Abstract
Background
Clinical indices to characterize the severity of inflammatory bowel disease (IBD) are widely used in clinical trials and real-world practice. However, there are few validated instruments for assessing IBD severity in administrative claims-based studies.
Methods
Patients (18–89 years) diagnosed with ulcerative colitis (UC) or Crohn’s disease (CD) and receiving ≥1 prescription claim for IBD therapy were identified using administrative claims data from the Optum Clinformatics, IMS PharMetrics, and Truven MarketScan databases (January 1, 2013–September 30, 2017). Regression modeling identified independent predictors of IBD-related hospitalization (inpatient stay or emergency department visit resulting in hospitalization), which were used to develop IBD severity indices. The index was validated against all-cause hospitalization and total cost and IBD-related hospitalization and total cost.
Results
There were 51,767 patients diagnosed with UC (n = 30,993) or CD (n = 20,774) who were initiated treatment with IBD therapy. Independent predictors of IBD-related hospitalization were Charlson Comorbidity Index score >1, anemia, weight loss, intravenous corticosteroid use, prior gastrointestinal-related emergency department visit and hospitalization, and unspecified disease location or more extensive disease. Female sex, renal comorbidities, intestinal fistula, and stricture were additional risk factors for patients with CD, whereas age <40 years was a UC-specific risk factor. Median IBD severity scores were 8 and 13 for UC and CD, respectively, from possible total scores of 51 and 37. Inflammatory bowel disease severity score correlated with significantly higher all-cause hospitalization and cost, all-cause total cost, IBD-related hospitalization cost, and total cost.
Conclusions
These validated UC and CD severity indices can be used to predict IBD-related outcomes using administrative claims databases.
Keywords: inflammatory bowel disease, severity score, risk score, hospitalization, administrative data
INTRODUCTION
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic, relapsing-remitting inflammatory condition of the gastrointestinal (GI) tract.1, 2 Inflammatory bowel disease currently affects more than 6.8 million individuals globally,3 with the United States having the highest age-standardized prevalence of IBD at 464.5 patients per 100,000 population.4
Treatment for IBD generally focuses on managing symptoms using conventional medications including aminosalicylates, corticosteroids, and thiopurines.1, 2, 5 Biological therapies, such as antitumor necrosis factor agents, vedolizumab, and ustekinumab or the oral Janus kinase (JAK) inhibitor tofacitinib are typically reserved for high-risk patients or those with moderate to severe disease who are intolerant to conventional therapy or for whom conventional therapy has failed.1, 2 However, the lack of curative therapy for IBD means that patients require lifelong treatment, with many undergoing surgery.1, 2
Inflammatory bowel disease is associated with a high economic burden stemming from increasing disease prevalence1, 2, 6 and both direct (hospitalization, surgery, pharmaceutical expenditure)7, 8 and indirect (productivity loss, premature retirement, premature mortality, out-of-pocket expenditure)6, 7 costs. Of note, medication and hospitalization account for up to 79% and 14%, respectively, of IBD-related health care expenditures.8 Furthermore, health care expenditures are significantly higher among patients with more severe disease or complications (eg, fistula). In addition, younger age (<40 years) and penetrating disease or strictures are predictors of higher health care costs in patients with UC and CD, respectively.7, 8
Indices used to assess disease severity in UC and CD have generally been developed in a research setting and classify patients based on clinical symptoms, patient-reported outcomes, and inflammatory burden.9, 10 However, most indices have not been validated,9, 10 so there is currently no gold standard IBD severity index.10 In addition, the development and utilization of clinical symptom and endoscopic indices can be time-consuming and expensive,9–11 and they often fail to correlate with laboratory data (eg, fecal calprotectin),1 limiting their applicability in clinical practice. Clinical IBD indices also tend to capture disease activity at a single point in time, failing to account for long-term disease burden.1, 2, 12
Administrative health claims databases are a key source of real-world data, including a large, diverse patient population and information on clinical parameters that is regularly collected during routine clinical practice.13 As such, claims databases facilitate retrospective and prospective investigation of the natural history of a disease, in addition to providing data on treatment safety, efficacy, and adherence over a long time period.13
Various algorithms have been used to assist researchers in identifying patients with UC or CD when performing insurance claims database studies.14 However, methods of identifying patients with severe IBD among records held in claims databases have not been reported. Accordingly, the present study aimed to develop and validate an IBD severity index using data from administrative claims databases.
MATERIALS AND METHODS
Data Sources
This was a retrospective cohort study of combined administrative claims data from the Optum Clinformatics (>10 million individual patients), IMS PharMetrics (>150 million), and Truven MarketScan (now known as IBM MarketScan; 69 million) databases from January 1, 2013, through September 30, 2017. These databases capture individual-specific administrative data, medical and pharmacy claims, laboratory analytic results, inpatient and outpatient diagnoses, and health care utilization.
Study Population and Cohort Selection
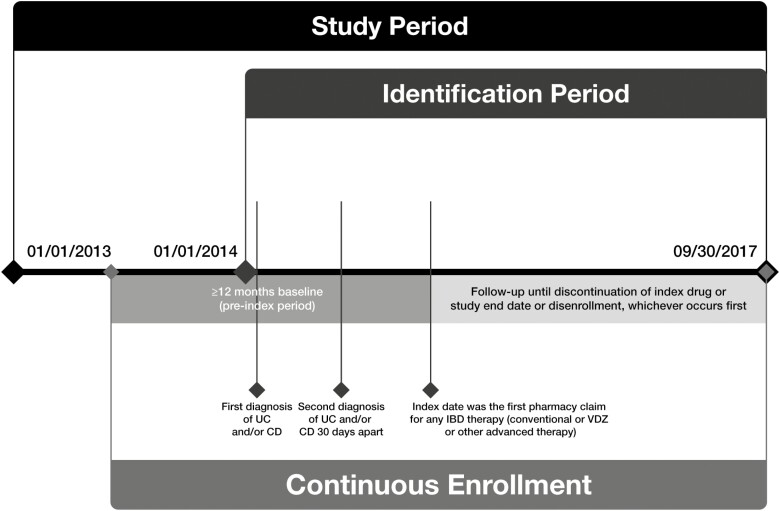
Eligible patients received ≥2 diagnoses of CD (International Classification of Diseases [ICD], Ninth/Tenth Revision, Clinical Modification [ICD-9/10-CM]: 555.xx; K50.xxx) or UC (ICD-9-CM: 556.xx except 556.4; ICD-10-CM: K51.xxx except K51.4xx) 30 days apart (diagnosis date was defined as the date of the first diagnosis) and had ≥1 prescription claim for conventional IBD therapies (eg, 5-aminosalicylic acid, immunomodulators) or advanced therapies (eg, vedolizumab, antitumor necrosis factor agents, tofacitinib, ustekinumab, natalizumab) after the diagnosis date (index date was defined as the first pharmacy claim for IBD therapy) during the identification period (January 1, 2014, to September 30, 2017; Fig. 1). Patients were also required to be 18 to 89 years of age on the index date, with continuous health plan enrollment for ≥12 months pre-index date. Patients were excluded from the study if they had a prior diagnosis of UC or CD or claims for IBD-related therapy 12 months before the first diagnosis of UC and/or CD; were diagnosed with ankylosing spondylitis, psoriasis, psoriatic arthritis, or rheumatoid arthritis at any time during the study period; or had a perianal fistula diagnosis claim (among patients with CD) any time before the diagnosis date or within 30 days of index therapy initiation. Patients with claims for both UC and CD were assigned to a single diagnostic category based on the diagnosis closest to the index date.
FIGURE 1.
Study design. Abbreviation: VDZ, vedolizumab.
Predictor Variables
Predictor variables included patients’ demographic characteristics, clinical characteristics, and IBD severity indicators. Patients’ demographic characteristics (age, sex) were collected on the index date, and clinical characteristics (Charlson Comorbidity Index [CCI] score, comorbidities, disease location) and IBD severity indicators (eg, anemia, malnutrition, prior GI-related emergency department [ED] visit, prior GI-related hospitalization) were assessed during the 12-month pre-index period. Time from diagnosis to first biologic therapy was also reported.
Outcome Variables
Outcome variables used for IBD severity index development were IBD-related hospitalization (inpatient stay or ED visit resulting in hospitalization but not surgery) and surgery (colectomy or small-bowel resection). The variables were analyzed from the index date until the outcome of interest (IBD-related hospitalization or surgery), disenrollment, or study end date, whichever occurred first. The following variables were used for IBD severity index validation: (1) all-cause hospitalization (any ED visit that resulted in hospitalization, excluding surgery-related hospitalization), (2) all-cause hospitalization and total costs, and (3) IBD-related hospitalization (any IBD-related ED visit that resulted in hospitalization but not surgery) and total costs. Costs were adjusted to 2017 US dollars using the annual medical care and drug costs components of the Consumer Price Index to reflect inflation.
Statistical Analysis
Patient demographic/clinical characteristics and all study variables were summarized using descriptive statistics. Logistic regression modeling was used to develop the IBD severity index, whereby 3 separate multivariate logistic regression models were developed with IBD-related hospitalization (yes/no) and surgery (colectomy [yes/no] or bowel resection [yes/no]) as the dependent variable and all demographic/clinical characteristics and IBD severity indicators as independent variables. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each model, and goodness of fit was estimated using concordance (C) statistics; the model with the highest number of significant covariates and best fit was selected as a proxy measure for IBD severity. A second logistic regression model was then developed using all significant predictors from the first model. Point values were assigned to each statistically significant predictor retained in the second model by multiplying the respective β-coefficient by 10 and then rounding to the nearest integer; the IBD risk score for an individual patient could be calculated by summing the scores for each predictor.
The IBD severity score was validated using the Pearson correlation coefficient (r) to test for concurrent validity between the index score and all-cause hospitalization, all-cause hospitalization and total costs, and IBD-related hospitalization and total costs. All statistical analyses were conducted using SAS version 9.3 (Cary, NC, USA).
ETHICAL CONSIDERATIONS
Patient privacy was preserved by the use of anonymized data in this retrospective, noninterventional cohort study.
RESULTS
A total of 51,767 patients diagnosed with UC (n = 30,993) and CD (n = 20,774) initiated treatment with advanced or conventional IBD therapy during the identification period. Across both groups, most patients were age ≥40 years (mean age of 46 and 43 years among patients with UC and CD, respectively) with a CCI score ≤1 (76% and 74% among patients with UC and CD, respectively; Table 1). Male and female patients were relatively equally represented (48% and 46% of UC and CD patients were male, respectively). Chronic obstructive pulmonary disease (COPD), diabetes mellitus, and liver disease were the most common comorbidities, affecting 7%–10% of patients with UC or CD combined. Around half of all patients had received intravenous (IV) corticosteroids for IBD. Patients diagnosed with UC had a shorter mean time to first biologic therapy at 128 days compared with 183 days for those diagnosed with CD.
TABLE 1.
Baseline Demographic and Clinical Characteristics
| UC (N = 30,993) | CD (N = 20,774) | |
|---|---|---|
| Age, years, mean (SD) | 45.87 (15.61) | 43.00 (15.78) |
| Age, years, n (%) | ||
| Age <40 | 11,607 (37.45) | 9315 (44.84) |
| Age ≥40 | 19,386 (62.55) | 11,459 (55.16) |
| Sex, n (%) | ||
| Male | 14,778 (47.68) | 9482 (45.64) |
| Female | 16,215 (52.32) | 11,292 (54.36) |
| CCI, n (%) | ||
| CCI score ≤1 | 23,701 (76.47) | 15,298 (73.64) |
| CCI score >1 | 7292 (23.53) | 5476 (26.36) |
| Other Individual Comorbidities, n (%) | ||
| Cardiac complications | 9 (0.03) | 11 (0.05) |
| Diabetes mellitus | 2841 (9.17) | 1665 (8.01) |
| Liver disease | 1806 (5.83) | 1813 (8.73) |
| Renal | 1409 (4.55) | 1139 (5.48) |
| COPD | 3001 (9.68) | 2326 (11.20) |
| IBD Severity Indicators, n (%) | ||
| Anemia | 4552 (14.69) | 4455 (21.45) |
| Clostridium difficile infection | 721 (2.33) | 404 (1.94) |
| Malnutrition | 3977 (12.83) | 3893 (18.74) |
| Pancolitis | 6902 (22.27) | 194 (0.93) |
| Weight loss | 2285 (7.37) | 2518 (12.12) |
| IV corticosteroid use | 13,999 (45.17) | 10,665 (51.34) |
| IV cyclosporine | 0 | 0 |
| Prior GI-related ED visit | 4021 (12.97) | 4006 (19.28) |
| Prior GI-related hospitalization | 4583 (14.79) | 5523 (26.59) |
| Prior surgeries | 761 (2.46) | 1697 (8.17) |
| Anal fistula | 128 (0.41) | 734 (3.53) |
| Fistula of the intestine | 36 (0.12) | 542 (2.61) |
| Intestinal stricture | 398 (1.28) | 3022 (14.55) |
| Location, n (%) | ||
| Pancolitis | 6902 (22.27) | N/A |
| Left-sided | 2918 (9.42) | N/A |
| Proctosigmoiditis | 1979 (6.39) | N/A |
| Proctitis | 5119 (16.52) | N/A |
| Other | 3284 (10.60) | N/A |
| Ileum/colon | N/A | 6518 (31.38) |
| Ileum | N/A | 5264 (25.34) |
| Colon | N/A | 3938 (18.96) |
| Unspecified | 10,791 (34.82) | 5054 (24.33) |
| Time From Diagnosis to First Biologic Treatment (days), n (SD) | 127.60 (224.94) | 182.79 (248.18) |
Abbreviation: N/A, not applicable.
IBD Severity Index Score Development
Selecting a proxy measure of IBD severity
We investigated 3 separate multivariate logistic regression models that used IBD-related hospitalization, colectomy, or bowel resection as a proxy for IBD severity in patients with UC (Supplementary Table 1) and CD (Supplementary Table 2). Inflammatory bowel disease–related hospitalization had the highest number of significant covariates (around double those identified in the other models), which were generally consistent across both the UC and the CD cohorts, and fitted the data well (C-statistics of 0.73 and 0.70 for UC and CD, respectively; Supplementary Tables 1 and 2). Therefore, IBD-related hospitalization was selected as a proxy for IBD severity.
Predictors of IBD-related hospitalization: Patients with ulcerative colitis
Significant predictors of IBD-related hospitalization for patients with UC included a CCI score >1, age <40 years, anemia, weight loss, IV corticosteroid use, prior GI-related ED visit and hospitalization, and time from diagnosis to first biologic therapy (Supplementary Table 1). Patients with proctosigmoiditis and proctitis had odds ratios for IBD-related hospitalization that were significantly less than 1, indicating a decreased likelihood of hospitalization. Sex, other individual comorbidities, Clostridium difficile infection, malnutrition, pancolitis, prior surgery, anal or intestinal fistula, intestinal stricture, pancolitis (location), and left-sided disease did not significantly predict IBD-related hospitalization in patients with UC (Supplementary Table 1).
Predictors of IBD-related hospitalization: Patients with Crohn’s disease
Among patients with CD, female sex, CCI score >1, renal comorbidity, anemia, weight loss, IV corticosteroid use, prior GI-related ED visit and hospitalization, intestinal fistula, intestinal stricture, and time from diagnosis to first biologic therapy were all significant predictors of IBD-related hospitalization (Supplementary Table 2). Patients with prior surgery and colon disease had odds ratios for IBD-related hospitalization that were significantly less than 1, indicating a decreased likelihood of hospitalization. As was observed in patients with UC, C. difficile infection, malnutrition, pancolitis, and anal fistula did not significantly predict IBD-related hospitalization in patients with CD (Supplementary Table 2). No association among IBD-related hospitalization and age, most comorbidities (cardiac complications, diabetes mellitus, liver disease, COPD), and ileum/colon and ileum disease location was observed in patients with CD (Supplementary Table 2).
IBD Severity Index Score Assignment and Validation
A final logistic regression model was developed for UC (Table 2) and CD (Table 3) using selected covariates that were significant predictors of IBD-related hospitalization in the first model. Risk scores were assigned to each variable.
TABLE 2.
Logistic Regression Model for Significant Predictors of IBD-Related Hospitalization and Risk Score Among Patients With UC
| OR Estimates (95% CI) | P | Risk Index | |
|---|---|---|---|
| CCI | |||
| CCI score ≤1 | Reference | 0 | |
| CCI score >1 | 1.22 (1.12–1.32) | <0.0001 | 2 |
| Age, years | |||
| Age <40 | 1.30 (1.21–1.40) | <0.0001 | 3 |
| Age ≥40 | Reference | 0 | |
| IBD Severity Indicators | |||
| Anemia | 1.37 (1.25–1.49) | <0.0001 | 3 |
| Weight loss | 1.27 (1.14–1.42) | <0.0001 | 2 |
| IV corticosteroid use | 1.36 (1.26–1.45) | <0.0001 | 3 |
| Prior GI-related ED visit | 1.26 (1.16–1.38) | <0.0001 | 2 |
| Prior GI-related hospitalization | 4.18 (3.85–4.54) | <0.0001 | 14 |
| Location | |||
| Pancolitis | 1.59 (1.40–1.80) | <0.0001 | 5 |
| Left-sided | 1.58 (1.36–1.84) | <0.0001 | 5 |
| Proctosigmoiditis | 1.32 (1.11–1.58) | 0.0019 | 3 |
| Proctitisa | Reference | 0 | |
| Other | 1.50 (1.30–1.74) | <0.0001 | 4 |
| Unspecified | 1.63 (1.44–1.83) | <0.0001 | 5 |
| C-statistic | 0.71 |
aProctitis was used as reference because it had the most negative β-coefficient value. Therefore, the scores of other locations were positive compared with proctitis.
TABLE 3.
Logistic Regression Model for Significant Predictors of IBD-Related Hospitalization and Risk Score Among Patients With CD
| OR Estimates (95% CI) | P | Risk Index | |
|---|---|---|---|
| Sex | |||
| Male | Reference | 0 | |
| Female | 1.08 (1.01–1.17) | 0.0349 | 1 |
| CCI | |||
| CCI score ≤1 | Reference | 0 | |
| CCI score >1 | 1.24 (1.14–1.35) | <0.0001 | 2 |
| Other Individual Comorbidities | |||
| Renal | 1.40 (1.21–1.61) | <0.0001 | 3 |
| IBD Severity Indicators | |||
| Anemia | 1.24 (1.13–1.35) | <0.0001 | 2 |
| Weight loss | 1.28 (1.15–1.42) | <0.0001 | 2 |
| IV corticosteroid use | 1.30 (1.21–1.41) | <0.0001 | 3 |
| Prior GI-related ED visit | 1.24 (1.13–1.35) | <0.0001 | 2 |
| Prior GI-related hospitalization | 2.93 (2.69–3.20) | <0.0001 | 11 |
| Prior surgeriesa | Reference | 0 | |
| Fistula of the intestine | 1.37 (1.12–1.67) | 0.0023 | 3 |
| Intestinal stricture | 1.47 (1.33–1.63) | <0.0001 | 4 |
| Location | |||
| Ileum/colon | 1.09 (0.97–1.21) | 0.1436 | 1 |
| Ileum | 1.11 (0.99–1.24) | 0.0812 | 1 |
| Colon | Reference | 0 | |
| Not specified | 1.20 (1.07–1.35) | 0.0020 | 2 |
| C-statistic | 0.69 |
aPrior surgeries were used as the reference because they had the most negative β-coefficient value. Therefore, scores of other severity indicators were positive compared with prior surgeries. P values in bold text are statistically significant.
Applying the severity index to all patients in the current data set established median IBD severity scores of 8 and 13 for patients with UC and CD, respectively (Table 4). Inflammatory bowel disease severity score was significantly positively correlated with all-cause hospitalization, all-cause hospitalization cost, all-cause total cost, IBD-related hospitalization cost, and IBD-related total cost in patients with UC and CD (Table 5). Among covariates, all-cause hospitalization most strongly correlated with the index for both UC (r = 0.29299; P < 0.0001) and CD (r = 0.19250; P < 0.0001; Table 5). These small correlation coefficients were deemed acceptable because of the large sample sizes used in our study.15
TABLE 4.
IBD Severity Scores in Patients With UC and CD
| UC (N = 30,993) | CD (N = 20,774) | |
|---|---|---|
| IBD Score | ||
| Mean (SD) | 9.81 (7.04) | 14.90 (6.75) |
| Median (range) | 8 (0–34) | 13 (0–38) |
| Quartile 1-Quartile 3 | 5–11 | 10–19 |
| IBD score ≤median, n (%) | 18,538 (59.81) | 11,816 (56.88) |
| IBD score >median, n (%) | 12,455 (40.19) | 8958 (43.12) |
Abbreviation: SD, standard deviation.
TABLE 5.
IBD Severity Index Validation
| Pearson Correlation Coefficients | |||||||
|---|---|---|---|---|---|---|---|
| Diagnosis | N | All-Cause Hospitalization | All-Cause Hospitalization Cost | All-Cause Total Cost | IBD-Related Hospitalization Cost | IBD-Related Total Cost | |
| IBD Severity Scorea | UC | 30,993 | 0.29299 | 0.07548 | 0.11832 | 0.06463 | 0.10037 |
| CD | 20,774 | 0.19250 | 0.12298 | 0.16271 | 0.11172 | 0.14562 | |
aAll P values < 0.0001.
DISCUSSION
Inflammatory bowel disease is a chronic, lifelong condition1, 2 that carries a significant economic burden largely due to costs associated with medication and hospitalization.7, 8 Furthermore, patients with more severe or extensive IBD have increased total health care costs.8
Administrative health claims databases provide a rich data set for exploring health care resource utilization by individual patients with IBD in real-world clinical practice.13 Although multiple clinical indices are available for assessing disease severity in IBD,9, 10 many remain unvalidated,9–11 and most are not suitable for claims-based research. Accordingly, this study describes the development and validation of an index to evaluate IBD severity among individual patients in retrospective claims databases.
The current analysis identified several predictive risk factors; IBD severity score correlated with all-cause and IBD-related hospitalization and total costs for patients with UC and CD. In particular, all-cause hospitalization had the greatest correlation with disease severity.
During index development, IBD-related hospitalization was selected over colectomy and bowel resection surgery as a proxy for IBD severity in regression modeling because hospitalization had the greatest number of significant predictive factors among patients with UC and CD: CCI score >1, anemia, weight loss, IV corticosteroid use, prior GI-related ED visit and hospitalization, and unspecified disease location were IBD severity risk factors shared by patients with UC and CD.
Additional CD-specific risk factors included female sex, renal comorbidities, intestinal fistula, and intestinal stricture, whereas younger age (<40 years) was a UC-specific risk factor. Many of these factors have previously been reported as predictors of poor outcomes for patients with IBD, including complicated disease, IBD-related hospitalizations/visits, and GI surgery.1, 2, 16, 17 Although the use of IBD-related hospitalization and surgery as outcome measures had many advantages, these measures may not reflect the entirety of IBD-related severity. They may also lead to overestimation of the severity of disease phenotypes historically associated with increased surgical risks, such as stenosing or penetrating CD compared with inflammatory CD.
The current study established median IBD severity scores of 8 and 13 in our UC and CD data set, respectively, from possible total cumulative scores of 51 and 37. About 41% of patients with IBD had severity scores greater than the median, which suggests that a score greater than the median could be predictive of patients with intermediate–severe disease, given that an earlier severity score developed by Ananthakrishnan and colleagues18 from retrospective administrative data for patients with CD classified 41% of patients as intermediate–high risk for severe hospitalization (requiring nonelective bowel surgery or >7 days of hospitalization), despite differences in the proxy measure of IBD severity and independent predictors of severity used in each index. Using a nationwide inpatient database, Ananthakrishnan and colleagues18 assigned each independent predictor of severe hospitalization among patients with CD (anemia, malnutrition, blood transfusion or total parenteral nutrition, C. difficile infection, transfer from another hospital or admission to a teaching hospital, and obstructing or fistulizing disease) a score of 0 to 13.18 Patients with a higher disease score had a significantly greater risk of severe hospitalization when validated in an independent cohort of patients.18
Compared with the CD severity score developed by Ananthakrishnan et al, our analysis utilized a greater number and range of administrative databases, captured almost double the number of patients (51,767 vs 25,938), incorporated the updated 10th revision of ICD diagnosis codes, had a longer duration of follow-up, and included a wide range of patients encountered during routine clinical practice, including outpatient care, rather than limiting our analysis to patients coded as having a CD-related hospitalization.18 Our severity index also features a wider range of possible total scores, thereby offering a greater level of granularity. However, severity levels cannot be easily delineated using the index developed in this current study; further work can be done in this area using appropriate algorithms to define severity levels, which may include the use of electronic medical records with doctor’s notes.
In this study, distinct severity indices were developed and validated for patients with UC and CD. Although UC and CD have overlapping features, it is recognized that they are distinct diseases with differing features and risk factors.12 For clinicians, unique disease severity indices may facilitate personalized prognosis and treatment plans for patients with IBD. Because the IBD severity score incorporates overall and long-term disease severity rather than current clinical disease activity, it may also enable earlier and appropriate intervention for patients at risk of severe IBD and IBD-related complications, including prescribing advanced therapies for patients at risk of severe disease.
This retrospective analysis of claims databases (which are not primarily intended for research) has several inherent limitations, including potential for information bias, selection bias, and confounding. Clinical conditions and diagnoses were identified using ICD-9/10-CM diagnosis codes, which are subject to potential miscoding. The presence of a diagnosis code does not guarantee that a patient has an active disease or condition. Similarly, a filled prescription claim does not indicate whether the medication was taken as prescribed. The study is also subject to possible selection bias (because only patients with continuous enrollment were eligible) and potential residual confounding (due to the retrospective nature of the study and unobserved clinical or other differences affecting care). The data collection period for this study ended in 2017, so newer therapies may be underrepresented. Furthermore, the generalizability of the study findings may be limited to commercially insured US-based patients with IBD aged ≥18 years because those who are uninsured and lack access to health care are not captured in this study. Health insurance, access to treatment and medication, and medical practices may vary across countries. Therefore, future studies should investigate additional risk factors to improve IBD severity score predictability and explore prospective validation in different patient populations and different health care environments to improve index generalizability.
CONCLUSION
We have developed and validated UC and CD severity scores to predict IBD-related outcomes using administrative claims databases. The severity index showed that all-cause hospitalization had the greatest correlation with disease severity. This simple IBD severity index provides a tool for exploring health outcomes and resource utilization in claims-based studies and may be useful for guiding clinical decision-making.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Sulena Shrestha and Yulin Shi, both former employees of STATinMED, for their contributions to this project. Writing assistance was provided by Marissa Scandlyn, PhD, and Reem Berro, PhD, on behalf of inVentiv Medical Communications, LLC, a Syneos Healt group company.
Author Contribution: GC, TL, and KD contributed to concept and design and supervised the study. CD and GC contributed to acquisition of data and administrative, technical, or logistic support. CD, GC, TL, and KD contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content. CD contributed to statistical analysis.
Supported by: This work is supported by Takeda Pharmaceuticals U.S.A., Inc.
Conflicts of Interest: CD is an employee of STATinMED Research, Plano, TX, and a paid consultant for Takeda Pharmaceuticals U.S.A., Inc. TL was employed by Takeda Pharmaceuticals U.S.A., Inc., at the time of the study; TL is currently an employee of Lilly Biomedicines at Eli Lilly and Company, Indianapolis, IN. GC and KD are current employees of Takeda Pharmaceuticals U.S.A., Inc., and own stock or stock options.
REFERENCES
- 1. Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet. 2017;389:1741–1755. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sales-Campos H, Basso PJ, Alves VB, et al. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuenzig ME, Lee L, El-Matary W, et al. The impact of inflammatory bowel disease in Canada 2018: indirect costs of IBD care. J Can Assoc Gastroenterol. 2019;2:S34-S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Valk ME, Mangen MJ, Severs M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis . Evolution of costs of inflammatory bowel disease over two years of follow-up. PLoS One. 2016;11:e0142481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pillai N, Dusheiko M, Maillard MH, et al. ; Swiss IBD Cohort Study Group . The Evolution of health care utilisation and costs for inflammatory bowel disease over ten years. J Crohns Colitis. 2019;13:744–754. [DOI] [PubMed] [Google Scholar]
- 9. Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14:348–354.e317. [DOI] [PubMed] [Google Scholar]
- 10. Alrubaiy L, Rikaby I, Sageer M, et al. Systematic review of the clinical disease severity indices for inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2460–2466. [DOI] [PubMed] [Google Scholar]
- 11. Alrubaiy L, Dodds P, Hutchings HA, et al. Development and validation of a new disease severity index: the Inflammatory Bowel Disease Index (IBDEX). Frontline Gastroenterol. 2015;6:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegel CA, Whitman CB, Spiegel BMR, et al. Development of an index to define overall disease severity in IBD. Gut. 2018;67:244–254. [DOI] [PubMed] [Google Scholar]
- 13. Blonde L, Khunti K, Harris SB, et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Y, Manne S, Bennett D. Identifying patients with inflammatory bowel diseases in an administrative health claims database: do algorithms generate similar findings? Inquiry. 2019;56:46958019887816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asuero AG, Sayago A, Gonzalez AG. The correlation coefficient: an overview. Crit Rev Anal Chem. 2006;36:41–59. [Google Scholar]
- 16. Long GH, Tatro AR, Oh YS, et al. Analysis of safety, medical resource utilization, and treatment costs by drug class for management of inflammatory bowel disease in the United States based on insurance claims data. Adv Ther. 2019;36:3079–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wanderås MH, Moum BA, Høivik ML, et al. Predictive factors for a severe clinical course in ulcerative colitis: results from population-based studies. World J Gastrointest Pharmacol Ther. 2016;7:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ananthakrishnan AN, McGinley EL, Binion DG, et al. A novel risk score to stratify severity of Crohn’s disease hospitalizations. Am J Gastroenterol. 2010;105:1799–1807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.