Abstract
Background
Many patients with Crohn’s disease (CD) who lose response to the standard ustekinumab dose interval of every 8 weeks (q8w) undergo dose intensification to q4w or q6w. However, baseline factors that predict success or failure after dose intensification are unknown. We sought to identify predictors of failure of ustekinumab after dose intensification for patients with CD.
Methods
This was a retrospective cohort study of adult CD patients undergoing ustekinumab dose intensification at a tertiary referral center between January 1, 2016, and January 31, 2019. Electronic health records were reviewed to obtain patient demographics, CD history, and laboratory data. The primary outcome was failure to achieve corticosteroid-free remission (Harvey-Bradshaw Index <5) within 12 months after intensification. The secondary outcome assessed was time to new biologic therapy after dose intensification. We used multivariable logistic regression and Cox regression to identify predictors of these outcomes.
Results
We included 123 patients who underwent ustekinumab dose intensification to q4w (n = 64), q5w (n = 1), q6w (n = 55), or q7w (n = 3). Multivariable logistic regression demonstrated that perianal disease, Harvey-Bradshaw Index, and opioid use at time of intensification were associated with failure to achieve remission. Cox regression demonstrated that perianal disease and corticosteroid use at time of intensification were associated with shorter time to a new biologic.
Conclusion
Perianal disease, Harvey-Bradshaw Index, current opioid use, and current corticosteroid use are associated with ustekinumab failure after dose intensification in CD. Larger, prospective studies are needed to corroborate these findings and guide therapeutic strategies for patients who lose response to standard ustekinumab dosing.
Keywords: ustekinumab, Crohn’s disease, dose escalation, optimization, IBD
INTRODUCTION
There is a rising need for individualized therapeutic approaches in the management of Crohn’s disease (CD). Crohn’s disease phenotypes vary widely from mild mucosal inflammation to fistulizing perianal disease. Consequently, sustained response to biologic therapy is variable. For patients starting antitumor necrosis factor (TNF) therapy, up to 40% will require dose intensification or change to an alternative biologic class.1, 2
Ustekinumab (UST) is a monoclonal antibody targeting interleukin (IL)-12 and IL-23 that was approved for the treatment of CD in 2016. The standard treatment regimen of UST for CD begins with an intravenous (IV) weight-based induction dose followed by subcutaneous (SQ) maintenance injections of 90 mg every 8 weeks (q8w). Though this regimen has been shown to be effective, many patients lose or have an inadequate clinical response to standard dosing.3, 4 For patients who lose response to anti-TNF therapy, therapeutic drug monitoring effectively guides dose intensification and has been shown to be associated with improved outcomes.5, 6 However, data regarding UST drug levels and clinical outcomes are limited.7, 8 Therefore, the best management approach for CD patients with suboptimal response to standard UST dosing is unclear, and many undergo empiric dose intensification.
Recent data suggest that UST dose intensification to 90 mg every 4 weeks (q4w) or every 6 weeks (q6w) is safe and effective for many patients.9, 10 However, the choice of dosing interval is often provider-dependent, and data directly comparing intensified dose schedules are lacking. Furthermore, although up to half of patients seem to respond to UST dose intensification, identification of factors associated with treatment failure after dose intensification is needed to tailor management strategies for high-risk patients.9, 10
In this study, we sought to compare baseline characteristics and outcomes among patients undergoing UST dose intensification to q4w or q6w from standard q8w dosing. We then attempted to identify factors associated with failure of UST therapy after dose intensification.
MATERIALS AND METHODS
Study Design and Patient Enrollment
This was a retrospective cohort study of patients 18 years of age and older with a diagnosis of CD (International Classification of Diseases, 10th Revision Clinical Modification code K50x) treated with UST at Massachusetts General Hospital or Brigham and Women’s Hospital in Boston, Massachusetts. Patients undergoing UST dose intensification from q8w to a shorter interval at the discretion of their provider between January 1, 2016, and January 31, 2019, were included. Electronic health records (EHRs) were manually reviewed to obtain patient demographics, CD history, medication history, and endoscopic, imaging, and laboratory data available through April 25, 2020. Crohn’s disease activity was measured using the Harvey-Bradshaw Index (HBI) obtained from clinic visits within 3 months before UST dose intensification and at visits within 12 months after intensification.
Independent Variables
Independent variables included age at time of dose intensification, sex, race, CD duration at time of intensification, time from UST initiation to dose intensification, substance use at time of intensification (eg, cigarette smoking, cannabis, and opioids), CD location (Montreal classification: L1 terminal ileum, L2 colon, L3 ileocolon, L4 upper GI), CD behavior (Montreal classification: B1 nonstricturing and nonpenetrating, B2 stricturing, and B3 penetrating), perianal disease including abscess, sinus tract, or fistula (determined by cross-sectional imaging, endoscopy, or physical examination), extraintestinal manifestations, UST dose intensification interval, prior IBD surgery, 2 or more prior anti-TNF therapies, prior anti-integrin therapy, corticosteroid use at time of intensification, Harvey-Bradshaw Index (HBI) within 3 months before intensification, elevated fecal calprotectin (>120 mcg/g) within 3 months before intensification, and mean albumin to C-reactive ratio (CRP) within 3 months before intensification. The albumin to CRP ratio was chosen because it has been shown to be a predictor of remission among Crohn’s disease patients receiving UST.11 Albumin values were converted from g/dL to mg/L by a multiplication factor of 100 before dividing by CRP values (mg/L).
Outcomes
The primary outcome was failure to achieve corticosteroid-free remission (HBI <5) within 12 months after dose intensification. Patients with HBI <5 and no use of corticosteroids at the time of intensification were excluded from the analysis of corticosteroid-free remission. The secondary outcome was discontinuation of UST with initiation of new biologic therapy. This was assessed as both a binary outcome limited to 12 months of follow-up after intensification and as a time-to-event analysis that was not limited to 12 months of follow-up. Additional outcomes assessed within 12 months after intensification included clinical response (ie, reduction of pre-intensification HBI by ≥3 points), improvement in endoscopic findings, improvement in computed tomography (CT) or magnetic resonance imaging (MRI) of abdomen/pelvis, patient-reported improvement in perianal disease, initiation of corticosteroids, IBD-related surgery, IBD-related hospitalization, reduction in CRP by ≥25%, normalization of elevated CRP (using upper limit of normal of assay), and normalization of fecal calprotectin (using upper limit of normal of assay). Patients who did not meet an outcome with less than 12 months of follow-up data available after intensification were excluded from the assessment of that outcome.
Statistical Analysis
Descriptive statistics were presented as means for continuous data and percentages for categorical data. Continuous and categorical data were compared using Student t test and Pearson χ 2 test, respectively. Multivariable logistic regression was used to identify predictors of failure to achieve corticosteroid-free remission within 12 months after UST dose intensification. To assess factors associated with time to new biologic therapy, a multivariable Cox proportional hazard model was generated, and a Kaplan-Meier curve was constructed. Patients were censored at loss of follow-up, time of UST discontinuation without initiation of new biologic therapy (ie, when patients self-discontinued therapy due to improvement of symptoms), or time of further ustekinumab dose intensification from every 6 weeks to every 4 weeks. Once the final Cox model was constructed, the proportional hazards assumption was tested using Martingale residuals. To avoid overfitting of the multivariable logistic and Cox regression models, candidate covariates were selected among variables using P < 0.20 on univariate comparisons by outcome. The final models were generated using forward selection among these covariates with a requirement of P < 0.05 to remain in the model. Stata/IC 15.1 (StataCorp, College Station, TX, USA) was used for all statistical analyses.
ETHICAL CONSIDERATIONS
This study was approved by the institutional review board of Mass General Brigham.
RESULTS
We identified 238 patients who were initiated on UST for CD between January 1, 2016, and January 31, 2019. Among these patients, 123 (51.7%) underwent dose intensification after a median of 307 (interquartile range, [IQR], 168–557) days from UST initiation. A median of 521 (IQR, 314–725) days of clinical follow-up was available after the time of dose intensification through April 25, 2020. Dose intensification schedules included q4w (n = 64), q5w (n = 1), q6w (n = 55), or q7w (n = 3). A total of 18 (14.6%) patients underwent further dose intensification from q6w to q4w; 13 of these patients were categorized as q6w because this frequency was utilized for >50% of the postintensification follow-up period. The remaining 5 patients were categorized as q4w. When comparing q4w with q6w dose intensification groups, baseline characteristics were similar, with the exception of cigarette smoking (4.7% q4w vs 18.2% q6w, P = 0.02) and current corticosteroid use at time of intensification (31.3% q4w vs 14.6% q6w, P = 0.03; Table 1).
TABLE 1.
Characteristics Compared by Every 4- and Every 6-Week Ustekinumab Intervals
| Characteristics | Ustekinumab Every 4 Weeks (n = 64) | Ustekinumab Every 6 Weeks (n = 55) | P a |
|---|---|---|---|
| Age, mean y | 42.2 | 40.3 | 0.44 |
| Sex, % | 0.27 | ||
| Female | 60.9 | 50.9 | |
| Male | 39.1 | 49.1 | |
| Race, % | 0.12 | ||
| Asian | 3.1 | 0.0 | |
| Black | 0.0 | 5.5 | |
| Hispanic | 0.0 | 1.8 | |
| Unknown | 0.8 | 3.6 | |
| White | 89.1 | 89.1 | |
| Age of IBD diagnosis, mean, y | 25.0 | 26.5 | 0.55 |
| Disease duration at ustekinumab start, mean, y | 15.5 | 12.7 | 0.08 |
| Time on ustekinumab before intensification, mean, d | 363.8 | 368.7 | 0.92 |
| Ustekinumab level before intensification, mean, ug/mL, n = 14, n = 4 | 2.0 | 1.3 | 0.54 |
| Current smoking, % | 4.7 | 18.2 | 0.02 |
| Current cannabis, % | 15.6 | 29.1 | 0.08 |
| Current opioids, % | 35.9 | 27.3 | 0.31 |
| HBI within 3 months before intensification %, n = 60 | 0.66 | ||
| Remission (<5) | 31.7 | 30.8 | |
| Mild (5–7) | 23.3 | 30.8 | |
| Moderate (8–16) | 43.3 | 34.6 | |
| Severe (>16) | 1.7 | 3.9 | |
| Extraintestinal manifestations, % | 54.7 | 47.3 | 0.42 |
| Prior IBD surgery, % | 64.1 | 63.6 | 0.96 |
| Prior anti-TNF, % | 100.0 | 100.0 | n/a |
| >1 prior anti-TNF, % | 81.3 | 67.3 | 0.08 |
| Prior anti-integrin, % | 48.4 | 47.3 | 0.90 |
| Current corticosteroids, % | 31.3 | 14.6 | 0.03 |
| CD Location (Montreal classification), % | 0.32 | ||
| L1 Terminal ileum | 10.9 | 16.4 | |
| L2 Colon | 18.8 | 25.5 | |
| L3 Ileocolon | 67.2 | 56.4 | |
| L4 Upper GI | 0.0 | 1.8 | |
| L3+L4 Ileocolon + Upper GI | 3.1 | 0.0 | |
| CD Behavior (Montreal classification), % | 0.36 | ||
| B1 Nonstricturing, nonpenetrating | 21.9 | 32.7 | |
| B2 Stricturing | 17.2 | 18.2 | |
| B3 Penetrating | 60.9 | 49.1 | |
| Perianal disease, % | 37.5 | 47.3 | 0.28 |
| Lab markers within 3 months before intensification | |||
| Fecal calprotectin >120 mcg/g, %, n = 25; n = 7 | 88.0 | 100.0 | 0.34 |
| Albumin to CRP ratio, mean, n = 54, n = 46 | 82.3 | 237.6 | 0.08 |
a Calculated by Student t test or Pearson χ 2 test
Within 12 months after dose intensification, 23 of 42 (54.8%) q4w patients and 18 of 33 (54.6%) q6w patients experienced corticosteroid-free remission (P = 0.99). Discontinuation of UST with initiation of new biologic therapy occurred in 8 of 44 (18.2%) q4w patients and 3 of 40 (7.5%) q6w patients (P = 0.15). All patients who discontinued UST did so due to treatment failure and not due to adverse reactions or insurance coverage. No UST drug levels were checked between UST intensification and discontinuation. Clinical response occurred in 23 of 38 (60.5%) q4w patients and 17 of 29 (58.6%) q6w patients (P = 0.24). Rates of other 12-month outcomes were similar between q4w and q6w groups (Table 2).
TABLE 2.
Twelve-month Outcomes Compared by Every 4- and Every 6-Week Ustekinumab Intervals
| Outcomes | Ustekinumab Every 4 Weeks | Ustekinumab Every 6 Weeks | P b |
|---|---|---|---|
| Corticosteroid-free remission (HBI <5)a, %, n = 42, n = 33 | 54.8 | 54.6 | 0.99 |
| Change to new biologic, %, n = 44, n = 40 | 18.2 | 7.5 | 0.15 |
| Clinical response (∆ HBI ≥3)c, %, n = 38, n = 29 | 60.5 | 58.6 | 0.24 |
| Endoscopic improvement, %, n = 27, n = 19 | 63.0 | 63.2 | 0.99 |
| Improvement in CT or MRI abdomen, %, n = 29, n = 14 | 41.4 | 35.7 | 0.72 |
| Improvement in perianal disease, %, n = 24, n = 26 | 16.7 | 7.7 | 0.33 |
| Start corticosteroids, %, n = 45, n = 49 | 15.6 | 15.0 | 0.94 |
| IBD surgery, %, n = 44, n = 40 | 11.4 | 5.0 | 0.29 |
| IBD hospitalization, %, n = 45, n = 40 | 17.8 | 7.5 | 0.16 |
| Reduction in CRP by 25%, %, n = 45, n = 39 | 40.0 | 53.9 | 0.20 |
| Normalization of elevated CRP, %, n = 38, n = 28 | 23.7 | 7.1 | 0.08 |
| Normalization of elevated fecal calprotectin, %, n = 14, n = 3 | 7.1 | 33.3 | 0.20 |
aExcludes patients with HBI <5 and no corticosteroid use at time of intensification
bCalculated by Student ttest or Pearson χ 2 test
cExcludes patients with HBI ≤5 at time of intensification
Though 92 patients (74.8%) in our cohort underwent dose intensification for loss of clinical response, we observed that 31 patients (25.2%) underwent UST dose intensification despite meeting criteria for clinical remission (ie, HBI <5 without use of systemic corticosteroids) at the time of dose intensification. To better understand the justification for dose intensification of these patients, we manually reviewed documentation from gastroenterology clinic assessments. The reasons for dose intensification included symptom recurrence before 8 weeks (10 of 31), endoscopic inflammation (8 of 31), low serum UST concentration (4 of 31), elevated fecal calprotectin (3 of 31), bowel inflammation on CT or MRI (3 of 31), perianal disease symptoms (2 of 31), and patient preference (1 of 31).
When comparing patients by corticosteroid-free remission vs no corticosteroid-free remission within 12 months after dose intensification, intensified dose interval frequency and baseline characteristics were similar, with the exception of perianal disease (33.3% vs 58.8%, P = 0.03), pre-intensification HBI (13.5% remission, 46.0% mild, 40.5% moderate, and 0.0% severe vs 0.0% remission, 30.3% mild, 63.6% moderate, and 6.1% severe; P = 0.02), and current opioid use (21.4% vs 50.0%; P < 0.01; Table 3). When comparing patients who changed to a new biologic vs those who did not change to a new biologic, characteristics were also similar with the exception of perianal disease (37.7% vs 64.7%; P = 0.04; Supplementary Table 1). The mean time from UST initiation to dose intensification was similar between those with and without perianal disease (359 days vs 372 days, P = 0.78) and those with and without current opioid use (410 days vs 347 days, P = 0.21).
TABLE 3.
Patient Characteristics Compared by Remission Status Within 12 Months After Ustekinumab Dose Intensification
| Characteristic | No Corticosteroid-free Remission (n = 34) | Corticosteroid-free Remissiona (n = 42) | P b |
|---|---|---|---|
| Age, mean y | 42.0 | 42.7 | 0.82 |
| Sex, % | 0.43 | ||
| Female | 52.9 | 61.9 | |
| Male | 47.1 | 38.1 | |
| Race, % | 0.31 | ||
| Asian | 0.0 | 2.4 | |
| Black | 0.0 | 7.1 | |
| Hispanic | 2.9 | 0.0 | |
| Unknown | 2.9 | 4.8 | |
| White | 94.1 | 85.7 | |
| Age of IBD diagnosis, mean, y | 26.0 | 26.1 | 0.97 |
| Disease duration at ustekinumab start, mean, y | 15.0 | 14.5 | 0.80 |
| Time on ustekinumab before intensification, mean, d | 347.6 | 345.1 | 0.97 |
| Ustekinumab level before intensification, mean, ug/mL, n = 2, n = 5 | 3.9 | 2.4 | 0.63 |
| Current smoking, % | 14.7 | 7.1 | 0.29 |
| Current cannabis, % | 26.5 | 14.3 | 0.18 |
| Current opioids, % | 50.0 | 21.4 | <0.01 |
| Intensification frequency, % | 0.66 | ||
| q4 weeks | 55.9 | 54.8 | |
| q5 weeks | 0.0 | 0.0 | |
| q6 weeks | 44.1 | 42.9 | |
| q7 weeks | 0.0 | 2.4 | |
| HBI within 3 months before intensification, %, n = 33, n = 37 | 0.02 | ||
| Remission (<5) | 0.0 | 13.5 | |
| Mild (5–7) | 30.3 | 46.0 | |
| Moderate (8–16) | 63.6 | 40.5 | |
| Severe (>16) | 6.1 | 0.0 | |
| Extraintestinal manifestations, % | 61.8 | 54.8 | 0.54 |
| Prior IBD surgery, % | 70.6 | 50.0 | 0.07 |
| Prior anti-TNF, % | 100.0 | 100.0 | n/a |
| >1 prior anti-TNF, % | 79.4 | 73.8 | 0.57 |
| Prior anti-integrin, % | 50.0 | 47.6 | 0.84 |
| Current corticosteroids, % | 29.4 | 28.6 | 0.94 |
| CD Location (Montreal classification), % | 0.41 | ||
| L1 Terminal ileum | 5.9 | 1.9 | |
| L2 Colon | 23.5 | 26.2 | |
| L3 Ileocolon | 67.6 | 57.1 | |
| L4 Upper GI | 2.9 | 0.0 | |
| L3+L4 Ileocolon + Upper GI | 0.0 | 4.8 | |
| CD Behavior (Montreal classification), % | 0.83 | ||
| B1 Nonstricturing, nonpenetrating | 26.5 | 26.2 | |
| B2 Stricturing | 11.8 | 16.7 | |
| B3 Penetrating | 61.8 | 57.1 | |
| Perianal disease, % | 58.8 | 33.3 | 0.03 |
| Lab markers within 3 months before intensification | |||
| Fecal calprotectin >120 mcg/g, %, n = 9, n = 12 | 64.3 | 85.7 | 0.19 |
| Albumin to CRP ratio, mean, n = 30, n = 36 | 217.1 | 137.9 | 0.55 |
aExcludes patients with both HBI <5 and no use of corticosteroids at time of intensification
bCalculated by Student t test or Pearson χ 2 test
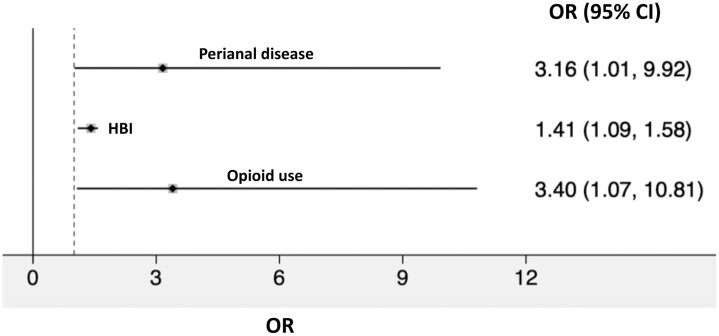
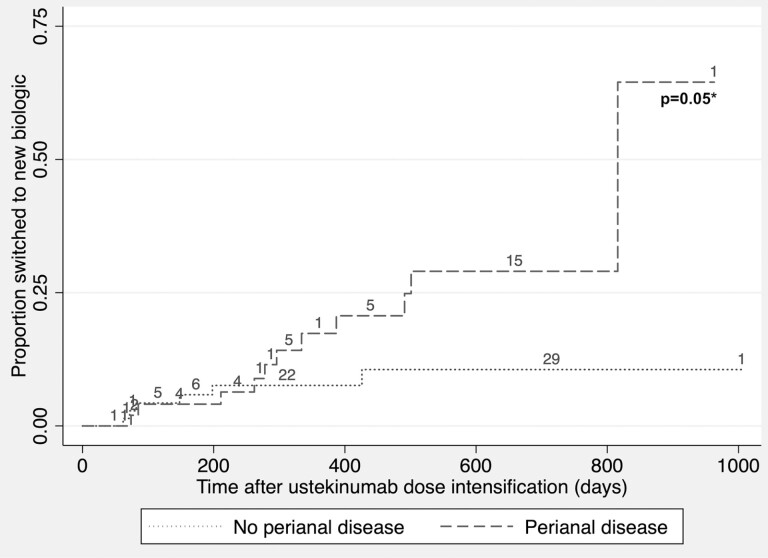
Multivariable logistic regression demonstrated that perianal disease (odds ratio [OR], 3.2; 95% CI, 1.0–9.9), HBI as a continuous variable (OR, 1.3; 95% CI, 1.1–1.6), and current opioid use (OR, 3.4; 95% CI, 1.1–10.8) at time of intensification were associated with failure to achieve remission (Fig. 1). Cox regression demonstrated that perianal disease (hazard ratio [HR], 3.0; 95% CI, 1.1–8.4) and current corticosteroid use (OR, 2.9; 95% CI, 1.0–7.9) at time of intensification were associated with shorter time to a new biologic. Kaplan-Meier analysis demonstrated a separation in the rate curves of time to new biologic stratified by perianal disease that did not reach statistical significance (log rank test P = 0.05; Fig. 2).
FIGURE 1.
Multivariable logistic regression model of failure to achieve corticosteroid-free remission. Model excludes patients who met criteria for corticosteroid-free remission (ie, HBI <5 and no use of systemic corticosteroids) at the time of ustekinumab dose intensification. Multivariable analysis assessed current opioid use, current corticosteroid use, perianal disease, HBI as a continuous variable, and prior surgery based on P-value <0.2 on univariate analysis. The final model includes only variables with P < 0.05 after forward selection.
FIGURE 2.
Kaplan-Meier analysis of time to new biologic therapy after ustekinumab dose intensification. *Calculated using log-rank test. Numbers represent censoring at loss of follow-up, time of ustekinumab discontinuation without initiation of new biologic therapy, or time of further ustekinumab dose intensification from every 6 weeks to every 4 weeks.
A sensitivity analysis of q4w vs q6w outcomes was performed after stratifying patients by metrics of disease severity at the time of intensification (Table 4). Among patients with pre-intensification HBI score of 5 to 7 (mild disease), rates of corticosteroid-free remission were similar. However, rates of changing to new biologic therapy were lower among q6w patients (40.0% q4w vs 0.0% q6w, P = 0.02). Among patients with HBI >7 (moderate or severe disease), rates of corticosteroid-free remission and changing to new biologic therapy were similar. After stratification of patients by corticosteroid use at the time of intensification, rates of remission and changing to new biologic therapy were similar. A second sensitivity analysis compared outcomes by number of prior biologic therapies (Supplementary Table 2). Patients who used 2 previous biologics were more likely to discontinue UST and start a new biologic after UST dose intensification than those who used 1 biologic or 3 or more biologics (25.7% vs 8.3% and 2.5%, P < 0.01).
TABLE 4.
Subgroup Analyses of 12-month Outcomes Compared by Every 4- and Every 6-Week Ustekinumab Intervals
| Corticosteroid-free Remission Stratified by Pre-intensification HBI | Every 4 Weeks | Every 6 Weeks | P a |
|---|---|---|---|
| HBI 5–7, %, n = 11, n = 15 | 54.6 | 66.7 | 0.53 |
| HBI >7, %, n = 23, n = 15 | 39.1 | 40.0 | 0.96 |
| Change to new biologic stratified by pre-intensification HBI | Every 4 weeks | Every 6 weeks | P a |
| HBI 5–7, %, n = 10, n = 12 | 40.0 | 0.0 | 0.02 |
| HBI >7, %, n = 20, n = 15 | 20.0 | 6.7 | 0.27 |
| Corticosteroid-free remission stratified by pre-intensification corticosteroid use | Every 4 weeks | Every 6 weeks | P a |
| No corticosteroid use, %, n = 27, n = 26 | 48.2 | 61.5 | 0.33 |
| Corticosteroid use, %, n = 15, n = 7 | 66.7 | 28.6 | 0.10 |
| Change to new biologic stratified by pre-intensification corticosteroid use | Every 4 weeks | Every 6 weeks | P a |
| No corticosteroid use, %, n = 34, n = 32 | 14.7 | 3.1 | 0.10 |
| Corticosteroid use, %, n = 10, n = 8 | 30.0 | 25.0 | 0.81 |
aCalculated using Pearson χ 2 test
DISCUSSION
Recapturing clinical response to a biologic agent continues to be a complex challenge in Crohn’s disease management. Though dose intensification has been shown to be effective among patients who lose response to TNF or integrin antagonists, the efficacy of dose intensification strategies using UST is less established.6, 12, 13 Additionally, it is unknown which baseline factors place patients at higher risk of failing UST after dose intensification. The results of our study suggest that perianal disease, HBI, and current opioid use were associated with failure to achieve remission, whereas perianal disease and current corticosteroid use were associated with shorter time to new biologic therapy.
Postintensification outcomes in the q4w and q6w groups suggest that these strategies are clinically effective, as more than 50% of patients in both groups achieved corticosteroid-free remission within 12 months. However, q4w patients had significantly greater use of systemic corticosteroids at the time of UST dose intensification. These patients may have had more complex disease at the time of intensification, as they had longer CD duration, greater proportion with use of 2 or more prior TNF antagonists, and greater proportion with penetrating disease, though these differences were not statistically significant. Therefore, we cannot unequivocally conclude that there are no differences in efficacy between q4w and q6w dosing.
Our study identified perianal disease as a predictor of both failure to achieve remission and need to change to alternative biologic after dose intensification. A recent study by Ollech and colleagues investigating the efficacy of UST dose escalation did not identify perianal disease as a predictor of clinical remission on univariate analysis.9 Patients with perianal disease comprised a larger proportion of our sample (44.7% among our cohort assessed for remission compared with 31.8% in Ollech et al) which may partly account for this discrepancy. Additionally, their entire cohort was escalated to q4w dosing, whereas 52% of our patients with perianal disease were escalated to q6w dosing with a lower rate of improvement in perianal symptoms compared with those escalated to q4w dosing (7.7% vs 16.7%). However, it is unclear if perianal disease is a driver of UST failure or rather a prognostic indicator of refractory CD. Perianal fistulas represent a disabling manifestation of CD and occur in up to 40% of patients by the time of CD diagnosis.14 Tumor necrosis factor antagonists are effective for the induction and maintenance of perianal CD, with up to 46% of patients demonstrating perianal fistula closure after 56 weeks of treatment with infliximab.15–18 Data regarding the efficacy of UST for perianal CD is less robust, with 36% of patients demonstrating resolution of perianal fistulae at 24 weeks in a small retrospective cohort study.19 The results of our study suggest that patients with perianal disease on UST may be less likely to experience remission after dose intensification. Because the majority of patients receiving UST in our health system ultimately required dose intensification, the presence of perianal disease should be considered before initiation of UST therapy, particularly if anti-TNF options have not been exhausted.
Current opioid use was also associated with lower odds of remission after dose intensification. Opioid use has been associated with severe infections and mortality among patients with CD; however, many patients with IBD receive opioid analgesics despite these risks.20, 21 The mechanisms by which opioid use increases the risk of IBD complications are not confirmed. However, opioids may mask symptoms of early flares and infections that lead to more severe presentations. Opioids also promote bowel hypomotility which can exacerbate obstructive symptoms related to CD. Opioid use in our study may be affected by confounding by indication because patients who require opioid analgesics may have more severe disease. However, we assessed metrics of CD severity including penetrating phenotype, prior biologic therapies, prior surgeries, HBI, and inflammatory markers that were not significantly different by remission status after intensification. It would be prudent to emphasize non-narcotic strategies of symptom management and wean opioid use in this population to unmask early symptoms of CD exacerbations. Our study would have benefited from understanding the dose relationship between opioid use and UST failure; however, limitations in EHR documentation precluded accurate assessment of total dose consumption.
This study has a number of strengths. Though prior investigations have emphasized the efficacy and safety of UST dose intensification, this study highlights factors associated with failure of intensification. These predictors were identified after assessing several metrics of CD severity. Although there seemed to be nonsignificant differences in prior IBD surgery by remission status, prior surgery was assessed in our multivariable analysis and did not remain in the final model due to lack of association with our outcome. If the factors we have identified are validated by larger studies, they can help providers risk stratify individuals before UST initiation or intensification. Additionally, our time to new biologic analysis applied rigorous censoring criteria for patients with limited follow-up data and UST discontinuation that was unrelated to treatment failure. We therefore have greater confidence that this outcome provides an objective assessment of UST failure that is measurable at a discrete time point. Our study also directly compares patient characteristics and outcomes by dose interval frequency, though our findings highlight the need for larger studies on this topic.
Our study has several limitations. This was a retrospective study utilizing EHR data, which is susceptible to incomplete data, documentation errors, and variable follow-up. In the absence of randomization, we cannot assume a causal relationship between dose intensification and clinical response among those with favorable outcomes. The observational nature of our study and small number of outcomes also limits our ability to control for all potential confounders. Our remission outcome relied on the HBI, which is a subjective scoring system that can vary between providers. Postintensification endoscopic and imaging data were present in less than 50% of our population, precluding multivariable assessment of more objective measures of remission. Though we assessed outcomes by dose interval frequency, our study was not powered to detect differences in these outcomes. We also identified various reasons for UST dose intensification in our cohort. It would be informative for clinicians to identify independent predictors of clinical response or failure in subgroups of patients with different indications for dose intensification, including those in whom a treat-to-target strategy was emphasized, those with postoperative disease recurrence, those with clinical loss of response, and those with partial response. However, larger studies are needed for this purpose.
The study took place at a tertiary referral center with a complex patient population; consequently, the majority of patients had failed at least 2 TNF antagonists before UST initiation. Therefore, our results cannot be generalized to patients who are treated with UST as a first- or second-line agent. Additionally, we are unable to track clinical data or medication dose adjustments that may have occurred at other centers. Our data also suggest that serum UST concentrations are uncommonly used to guide decisions regarding dose intensification (n = 18 of 123 patients), and the scarcity of this data precluded our ability to assess the efficacy of dose intensification for patients with lower UST levels.
CONCLUSION
In summary, we demonstrated that UST dose intensification to either q4w and q6w intervals was effective in achieving corticosteroid-free remission for nearly 55% of patients previously receiving standard q8w dosing. However, perianal disease, HBI, current opioid use, and current corticosteroid use are associated with UST failure after dose intensification. These factors should be considered when determining the appropriateness of q4w or q6w dosing. Alternative strategies, such as IV reinduction of UST followed by q4w dosing, may be more effective for patients with perianal disease or those requiring corticosteroids. The efficacy and safety of UST reinduction therapy for patients with moderate to severe CD is currently being evaluated as a randomized controlled trial (NCT03782376). Larger, prospective studies are needed to corroborate our findings and assess the relative efficacies and cost effectiveness of different dose intensification strategies among patients with variable CD characteristics and complexity. Such data will be critical to help providers tailor management approaches for patients who lose response to standard UST dosing.
Supplementary Material
Author Contribution: RSD contributed to the study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, and drafting of manuscript. CN contributed to the acquisition of data, acquisition of data, critical revision of the manuscript for important intellectual content. JM and SG critical revision of the manuscript for important intellectual content. JRA contributed to the study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision.
Supported by: This work was supported in part by training grant T32DK007533-35 (RSD and SG) from the National Institute of Diabetes and Digestive and Kidney Diseases, awarded to Brigham and Women’s Hospital.
Conflicts of Interest: JRA serves as a consultant for Takeda, Janssen, Pfizer, Pandion, Servatus, Finch Therapeutics, Iterative Scopes, and Artugen and has grant support from Merck. RD, CN, JM, and SG have no financial or personal conflicts of interest to disclose.
REFERENCES
- 1. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767. [DOI] [PubMed] [Google Scholar]
- 2. Danese S, Fiorino G, Reinisch W. Review article: Causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-α therapy. Aliment Pharmacol Ther. 2011;34:1–10. [DOI] [PubMed] [Google Scholar]
- 3. Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology. 2018;155:1045–1058. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Gasink C, Gao LL, et al. ; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Horin S, Chowers Y. Tailoring anti-TNF therapy in IBD: drug levels and disease activity. Nat Rev Gastroenterol Hepatol. 2014;11:243–255. [DOI] [PubMed] [Google Scholar]
- 6. Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:550–557.e2. [DOI] [PubMed] [Google Scholar]
- 7. Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2017;15:1427–1434.e2. [DOI] [PubMed] [Google Scholar]
- 8. Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology. 2018;154:1660–1671. [DOI] [PubMed] [Google Scholar]
- 9. Ollech JE, Normatov I, Peleg N, et al. Effectiveness of ustekinumab dose escalation in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kopylov U, Hanzel J, Liefferinckx C, et al. Effectiveness of ustekinumab dose escalation in Crohn’s disease patients with insufficient response to standard-dose subcutaneous maintenance therapy. Aliment Pharmacol Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 11. Waljee AK, Wallace BI, Cohen-Mekelburg S, et al. Development and validation of machine learning models in prediction of remission in patients with moderate to severe Crohn disease. JAMA Netw Open. 2019;2:e193721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shmidt E, Kochhar G, Hartke J, et al. Predictors and management of loss of response to vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:2461–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qiu Y, Chen BL, Mao R, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol. 2017;52:535–554. [DOI] [PubMed] [Google Scholar]
- 14. Park SH, Aniwan S, Scott Harmsen W, et al. Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort. Inflamm Bowel Dis. 2019;25:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405. [DOI] [PubMed] [Google Scholar]
- 16. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 17. Castaño-Milla C, Chaparro M, Saro C, et al. Effectiveness of adalimumab in perianal fistulas in crohn’s disease patients naive to anti-TNF therapy. J Clin Gastroenterol. 2015;49:34–40. [DOI] [PubMed] [Google Scholar]
- 18. Rasul I, Wilson SR, MacRae H, et al. Clinical and radiological responses after infliximab treatment for perianal fistulizing Crohn’s disease. Am J Gastroenterol. 2004;99:82–88. [DOI] [PubMed] [Google Scholar]
- 19. Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn’s disease: results of the ICC registry, a nationwide prospective observational cohort study. J Crohns Colitis. 2020;14:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dalal RS, Palchaudhuri S, Snider CK, et al. Exposure to intravenous opioids is associated with future exposure to opioids in hospitalized patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18:2269–2278.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.