Abstract
Brackground
We aimed to examine associations between elevated symptoms of depression and anxiety and disease activity in inflammatory bowel disease (IBD). Previous findings have been inconsistent and have not accounted for variability in the courses of these conditions over time.
Methods
We followed 247 participants with IBD (153 Crohn’s disease [CD], 94 ulcerative colitis [UC]) for 3 years. Annually, participants underwent an abdominal examination, reported therapies used for IBD, and completed the Hospital Anxiety and Depression Scale (HADS) questionnaire. We evaluated associations of elevated symptoms (scores ≥11) of anxiety (HADS-A) and depression (HADS-D) with the presence of active IBD as measured using the Powell Tuck Index for UC and the Harvey-Bradshaw Disease Activity Index for CD. We employed logistic regression with generalized estimating equations, simultaneously estimating between-person and within-person effects.
Results
Of 247 participants, 15 (6.1%) had elevated symptoms of depression (HADS-D ≥11) at enrollment, 41 (16.6%) had elevated symptoms of anxiety (HADS-A ≥11), and 101 (40.9%) had active IBD. On average, individuals with elevated symptoms of depression (odds ratio [OR], 6.27; 95% CI, 1.39–28.2) and anxiety (OR, 2.17; 95% CI, 1.01–4.66) had increased odds of active IBD. Within individuals, elevations in symptoms of depression over time were associated with increased odds of active IBD (OR, 2.70; 95% CI, 1.15–6.34), but elevated symptoms of anxiety were not. After adjustment for covariates (including disease activity), elevated symptoms of depression were also associated with increased odds of biologic therapy use (OR, 2.02; 95% CI, 1.02–4.00).
Conclusion
Symptoms of depression and anxiety are associated with disease activity in IBD over time. Reducing these symptoms should be incorporated into the management of IBD.
Keywords: IBD, depression, anxiety, mood disorders, symptoms, self-reported outcomes
Introduction
Persons with inflammatory bowel disease (IBD) have an increased risk of comorbid depression and anxiety.1, 2 Under-recognized or undertreated mental health problems in IBD patients may lead to increased disability, poorer medication adherence, and increased health care utilization and costs.2 However, findings regarding the effect of psychiatric comorbidity on disease outcomes in IBD have been inconsistent. Two recent studies reported that adverse IBD outcomes and high health care utilization were associated with diagnosed psychiatric disorders.3, 4 Both were conducted in tertiary care centers, and neither reported on symptom severity of the psychiatric disorder. Importantly, psychiatric disorders such as depression or anxiety may remit and relapse, just as with IBD. Thus, a past psychiatric diagnosis does not equate to having current active symptoms. A systematic review of 11 studies assessing the level of depressive symptoms and disease course in IBD found that 5 studies reported an association—but only in persons with Crohn’s disease (CD).5 This association was not apparent in participants whose IBD was in remission at baseline. Thus, it is unclear how differences in the severity of depressive symptoms between people influence outcomes in IBD. Moreover, studies have not yet assessed elevated symptoms of anxiety, which are also common in IBD and are frequently comorbid with elevated depressive symptoms. Nor have they evaluated whether changes in the symptoms of depression or anxiety within an individual over time influence disease activity. This is particularly important for determining the potential benefits of effective identification and treatment of depression and anxiety in IBD.
We aimed to examine associations between disease activity in IBD and elevated symptoms of depression and anxiety. We hypothesized that within-person fluctuations in symptoms of depression or anxiety over time would be associated with changes in disease activity. The novelty of our study is that we explored the impact of active depression and anxiety symptoms on IBD symptoms over time in persons who did not have these mental health symptoms at baseline (“within” analysis), in addition to comparing outcomes of persons with IBD with depression and anxiety symptoms with those who did not have these symptoms (“between” analysis).
METHODS
Participants
As detailed elsewhere, we conducted this longitudinal cohort study in Manitoba, Canada.6 As part of a larger study, we recruited participants with IBD (Crohn’s disease or ulcerative colitis [UC]) through academic and community-based gastroenterology clinics and a provincial research registry between November 2014 and July 2016. We confirmed diagnoses of IBD by medical records review or with the treating gastroenterologist. All participants were 18 years or older, provided written informed consent, and had sufficient knowledge of the English language to complete written questionnaires and a structured clinical interview. We obtained ethics approval from the University of Manitoba Health Research Ethics Board.
Participants completed an enrollment visit followed by 3 follow-up visits at yearly intervals. At each visit participants completed validated self-report measures and underwent clinical assessments.
Measures
Psychiatric morbidity
We used the Hospital Anxiety and Depression Scale (HADS) to assess the severity of current symptoms of anxiety (HADS-A) and depression (HADS-D). The HADS includes 14 items, 7 for anxiety and 7 for depression; total scores on each subscale range from 0 to 21.7 The HADS has been validated in IBD.8 Based on their high specificity in IBD8 and the recommendation as indicating severe symptoms in medically ill populations, we used the cut point of ≥11 to identify elevated symptoms of depression and anxiety.7
Inflammatory bowel disease
We classified disease phenotype and progression using the Montreal Classification.9 We recorded current use of immune therapies (ITs) including thiopurines, methotrexate, TNF-alpha antagonists, ustekinumab, vedoliuzmab, and corticosteroids; we also recorded use of 5-aminosalicylates (5-ASA). These were categorized as “any IT” or “no IT” at each visit. We assessed symptomatic disease activity using the Powell Tuck Index for UC and the Harvey-Bradshaw Disease Activity Index for CD.10, 11 Both indices were recorded by trained personnel; a score of ≥5 on each index indicates active disease. This served as our primary outcome.
Covariates
Participants reported their gender (male or female), date of birth, race/ethnicity (using Statistics Canada categories), level of education, smoking status, and comorbid conditions. Based on the distribution of responses, we categorized race as white or nonwhite. Highest level of education was categorized as high school or below, or more than high school. Annual household income was categorized as <$50,000/year, ≥$50,000, or declined to answer. We classified current smoking status as yes or no. Participants also reported comorbid chronic health conditions diagnosed by a physician including hypertension, hyperlipidemia, heart disease, peripheral vascular disease, chronic lung disease, diabetes, migraine, autoimmune thyroid disease, systematic lupus erythematosus, cancer (breast, lung, colorectal, skin, other), arthritis, osteoporosis, fibromyalgia, renal disease, peptic ulcer disease, liver disease, irritable bowel syndrome (IBS), and seizure disorders. With the exception of psychiatric comorbidities, these were summarized as a count, categorized as 0, 1 or ≥2.
Analyses
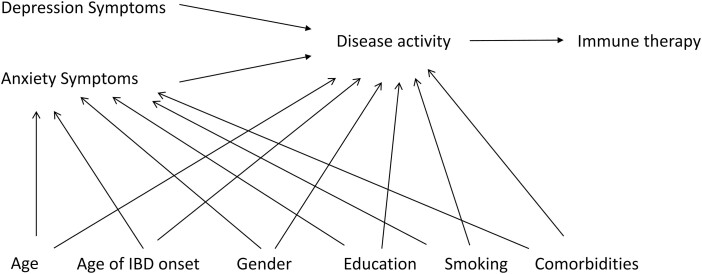
We summarized the characteristics of the study population using mean (standard deviation), median (interquartile range [IQR]), and frequency (percentage). Missing data were not imputed. Univariate analyses used Student t tests, Wilcoxon and Kruskal-Wallis tests, and χ 2 tests, as appropriate. To determine the effect of clinically meaningful elevated symptoms of depression and anxiety (ie, HADS-D or HADS-A ≥11) on disease activity, we used a logistic model with generalized estimating equations with an exchangeable correlation matrix to account for dependence of repeated measures within individuals. These models produce population averages of within-person and between-person effects but can be parameterized to separate these effects using separate variables12; thus we included 4 variables for depression and anxiety, including HADS-D_within, HADS-D_between, HADS-A_within, and HADS-A_between. Thus the HADS-D_within variable would indicate the effect on disease activity of a change from no elevation in symptoms of depression to elevated symptoms of depression within an individual from one visit to another. The HADS-D_between variable would indicate the effect on disease activity of an individual having elevated symptoms of depression compared with an individual who did not have elevated symptoms of depression. The HADS-A variables were analogous to those for depression. We included gender, age (as the time scale), race, education, income, smoking status, age at IBD onset, and number of comorbidities as covariates, updating the status of each covariate as reported or measured at each visit. Based on our postulated causal pathway (Fig. 1), we did not include use of immune therapy as a covariate. Expecting that symptoms of depression and anxiety would be associated with increased disease activity, we also used a logistic model to test whether these symptoms were associated with use of biologic therapy. The logistic model was the same as previously described. We tested for effect modification by type of IBD in these models by including interaction terms between type and each of the HADS variables. We report effect measures as odds ratios (OR; 95% confidence intervals [CI]).
Figure 1.
Directed acyclic graph of the hypothesized association between symptoms of depression and anxiety and inflammatory bowel disease activity.
Complementary Analyses
To address the possible contribution of mood-related increases in functional symptoms among participants with self-reported comorbid IBS, we repeated the analyses for disease activity and biologic use, excluding individuals who may have had comorbid IBS. Given that tumor necrosis factor (TNF)-alpha may be associated with depression, we repeated our analyses for use of biologic therapy, excluding individuals using non-TNF biologics.13
Statistical analyses were conducted using SAS V9.4 (SAS Institute Inc., Cary, NC).
RESULTS
We enrolled 247 persons with IBD, of whom 216 (87.4%) completed all 4 study visits. A further 7 participants died before study completion; thus 24 participants were lost to follow-up. The median (IQR) follow-up period was 2.99 (2.95–3.05) years. Two thirds of participants had Crohn’s disease, and two thirds were women. Most participants had more than a high school education and an annual household income of $50,000 or more per annum. At baseline, persons with Crohn’s disease were more likely to have active disease and use biological therapy than persons with ulcerative colitis. They tended to have more physical comorbidities and a higher baseline HADS score for depression (Table 1). Overall, use of corticosteroids was low.
Table 1.
Characteristics of Study Participants
| Variable | Total IBD N = 247 | CD N = 153 | UC N = 94 |
|---|---|---|---|
| Age (yrs), mean (SD) | 47.4 (14.8) | 48.2 (14.5) | 46.2 (15.3) |
| Women, n (%) | 156 (63.2) | 102 (66.7) | 54 (57.5) |
| White, n (%) | 210 (85.0) | 132 (86.8) | 78 (83.0) |
| >High school education, n (%) | 171 (69.2) | 109 (71.2) | 62 (66.0) |
| Annual Income, n (%) | |||
| <$50,000 | 58 (23.5) | 44 (28.8) | 14 (14.9) |
| ≥$50,000 | 171 (69.2) | 101 (66.0) | 70 (74.5) |
| Declined | 18 (7.3) | 8 (5.2) | 10 (10.6) |
| Immune therapy, n (%) | |||
| Any | 185 (74.9) | 116 (75.8) | 69 (73.4) |
| Biologic | 80 (32.4) | 70 (45.8) | 10 (10.6) |
| Oral corticosteroid | 9 (3.6) | 5 (3.3) | 4 (4.3) |
| Disease duration (yrs), mean (SD) | 20.8 (12.4) | 22.5 (12.1) | 18.2 (12.4) |
| Age of IBD onset (yrs), n (%) | |||
| ≤17 | 32 (13.0) | 20 (13.1) | 12(12.8) |
| 17–40 | 164 (66.4) | 103 (67.3) | 61 (64.9) |
| ≥40 | 51 (20.6) | 30 (19.6) | 21 (22.3) |
| Montreal Classification UC, n (%) | |||
| E1 | 14 (15.1) | ||
| E2 | 38 (40.9) | ||
| E3 | 41 (44.1) | ||
| Montreal Classification CD, n (%) | |||
| Localization | |||
| L1 | 66 (43.1) | ||
| L2 | 22 (13.7) | ||
| L3 | 67 (43.1) | ||
| L4 | 0 (0) | ||
| Behaviour | |||
| B1 | 65 (42.8) | ||
| B2 | 53 (34.9) | ||
| B3 | 34 (22.4) | ||
| Active diseasea, n (%) | 101 (41.7) | 75 (50.3) | 26 (28.0) |
| IBS, n (%) | 62 (25.1) | 44 (28.8) | 18 (19.2) |
| HADS-D ≥ 11 | 15 (6.1) | 11 (7.2) | 5 (5.32) |
| HADS-Ab ≥11 | 41 (16.6) | 27 (17.8) | 14 (14.9) |
| HADS-D, mean (SD) | 4.0 (3.7) | 4.3 (3.8) | 3.4 (3.5) |
| HADS-Ab, mean (SD) | 6.3 (4.1) | 6.6 (4.1) | 6.0 (4.2) |
| Current smoker, n (%) | 42 (16.6) | 29 (18.9) | 13 (13.8) |
| Physical comorbidity, n (%) | |||
| 0 | 81 (32.8) | 43 (28.1) | 38 (40.4) |
| 1 | 58 (23.5) | 36 (23.5) | 22 (23.4) |
| ≥2 | 108 (43.7) | 74 (48.4) | 34 (36.2) |
a5 missing; b1 missing
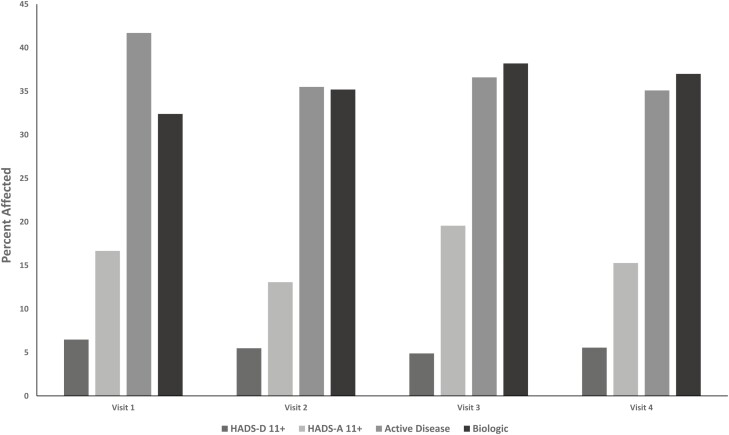
At the population-level, the percentage of participants with clinically meaningful elevations in symptoms of depression or anxiety fluctuated only slightly across visits (Fig. 2). Over the study period, 30 (12.2%) of participants experienced a change in the presence or absence of depression symptoms, and 55 (22.4%) experienced a change in the presence or absence of anxiety symptoms. Elevated symptoms of anxiety were more common than elevated symptoms of depression. At enrollment, participants with elevated symptoms of depression did not differ with respect to demographic or clinical characteristics from participants without elevated symptoms (Supplemental Table 1). Compared with participants without elevated symptoms of anxiety, those with elevated symptoms were more likely to smoke and tended to be more likely to have irritable bowel syndrome.
Figure 2.
Percentage of participants with clinically meaningful symptoms of depression or anxiety and inflammatory bowel disease activity by study visit.
Over one third of participants had active disease at each visit, and about one third used biologic therapy at each visit. On average, compared with participants with inactive disease, those with active disease had lower annual incomes, were more likely to have Crohn’s disease, were more likely to use biologic therapy, were more likely to have 2 or more physical comorbidities, and were more likely to have elevated symptoms of depression and anxiety (Table 2).
Table 2.
Participant Characteristics Stratified According to the Presence or Absence of Disease Activity
| Characteristic | Inactive N = 141 | Active N = 101 | P |
|---|---|---|---|
| Age (years), mean (SD) | 46.4 (15.4) | 49.0 (14.0) | 0.17 |
| Women, n (%) | 87 (61.7) | 66 (65.4) | 0.56 |
| White, n (%) | 122 (87.1) | 84 (83.2) | 0.39 |
| >High school education, n (%) | 100 (70.9) | 70 (69.3) | 0.79 |
| Annual Income, n (%) | 0.006 | ||
| <$50,000 | 22 (15.6) | 33 (32.7) | |
| ≥$50,000 | 106 (75.2) | 63 (62.4) | |
| Declined | 13 (9.2) | 5 (4.9) | |
| IBD Subtype, n (%) | |||
| Crohn’s disease | 74 (52.5) | 75 (74.3) | <0.001 |
| Ulcerative colitis | 67 (47.5) | 26 (25.7) | |
| Immune therapy, n (%) | |||
| Any | 106 (75.2) | 76 (75.3) | 0.99 |
| Biologic | 36 (25.5) | 41 (40.6) | 0.013 |
| Oral corticosteroid | 3 (2.1) | 6 (5.9) | 0.17 |
| Irritable bowel syndrome, n (%) | 27 (19.2) | 34 (33.7) | 0.01 |
| HADS-D ≥ 11 | 4 (2.8) | 12 (11.9) | 0.005 |
| HADS-A ≥ 11 | 16 (11.4) | 24 (24.0) | 0.009 |
| HADS-D, mean (SD) | 2.9 (3.1) | 5.3 (3.9) | <0.001 |
| HADS-A, mean (SD) | 5.6 (3.9) | 7.4 (4.2) | <0.001 |
| Current smoker, n (%) | 18 (12.8) | 24 (23.8) | 0.026 |
| Physical comorbidity, n (%) | 0.017 | ||
| 0 | 55 (39.0) | 23 (22.8) | |
| 1 | 33 (23.4) | 24 (23.8) | |
| ≥2 | 53 (37.6) | 54 (53.5) |
Disease Activity
In unadjusted analyses on average at any given point in time, elevated symptoms of depression (OR, 8.41; 95% CI, 1.19–36.9) or anxiety (OR, 2.45; 95% CI, 1.16–5.18) were associated with increased odds of active disease (Table 3). Within persons, elevated symptoms of depression over time were associated with over two-fold increased odds of active disease (2.45; 95% CI, 1.17, –5.16; Table 3). However, similar changes in symptoms of anxiety were not associated with the odds of active disease (OR, 0.90; 95% CI, 0.51–1.57). After adjustment for age, age at IBD onset, gender, education, smoking status, and number of physical comorbidities, the findings were only slightly attenuated. We did not observe any interactions between type of IBD and elevated symptoms of depression or anxiety on disease activity. When we repeated the analysis after excluding those 25% of participants with IBS, our findings were similar; as expected, the confidence intervals were broader, and the within-person effect of depression was no longer statistically significant (Supplemental Table 2).
Table 3.
Association of Within-individual and Between-individual Effects of Anxiety and Depression With Disease Activity
| Depression | Anxiety | |||
|---|---|---|---|---|
| Between-effect OR (95% CI) | Within-effect OR (95% CI) | Between-effect OR (95% CI) | Within-effect OR (95% CI) | |
| Unadjusted | 8.41 (1.91–36.9) | 2.45 (1.17–5.16) | 2.45 (1.16–5.18) | 0.90 (0.51–1.57) |
| Adjusteda | 6.27 (1.39–28.2) | 2.70 (1.15–6.34) | 2.17 (1.01–4.66) | 1.03 (0.56–1.89) |
For depression, the within-effect indicates the effect on disease activity of a change from no elevation in symptoms of depression to elevated symptoms of depression within an individual from one visit to another. The between-effect indicates the effect on disease activity of an individual having elevated symptoms of depression compared with an individual who did not have elevated symptoms of depression. The anxiety variables are analogous to those for depression. aAdjusted for age, age at IBD onset, gender, education, current smoking status, and number of physical comorbidities.
Biologic Therapy Use
In unadjusted analyses on average at any given point in time, elevated symptoms of depression or anxiety were not associated with the odds of using biologic therapy (Table 4). However within-persons, elevation in symptoms of depression over time was associated with 1.88-fold increased odds of using biologic therapy (OR, 1.88; 95% CI, 1.13–3.13). Changes in symptoms of anxiety over time were not associated with use of biologic therapy. After adjustment for age, age at IBD onset, gender, education, smoking status, and number of physical comorbidities, the point estimate for the association of within-person changes in depression symptoms with biologic therapy use was unchanged, but the confidence intervals were slightly broader and the association became nonsignificant (OR, 1.86; 95% CI, 0.98–3.54). After further adjustment for disease activity, the point estimate increased slightly (OR, 2.02; 95% CI, 1.02–4.00). We observed an interaction between IBD type and between-person differences in depression symptoms. Among participants with CD on average, elevated symptoms of depression were not associated with increased odds of using biologic therapy. In contrast, among participants with UC, elevated symptoms of depression were associated with increased odds of using biologic therapy.
Table 4.
Association of Within-individual and Between-individual Effects of Anxiety and Depression With Use of Biologic Therapy
| Depression | Anxiety | |||
|---|---|---|---|---|
| Between-effect OR (95% CI) | Within-effect OR (95% CI) | Between-effect OR (95% CI) | Within-effect OR (95% CI) | |
| Unadjusted | 0.78 (0.18–3.46) | 1.88 (1.13–3.13) | 1.79 (0.75–4.27) | 0.96 (0.77–1.20) |
| Adjusteda | 0.58 (0.089–3.80) | 1.86 (0.98–3.54) | 1.66 (0.66–4.16) | 0.95 (0.72–1.25) |
| CD | 0.26 (0.04–2.04) | |||
| UC | 49.9 (4.30–580.7) | |||
| Adjusteda,b | 0.76 (0.11–5.24) | 2.02 (1.02–4.00) | 1.83 (0.67–4.97) | 0.98 (0.74–1.30) |
| CD | 0.37 (0.044–3.18) | |||
| UC | 71.0 (6.65–757.9) |
For depression, the within-effect indicates the effect on disease activity of a change from no elevation in symptoms of depression to elevated symptoms of depression within an individual from one visit to another. The between-effect indicates the effect on disease activity of an individual having elevated symptoms of depression compared with an individual who did not have elevated symptoms of depression. The anxiety variables are analogous to those for depression. aAdjusted for age, age at IBD onset, gender, education, current smoking status, number of physical comorbidities. bAdjusted for disease activity
When we repeated the analysis after excluding the 25% of participants with IBS, our findings were consistent with those of the primary analysis (Supplemental Table 3). Specifically, within-persons, elevation in symptoms of depression over time was associated with increased odds of using biologic therapy—whether we adjusted for disease activity (OR, 2.72; 95% CI, 1.14–6.50) or not (OR, 2.72; 95% CI, 1.14–6.50). At enrollment, only 1 participant was using a non-TNF biologic therapy, but this reached 15 participants by the final visit. When we repeated the analysis excluding participants who used a non-TNF biologic therapy, within-persons elevation in symptoms of depression over time was associated with increased odds of using TNF therapy (OR, 2.36; 95% CI, 1.33–4.20).
Discussion
In this longitudinal cohort study, we tested the association between elevated symptoms of depression and anxiety and disease activity in IBD. Elevated symptoms of depression affected nearly 1 in 17 participants at any assessment time, whereas elevated symptoms of anxiety affected 1 in 5 to 6, regardless of the subtype of IBD. We found that elevated symptoms of depression and anxiety were much more common among persons with active disease than those with inactive disease. On average, participants with elevated depression symptoms were six-fold more likely to have active disease than participants without elevated symptoms, but elevated anxiety symptoms were only two-fold as likely to be associated with active disease. Strikingly, within-person fluctuations of depressive symptoms were also strongly associated with increased odds of disease activity and of using biologic therapy.
In a systematic review, earlier studies (generally with follow-up periods of 1–2 years) were found to report variable associations between depressive symptoms and disease activity or course in IBD.5 Among 3 prospective studies in ulcerative colitis, depressive symptoms were not associated with disease relapse. Among 4 prospective studies in Crohn’s disease, 3 found that elevated depressive symptoms at enrollment were associated with active disease, measured using the Crohn’s Disease Activity Index. Four additional studies enrolled participants with ulcerative colitis and Crohn’s disease, with mixed results. Overall, only 3 studies measured symptoms of depression and anxiety,14–16 all using the same instrument (HADS) that we used. In the smallest of these studies, which enrolled 75 persons with UC who were in remission, depression was not associated with time to relapse over 1 year.15 Among 468 participants with Crohn’s disease from Switzerland followed for 18 months, anxiety was associated with increased 1.32-fold odds of disease exacerbation after accounting for perceived stress.14 In a separate model, depression was associated with 1.78-fold increased odds of disease exacerbation. In another Swiss study involving 2870 participants with IBD, elevated symptoms of depression predicted symptomatic relapse as measured by the Crohn’s Disease Activity Index for Crohn’s disease and the Modified Truelove and Witts Activity Index for ulcerative colitis.16 Elevated symptoms of anxiety also predicted relapse in Crohn’s disease.16 In an online study from the United States, depression symptoms as measured by the Patient Health Questionnaire-5 at baseline were associated with an increased risk for symptomatic flare, hospitalizations, and surgeries at 22 months in persons with CD.17
We did not assess active inflammation using a biomarker; therefore, it is uncertain whether active disease reflected active symptoms and inflammation or simply active symptoms. There is a moderate association between active inflammation and active symptoms in ulcerative colitis, but this association is weak in Crohn’s disease.18 Persons with active disease were more likely to have self-reported IBS (33% vs 19%), which may be associated with gastrointestinal symptoms in the absence of inflammation. However, when we excluded persons with IBS, an increase in depression symptoms within-persons remained associated, with a two-fold to three-fold increased odds of active disease.
Symptoms in Crohn’s disease can also be associated with noninflammatory strictures or postoperative adhesions or bacterial overgrowth. Yet even if active disease in our study only reflected active symptoms, the strong association with elevated depression symptoms is important because symptoms drive health care utilization and negatively impact quality of life.19 Some of our findings suggest that depressive symptoms were indeed associated with active inflammation. Specifically within-persons, the development of elevated depressive symptoms was associated with two-fold increased odds of using biologic therapy, which presumably would have been prescribed for at least moderately active inflammation.
Previous research from Manitoba has shown that high perceived stress was the only significant predictor of a flare of symptoms over the 3 months after a baseline assessment.18,20 This association between high perceived stress and active symptoms was bidirectional.21 Hence in this current study, it is also possible that high disease activity was enhancing depression symptoms in those with a tendency to develop depression symptoms.
Strengths of this study include the longitudinal design, inclusion of participants from community and tertiary care centers, high retention rate, use of validated measures with IBD-confirmed cut points for assessing symptoms of depression and anxiety, and consideration of between-person and within-person associations between psychiatric symptoms and disease activity. We also accounted for potentially important confounders including sociodemographic characteristics, health behaviors, and physical comorbidities. Limitations should also be noted. As described previously, we did not capture a biomarker of active inflammation; therefore, it is uncertain if the active disease reflected active symptoms and active inflammation or simply active symptoms. Limited data suggest that antidepressants may influence disease activity.22 We did not capture use of psychotropic therapies for depression and anxiety; however, these therapies lie in the causal pathway between symptoms of depression and anxiety and disease activity, and their inclusion in the regression models would have led to overadjustment bias.23 We examined the association between changes in depression and anxiety symptoms and disease activity in a prevalent cohort. Future work should examine these relationships in an incident cohort, including whether the influence of depression and anxiety symptoms at time of IBD onset or diagnosis on disease activity differs from the influence of these symptoms developing later in the disease course. Finally, we may not have measured all relevant confounders; however in the within-person analysis, each participant effectively acts as their own control, accounting for fixed, measured, and unmeasured characteristics of that person.
Debate persists as to the value of universal screening for depression in chronic disease populations,24, 25 but enhanced case finding, using either formal or informal approaches, is likely to be valuable. In a Spanish study, only 25% of patients with heart disease, diabetes, or stroke reported that their physicians ever asked them about their mental health.26 Similarly, we have found that depression and anxiety disorders are underdiagnosed in IBD.27 Our findings and those of other researchers indicate that elevated symptoms of depression and anxiety have broad consequences for IBD, therefore reducing symptoms of depression and anxiety should also be a goal of IBD management. Although it has not been well studied whether treating abnormal mental health in IBD will improve the course of disease, these symptoms are modifiable. Smoking is one of the few risk factors shown to be associated with a worse course of Crohn’s disease in Western countries, and there is uniform agreement that smoking cessation is an important goal in Crohn’s disease management.28 Multiple simple mental health assessment tools are highly sensitive and specific in the IBD population.8 Because screening is not helpful unless it is coupled with effective symptom management, our findings also highlight the need for integrated models of care in the management of IBD.29 Prevention and early detection of depression could potentially reduce not only an individual patient’s suffering but also health care utilization and associated costs.30
Supplementary Material
Acknowledgements
Members of the CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease are Charles N. Bernstein, Ruth Ann Marrie, James M Bolton, Jitender Sareen, John R. Walker, Scott B. Patten, Alexander Singer, Lisa M. Lix, Carol A. Hitchon, Renée El-Gabalawy, Alan Katz, John D. Fisk, Lesley Graff, Lindsay Berrigan, Ryan Zarychanski, Christine Peschken, James Marriott, and Kaarina Kowalec. Dr. Lix is the Canada Research Chair. Dr. El-Gabalawy has received funding from University of Manitoba Start-Up Funding and the CIHR Chronic Pain SPOR Network. Dr. Sareen has received CIHR grant #333252. The sponsors had no role in the design and conduct of the study, collection and interpretation of the data, nor in the decision to submit the manuscript for publication. The authors acknowledge the Manitoba Centre for Health Policy for use of the Manitoba Population Research Data Repository. The results and conclusions presented are those of the authors, and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred.
Supported by: This study was funded by the Waugh Family Foundation MS Society of Canada Operating Grant (EGID 2639), Canadian Institutes of Health Research (THC-135234), Crohn’s and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis (RM), and a Research Manitoba Chair (RM). CB is supported in part by the Bingham Chair in Gastroenterology. SP holds the Cuthbertson & Fischer Chair in Pediatric Mental Health at the University of Calgary.
Author Contribution: RAM, LAG, JDF, SBP, and CNB contributed to the study concept and design. RAM, LAG, JDF, SBP, and CNB contributed to the data analysis and interpretation of the data. RAM and CNB wrote the initial draft of manuscript. RAM, LAG, JDF, SBP, and CNB critically revised the manuscript. RAM and CNB obtained study funding. All authors read and approved the final manuscript.
Conflicts of Interest: CB is on advisory boards for Abbvie Canada, Janssen Canada, Takeda Canada, and Pfizer Canada; he is a consultant for Mylan Pharmaceuticals; he receives educational grants from Abbvie Canada, Pfizer Canada, Shire Canada, Takeda Canada, and Janssen Canada; he is on the speaker’s panels for Abbvie Canada, Janssen Canada, Takeda Canada, and Medtronic Canada and has received research funding from Abbvie Canada. The other authors have no conflicts to report.
REFERENCES
- 1. Marrie RA, Walld R, Bolton JM, et al. ; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease . Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosom Res. 2017;101:17–23. [DOI] [PubMed] [Google Scholar]
- 2. Szigethy EM, Allen JI, Reiss M, et al. White paper AGA: the impact of mental and psychosocial factors on the care of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017;15:986–997. [DOI] [PubMed] [Google Scholar]
- 3. Click B, Ramos Rivers C, Koutroubakis IE, et al. Demographic and clinical predictors of high healthcare use in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Limsrivilai J, Stidham RW, Govani SM, et al. Factors that predict high health care utilization and costs for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2017;15:385–392.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alexakis C, Kumar S, Saxena S, et al. Systematic review with meta-analysis: the impact of a depressive state on disease course in adult inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:225–235. [DOI] [PubMed] [Google Scholar]
- 6. Marrie RA, Graff L, Walker JR, et al. Effects of psychiatric comorbidity in immune-mediated inflammatory disease: protocol for a prospective study. JMIR Res Protoc. 2018;7:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 8. Bernstein CN, Zhang L, Lix LM, et al. ; CIHR Team in Defining the Burden and Managing the Effects of Immune-mediated Inflammatory Disease . The validity and reliability of screening measures for depression and anxiety disorders in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- 10. Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13:833–837. [DOI] [PubMed] [Google Scholar]
- 11. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 12. Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Himmerich H, Fulda S, Linseisen J, et al. Depression, comorbidities and the TNF-alpha system. Eur Psychiatry. 2008;23:421–429. [DOI] [PubMed] [Google Scholar]
- 14. Cámara RJ, Schoepfer AM, Pittet V, et al. ; Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS) Group . Mood and nonmood components of perceived stress and exacerbation of Crohn’s disease. Inflamm Bowel Dis. 2011;17:2358–2365. [DOI] [PubMed] [Google Scholar]
- 15. Langhorst J, Hofstetter A, Wolfe F, et al. Short-term stress, but not mucosal healing nor depression was predictive for the risk of relapse in patients with ulcerative colitis: a prospective 12-month follow-up study. Inflamm Bowel Dis. 2013;19:2380–2386. [DOI] [PubMed] [Google Scholar]
- 16. Mikocka-Walus A, Pittet V, Rossel JB, von Känel R; Swiss IBD Cohort Study Group . Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:829–835.e1. [DOI] [PubMed] [Google Scholar]
- 17. Kochar B, Barnes EL, Long MD, et al. Depression is associated with more aggressive inflammatory bowel disease. Am J Gastroenterol. 2018;113:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Targownik LE, Sexton KA, Bernstein MT, et al. The relationship among perceived stress, symptoms, and inflammation in persons with inflammatory bowel disease. Am J Gastroenterol. 2015;110:1001–12; quiz 1013. [DOI] [PubMed] [Google Scholar]
- 19. Shafer LA, Walker JR, Chhibba T, et al. Independent validation of a self-report version of the IBD disability index (IBDDI) in a population-based cohort of IBD patients. Inflamm Bowel Dis. 2018;24:766–774. [DOI] [PubMed] [Google Scholar]
- 20. Bernstein CN, Singh S, Graff LA, et al. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. 2010;105:1994–2002. [DOI] [PubMed] [Google Scholar]
- 21. Sexton KA, Walker JR, Graff LA, et al. Evidence of bidirectional associations between perceived stress and symptom activity: a prospective longitudinal investigation in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:473–483. [DOI] [PubMed] [Google Scholar]
- 22. Macer BJ, Prady SL, Mikocka-Walus A. Antidepressants in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2017;23:534–550. [DOI] [PubMed] [Google Scholar]
- 23. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levis B, Benedetti A, Ioannidis JPA, et al. Patient Health Questionnaire-9 scores do not accurately estimate depression prevalence: individual participant data meta-analysis. J Clin Epidemiol. 2020;122:115–128.e1. [DOI] [PubMed] [Google Scholar]
- 25. Jani BD, Purves D, Barry S, et al. Challenges and implications of routine depression screening for depression in chronic disease and multimorbidity: a cross sectional study. Plos One. 2013;8:e74610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marín-Jiménez I, Gobbo Montoya M, Panadero A, et al. Management of the psychological impact of inflammatory bowel disease: perspective of doctors and patients-the ENMENTE project. Inflamm Bowel Dis. 2017;23:1492–1498. [DOI] [PubMed] [Google Scholar]
- 27. Lewis K, Marrie RA, Bernstein CN, et al. ; CIHR Team in Defining the Burden and Managing the Effects of Immune-Mediated Inflammatory Disease . The prevalence and risk factors of undiagnosed depression and anxiety disorders among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1674–1680. [DOI] [PubMed] [Google Scholar]
- 28. Alexakis C, Saxena S, Chhaya V, et al. Smoking status at diagnosis and subsequent smoking cessation: associations with corticosteroid use and intestinal resection in Crohn’s disease. Am J Gastroenterol. 2018;113:1689–1700. [DOI] [PubMed] [Google Scholar]
- 29. Schoenfeld R, Nguyen G, Bernstein CN. Integrated care models: optimizing adult ambulatory care in inflammatory bowel disease. J Can Assoc Gastroenterol 2020;3:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lores T, Goess C, Mikocka-Walus A, et al. Integrated psychological care reduces healthcare costs at a hospital-based inflammatory bowel disease service. Clin Gastroenterol Hepatol 2021;19(1):96–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.