Abstract
Objective
To assess the diagnostic test accuracy of two rapid antigen tests in asymptomatic and presymptomatic close contacts of people with SARS-CoV-2 infection on day 5 after exposure.
Design
Prospective cross sectional study.
Setting
Four public health service covid-19 test sites in the Netherlands.
Participants
4274 consecutively included close contacts (identified through test-and-trace programme or contact tracing app) aged 16 years or older and asymptomatic for covid-19 when requesting a test.
Main outcome measures
Sensitivity, specificity, and positive and negative predictive values of Veritor System (Beckton Dickinson) and Biosensor (Roche Diagnostics) rapid antigen tests, with reverse-transcriptase polymerase chain reaction (RT-PCR) testing as reference standard. The viral load cut-off above which 95% of people with a positive RT-PCR test result were virus culture positive was used as a proxy of infectiousness.
Results
Of 2678 participants tested with Veritor, 233 (8.7%) had a RT-PCR confirmed SARS-CoV-2 infection of whom 149 were also detected by the rapid antigen test (sensitivity 63.9%, 95% confidence interval 57.4% to 70.1%). Of 1596 participants tested with Biosensor, 132 (8.3%) had a RT-PCR confirmed SARS-CoV-2 infection of whom 83 were detected by the rapid antigen test (sensitivity 62.9%, 54.0% to 71.1%). In those who were still asymptomatic at the time of sampling, sensitivity was 58.7% (51.1% to 66.0%) for Veritor (n=2317) and 59.4% (49.2% to 69.1%) for Biosensor (n=1414), and in those who developed symptoms were 84.2% (68.7% to 94.0%; n=219) for Veritor and 73.3% (54.1% to 87.7%; n=158) for Biosensor. When a viral load cut-off was applied for infectiouness (≥5.2 log10 SARS-CoV-2 E gene copies/mL), the overall sensitivity was 90.1% (84.2% to 94.4%) for Veritor and 86.8% (78.1% to 93.0%) for Biosensor, and 88.1% (80.5% to 93.5%) for Veritor and 85.1% (74.3% to 92.6%) for Biosensor, among those who remained asymptomatic throughout. Specificities were >99%, and positive and negative predictive values were >90% and >95%, for both rapid antigen tests in all analyses.
Conclusions
The sensitivities of both rapid antigen tests in asymptomatic and presymptomatic close contacts tested on day 5 onwards after close contact with an index case were more than 60%, increasing to more than 85% after a viral load cut-off was applied as a proxy for infectiousness.
Introduction
The cornerstone of control during the covid-19 pandemic has been the implementation of generic infection control measures (hand hygiene, physical distancing, and staying at home when symptoms develop) combined with test-and-trace programmes. Mathematical modelling studies have shown that test-and-trace programmes in combination with generic infection control measures can successfully control SARS-CoV-2 epidemics, even when assuming that at least 40% of transmissions might result arise from asymptomatic people or those whose symptoms have not yet developed.1 2 Such measures can only reduce the reproductive number below 1.0, however, when delays in test and trace are minimised.3 4 In test-and-trace programmes, contacts of infected people are actively traced and offered testing, initially only when symptoms develop, but increasingly also when asymptomatic or before symptoms develop.5
In the first phase of the pandemic, only people with symptoms had access to (free-of-charge) testing at Dutch public health service test sites, and testing was performed using reverse-transcriptase polymerase chain reaction (RT-PCR) of combined oral and nasal or nasopharyngeal swabs. The sensitivities of these tests increase as the upper respiratory tract viral load increases, which is known to reach a high plateau on day 5 after infection.6 7 8 9 From 1 December 2020 onwards, it was possible to test asymptomatic close contacts of index cases on day 5 after exposure.
Although RT-PCR is considered the reference test for SARS-CoV-2, it also has disadvantages. RT-PCR testing platforms are typically only available in centralised laboratories and require sample batching, thereby introducing delays in testing. Point-of-care SARS-CoV-2 tests soon became available and, of these, rapid lateral flow antigen diagnostic tests are promising.10 These tests require no or minimal equipment, provide a result within minutes, and can be performed in a range of settings with relatively little training.
Multiple studies have now compared rapid antigen tests with RT-PCR testing.11 Based on these studies, the Dutch Ministry of Health concluded at the end of 2020 that the performance of rapid antigen tests was sufficient to be used in the Dutch public health service test sites in those with mild to moderate symptoms without the need for retesting with RT-PCR.12 The ministry recommended to continue RT-PCR testing in those with severe symptoms, at risk medical groups, people working in high risk settings such as hospitals, and asymptomatic or presymptomatic close contacts.13 The reason being that the diagnostic accuracies of rapid antigen tests were expected to be lower in asymptomatic people and in samples containing lower SARS-CoV-2 viral loads.11 14 This is not necessarily problematic if lower viral load translates into lower subsequent expected infectiousness.15 A recently published Cochrane review showed that 12 evaluations of rapid antigen tests in asymptomatic people had been performed up to 30 September 2020, of which only four took into account exposure to an index case.16 The identified studies were small and did not perform virus culture. Evaluations published since 1 October 2020 had the same limitations.11 17 18 19 In the current study we quantified the accuracy of two rapid antigen tests for detecting SARS-CoV-2 infection with RT-PCR testing as the reference standard in asymptomatic and presymptomatic close contacts of index cases.
Methods
Study design and population
This prospective cross sectional diagnostic test accuracy study was embedded within the Dutch routine testing infrastructure. In the Netherlands, asymptomatic and presymptomatic close contacts can be identified by either the Dutch public health service test-and-trace programme, the Dutch contact tracing mobile phone application (the CoronaMelder app), an individual with confirmed SARS-CoV-2 infection (index case), or a combination of these. Between 1 December 2020 and the end of the study period, Dutch testing policy stipulated that asymptomatic and presymptomatic close contacts should schedule an RT-PCR test from the fifth day onwards after the last exposure. As individuals are generally not tested on the day of their test request, some might develop symptoms by the time of sampling. Close contacts who were still asymptomatic at the time of sampling and had a negative RT-PCR test result ≥5 days after exposure were encouraged to get retested if they developed symptoms and to avoid close contact with vulnerable people.20
Participants were recruited consecutively at four Dutch public health service test sites, located in the West-Brabant region (Raamsdonksveer and Roosendaal) and in the city of Rotterdam (Rotterdam Ahoy and Rotterdam The Hague Airport; travellers were excluded). Close contacts, presenting at these test sites, were considered eligible if they were aged 16 years or older, scheduled for a test ≥5 days after exposure, asymptomatic at the time of the test request, and willing and able to sign an informed consent in Dutch.
Inclusion procedure
Participants arrived at the test sites by car (West-Brabant) or on foot (Rotterdam). Test site staff verbally verified study eligibility. Eligible individuals received a study flyer and a participant information letter. After signing the informed consent form, a short questionnaire on presence, type, and onset of symptoms (see supplementary material 1) was self-completed by participants (West-Brabant) or by test site staff (Rotterdam) while participants waited for sampling. Two people independently extracted questionnaire data in duplicate.
Specimen collection and testing and virus culture procedures
Supplementary material 2 provides a detailed description of how the specimens were collected and tested, including culturing. A trained staff member took two combined oropharyngeal-nasal (West-Brabant) or oronasopharyngeal (Rotterdam) swabs from the study participant: one for RT-PCR testing and the other for rapid antigen testing. Swabs were transported to relevant offsite and onsite laboratories, respectively.
During the study period, all study sites were using Roche cobas 6800/8800 platforms for RT-PCR testing (supplementary material 2). The sites in West-Brabant were using the BD Veritor System (Becton Dickinson, Franklin Lakes, NJ) and the Rotterdam sites the Biosensor test (Roche Diagnostics, Basel, Switzerland). Both tests were applied according to the manufacturer’s instructions. The results of Veritor were determined visually instead of using a Veritor Plus Analyzer. Interpretation of the rapid antigen test results was always done before (thus staff were blinded) the RT-PCR result. Similarly, the results of the rapid antigen tests were not available to those assessing the RT-PCR results. Participants received the RT-PCR test result, but not the rapid antigen test result, to direct further management (such as quarantaine advice).
At the Erasmus Medical Center Viroscience diagnostic laboratory, samples from participants in Rotterdam with a positive RT-PCR test result were cultured for seven days. Once cytopathic effects were visible, the presence of SARS-CoV-2 was confirmed with immunofluorescent detection of SARS-CoV-2 nucleocapsid protein (Rabbit polyclonal antibody; Sino Biological, Eschborn, Germany; supplementary material 2). Samples from participants in West-Brabant were not cultured.
Outcomes and statistical analyses
The primary outcome was the diagnostic accuracy (sensitivity, specificity, and positive and negative predictive values with corresponding 95% confidence intervals) of each rapid antigen test, with RT-PCR as reference standard. The Roche cobas platforms for RT-PCR testing were used according to the manufacturer’s instructions; amplification curves and cycle threshold values were interpreted using the manufacturer’s interpretation algorithms, which complied with the European in-vitro diagnostic devices directive. As the number of individuals without RT-PCR or rapid antigen test results was low (n=21 (0.5%); fig 1), we performed a complete case analysis.
Fig 1.
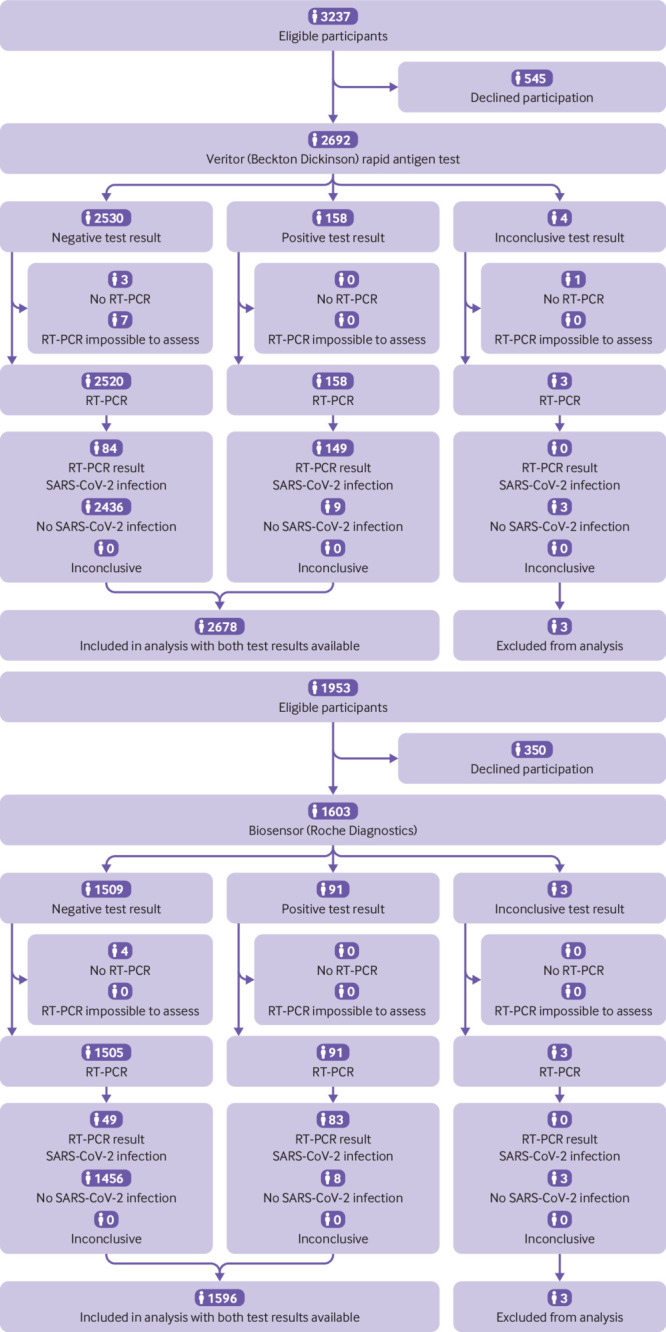
Flow of study participants. RT-PCR=reverse-transcriptase polymerase chain reaction
Secondary outcomes included the diagnostic accuracy variables stratified for presence of covid-19-like symptoms between the test request and time of sampling (yes or no), number of days between last contact and date of sampling (<5, 5, and >5 days), and different viral load cut-offs and viral load cut-off above which 95% of people with a positive RT-PCR test result had a positive culture as a proxy of infectiousness. Cycle threshold values were first converted into viral loads (SARS-CoV-2 E gene copies/mL) using a standard curve (supplementary material 2). The infectiousness cut-off was defined as the viral load above which 95% of people with a RT-PCR test result showed in vitro infectivity in cell culture.
Finally, to capture any missed infections by the day 5 RT-PCR test, we prespecified the use of the SARS-CoV-2 test result databases of participating public health services to determine through pseudonymised linkage whether study participants with a negative day 5 RT-PCR test result had tested positive in the subsequent 10 days by either RT-PCR test or a rapid antigen test.
Sample size calculation
Previous performance studies of rapid antigen tests in people with covid-19 symptoms found sensitivities of around 85%.11 15 21 22 We based our sample size calculation on an expected sensitivity of 80%, with a margin of error of 7%, type I error of 5%, and power of 90%. We therefore aimed for 140 positive RT-PCR test results for each rapid antigen test compared with RT-PCR test. In our target population, we anticipated a SARS-CoV-2 prevalence (based on RT-PCR testing) of 10%, and closely monitored RT-PCR test positivity proportion over time to prolong recruitment if needed.
Patient and public involvement
Patients and the public were indirectly involved in this research. Strong signals and requests were made by the public via news outlets and social media to determine whether rapid antigen tests, which provide a test result quicker than RT-PCR testing, can also be used to test close contacts of individuals already infected with SARS-CoV-2 at day 5 since the contact with the index case. Because the pandemic was at its height in the Netherlands, the urgency of the study, and the short time from conception to conduct of the study, we did not reach out actively to individuals outside our large, multidisciplinary study group.
Results
Between 14 December 2020 and 6 February 2021, 5190 people were considered eligible for participation of whom 4295 participated (fig 1). Results for both RT-PCR and rapid antigen tests were available for 2678 (99.5%) in the Veritor group and 1596 (99.6%) in the Biosensor group. The Veritor and Biosensor groups were similar: the mean ages were 45.9 (SD 17.6) years and 40.7 (SD 16.4) years, respectively, 1370 (51.3%) and 751 (47.3%) were female participants, and 219 (8.6%) and 158 (10.1%) had developed symptoms at the time of sampling (supplementary table S1).
In the Veritor group, 233 (8.7%) participants had an RT-PCR confirmed SARS-CoV-2 infection; of these, 149 were also detected by Veritor resulting in an overall sensitivity of 63.9% (95% confidence interval 57.4% to 70.1%; table 1). Specificity and positive and negative predictive values were 99.6% (99.3% to 99.8%), 94.3% (89.5% to 97.4%), and 96.7% (95.9% to 97.3%), respectively. In the Biosensor group, 132 (8.3%) participants had an RT-PCR confirmed SARS-CoV-2 infection; of these, 83 were also detected by Biosensor resulting in an overall sensitivity of 62.9% (54.0% to 71.1%). Specificity and positive and negative predictive values were 99.5% (98.9% to 99.8%), 91.2% (83.4% to 96.1%), and 96.7% (95.7% to 97.6%), respectively.
Table 1.
Diagnostic accuracy variables of two rapid antigen tests. Values are percentages (95% confidence interval) unless stated otherwise
| Analysis | No | Prevalence* (%) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Veritor System (Beckton Dickinson) | ||||||
| Primary analysis | 2678 | 8.7 | 63.9 (57.4 to 70.1) | 99.6 (99.3 to 99.8) | 94.3 (89.5 to 97.4) | 96.7 (95.9 to 97.3) |
| Secondary (stratified) analysis: | ||||||
| Infectiousness viral load cut-off† | 2677‡ | 5.7 | 90.1 (84.2 to 94.4) | 99.2 (98.8 to 99.5) | 87.3 (81.0 to 92.0) | 99.4 (99.0 to 99.7) |
| Symptoms present at sampling§: | ||||||
| Yes | 219 | 17.4 | 84.2 (68.7 to 94.0) | 99.4 (97.0 to 100) | 97.0 (84.2 to 99.9) | 96.8 (93.1 to 98.8) |
| No | 2317 | 7.7 | 58.7 (51.1 to 66.0) | 99.6 (99.3 to 99.8) | 92.9 (86.5 to 96.9) | 96.6 (95.8 to 97.4) |
| Interval (days) between sampling and last contact with index case¶: | ||||||
| <5 | 379 | 14.8 | 69.6 (55.9 to 81.2) | 99.7 (98.3 to 100) | 97.5 (86.8 to 99.9) | 95.0 (92.1 to 97.1) |
| 5 | 1303 | 6.5 | 62.4 (51.2 to 72.6) | 99.9 (99.5 to 100) | 98.1 (90.1 to 100) | 97.4 (96.4 to 98.2) |
| >5 | 511 | 9.0 | 56.5 (41.1 to 71.1) | 99.1 (97.8 to 99.8) | 86.7 (69.3 to 96.2) | 95.8 (93.7 to 97.4) |
| Biosensor (Roche Diagnostics) | ||||||
| Primary analysis | 1596 | 8.3 | 62.9 (54.0 to 71.1) | 99.5 (98.9 to 99.8) | 91.2 (83.4 to 96.1) | 96.7 (95.7 to 97.6) |
| Secondary (stratified) analysis: | ||||||
| Infectiousness viral load cut-off† | 1596 | 5.7 | 86.8 (78.1 to 93.0) | 99.2 (98.6 to 99.6) | 86.8 (78.1 to 93.0) | 99.2 (98.6 to 99.6) |
| Symptoms present at sampling§: | ||||||
| Yes | 158 | 19.0 | 73.3 (54.1 to 87.7) | 98.4 (94.5 to 99.8) | 91.7 (73.0 to 99.0) | 94.0 (88.6 to 97.4) |
| No | 1414 | 7.1 | 59.4 (49.2 to 69.1) | 99.5 (99.0 to 99.8) | 90.9 (81.3 to 96.6) | 97.0 (95.9 to 97.8) |
| Interval (days) between sampling and last contact with index case¶: | ||||||
| <5 | 153 | 13.1 | 75.0 (50.9 to 91.3) | 99.2 (95.9 to 100) | 93.8 (69.8 to 99.8) | 96.4 (91.7 to 98.8) |
| 5 | 1095 | 7.8 | 61.2 (50.0 to 71.6) | 99.5 (98.9 to 99.8) | 91.2 (80.7 to 97.1) | 96.8 (95.6 to 97.8) |
| >5 | 205 | 6.3 | 69.2 (38.6 to 90.9) | 99.5 (97.1 to 100) | 90.0 (55.5 to 99.7) | 97.9 (94.8 to 99.4) |
PPV=positive predictive value; NPV=negative predictive value.
SARS-CoV-2 infection based on reverse-transcriptase polymerase chain reaction (RT-PCR) test result.
Viral load cut-off for infectiousness, defined as viral load above which 95% of people with a positive RT-PCR test result had a positive viral culture, was 5.2 log10 SARS-CoV-2 E gene copies/mL.
Viral load unavailable for one participant in Veritor group with a positive RT-PCR test result.
Symptoms not available for 142 participants in Veritor group and 24 in Biosensor group.
Interval between moment of sampling and last contact with an infected individual was not available for 488 participants in Veritor group and 143 in Biosensor group, mainly because this question was added to the questionnaire later in study. Initially, a three item questionnaire was used. Questions 1 and 2 in the five item questionnaire (see supplementary material 1) were added after the first week of the study. The time interval between the last contact and time of sampling is not the same as the time between the test request and time of sampling.
In participants who developed symptoms between the test request and time of sampling, sensitivity was 84.2% (68.7% to 94.0%) for Veritor (n=219; prevalence 17.4%) and 73.3% (54.1% to 87.7%) for Biosensor (n=158; prevalence 19.0%). Specificity and positive and negative predictive values were 99.4% (97.0% to 100%), 97.0% (84.2% to 99.9%), and 96.8% (93.1% to 98.8%) for Veritor, and 98.4% (94.5% to 99.8%), 91.7% (73.0% to 99.0%), and 94.0% (88.6% to 97.4%) for Biosensor, respectively.
In participants who remained asymptomatic up to the time of sampling, sensitivity was 58.7% (51.1% to 66.0%) for Veritor (n=2317; prevalence 7.7%) and 59.4% (49.2% to 69.1%) for Biosensor (n=1414; prevalence 7.1%). Specificity and positive and negative predictive values were 99.6% (99.3% to 99.8%), 92.9% (86.5% to 96.9%), and 96.6% (95.8% to 97.4%) for Veritor, and 99.5% (99.0% to 99.8%), 90.9% (81.3% to 96.6%), and 97.0% (95.9% to 97.8%) for Biosensor, respectively.
Table 1 shows the results of additional secondary analyses. Supplementary tables S2 and S3 show the results for 2×2 tables of all primary and secondary analyses.
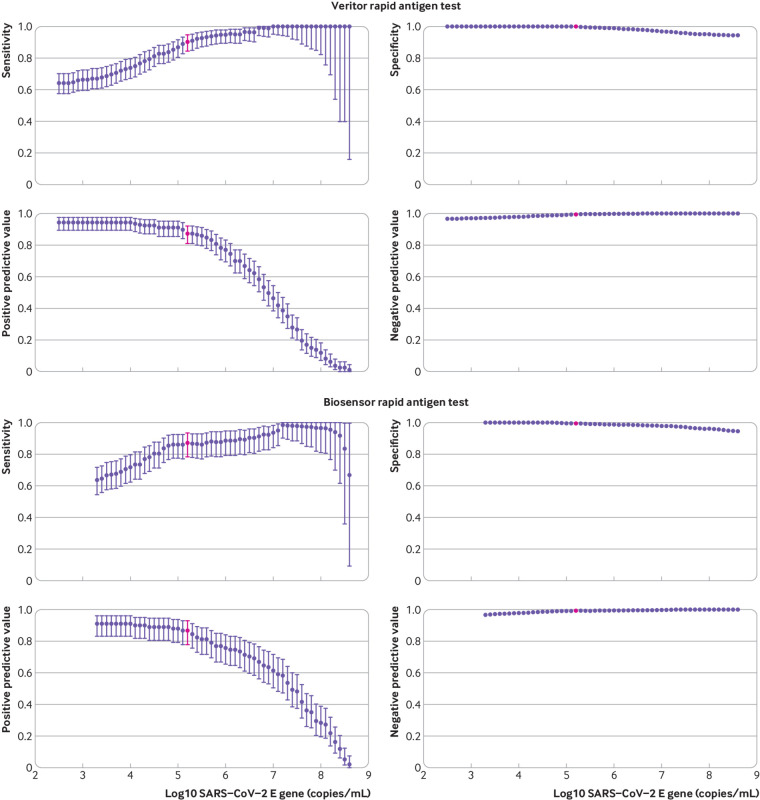
Figure 2 shows the distribution of viral loads in participants with a positive RT-PCR test result, stratified by a combination of the Biosensor test result and the ability to culture SARS-CoV-2. When viral load was ≥5.2 log10 SARS-CoV-2 E gene copies/mL, the specimens of 95% of participants with positive RT-PCR test results could be cultured. The percentage of participants with a viral load ≥5.2 log10 SARS-CoV-2 E gene copies/mL was 5.7% in both groups (152/2677 for Veritor and 91/1596 for Biosensor), and among those with a positive RT-PCR test result, 65.2% (152/233) in the Veritor group and 68.9% (91/132) in the Biosensor group. Using that viral load cut-off as a proxy for infectiousness, sensitivity was 90.1% (84.2% to 94.4%) for Veritor and 86.8% (78.1% to 93.0%) for Biosensor. Specificity and positive and negative preditive values were 99.2% (98.8% to 99.5%), 87.3% (81.0% to 92.0%), and 99.4% (99.0% to 99.7%) for Veritor and 99.2% (98.6% to 99.6%), 86.8% (78.1% to 93.0%), and 99.2% (98.6% to 99.6%) for Biosensor, respectively. Figure 3 shows diagnostic accuracy variables stratified by different viral load cut-offs. The sensitivity of both rapid antigen tests at the infectious viral load cut-off in participants without symptoms at the time of sampling was 88.1% (80.5% to 93.5%) for Veritor (n=2317; prevalence 4.7%) and 85.1% (74.3% to 92.6%) for Biosensor (n=1414; prevalence 4.7%). Specificity and positive and negative predictive values were 99.2% (98.8% to 99.6%), 85.0% (77.0% to 91.0%), and 99.4% (99.0% to 99.7%) for Veritor and 99.3% (98.7% to 99.7%), 86.4% (75.7% to 93.6%), and 99.3% (98.6% to 99.6%) for Biosensor, respectively. Supplementary figure 1 shows the diagnostic accuracy variables for this group at varying viral load cut-offs.
Fig 2.
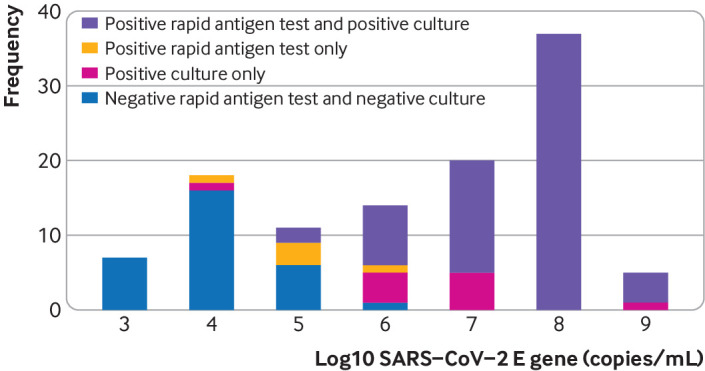
Distribution of viral loads in participants with a positive reverse-transcriptase polymerase chain reaction (RT-PCR) test result, stratified by Biosensor rapid antigen test result (negative or positive) and ability to culture, or not culture, SARS-CoV-2
Fig 3.
Diagnostic accuracy variables of both rapid antigen tests for different definitions of reverse-transcriptase polymerase chain reaction (RT-PCR) test positivity based on viral load cut-offs, where a positive RT-PCR test result with a viral load below the viral load cut-off threshold is considered a negative result. Points highlighted in red indicate a viral load cut-off of 5.2 log10 SARS-CoV-2 E gene copies/mL, which was considered the viral load cut-off for infectiousness as determined by viral culture. PPV=positive predictive value; NPV=negative predictive value
After linkage with test result databases of participating public health services, only 57 (1.6%) participants were found to have a positive SARS-CoV-2 test result within 10 days after their negative day 5 test result by RT-PCR. None of them had a positive day 5 test result by rapid antigen test.
Discussion
The Veritor and Biosensor lateral flow rapid antigen tests are among the most used diagnostic rapid tests for SARS-CoV-2 in the Netherlands but have only been evaluated in people with symptoms of covid-19. We determined the performance of these tests in asymptomatic and presymptomatic close contacts on the fifth day after exposure to an index case. At the time of the study, the prevalence of SARS-CoV-2 in our study population was around 8.5% and in the Dutch testers population as a whole (people with symptoms and asymptomatic or presymptomatic testers combined) around 18%.23 Both tests had a sensitivity of about 63% compared with RT-PCR testing. The sensitivity increased to 87-90% when a viral load cut-off of ≥5.2 log10 SARS-CoV-2 E gene copies/mL was used as a proxy for infectiousness. Specificities and positive and negative predictive values were high in analyses for both tests.
Comparison with other studies
The sensitivities of the tests were expectedly lower than those generally reported for people with symptoms and higher than those for asymptomatic people who are tested at random.11 Our study population consisted of participants who were asymptomatic at test request and developed symptoms between test request and actual testing (about 10%) and those who did not. The sensitivities of the rapid antigen tests were close to 90% in the former group, which is comparable to previous studies of people with symptoms.11 16 The sensitivities were close to 60% in the latter group, which is higher than those reported in previous studies of asymptomatic people.11 16 However, those studies generally focused on those who were tested at random and not because they had been exposed to an index case (close contacts). At the time of our study, the prevalence of SARS-CoV-2 in the Dutch population as a whole was about 2%,17 which is more than four times lower than the prevalence in our study population of those with known exposures to index cases.
RT-PCR as reference standard
We used RT-PCR on Roche cobas platforms as the reference test. RT-PCR tests are considered the preferred reference tests,24 but with one important caveat: previous studies have shown that, on average, viral load and RT-PCR test sensitivity gradually increase in the 5-7 days after infection, reach a plateau that lasts for 1-2 weeks, and then decline.7 By the time people develop symptoms, they generally have a sufficiently high viral load for RT-PCR test sensitivity to be considered optimal. However, the viral load in most people who do not (yet) have symptoms is lower, and RT-PCR test sensitivity might therefore be suboptimal. At the same time, many of these people are able to transmit SARS-CoV-2 to others. The Dutch Ministry of Health dealt with this delicate balance between infectiousness and RT-PCR test sensitivity by encouraging asymptomatic and presymptomatic close contracts of index cases to be tested from the fifth day after exposure to an index case. Our data suggest that this is appropriate because only 1.6% of those with a negative RT-PCR and rapid antigen test result on the fifth day after exposure had a positive test result within the subsequent 10 days.
Virus culture result as proxy for infectiousness
We used the viral load cut-off above which 95% of people with a positive RT-PCR test result had a positive virus culture as a proxy of infectiousness. Although this cut-off is not fully evidence based, it is a best guess based on current knowledge and is less arbitrary than using RT-PCR cycle threshold value cut-offs of 25 or 30, as is often done.21 25 Animal models have provided some evidence for an association between SARS-CoV-2 infectiousness and the ability to culture virus. For example, in a golden hamster model, infectiousness correlated with the detection of infectious virus in culture but not with detection of viral RNA.26 Data from human studies are limited but mounting. Correlations between infectivity in culture and viral load, and between viral load and secondary attack rate, have been established, but variability between laboratories and studies was high.27 28 29 30 Furthermore, the exact upper respiratory tract viral load cut-off below which no transmissions take place is still not known; some reports have suggested infectiousness at viral loads as low as 10 000 SARS-CoV-2 RNA copies/mL.31
The ability to culture virus is not only affected by the viral load of the sample but also by other factors related to the host, type of viral culture kits and methods used, and experience level of the laboratory team. An important host factor is the presence of SARS-CoV-2 neutralising antibodies.25 To address concerns about high variability between laboratories, we performed all virus cultures in one experienced laboratory (at the Erasmus Medical Center Viroscience diagnostic laboratory in Rotterdam)15 21 25 on fresh material (no freeze-thaw cycles). Therefore, only specimens collected by the Rotterdam study sites were cultured, and the infectiousness cut-off was extrapolated to specimens collected by the West-Brabant sites. Reassuringly, the two laboratories had similar RT-PCR test calibration curves, indicating that cycle threshold values corresponded to similar viral loads.
Strengths and limitations of this study
Strengths of our study include the well defined study population, large sample size, collection of samples for the reference and index test at the same time, and reference and index tests performed by trained staff who were blinded to the result of the other test. We also consider the use of virus culture results in our definition of infectiousness to be a strength, despite some of the limitations, because RT-PCR cycle values are even more influenced by laboratory workflow than the methods that we used.
Our study also has limitations. Firstly, we did not assess the type of the close contacts (eg, at home, at work, at school, or on public transport) or duration of the close contacts. This is especially problematic for household contacts, because exposure might take place over a prolonged period, which is associated with a high probability of testing positive (20% v 10%).23 In our study, 12% of the participants reported that their last contact was within the past five days, and this group had a higher RT-PCR test positivity percentage than the study population as a whole. We suspect that they might have been household contacts with prolonged exposure but this cannot be verified. Secondly, we did not actively follow-up participants who had a negative RT-PCR test result at day 5, but we explicitly designed per protocol to apply pseudonomysed linkage of these participants to the test result databases of the participating public health services. The 1.6% of infected close contacts who according to that database had a positive test result within 10 days after their negative day 5 RT-PCR test result in our study likely represent only those who developed symptoms and requested a new test for that reason at one of the participating public health services. Active follow-up, including repeat testing in all study participants, would have reduced the uncertainty around false negative RT-PCR test results completely, as was also recommended in a recent guidance paper.24 Unfortunately, we could not implement this for ethical and logistic constraints, as our study was embedded in busy public health service test sites during the height of the second wave in the Netherlands.
Policy implications
As a result of this study, early in 2021 Dutch test sites implemented the two (and other nationally approved) rapid antigen tests for testing of close contacts, even when they have not (yet) developed symptoms. Close contacts regardless of symptoms are encouraged to get tested as soon as possible after known exposure to avoid delays in identifying people who are positive for SARS-CoV-2. However, if they are tested before the fifth day after exposure, they are retested on the fifth day and remain in quarantine until the fifth day test result is negative. Rapid antigen tests are, however, still not used in high risk situations, such as testing of vulnerable people in care facilities, severly ill patients, or healthcare workers.
The advantages of rapid antigen testing compared with RT-PCR testing include simplified logistics and reduced dependence on equipment (which in turn allow for testing in the community and for self-testing) and reduced delays. The extent to which these advantages outweigh the lower sensitivity compared with RT-PCR testing is currently unknown. With the increasing use of rapid antigen tests instead of RT-PCR testing, we expect the number of missed infections to increase. This underlines the importance of immediate self-quarantine and repeat testing when symptoms develop after a negative result by rapid antigen test or RT-PCR. Furthermore, false positivity of rapid antigen test results was rare in our study but might become a larger issue as the prevalence of SARS-CoV-2 declines. In that case, positive results with rapid antigen tests might have to be confirmed by RT-PCR.32 We will continue to monitor the advantages and disadvantages of rapid antigen tests compared with RT-PCR testing utilising national test-and-trace databases and mathematical modelling.
Conclusions
The sensitivities of both rapid antigen tests compared with RT-PCR tests in asymptomatic and presymptomatic close contacts on the fifth day after exposure to the index case was more than 60%, increasing to more than 85% after a viral load cut-off was applied as a proxy of infectiousness. The Veritor and Biosensor rapid antigen tests can therefore reliably be used to test close contacts for infectiousness from the fifth day after infection, even when they have not (yet) developed symptoms, but the tests should not be used when the consequences of missed infections will be severe.
What is already known on this topic
At the end of 2020, rapid antigen tests had been evaluated and considered sufficient to be used in the Dutch public health service test sites in people with mild to moderate symptoms of covid-19 without the need for retesting with reverse-transcriptase polymerase chain reaction
The few evaluations of rapid antigen tests in asymptomatic people at that time were of small sample size, largely did not account for whether the tested individual had been exposed to an index case, and did not perform virus culture
What this study adds
The sensitivity of both the Veritor (Beckton Dickinson) and the Biosensor (Roche Diagnostics) for detecting SARS-CoV-2 in this population was more than 60%, increasing to more than 85% after a viral load cut-off was applied as a proxy of infectiousness
The results suggest that close contacts of people with confirmed SARS-CoV-2 infection can accurately be tested for SARS-CoV-2 using either rapid antigen test from day 5 onwards, even when they have not (yet) developed symptoms
The tests should not be used when the consequences of missed infections will be severe
Acknowledgments
We thank the participants, study staff at the local public health service test sites, staff at the participating labs, and study staff at the National Institute for Public Health and the Environment (RIVM) who helped process the questionnaires and design and distribute the study forms. A special thanks to Esther Stiefelhagen, Roel Ensing, and Wendy Mouthaan for their efforts in the logistics towards and at the local test sites. Written permission was obtained from all three to include their names. ES, RE, and WH did not receive any compensation for their contributions.
Web extra.
Extra material supplied by authors
Supplementary file: Tables S1-S3, figure S1, and supplementary material 1 and 2
Supplementary file: Excel file showing calculation of 2×2 tables based on diagnostic accuracy of both tests with differing prevalence or sample size
Contributors: KGMM and JHHMvdW initiated the study. ES, IKV, RPV, WvdB, EL, RM, GJS, KB, LH, JHHMvdW, SvdH, and KGMM designed the study. IKV coordinated the study. WvdB, SDP, RM, JV, and RCH were responsible for laboratory analyses and data processing. CHGvK performed virus culture. ES and IKV verified the underlying data. ES performed the statistical analysis in close collaboration with IKV and KGMM. ES, IKV, RPV, SvdH, and KGMM drafted the first version of the manuscript. All authors critically read the manuscript and provided feedback. All authors approved the submission of the current version of the manuscript. ES and IKV contributed equally as first authors. SvdH and KGMM contributed equally as senior authors. KGMM is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Dutch Ministry of Health, Welfare, and Sport for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Funding: This study was funded by the Dutch Ministry of Health, Welfare, and Sport. The funder had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the paper for publication.
The corresponding author (KGMM; the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The Dutch Outbreak Management Team that provides guidance to the Ministry of Health, Welfare, and Sport on policy regarding covid-19, advised, based on the results of this study, that close contacts of people with a confirmed SARS-CoV-2 infection can be tested for SARS-CoV-2 using a rapid antigen test from day 5 onwards, even when they have not (yet) developed covid-19 symptoms. As such, the results of our study have been disseminated and are currently incorporated in a nationwide testing policy. At the time, this change in policy has been covered by different news outlets.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required because the study is outside the scope of the Dutch Medical Research Involving Human Subjects Act (protocol No 20/750). All participants signed an informed consent form before any study procedure.
Data availability statement
Individual participant data collected during the study will be available, after deidentification, of all participants. Data will be available to researchers who provide a methodologically sound proposal to achieve the aims in the approved proposal. Proposals should be directed to the corresponding author to gain access to the data. Data requestors will need to sign a data access agreement.
References
- 1. Ferrari A, Santus E, Cirillo D, et al. Simulating SARS-CoV-2 epidemics by region-specific variables and modeling contact tracing app containment. NPJ Digit Med 2021;4:9. 10.1038/s41746-020-00374-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kucharski AJ, Klepac P, Conlan AJK, et al. CMMID COVID-19 working group . Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis 2020;20:1151-60. 10.1016/S1473-3099(20)30457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferretti L, Wymant C, Kendall M, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020;368:eabb6936. 10.1126/science.abb6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kretzschmar ME, Rozhnova G, Bootsma M, van Boven M, van de Wijgert J, Bonten M. Time is of the essence: impact of delays on effectiveness of contact tracing for COVID-19, a modelling study. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 5.Infectieziektebestrijding RC. COVID-19 Test-and-tracing protocol 2021 [updated 26 Feb 2021. https://lci.rivm.nl/COVID-19-bco.
- 6. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021;2:e13-22. 10.1016/S2666-5247(20)30172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann Intern Med 2020;173:262-7. 10.7326/M20-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauer SA, Grantz KH, Bi Q, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med 2020;172:577-82. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallett S, Allen AJ, Graziadio S, et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med 2020;18:346. 10.1186/s12916-020-01810-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol 2021;19:171-83. 10.1038/s41579-020-00461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RIVM Centrum Infectieziektebestrijding. Status validatie SARS-CoV-2 antigeen sneltesten, 10 Mar 2021, 2021. https://lci.rivm.nl/antigeensneltesten
- 12. WHO . SARS-CoV-2 antigen-detecting rapid diagnostic tests: An implementation guide. WHO, 2020: 48. [Google Scholar]
- 13.RIVM Centrum Infectieziektebestrijding. OMT (Outbreak Management Team) 87 2021 [updated 27 November 2020]. https://www.rivm.nl/omt89 accessed 8 June 2021.
- 14. Boehme C, Hannay E, Sampath R. SARS-CoV-2 testing for public health use: core principles and considerations for defined use settings. Lancet Glob Health 2021;9:e247-9. 10.1016/S2214-109X(21)00006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Beek JI, Igloi Z, Boelsums T, Fanoy E, Gotz H, Molenkamp R, van Kampen J, GeurtsvanKessel C, van der Eijk A, van de Vijver D, Koopmans M. From more testing to smart testing: data-guided SARS-CoV-2 testing choices. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 16. Dinnes J, Deeks JJ, Berhane S, et al. Cochrane COVID-19 Diagnostic Test Accuracy Group . Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2021;3:CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pray IW, Ford L, Cole D, et al. CDC COVID-19 Surge Laboratory Group . Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep 2021;69:1642-7. 10.15585/mmwr.mm695152a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stohr JJJMZ. V.F.; Goderski, G.; Meijer, A.; Nagel-Imming, C.R.S.; Kluytmans-van den Bergh, M.F.Q.; Pas, S.D.; van den Oetelaar, F.; Hellwich, M.; Gan, K.H.; Rietveld, A.; Verweij, J.J.; Murk, J.L.; van den Bijllaardt, W.; Kluytmans, J.A.J.W. Self-testing for the detection of SARS-CoV-2 infection with rapid antigen tests. medRxiv 2021. [DOI] [PMC free article] [PubMed]
- 19. Winkel BMFS. E.; Gremmels, H.; Debast, S.B.; Schuurman, R.; Wensing, A.M.J.; Bonten, M.J.M.; Goedhart, E.; Hofstra, M.; Antigen Rapid Test Validation Group. Screening for SARS-CoV-2 infection in asymptomatic individuals using the Panbio™ COVID-19 Antigen Rapid Test (Abbott) compared to RT-qPCR. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 20.RIVM Centrum Infectieziektebestrijding. COVID-19: What (groups of) persons can be tested using which test(s)? Attachment to Outbreak Management Team advice 94 [Dutch] 2020 [updated 31 December 2020]. https://lci.rivm.nl/sites/default/files/2021-01/COVID-19%20Welke%20testen%20bij%20welke%20groepen.pdf accessed 8 June 2021.
- 21. Igloi Z, Velzing J, van Beek J, et al. Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands. Emerg Infect Dis 2021;27:1323-9. 10.3201/eid2705.204688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van der Moeren N, Zwart VF, Lodder EB, et al. Performance evaluation of a SARS-CoV-2 Rapid antigen test: test performance in the community in The Netherlands. medRxiv 2020.
- 23. RIVM Centrum Infectieziektebestrijding . 2021. https://www.rivm.nl/coronavirus-covid-19/actueel/wekelijkse-update-epidemiologische-situatie-covid-19-in-nederland.
- 24. Doust JA, Bell KJL, Leeflang MMG, et al. Guidance for the design and reporting of studies evaluating the clinical performance of tests for present or past SARS-CoV-2 infection. BMJ 2021;372:n568. 10.1136/bmj.n568 [DOI] [PubMed] [Google Scholar]
- 25. van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun 2021;12:267. 10.1038/s41467-020-20568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020;583:834-8. 10.1038/s41586-020-2342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deeks JJ, Raffle AE. Lateral flow tests cannot rule out SARS-CoV-2 infection. BMJ 2020;371:m4787. 10.1136/bmj.m4787 [DOI] [PubMed] [Google Scholar]
- 28. Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral cultures for COVID-19 infectious potential assessment - a systematic review. Clin Infect Dis 2020;ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee LYW, Rozmanowski S, Pang M, et al. SARS-CoV-2 infectivity by viral load, S gene variants and demographic factors and the utility of lateral flow devices to prevent transmission. Clin Infect Dis 2021;ciab421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marks M, Millat-Martinez P, Ouchi D, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis 2021;21:629-36. 10.1016/S1473-3099(20)30985-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ladoy A, Opota O, Carron PN, et al. Size and duration of COVID-19 clusters go along with a high SARS-CoV-2 viral load: A spatio-temporal investigation in Vaud state, Switzerland. Sci Total Environ 2021;787:147483. 10.1016/j.scitotenv.2021.147483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins S. COVID-19: Reintroducing confirmatory PCR testing Public Health England, 2021 [updated 30 March 2021]. https://publichealthmatters.blog.gov.uk/2021/03/30/covid-19-reintroducing-confirmatory-pcr-testing/ accessed 8 June 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file: Tables S1-S3, figure S1, and supplementary material 1 and 2
Supplementary file: Excel file showing calculation of 2×2 tables based on diagnostic accuracy of both tests with differing prevalence or sample size
Data Availability Statement
Individual participant data collected during the study will be available, after deidentification, of all participants. Data will be available to researchers who provide a methodologically sound proposal to achieve the aims in the approved proposal. Proposals should be directed to the corresponding author to gain access to the data. Data requestors will need to sign a data access agreement.