Abstract
Background
The incidence and prevalence of inflammatory bowel diseases (IBD) in older adults are rising. There is a limited comparative assessment of risk of disease- and treatment-related complications in older patients (older than 60 years) with adult-onset (age at disease onset, 18–59 years; AO-IBD) vs elderly-onset IBD (age at disease onset, older than 60 years; EO-IBD). We compared clinical outcomes in older patients with IBD with AO-IBD vs EO-IBD.
Methods
We conducted a retrospective cohort study comparing risk of disease-related complications (IBD-related surgery, hospitalization, treatment escalation, clinical flare, or disease complication) and treatment-related complications (serious infection, malignancy, or death) in older patients with AO-IBD vs EO-IBD through Cox proportional hazard analysis, adjusting for age at cohort entry, disease phenotype, disease duration, prior surgery and/or hospitalization, medication use, disease activity at cohort entry, and comorbidities.
Results
We included 356 older patients with IBD (AO-IBD, 191 patients, 67 ± 5 y at cohort entry; EO-IBD, 165 patients, 72 ± 8 y at cohort entry). No significant differences were observed in the risk of disease-related complications in older patients with prevalent vs incident IBD (adjusted hazard ratio [aHR], 0.85; 95% CI, 0.58–1.25), although risk of IBD-related surgery was lower in older patients with prevalent IBD (aHR, 0.47; 95% CI, 0.25–0.89). Older patients with prevalent IBD were significantly less likely to experience treatment-related complications (aHR, 0.58; 95% CI, 0.39–0.87).
Conclusion
Patients with AO-IBD have lower risk of treatment-related complications as they age compared with patients with EO-IBD, without a significant difference in risk of disease-related complications.
Keywords: Crohn’s disease, aging, infections, biologics, colitis
INTRODUCTION
The incidence and prevalence of inflammatory bowel diseases (IBD) in older adults is rising, and it is expected that by 2030, over one third of patients with IBD will be older than 60 years old.1, 2 Though approximately 10%–15% of new IBD diagnoses occur in individuals older than 60 years (elderly-onset IBD [EO-IBD]), the majority of older patients with IBD have prevalent IBD (ie, diagnosed in adulthood, referred to as adult-onset IBD [AO-IBD], diagnosed between 18–59 years old) and age with IBD.3, 4 However, there is very limited evidence-based treatment guidance for older patients with IBD.
Age is an important factor that influences disease course, treatment effectiveness, and safety in patients with IBD.5–8 Prior studies have suggested systematic differences in risk of disease and treatment outcomes between older patients (with incident or prevalent IBD) and younger patients with IBD. Population-based studies have suggested that risk of disease-related complications (risk of surgery, hospitalization, and disease progression) is similar between EO-IBD and AO-IBD, but treatment pattern is significantly different, with lower cumulative use of immunosuppressive and biologic agents in patients with EO-IBD vs AO-IBD.9–11 Retrospective cohort studies have suggested that older patients are more susceptible to treatment side effects, with higher rates of thiopurine and/or tumor necrosis factor-α antagonist (TNFα) discontinuation due to intolerance as compared with younger patients.12–14 However, studies have not examined whether the risk of disease- and treatment-related complications is different in aging adults with AO-IBD vs patients with EO-IBD. It is conceivable disease- and treatment-experienced older patients with prevalent AO-IBD may tolerate therapy better compared with adults with EO-IBD.
To better risk-stratify and inform treatment approach in older patients with IBD, we conducted a retrospective cohort study comparing risk of disease- and treatment-related complications in older patients with IBD (age older than 60 years at cohort entry) with prevalent AO-IBD vs EO-IBD. We hypothesized that aging patients with AO-IBD would have lower risk of treatment-related complications as compared with patients with EO-IBD, with a comparable risk of disease-related complications.
METHODS
Study Design
We conducted a single-center, retrospective cohort study in patients with IBD seen and followed at University of California San Diego (UCSD). The study was approved by the UCSD institutional review board (IRB #190418).
Patients
We included patients IBD 60 years and older, with either prevalent AO-IBD or EO-IBD, who were treated and followed at UCSD for at least 6 months between Januar 1, 2011, and June 30, 2019. Patients younger than 60 years throughout their follow-up were excluded. Cohort entry was defined as first encounter for the patient in the IBD clinic at UCSD.
Exposure and Comparator
The primary predictor variable was age at onset of IBD, categorically classified as prevalent AO-IBD (age at IBD diagnosis, 18–59 years old; exposure) or EO-IBD (age at IBD diagnosis, 60 years or older; comparator). We also included 11 patients with pediatric-onset IBD (age at IBD diagnosis, younger than 18 years) and grouped them with AO-IBD.
Outcomes
Primary safety outcome focused on risk of treatment-related complications, defined as a composite of time to serious infection, malignancy, or death after cohort entry. Primary effectiveness outcome focused on risk of disease-related complications, defined as a composite of time to IBD-related surgery, all-cause hospitalization, disease complication (new stricture, fistula, perianal disease, or extraintestinal manifestations), clinical flare (based on physician global assessment), and escalation of therapy. Individual components of primary safety and effectiveness outcomes were also evaluated. In addition, we evaluated factors associated with composite risk of treatment- and disease-related complications separately in patients with AO-IBD and EO-IBD.
Data Source and Abstraction
A single reviewer (JJR) abstracted data through medical record review using a piloted data abstraction form, with constant feedback from a second gastroenterologist reviewer (SS). Besides exposures and outcomes detailed previously, the following additional data were abstracted: (1) patient characteristics—age at cohort entry, sex, race/ethnicity, smoking status (current or not current), body mass index (BMI), and other comorbidities; (2) disease characteristics at time of cohort entry—type of IBD, age at disease onset, disease duration, disease extent and behavior, and clinical and endoscopic status at cohort entry; (3) treatment characteristics—current and prior IBD-related medications; and (4) outcomes characteristics—history and dates of gastrointestinal surgery, hospitalization, infection requiring hospitalization in the past 5 years, and malignancy other than nonmelanoma skin cancers.
Statistical Analysis
We performed a time-to-event analysis using Kaplan-Meier curves to evaluate the association between aging patients with AO-IBD and EO-IBD and the risk of disease- and treatment-related complications. Patients were followed from time of cohort entry until occurrence of disease- and treatment-related complications, loss to follow-up, or study completion (June 30, 2019). To evaluate the independent association between AO-IBD and EO-IBD in older patients on different outcomes, we conducted a multivariable Cox proportional hazard analysis with backward variable selection at a P value threshold of <0.20 in univariate analysis among age at cohort entry, sex, race/ethnicity (white vs non-white), smoking status (current vs prior/never), BMI (continuous variable), type of IBD (CD or UC), disease activity at cohort entry, disease duration, disease extent, prior history of hospitalization, prior history of IBD-related surgery, major comorbidities, IBD-related medications at cohort entry (none/5-aminosalicylates vs immunosuppressive agents including thiopurines, methotrexate or biologic agents), and corticosteroid use at cohort entry.
Additionally, to evaluate factors associated with composite risk of treatment- and disease-related complications separately in patients with AO-IBD and EO-IBD and separately in patients with CD and UC, we performed Cox proportional hazard analysis with previously mentioned covariates. All hypothesis testing was performed using a 2-sided P value with a statistically significant threshold of P < 0.05. Analyses were performed using R (www.R-project.org, Vienna, Austria).
RESULTS
Patient Characteristics
Our cohort included 356 older patients with IBD, of whom 191 (54%) had AO-IBD at cohort entry (age at cohort entry, 67 ± 5 years; 45% female; 57% with CD; disease duration, 29.7 ± 15.0 years), and 165 (43%) had EO-IBD (age at cohort entry, 72 ± 8 years; 50% female; 48% with CD; disease duration, 3.1 ± 4.6 years). Patients were followed over median 3.6 years after cohort entry, and this follow-up time was comparable in patients with AO-IBD (median, 3.8 years; interquartile range [IQR], 4.8) and EO-IBD (median, 3.5 years; IQR,3.9). Table 1 shows detailed baseline characteristics.
TABLE 1.
Patient Baseline Characteristics, Grouped by Adult-onset and Elderly-onset Disease
| Patient Characteristics at Cohort Entry | Adult-Onset IBD (N = 191) | Elderly-Onset IBD (N = 165) | P |
|---|---|---|---|
| Age at cohort entry, mean years (SD) | 67.2 (5.3) | 71.7 (7.9) | <0.01 |
| Age at diagnosis, mean years (SD) | 37.5 (14) | 68.6 (7.4) | <0.01 |
| Sex, male n (%) | 105 (55) | 83 (50.3) | 0.38 |
| Smoking, n (%) | <0.01 | ||
| Current | 12 (6.3) | 2 (1.2) | |
| Former | 77 (40.3) | 84 (51.2) | |
| Never | 102 (53.4) | 78 (47.6) | |
| Race/ethnicity, n (%) | 0.09 | ||
| African American | 4 (2.1) | 6 (3.8) | |
| Asian | 5 (2.7) | 11 (6.9) | |
| White | 166 (88.8) | 132 (82.5) | |
| Hispanic | 3 (1.6) | 7 (4.4) | |
| Other | 9 (4.8) | 4 (2.5) | |
| BMI, mean (SD) | 25.7 (5.2) | 25.9 (5.2) | 0.70 |
| Proportion obese, n (%) | 37 (19.4) | 27 (16.4) | 0.46 |
| Comorbidities, n (%) | |||
| Diabetes | 30 (15.7) | 22 (13.3) | 0.53 |
| Coronary artery disease | 19 (9.9) | 28 (17) | 0.051 |
| Congestive heart failure | 11 (5.8) | 7 (4.2) | 0.51 |
| Stroke/TIA or vascular disease | 12 (6.3) | 16 (9.7) | 0.23 |
| COPD | 5 (2.6) | 10 (6.1) | 0.11 |
| Hypertension | 93 (48.7) | 89 (53.9) | 0.32 |
| Hyperlipidemia | 65 (34) | 71 (43) | 0.08 |
| Chronic kidney disease | 25 (13.1) | 12 (7.3) | 0.07 |
| Type of IBD, n (%; n = 66) | |||
| CD | 108 (56.5) | 75 (45.5) | 0.04 |
| UC | 83 (43.5) | 90 (54.5) | |
| Disease extent: UC, n (%) | 66 | 82 | 0.94 |
| E1 | 5 (7.6) | 7 (8.5) | |
| E2 | 19 (28.8) | 25 (30.5) | |
| E3 | 42 (63.6) | 50 (61) | |
| Disease extent: CD, n (%) | 103 | 75 | <0.01 |
| L1 | 18 (17.5) | 24 (32) | |
| L2 | 16 (15.5) | 22 (29.3) | |
| L3 | 69 (67) | 29 (38.7) | |
| CD behavior: stricturing, n (%) | 65 (60.7) | 25 (33.3) | <0.01 |
| CD behavior: penetrating, n (%) | 39 (36.4) | 16 (21.3) | 0.03 |
| CD behavior: perianal, n (%) | 26 (13.8) | 11 (6.7) | 0.03 |
| Current IBD medications, n (%) | |||
| 5-ASA | 72 (37.7) | 81 (49.1) | 0.03 |
| Corticosteroids | 41 (21.5) | 52 (31.5) | 0.03 |
| Immunomodulators | 37 (19.4) | 18 (10.9) | 0.03 |
| Biologics | 39 (20.4) | 22 (13.3) | 0.07 |
| Prior IBD medications, n (%) | |||
| 5-ASA | 154 (81.1) | 115 (69.7) | 0.01 |
| Corticosteroids | 140 (73.7) | 93 (56.4) | <0.01 |
| Immunomodulators | 94 (49.5) | 40 (24.2) | <0.01 |
| Biologics | 73 (38.2) | 35 (21.2) | <0.01 |
| Prior serious infection, n (%) | 20 (10.5) | 25 (15.2) | 0.19 |
| Prior malignancy, n (%) | 45 (23.6) | 41 (24.8) | 0.78 |
| Type of malignancy, n (%) | 0.80 | ||
| Hematologic | 5 (11.40 | 6 (14.6) | |
| Melanoma | 6 (13.6) | 4 (9.8) | |
| Solid | 33 (75) | 31 (75.6) | |
| Current remission, n (%) | 142 (74.4) | 133 (80.5) | 0.51 |
| Prior GI surgery, n (%) | 99 (51.8) | 29 (17.6) | <0.01 |
| Prior hospitalization, n (%) | 149 (78) | 118 (71.5) | 0.16 |
Abbreviations: TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; 5-ASA, 5-aminosalicylates; GI, gastrointestinal
The burden of major comorbidities was similar between aging patients with AO-IBD vs EO-IBD, including diabetes (15.7% vs 13.3%, P = 0.53), coronary artery disease (9.9% vs 17.0%, P = 0.051), stroke (6.3% vs 9.7%, P = 0.23), chronic obstructive pulmonary disease (2.6% vs 6.1%, P = 0.11), or chronic kidney diseases (13.1% vs 7.3%, P = 0.07). With regard to IBD characteristics, patients with AO-IBD were more likely to have undergone IBD-related surgery (51.8% vs 17.6%, P < 0.01) and been exposed to thiopurines or methotrexate (49.5% vs 24.2%, P < 0.01) or biologic agents (38.2% vs 21.2%, P < 0.01) compared with patients with EO-IBD. However, no differences were observed in rates of hospitalization (78.0% vs 71.5%, P = 0.16), serious infection (10.5% vs 15.2%, P = 0.19), and malignancy (23.6% vs 24.8%, P = 0.78) within the preceding 5 years before cohort entry. Among patients with CD, patients with AO-IBD were more likely to have stricturing (60.7% vs 33.3%, P < 0.01) and/or penetrating behavior (36.4% vs 21.3%, P = 0.03) and perianal disease (13.8% vs 6.7%, P = 0.03); no differences were observed in disease extent in patients with UC. At the time of cohort entry, patients with AO-IBD were less likely to be in clinical flare (46.1% vs 66.7%, P < 0.01) or on corticosteroids (21.5% vs 31.5%, P = 0.03) compared with patients with EO-IBD.
Risk of Treatment-related Complications
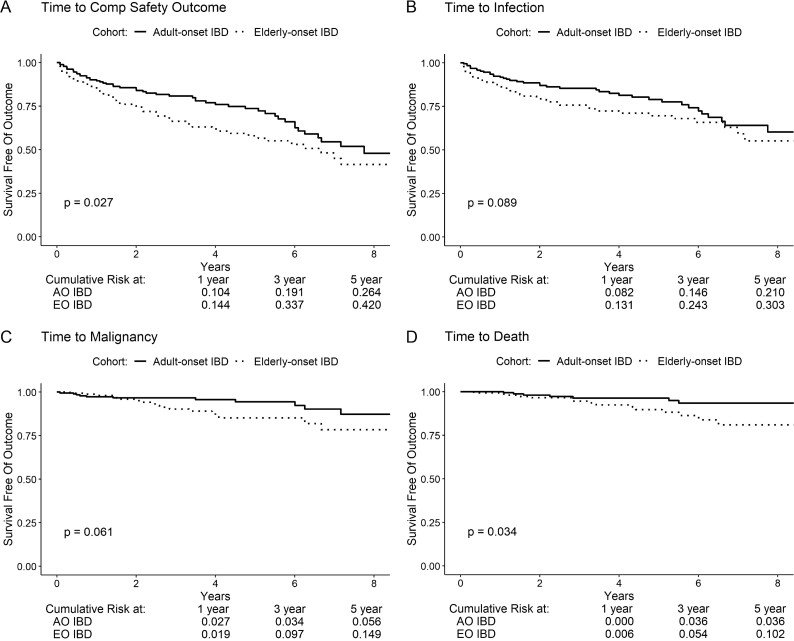
Overall, 113 composite safety events were noted (AO-IBD vs EO-IBD, 51 vs 61), including 85 serious infections (39 vs 45), 29 malignancy events (11 vs 18), and 22 deaths (7 vs 15). On survival analysis, cumulative risk of treatment-related complications (composite of serious infection, malignancy, or death) was significantly lower in older adults with AO-IBD vs EO-IBD (5-year risk, 26.4% vs 42.0%, P = 0.03; Fig. 1A–D). Individually, older adults with AO-IBD had a numerically lower risk of serious infections (5-year risk, 21.0% vs 30.3%, P = 0.09), malignancy (5-year risk, 5.6% vs 14.9%, P = 0.06), and a significantly lower risk of death (5-year risk, 3.6% vs 10.2%, P = 0.03) after cohort entry.
FIGURE 1.
Kaplan-Meier analysis for event-free survival of (A) composite safety, (B) infection, (C) malignancy, and (D) death in adult-onset vs elderly-onset inflammatory bowel diseases. Composite safety is the first of any infection, malignancy, or death.
Overall, 137 composite effectiveness events were observed (AO-IBD vs EO-IBD, 87 vs 49), including 45 patients who underwent abdominal surgery (20 vs 25), 193 hospitalization events (93 vs 99), and 89 clinical flare events (55 vs 34). On multivariable Cox proportional hazard analysis, older adults with prevalent AO-IBD had a 42% lower risk of experiencing treatment-related complications (adjusted hazard ratio [HR], 0.58; 95% confidence interval [95% CI], 0.39–0.87) compared with patients with EO-IBD, after adjusting for covariates (Table 2, Supplementary Table 1); none of the other factors including prior hospitalization, prior IBD-related surgery, immunosuppressive and/or corticosteroid use at cohort entry, and IBD type were associated with risk of treatment-related complications.
TABLE 2.
Multivariate Cox Proportional Hazards Analysis With Backward Covariate Selection for Clinical Factors Associated with Composite Safetya
| Covariatesb | Hazard Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Adult-onset IBD c | 0.580 | 0.386–0.871 | <0.01 |
| Prior GI Surgery | 1.440 | 0.913–2.270 | 0.12 |
| Prior Hospitalization | 1.455 | 0.881–2.402 | 0.14 |
| Medication at Cohort Entryd | 1.352 | 0.895–2.042 | 0.15 |
| Corticosteroids at Cohort Entry | 1.359 | 0.889–2.079 | 0.16 |
| Type of IBDe | 1.294 | 0.876–1.911 | 0.20 |
aComposite safety defined as the first occurrence of infection, malignancy, or death following cohort entry.
bCovariates were selected via backward regression with P cutoff of 0.2.
cAdult-onset IBD defined as age of IBD diagnosis younger than 60 years, compared with elderly-onset IBD (60 years and older at diagnosis) as baseline.
dMedication at cohort entry defined as a binary categorical variable: either taking no medications/5-aminosalicylates at entry (baseline) or taking immunomodulators/biologics at entry.
eType of IBD defined as a binary categorical variable: either ulcerative colitis or Crohn’s disease, with Crohn’s disease as baseline
Abbreviations: IBD, inflammatory bowel diseases; GI, gastrointestinal
Risk of Disease-related Complications
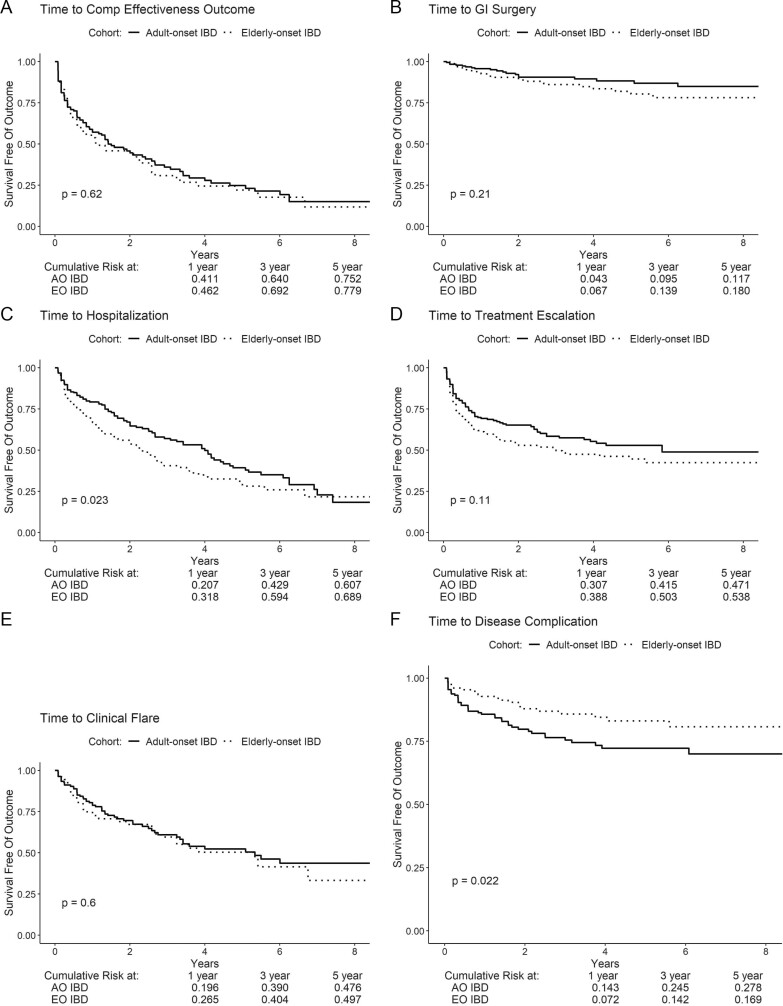
No significant differences were observed in the cumulative risk of disease-related complications between older patients with AO-IBD vs EO-IBD (5-year risk, 79.6% vs 78.1%, P = 0.96; Fig. 2A–F). Individually, no significant differences were observed in the risk of IBD-related surgery and clinical flare, although patients with AO-IBD had a shorter time to disease complication (new stricture, fistula, perianal disease among patients with CD, or extraintestinal manifestations) but a longer time to hospitalization compared with patients with EO-IBD.
FIGURE 2.
Kaplan-Meier analysis for event-free survival of (A) composite effectiveness, (B) IBD-related surgery, (C) all-cause hospitalization, (D) treatment escalation, (E) clinical flare, and (F) disease complications. Composite effectiveness is the first of any IBD-related surgery, hospitalization from any cause, treatment escalation, or disease complication. Treatment escalation included any escalation in therapy to immunomodulating, biologic, combination thereof, or other immunosuppressing medications.
On multivariable Cox proportional hazard analysis, no significant differences were observed in the risk of disease-related complications between older adults with prevalent AO-IBD vs EO-IBD, after adjusting for important covariates (adjusted HR, 0.85; 95% CI, 0.58–1.25; Table 3, Supplementary Table 1). Active disease and being on corticosteroids at cohort entry were associated with risk of subsequent disease-related complications. Individually, older patients with AO-IBD were significantly less likely to undergo IBD-related surgery (adjusted HR, 0.470; 95% CI, 0.25–0.89) compared with patients with EO-IBD.
TABLE 3.
Multivariate Cox Proportional Hazards Analysis With Backward Covariate Selection for Clinical Factors Associated with Composite Effectivenessa
| Covariatesb | Hazard Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Adult-onset IBDc | 0.846 | 0.575–1.245 | 0.40 |
| >1 Comorbidity at Entry | 1.320 | 0.918–1.898 | 0.13 |
| Prior Hospitalization | 1.426 | 0.895–2.270 | 0.14 |
| Clinical Flare at Cohort Entry | 1.520 | 1.037–2.228 | 0.03 |
| Medication at Cohort Entryd | 1.378 | 0.948 0 2.004 | 0.09 |
| Corticosteroids at Cohort Entry | 1.621 | 1.048–2.509 | 0.03 |
| Prior GI Surgery | 1.396 | 0.910–2.141 | 0.13 |
aComposite effectiveness defined as the first occurrence of IBD-related surgery, hospitalization, treatment escalation, clinical flare, or disease complication following cohort entry. Treatment escalation defined as any change to higher class of medication; increases in dosage/frequency; or changes within a class of medications. Disease complication defined as any new stricture, fistula, or perianal behavior.
bCovariates were selected via backward regression with p-cutoff of 0.2.
cAdult-onset IBD defined as age of IBD diagnosis younger than 60 years, compared with elderly-onset IBD (60 years and older at diagnosis) as baseline.
dMedication at cohort entry defined as a binary categorical variable: either taking no medications/5-aminosalicylates at entry (baseline) or taking immunomodulators/biologics at entry.
Abbreviations: IBD, inflammatory bowel diseases; GI, gastrointestinal
Risk Factors for Complications in Older Patients With Adult-onset IBD
Corticosteroid use at cohort entry was independently associated with increased risk of treatment-related complications (adjusted HR, 3.17; 95% CI, 1.23–8.16) in aging patients with AO-UC, whereas prior IBD-related surgery was associated with increased risk of treatment-related complications in older patients with AO-CD (adjusted HR, 2.78; 95% CI, 1.04–7.43; Supplementary Table 2). In contrast, in aging patients with AO-UC, older age at diagnosis (adjusted HR, 3.17; 95% CI, 1.23–8.16), longer disease duration (adjusted HR, 1.11; 95% CI, 1.03–1.20), and prior IBD-related surgery (adjusted HR, 4.06; 95% CI, 1.74–9.50) were associated with increased risk of disease-related complications, whereas corticosteroid use at cohort entry (adjusted HR, 2.53; 95% CI, 1.23–5.21) and stricturing disease (adjusted HR, 1.91; 95% CI, 1.07–3.41) were associated with increased risk of disease-related complications in older patients with AO-CD.
Risk Factors for Complications in Patients With Elderly-onset IBD
In contrast to older patients with AO-UC, presence of multiple major comorbidities was independently associated with increased risk of treatment-related complications (adjusted HR, 2.26; 95% CI, 1.11–4.59) in patients with EO-UC (Supplementary Table 2). Shorter disease duration (adjusted HR, 0.82; 95% CI, 0.69–0.97) and presence of multiple comorbidities (adjusted HR, 5.65; 95% CI, 1.98–16.13) were associated with increased risk of disease-related complications in patients with EO-UC. No specific factors were independently associated with increased risk of treatment- and disease-related complications in patients with EO-CD.
DISCUSSION
The management of older patients with IBD poses unique challenges related to balancing risk of disease- and treatment-related complications in the context of overall health. As the prevalence of IBD in older adults rises, it is critical to identify patients who may warrant and/or tolerate aggressive therapy. In this single-center retrospective cohort study of 356 older patients with IBD, we made several key observations that may inform treatment. First, we observed that older adults with established AO-IBD have lower risk of treatment-related complications (composite of serious infections, malignancy, and death) compared with patients with EO-IBD, even after adjustment for age, comorbidities, and other aspects of disease and pharmacotherapy. Second, risk of disease-related complications (composite of surgery, hospitalization, disease complications, clinical flare warranting treatment escalation) was comparable in aging adults with established AO-IBD and EO-IBD. Third, in disease- and treatment-experienced older patients with AO-IBD, conventional factors like corticosteroid exposure and prior surgery were associated with increased risk of treatment-related complications. Whereas in patients with EO-IBD, presence of multiple major comorbidities was associated with increased risk of treatment-related complications, which may be related to greater vulnerability to treatment insults in these patients. Overall, these findings suggest that among older patients with IBD, aging adults with established AO-IBD who are susceptible to disease complications may tolerate aggressive immunosuppressive therapy well and may be treated similar to younger patients; in contrast, patients with EO-IBD may warrant a more careful assessment of balance between disease and treatment complications.
Older patients with IBD represent a vulnerable population with higher rates of hospitalization, inpatient mortality, serious infections, and longer length of stay and costs of hospitalization.6, 10, 15 Several prior studies have confirmed higher risk of serious infections, malignancy, and intolerance to biologic agents and immunosuppressive therapy in older adults with IBD compared with younger patients.7, 12–15 However, there has been limited evaluation of which older adults may be more susceptible to treatment-related complications. Chronological age is an inadequate metric to ascertain risk-benefit trade-offs of different therapies. Beyond chronological age, biologic reserve and functional status may be more predictive of overall risks of adverse health outcomes, particularly hospitalized infections.16 We hypothesize that aging adults with prevalent AO-IBD who are more disease- and treatment-experienced may have adapted and built up adequate biologic and functional reserve to cope with IBD compared with patients with EO-IBD where disease onset at an advanced age may contribute to frailty and increased susceptibility to treatment-related complications. Risk of serious infections with biologic therapy is highest in the first year after therapy initiation, and once patients achieve durable remission, risk of infections decreases.17
We observed that the risk of disease-related complications was comparable between aging adults with established IBD and patients with EO-IBD, with a cumulative 5-year risk of hospitalization of >60% and risk of IBD-related surgery of 12%–18% after cohort entry. Prior studies have confirmed that despite a suggestion of a relatively milder phenotype in EO-IBD, rates of surgery, hospitalization, and progression to disease-related complications are similar to AO-IBD.9–11 Though we did not analyze cumulative incidence of biologic and/or immunosuppressive agent use in our cohort, prior studies have suggested that despite comparable risk of corticosteroid exposure and disease-related complications, patients with EO-IBD have significantly lower rates of exposure to biologic agents; whether this pattern holds true even among aging adults with AO-IBD is unclear.9, 10
While ours is one of the first studies comparing disease- and treatment-related complications in older patients with prevalent AO-IBD vs patients with EO-IBD, there are several limitations to consider. First, we did not examine the utilization, effectiveness, and safety of specific therapies in older patients with IBD to inform comparative safety of these agents. Rather, we sought to examine overall clinical course and outcomes in older patients based on age of disease onset to help inform treatment approach. We have previously demonstrated that strategy of algorithmic early combined immunosuppression strategy was equally effective in older vs younger patients and was more effective than conventional management in maintaining corticosteroid-free clinical remission and delaying risk of CD-related surgery, hospitalization, and serious disease-related complications.18 Though patients were not classified by age at disease onset in the trial, our findings suggest that older patients with prevalent IBD at high risk of disease-related complications may be treated with algorithmic biologic-based step therapy. Second, we are unable to ascertain time-varying exposures after cohort entry and how they may have informed risk of complications. With generally higher preponderance of chronic corticosteroid use in patients with EO-IBD vs AO-IBD, it is possible that patients with EO-IBD may have excess corticosteroid exposure and, hence, be more susceptible to infections.8 Third, it is hard to directly attribute outcomes as being disease-related vs treatment-related complications inasmuch as some outcomes may not be directly and consistently attributable to disease or treatment; we relied on pragmatic patient-centered classification of outcomes. Fourth, though our study was well-powered to examine composite outcomes, we were not adequately powered to evaluate specific individual outcomes.
In conclusion, among older patients with IBD, those with established AO-IBD may have lower risk of treatment-related complications compared with patients with EO-IBD. Future research is needed to more accurately identify older patients with IBD who may be at higher risk of disease- or treatment-related complications. A pragmatic, informed approach that balances individuals’ risk of disease- and treatment-related complications is warranted in older patients with IBD.
Supplementary Material
Author Contribution: SS conceived and designed the study. JJR, PSD, LM, AC, BSB, WJS, and SS acquired the data. JJR, JL, and SS analyzed and interpreted the data. JJR and SS drafted the article. JL, PSD, LM, AC, BSB, and WJS critically revised the manuscript for important intellectual content. JJR, JL, PSD, LM, AC, BSB, WJS, and SS approved the final version of the article. Supported by: WS is partially supported by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515). BSB is supported by National Institute of Diabetes and Digestive and Kidney Diseases K23DK123406. SS is supported by National Institute of Diabetes and Digestive and Kidney Diseases K23DK117058, American College of Gastroenterology Junior Faculty Development Award #144271, Crohn’s and Colitis Foundation Career Development Award #404614. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest: JR, JL, and LP report no conflicts. PD reports research support from Takeda, Pfizer, Abbvie, Janssen, Polymedco, ALPCO, Buhlmann, and Prometheus and consulting fees from Takeda, Pfizer, Abbvie, and Janssen. AC reports consulting and speaking fees from Abbvie, Janssen, and Takeda. BB reports research grants from Prometheus Biosciences and personal fees from Pfizer. WS has received research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos, Pfizer, and Prometheus Laboratories (now Prometheus Biosciences); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, and Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse is a consultant and has stock options for Opthotech and Progenity and is an employee and has stock options for Oppilan Pharma, Escalier Biosciences, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Ventyx Biosciences, and Vimalan Biosciences. SS has received research grants from AbbVie and Janssen and personal fees from Pfizer.
REFERENCES
- 1. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156:1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 3. Ananthakrishnan AN, Donaldson T, Lasch K, et al. Management of inflammatory bowel disease in the elderly patient: challenges and opportunities. Inflamm Bowel Dis. 2017;23:882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–477. [DOI] [PubMed] [Google Scholar]
- 5. Beaugerie L, Kirchgesner J. Balancing benefit vs risk of immunosuppressive therapy for individual patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:370–379. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen NH, Ohno-Machado L, Sandborn WJ, et al. Infections and cardiovascular complications are common causes for hospitalization in older patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2018;24:916–923. [DOI] [PubMed] [Google Scholar]
- 7. Borren NZ, Ananthakrishnan AN. Safety of biologic therapy in older patients with immune-mediated diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1736–1743.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brassard P, Bitton A, Suissa A, et al. Oral corticosteroids and the risk of serious infections in patients with elderly-onset inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1795–1802; quiz 1803. [DOI] [PubMed] [Google Scholar]
- 9. Everhov ÅH, Halfvarson J, Myrelid P, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology. 2018;154:518–528.e15. [DOI] [PubMed] [Google Scholar]
- 10. Rozich JJ, Dulai PS, Fumery M, et al. Progression of elderly onset inflammatory bowel diseases: a systematic review and meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2020;18:2437–2447.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–432. [DOI] [PubMed] [Google Scholar]
- 12. Calafat M, Mañosa M, Cañete F, et al. ; ENEIDA registry of GETECCU . Increased risk of thiopurine-related adverse events in elderly patients with IBD. Aliment Pharmacol Ther. 2019;50:780–788. [DOI] [PubMed] [Google Scholar]
- 13. de Jong ME, Smits LJT, van Ruijven B, et al. Increased discontinuation rates of anti-TNF therapy in elderly inflammatory bowel disease patients. J Crohns Colitis. 2020;14:888–895. [DOI] [PubMed] [Google Scholar]
- 14. Porcari S, Viola A, Orlando A, et al. ; Sicilian Network for Inflammatory Bowel Diseases (SN-IBD) . Persistence on anti-tumour necrosis factor therapy in older patients with inflammatory bowel disease compared with younger patients: data from the Sicilian Network for Inflammatory Bowel Diseases (SN-IBD). Drugs Aging. 2020;37:383–392. [DOI] [PubMed] [Google Scholar]
- 15. Piovani D, Danese S, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: biologics and risk of infection or cancer in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:820–830. [DOI] [PubMed] [Google Scholar]
- 16. Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. [DOI] [PubMed] [Google Scholar]
- 17. Nyboe Andersen N, Pasternak B, Friis-Møller N, et al. Association between tumour necrosis factor-α inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ. 2015;350:h2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh S, Stitt LW, Zou G, et al. Early combined immunosuppression may be effective and safe in older patients with Crohn’s disease: post hoc analysis of REACT. Aliment Pharmacol Ther. 2019;49:1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.