in individual patients. Reactive TDM is supported by guidelines and is currently being widely adopted in clinical practice. Proactive TDM has current uncertainties, but data are emerging that indicate that it is an effective strategy for the improved retention of TNF antagonist therapy.
ACKNOWLEDGMENTS
The authors acknowledge Fiona Nitsche, PhD, CMPP and Richard Fay, PhD, CMPP of Evidence Scientific Solutions (London, UK and Philadelphia, PA) for writing and editorial assistance, which was funded by UCB Pharma. The authors also acknowledge Mylene Serna, PharmD (UCB Pharma, Smyrna, GA) for publication coordination.
Funding: NVC holds a Research Scholar Award from the American Gastroenterological Association. Funding for this review article was provided by UCB Pharma.
Conflicts of interest: NVC reports grants from R-Biopharm, grants and personal fees from Takeda and UCB, and personal fees from Celltrion and Prometheus outside the submitted work. BF is a speakers’ bureau member for Abbott/AbbVie, Johnson & Johnson/Janssen Pharmaceuticals, Takeda, UCB Pharma, and Warner Chilcott; served as a consultant and advisory board member for Abbott/AbbVie, ActoGeniX, Albireo Pharma, Amgen, AstraZeneca, Avaxia Biologics, Axcan Pharma, Baxter Healthcare, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharmaceuticals, Gicare Pharma, Gilead, Given Imaging, GlaxoSmithKline, Ironwood Pharmaceuticals, Janssen Biotech, Johnson & Johnson/Janssen Pharmaceuticals, Kyowa Hakko Kirin, Lexicon Pharmaceuticals, Lilly, Merck, Millennium, Nektar, Novo Nordisk, Pfizer, Prometheus Laboratories, Protagonist Therapeutics, Receptos, Roche/Genentech, Salix, Serono, Shire, Sigmoid Pharma, Synergy, Takeda, Teva, Tillotts Pharma, Vertex, Warner Chilcott, Wyeth, Zealand Pharma, and Zyngenia; and has contracted research with Abbott/AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen Biotech, Johnson & Johnson/Janssen Pharmaceuticals, Millennium, Pfizer, Receptos, Roche/Genentech, Sanofi, Santarus, Tillotts Pharma, and UCB Pharma. DW has served as a speaker, consultant, or advisory board member for AbbVie (Abbott), Celgene, Janssen, Pfizer, Prometheus, Takeda, and UCB Pharma. AP and MY are employees and stockholders of UCB Pharma. SH has served as a consultant or advisory board member for Janssen, UCB, Boehringer Ingelheim, and Gilead. TR has served as a speaker, consultant, or advisory board member for AbbVie, Arena, Boehringer Ingelheim, Celgene, Eli Lilly, Ferring, Genentech, Gilead, Ironwood, Janssen, Pfizer, Prometheus, and Takeda. WJS reports research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, Celgene/Receptos, Pfizer, and Prometheus Laboratories (now Prometheus Biosciences); consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, and Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. WJS reports that his spouse has been a consultant for Opthotech and Progenity; has had stock options from Escalier Biosciences, Oppilan Pharma, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Ventyx Biosciencesm and Vimalan Biosciences; and has been an employee of Escalier Biosciences, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Ventyx Biosciences, and Vimalan Biosciences.
REFERENCES
- 1. Lin K, Mahadevan U. Pharmacokinetics of biologics and the role of therapeutic monitoring. Gastroenterol Clin North Am. 2014;43:565–579. [DOI] [PubMed] [Google Scholar]
- 2. Vande Casteele N, Gils A. Pharmacokinetics of anti-TNF monoclonal antibodies in inflammatory bowel disease: adding value to current practice. J Clin Pharmacol. 2015;55:S39–S50. [DOI] [PubMed] [Google Scholar]
- 3. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–132 9.e3. [DOI] [PubMed] [Google Scholar]
- 4. Lab Corp. Certolizumab and anti-certolizumab antibody, DoseASSURE™ CTZ. Accessed December 31, 2019. https://www.labcorp.com/test-menu/46121/certolizumab-and-anti-certolizumab-antibody-idose-iassure%E2%84%A2-ctz#.
- 5. Mayo Clinic Laboratories. Test ID: FCERT–certolizumab pegol and anti-certolizumab antibodies, serum. Accessed December 31, 2019. https://qa.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/75198.
- 6. van Schouwenburg PA, Bartelds GM, Hart MH, et al. A novel method for the detection of antibodies to adalimumab in the presence of drug reveals “hidden” immunogenicity in rheumatoid arthritis patients. J Immunol Methods. 2010;362:82–88. [DOI] [PubMed] [Google Scholar]
- 7. Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods. 2012;382:177–188. [DOI] [PubMed] [Google Scholar]
- 8. Wang SL, Hauenstein S, Ohrmund L, et al. Monitoring of adalimumab and antibodies-to-adalimumab levels in patient serum by the homogeneous mobility shift assay. J Pharm Biomed Anal. 2013;78-79:39–44. [DOI] [PubMed] [Google Scholar]
- 9. Patton A, Mullenix MC, Swanson SJ, Koren E. An acid dissociation bridging ELISA for detection of antibodies directed against therapeutic proteins in the presence of antigen. J Immunol Methods. 2005;304:189–195. [DOI] [PubMed] [Google Scholar]
- 10. Marini JC, Sendecki J, Cornillie F, et al. Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of Remicade®. Aaps J. 2017;19:161–171. [DOI] [PubMed] [Google Scholar]
- 11. Bodini G, Giannini EG, Furnari M, et al. Comparison of two different techniques to assess adalimumab trough levels in patients with Crohn’s disease. J Gastrointestin Liver Dis. 2015;24:451–456. [DOI] [PubMed] [Google Scholar]
- 12. Paul S, Smeraglia J, de Longueville M, et al. Comparison of two enzyme-linked immunosorbent assays used for drug concentration monitoring in psoriatic arthritis patients treated with certolizumab pegol. Paper presented at: American College of Rheumatology Annual Meeting; November 11-16, 2016; Washington, DC. [Google Scholar]
- 13. Clarke WT, Papamichael K, Vande Casteele N, et al. Infliximab and adalimumab concentrations may vary between the enzyme-linked immunosorbent assay and the homogeneous mobility shift assay in patients with inflammatory bowel disease: a prospective cross-sectional observational study. Inflamm Bowel Dis. 2019;25:e143–e145. [DOI] [PubMed] [Google Scholar]
- 14. Afonso J, Lopes S, Gonçalves R, et al. ; Portuguese IBD Study Group (GEDII) . Proactive therapeutic drug monitoring of infliximab: a comparative study of a new point-of-care quantitative test with two established ELISA assays. Aliment Pharmacol Ther. 2016;44:684–692. [DOI] [PubMed] [Google Scholar]
- 15. Van Stappen T, Bollen L, Vande Casteele N, et al. Rapid test for infliximab drug concentration allows immediate dose adaptation. Clin Transl Gastroenterol. 2016;7:e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feuerstein JD, Nguyen GC, Kupfer SS, et al. ; American Gastroenterological Association Institute Clinical Guidelines Committee . American Gastroenterological Association Institute Guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 17. Gomollón F, Dignass A, Annese V, et al. ; ECCO . 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 18. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 19. Mitrev N, Vande Casteele N, Seow CH, et al. ; IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group . Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46:1037–1053. [DOI] [PubMed] [Google Scholar]
- 20. Steinhart AH, Panaccione R, Targownik L, et al. Clinical practice guideline for the medical management of perianal fistulizing Crohn’s disease: the Toronto consensus. Inflamm Bowel Dis. 2019;25:1–13. [DOI] [PubMed] [Google Scholar]
- 21. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153:835–857.e6. [DOI] [PubMed] [Google Scholar]
- 22. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67:818–826. [DOI] [PubMed] [Google Scholar]
- 23. Melmed GY, Irving PM, Jones J, et al. Appropriateness of testing for anti-tumor necrosis factor agent and antibody concentrations, and interpretation of results. Clin Gastroenterol Hepatol. 2016;14:1302–1309. [DOI] [PubMed] [Google Scholar]
- 24. Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1655–1668.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papamichael K, Vande Casteele N, Ferrante M, et al. Therapeutic drug monitoring during induction of anti-tumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis. 2017;23:1510–1515. [DOI] [PubMed] [Google Scholar]
- 26. El-Matary W, Walters TD, Huynh HQ, et al. Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal Crohn’s disease in children. Inflamm Bowel Dis. 2019;25:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45:933–940. [DOI] [PubMed] [Google Scholar]
- 28. Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–553. [DOI] [PubMed] [Google Scholar]
- 29. Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254. [DOI] [PubMed] [Google Scholar]
- 30. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 31. Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40–4 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vande Casteele N, Gils A, Ballet V, et al. Randomized controlled trial of drug level versus clinically based dosing of infliximab maintenance therapy in IBD: final results of the TAXIT study [UEGW abstract UEG13-ABS-2468]. Presented at 21st United European Gastroenterology Week; October 12-16, 2013; Berlin, Germany. [Google Scholar]
- 34. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group . Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 35. Fasanmade AA, Adedokun OJ, Blank M, et al. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33:946–964. [DOI] [PubMed] [Google Scholar]
- 36. Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. [DOI] [PubMed] [Google Scholar]
- 37. Petitcollin A, Leuret O, Tron C, et al. Modeling immunization to infliximab in children with Crohn’s disease using population pharmacokinetics: a pilot study. Inflamm Bowel Dis. 2018;24:1745–1754. [DOI] [PubMed] [Google Scholar]
- 38. Vande Casteele N, Feagan BG, Gils A, et al. Therapeutic drug monitoring in inflammatory bowel disease: current state and future perspectives. Curr Gastroenterol Rep. 2014;16:378. [DOI] [PubMed] [Google Scholar]
- 39. Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohns Colitis. 2013;7:736–743. [DOI] [PubMed] [Google Scholar]
- 40. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reinisch W, Feagan BG, Rutgeerts PJ, et al. Infliximab concentration and clinical outcome in patients with ulcerative colitis. Gastroenterology. 2012;142:S114. [Google Scholar]
- 43. Vande Casteele N, Jeyarajah J, Jairath V, et al. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17:1814–1821.e1. [DOI] [PubMed] [Google Scholar]
- 44. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543–549. [DOI] [PubMed] [Google Scholar]
- 45. Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19:2568–2576. [DOI] [PubMed] [Google Scholar]
- 46. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–927. [DOI] [PubMed] [Google Scholar]
- 47. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualized therapy is a long-term cost-effective method compared to dose intensification in Crohn’s disease patients failing infliximab. Dig Dis Sci. 2015;60:2762–2770. [DOI] [PubMed] [Google Scholar]
- 48. Pouillon L, Ferrante M, Van Assche G, et al. Mucosal healing and long-term outcomes of patients with inflammatory bowel diseases receiving clinic-based vs trough concentration-based dosing of infliximab. Clin Gastroenterol Hepatol. 2018;16:1276–1283.e1. [DOI] [PubMed] [Google Scholar]
- 49. D’Haens G, Vermeire S, Lambrecht G, et al. ; GETAID . Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn’s disease. Gastroenterology. 2018;154:1343–1351.e1. [DOI] [PubMed] [Google Scholar]
- 50. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:514–521.e4. [DOI] [PubMed] [Google Scholar]
- 52. Colombel JF, Adedokun OJ, Gasink C, et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin Gastroenterol Hepatol. 2019;17:1525–1532.e1521. [DOI] [PubMed] [Google Scholar]
- 53. Vande Casteele N, Baert F, Bian S, et al. Subcutaneous absorption contributes to observed interindividual variability in adalimumab serum concentrations in Crohn’s disease: a prospective multicentre study. J Crohns Colitis. 2019;13:1248–1256. [DOI] [PubMed] [Google Scholar]
- 54. Bodini G, Giannini EG, Savarino V, et al. Adalimumab trough serum levels and anti-adalimumab antibodies in the long-term clinical outcome of patients with Crohn’s disease. Scand J Gastroenterol. 2016;51:1081–1086. [DOI] [PubMed] [Google Scholar]
- 55. Chiu YL, Rubin DT, Vermeire S, et al. Serum adalimumab concentration and clinical remission in patients with Crohn’s disease. Inflamm Bowel Dis. 2013;19:1112–1122. [DOI] [PubMed] [Google Scholar]
- 56. Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther. 2014;40:620–628. [DOI] [PubMed] [Google Scholar]
- 57. Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:80–84.e2. [DOI] [PubMed] [Google Scholar]
- 58. Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:409–415. [DOI] [PubMed] [Google Scholar]
- 59. Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut. 2016;65:1126–31. [DOI] [PubMed] [Google Scholar]
- 60. Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137:1628–1640. [DOI] [PubMed] [Google Scholar]
- 61. Paul S, Moreau AC, Del Tedesco E, et al. Pharmacokinetics of adalimumab in inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:1288–1295. [DOI] [PubMed] [Google Scholar]
- 62. Panés J, Colombel J-F, D’Haens GR, et al. High versus standard adalimumab induction dosing regimens in patients with moderately to severely active ulcerative colitis: results from the SERENE-UC induction study. United European Gastroenterol J. 2019;7:118. [Google Scholar]
- 63. Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn’s disease compared with reactive monitoring. Gastroenterology. 2019;157:985–996.e982. [DOI] [PubMed] [Google Scholar]
- 64. Ramos GP, Al-Bawardy B, Willrich MAV, et al. Certolizumab trough levels and antibodies in inflammatory bowel disease: a single-center experience. Gastroenterology. 2018;154:S826–S827. [Google Scholar]
- 65. Sandborn WJ, Feagan BG, Stoinov S, et al. ; PRECISE 1 Study Investigators . Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. [DOI] [PubMed] [Google Scholar]
- 66. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. ; PRECISE 2 Study Investigators . Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250. [DOI] [PubMed] [Google Scholar]
- 67. Vande Casteele N, Mould DR, Coarse J, et al. Accounting for pharmacokinetic variability of certolizumab pegol in patients with Crohn’s disease. Clin Pharmacokinet. 2017;56:1513–1523. [DOI] [PubMed] [Google Scholar]
- 68. Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:423–431.e421. [DOI] [PubMed] [Google Scholar]
- 69. Sandborn WJ, Wolf DC, Kosutic G, et al. Effects of transient and persistent anti-drug antibodies to certolizumab pegol: longitudinal data from a 7-year study in Crohn’s disease. Inflamm Bowel Dis. 2017;23:1047–1056. [DOI] [PubMed] [Google Scholar]
- 70. Vande Casteele N, Feagan BG, Vermeire S, et al. Exposure-response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn’s disease. Aliment Pharmacol Ther. 2018;47:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ward MG, Thwaites PA, Beswick L, et al. Intra-patient variability in adalimumab drug levels within and between cycles in Crohn’s disease. Aliment Pharmacol Ther. 2017;45:1135–1145. [DOI] [PubMed] [Google Scholar]