Abstract
The midcycle luteinizing hormone (LH) surge initiates a cascade of events within the ovarian follicle which culminates in ovulation. Only mural granulosa cells and theca cells express large numbers of LH receptors, and LH-stimulated paracrine mediators communicate the ovulatory signal within the follicle. Recent reports identified the neuropeptide neurotensin (NTS) as a product of granulosa cells. Here, we demonstrate that granulosa cells were the primary site of NTS expression in macaque ovulatory follicles. Granulosa cell NTS mRNA and protein increased after human chorionic gonadotropin (hCG) administration, which substitutes for the LH surge. To identify ovulatory actions of NTS, a NTS-neutralizing antibody was injected into preovulatory macaque follicles. hCG administration immediately followed, and ovaries were removed 48 hours later to evaluate ovulatory events. Follicles injected with control IgG ovulated normally. In contrast, 75% of NTS antibody-injected follicles failed to ovulate, containing oocytes trapped within unruptured, hemorrhagic follicles. Serum progesterone was unchanged. Of the three NTS receptors, SORT1 was highly expressed in follicular granulosa, theca, and endothelial cells; NTSR1 and NTSR2 were expressed at lower levels. Excessive blood cells in NTS antibody-injected follicles indicated vascular anomalies, so the response of monkey ovarian endothelial cells to NTS was evaluated in vitro. NTS stimulated endothelial cell migration and capillary sprout formation, consistent with a role for NTS in vascular remodeling associated with ovulation. In summary, we identified NTS as a possible paracrine mediator of ovulation. Further investigation of the NTS synthesis/response pathway may lead to improved treatments for infertility and novel targets for contraception.
Keywords: angiogenesis, fertility, ovary, primate, sortilin
1 |. INTRODUCTION
Ovulation is the release of a mature, fertilizable oocyte from the ovary. The primary endocrine signal for ovulation is the midcycle surge of luteinizing hormone (LH). Many cells of the ovulatory follicle do not express LH receptors (LHCGRs). Only theca cells and granulosa cells closest to the follicle basement membrane express biologically meaningful numbers of LHCGRs.1,2 The ovulatory LH signal acts at these cells to regulate production of paracrine mediators, which communicate the ovulatory signal to the remainder of the follicle.
Neurotensin (NTS) is a small protein that was recently identified as a candidate paracrine mediator of ovulation. The ovulatory gonadotropin surge increases granulosa cell expression of NTS.3,4 NTS was originally characterized as a neuropeptide, involved in appetite, pain, and certain neurological disorders.5 More recent studies have demonstrated a role for NTS in the gastrointestinal tract, modulating both motility and absorption.6,7 Information on NTS and female reproductive function is limited. NTS and its receptors exhibited differential expression between postpartum and virgin female mice across multiple brain regions, supporting its role for potential involvement in maternal behaviors.8 To our knowledge, there are no published reports describing NTS action in the ovarian follicle, including during the ovulatory period.
Neurotensin acts at its target cells through multiple plasma membrane receptors. NTSR1 and NTSR2 are members of the seven transmembrane domain, G protein-coupled family of receptors.9 A third NTS receptor, SORT1, is also known as sortilin or NTSR3. SORT1 has a single transmembrane domain, with NTS binding to the large extracellular domain.10 Signal transduction pathways activated in response to NTS binding to SORT1 are poorly understood.10 Expression and activity of these NTS receptors in the ovary, and in particular the ovulatory follicle, has not been explored.
These studies were undertaken to determine if NTS is an intrafollicular mediator of ovulation in macaques, a primate species with ovarian function highly similar to that of women. A model of gonadotropin administration and controlled ovulation, along with intrafollicular administration of a NTS-neutralizing antibody,11 was employed. This allowed for evaluation of NTS action in the ovulatory follicle while bypassing possible NTS action in the hypothalamus and anterior pituitary.12,13 These studies also localize expression of NTS and its three receptors to key cell types of the ovulatory follicle. Our studies demonstrate that cells of the ovarian follicle express both NTS and NTS receptors concomitantly during the ovulatory interval, and that NTS may mediate essential events of the ovulatory cascade in primates.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Whole ovaries and ovarian biopsies were obtained from adult female cynomolgus macaques (Macaca fascicularis, aged 4–8 years) at Eastern Virginia Medical School (Norfolk, VA). Animal protocols were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Eastern Virginia Medical School Animal Care and Use Committee. Serum estradiol and progesterone levels were determined using the Immulite 1000 immunoassay system (Siemens, Rockville, MD). Aseptic surgeries were performed under isoflurane anesthesia, followed by postoperative analgesia (buprenorphine and either ketoprofen or meloxicam).14 Non-ovarian tissues (heart, pancreas, adrenal, kidney, and thyroid) were obtained from adult female macaques at necropsy.
Adult baboon adrenal tissue for immunostaining was a gift from Dr Gerald Pepe, Eastern Virginia Medical School, and was obtained as previously detailed.15
2.2 |. Ovarian stimulation
An ovarian stimulation model was used to obtain ovaries with multiple ovulatory follicles.14 Briefly, monkeys received FSH (Merck and Co., Inc, Kenilworth, NJ) and LH (Serono Reproductive Biology Institute, Rockland, MA) to stimulate the growth of multiple follicles. Animals also received a GnRH antagonist (Ganirelix; Merck) daily to prevent an endogenous ovulatory LH surge. At surgery, either ovariectomy or aspiration of ovarian follicles greater than 4 mm was performed before (0), 12, 24, or 36 hours after administration of hCG (Serono). For follicle aspirates, oocytes were removed mechanically, and remaining cells subjected to Percoll gradient centrifugation to enrich for granulosa cells as previously described.16
2.3 |. Controlled ovulation with follicle injection
Controlled ovulation with follicle injection was used to introduce an antibody into the ovulatory follicle as previously detailed.11 Animals were monitored for rising serum estradiol to indicate development of a large preovulatory follicle. Animals then received a GnRH antagonist (Acyline; NICHD, Rockville, MD) to prevent endogenous LH surge concomitant with follicle stimulating hormone (FSH; Merck) and LH (Serono) for 2 days to maintain healthy development of the follicle. On the next day, intrafollicular injection of a rabbit antibody against NTS (ImmunoStar, Hudson, WI; n = 4) or control IgG (Abbiotec, San Diego, CA; n = 5) was performed during aseptic surgery; an estimated 10 micrograms of antibody protein was delivered to each follicle. Immediately postoperatively, hCG (Serono) was administered to initiate ovulatory events. Ovariectomy was performed 48 hours after hCG, with ovulation anticipated at about 40 hours.17 Ovaries were photographed in situ before ovariectomy. Evaluation of ovulation in control IgG-injected ovaries has been previously reported.18,19
2.4 |. Monkey ovarian microvascular endothelial cells (mOMECs)
Replicating populations of ovarian microvascular endothelial cells (mOMECs) were obtained from monkeys experiencing ovarian stimulation and hCG administration. mOMECs were isolated from follicular aspirates or whole ovaries, characterized, and maintained as previously described.20 Cells were amplified in media optimized for growth of microvascular endothelial cells (EGM-2MV, Lonza, Salisbury, MD) and switched to media lacking growth factors and serum (basal medium EMB-2, Lonza) overnight before use in experiments. All treatments were performed in EMB-2 medium. Replicate experiments were performed with mOMECs isolated from different animals.
For isolation of RNA, mOMECs were treated with PGE2 (1 μM; Cayman Chemical, Ann Arbor, MI) or VEGFA (5 ng/mL; R&D Systems, Minneapolis, MN). Cells were harvested 4 or 24 hours after initiation of treatments by lysing for RNA preparation (below).
For the migration assay,20 mOMECs were seeded directly onto cell culture inserts containing membranes with 8 μm pores (BD Biosciences, San Jose, CA), and recombinant human NTS (0–50 μM; Bachem, Torrance, CA) was added to basal medium in the well of the culture plate. After 24 hours, membranes were stained with hematoxylin and eosin (Sigma). Images of migrated cells (five images per membrane) were counted and averaged.
For assessment of proliferation,20 mOMECs were plated onto glass chamber slides (Nunc, Thermo Fisher) until 50% confluent. After overnight incubation with basal medium, medium containing NTS (0–5 μM) was added. Slides were fixed in 10% formalin after 24 hours and used for detection of Ki67 by immunohistochemistry.20 Images (four per chamber) were collected, the status of each cell (Ki67+/Ki67−) was recorded, and an average percent positive was determined.
Sprout formation was assessed by coating microcarrier beads with mOMECs and suspending the cell-coated beads in a three-dimensional fibrin matrix as previously described.20 Cultures were treated with NTS (0–50 μM), and five beads/treatment were photographed after 2 days in culture. Images were assessed for the number of sprouts per bead and the length of each sprout. To confirm that the NTS antibody-neutralized NTS activity, NTS human recombinant protein (500 μM), NTS antibody (1:1000 dilution; ImmunoStar), and NTS + antibody were incubated for 2 hours at room temperature, then diluted for use in the sprout formation assay so that the final concentration of NTS was 5 μM. Photographs were taken after 1 day of culture.
2.5 |. Monkey theca cells
Replicating populations of primary monkey theca cells were isolated from monkey ovaries after a modified ovarian stimulation protocol.21 Briefly, FSH was administered for 6 days, hCG was administered, and ovaries were recovered 48 hours later. Monkey theca cells were isolated from small antral follicles (1–3 mm), cultured, and phenotype verified as previously described.21,22 Theca cells were studied during passages 3–5. Cells were switched to a minimal medium22 overnight before treatment with hCG (20 IU/mL; Sigma-Aldrich, St. Louis, MO) or forskolin (20 μM; Sigma-Aldrich). Cells were harvested 4 or 24 hours after initiation of treatment by lysing for RNA preparation (below).
2.6 |. RNA and quantitative PCR (qPCR)
Total RNA was obtained from granulosa cells, pancreas, and heart using TRIzol reagent (Thermo Fisher, Fair Lawn, NJ) according to manufacturer’s instructions, while total RNA was obtained from cultured monkey theca and endothelial cells using the Qiagen RNeasy Mini Kit (Germantown, MD) according to kit instructions. All RNA was treated with deoxyribonuclease and reverse transcribed as previously described.21 Levels of mRNA for NTS, NTSR1, NTSR2, and SORT1 were assessed by qPCR using a Roche Lightcycler (Roche Diagnostics, Atlanta, GA) and the FastStart DNA Master SYBR Green I kit (Roche) following manufacturer’s instructions. Primers were designed based on human or monkey sequences and span an intron to prevent undetected amplification of genomic DNA (Table S1). PCR products were sequenced (Genewiz, South Plainfield, NJ) to confirm amplicon identity. Sequenced amplicons were quantified and re-amplified to generate a standard curve of known copy number for each PCR assay. All data are initially expressed as the ratio of mRNA of interest to ACTB mRNA for each sample, where a value of 1.0 indicates the same number of copies of mRNA of interest and copies of ACTB in each mRNA sample. For NTS in granulosa cells and all cultured cells, the ratio of mRNA of interest/ACTB for basal samples was set equal to 1.0, and all treatments were expressed relative to basal expression.
2.7 |. Histology
All ovaries were fixed in 10% formalin for 24 hours, embedded in paraffin, and sectioned at 5 μm. Ovaries obtained after follicle injection were embedded in a specific orientation such that sections included the follicle apex and follicle wall opposite the apex at the maximal follicle diameter in order to ensure optimal view of the follicle apex.18 Follicle-injected ovaries were serially sectioned, and every fifth section was stained with hematoxylin and eosin (Sigma) and evaluated by at least two independent observers to identify the oocyte and (if present) condition of the cumulus as well as presence/absence of a rupture site. Rupture site size was quantified by measuring the width on the section with the largest rupture site and counting the number of 5 μm sections where the rupture site was present to calculate the area of an oval. Unruptured follicles were assigned a rupture area of 0 mm2. Whole ovary images were assembled from multiple microscopic images of a single tissue section using Image Composite Editor (Microsoft Corp., Redmond, WA). Granulosa cell layer thickness was assessed as a quantitative metric of luteinization as previously described.18 Briefly, a stained ovarian section was selected which included the maximal diameter of the follicle and the rupture site (if rupture occurred) or thinnest portion of the remaining follicle wall (if rupture did not occur). The granulosa cell layer opposite the apex or thinnest portion of the remaining follicle wall was assessed by determining distance from granulosa cell basement membrane to antral edge of granulosa cells, with eight replicate measurements made for each ovarian tissue section. Additional tissue sections from follicle injection ovaries were stained using the Ayoub-Shklar method, which yields blue collagen fibers and bright red erythrocytes.23 Briefly, sections were stained in 5% of acid fuchsin (Sigma) in water, then dual stained in a solution of 0.5% aniline blue (Santa Cruz Biotech, Dallas, TX), 2% Orange G (Thermo Fisher), and 1% phosphotungstic acid (Sigma) before dehydration and mounting.
2.8 |. Immunohistochemistry
Ovaries obtained after controlled ovarian stimulation were sectioned for immunostaining. Immunostaining was performed on paraffin-embedded tissues sectioned at 5 μm as previously described.18 Immunodetection of NTS and NTSR2 were performed after acidic antigen retrieval (10 mM sodium citrate, 0.05% Tween20; 18), and SORT immunodetection was performed after basic antigen retrieval (10 mM Tris base, 1 mM EDTA, 0.05% Tween20; 18). Slides were blocked, then incubated overnight with rabbit primary antibodies against NTS (ImmunoStar #20072; 1:100 dilution), NTSR1 (Thermo Fisher #PA3–214; 1:400 dilution), NTSR2 (EMD Millipore #AB15134; 20 μg/mL), or SORT1 (Sigma #HPA006889; 4 μg/mL). Omission of the primary antibody served as a negative control. Slides were incubated with DAB substrate using the Vectastain Rabbit ABC kit (Vector Laboratories, Burlingame, CA) according to manufacturer instructions, then counterstained in hematoxylin.
2.9 |. Western blot
Tissue and granulosa cell lysate preparation and western blots were performed essentially as previously described.20 Protein from lysates of tissue (20 μg) or granulosa cells (15 μg) were loaded onto a 3%-8% polyacrylamide gradient gel (Thermo Fisher, Waltham, MA). Proteins were transferred to a polyvinylidene fluoride membrane (Immobilon; Millipore, Billerica, MA). For detection of NTS, the membrane was blocked in 3% of bovine serum albumin, 0.25% of gelatin, and 0.05% of Triton in 10X Tris-buffered saline (TBS) (Santa Cruz Biotechnology SC-24951), while the NTSR2 and SORT1 membranes were blocked in 5% of nonfat dry milk in 10X TBS with 0.1% Tween. Membranes were probed with antibodies against NTS (1:5000; ImmunoStar), NTSR2 (2 μg/mL; EMD Millipore AB15134), or SORT1 (0.4 μg/mL; Sigma HPA006889), then incubated with an anti-rabbit HRP-conjugated secondary antibody (1:10 000; Vector Labs PI-1000). Protein bands were visualized with Amersham ECL Western Blotting Detection Reagents (Sigma, St. Louis, MO).
2.10 |. Data analysis
Data were assessed for heterogeneity of variance by Bartlett’s tests. Data were log transformed when Bartlett’s test yielded P < .05; log-transformed data were subjected to Bartlett’s test to confirm that P >.05. All data sets were assessed by unpaired t test, paired t test, or ANOVA (without or with repeated measures) as indicated in the text and figure legends. ANOVA was followed by Duncan’s multiple range test when P < .05. Statistics were performed using StatPak version 4.12 software; Northwest Analytical, Portland, OR. Significance was assumed at P < .05. Data are expressed as mean ± SEM.
3 |. RESULTS
3.1 |. The ovulatory gonadotropin surge increases NTS expression
Published reports indicate that NTS is expressed by the granulosa cells of the ovulatory follicle and show massive upregulation of NTS mRNA in response to the ovulatory gonadotropin surge.3,4 This finding was confirmed in granulosa cells of monkey ovulatory follicles. Granulosa cells were obtained after ovarian stimulation, either in the absence of hCG (0 hour) or 12, 24, or 36 hours after an ovulatory dose of hCG. NTS mRNA was low-to-nondetectable before administration of an ovulatory dose of hCG (Figure 1A). In response to hCG administration, NTS mRNA levels rose 30-fold, and NTS mRNA remained high through the remainder of the ovulatory interval.
FIGURE 1.
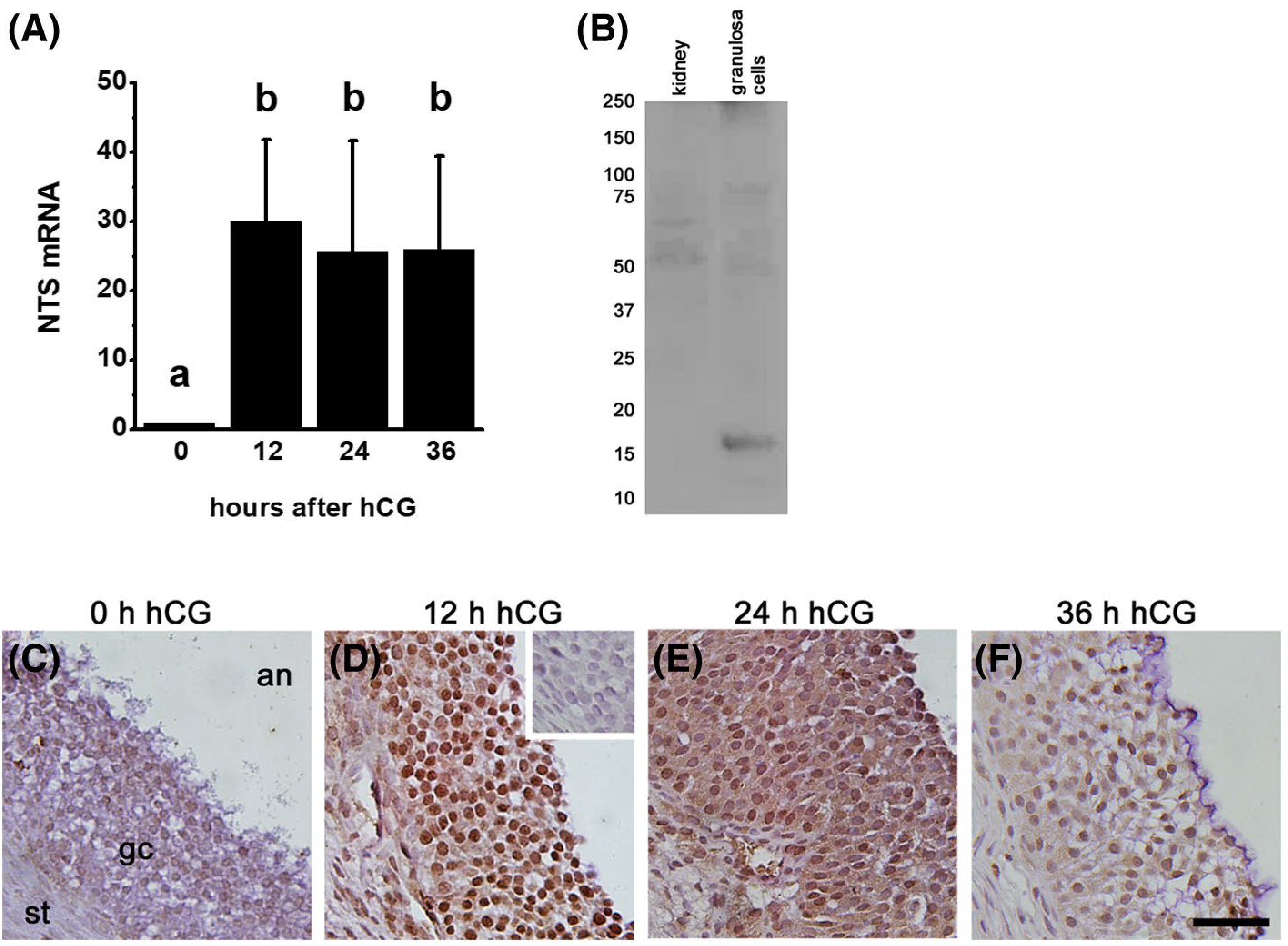
NTS Expression in Monkey Granulosa Cells. A. Granulosa cells collected from monkeys experiencing ovarian stimulation and either 0 (no hCG), 12, 24, or 36 hours after an ovulatory dose of hCG were used for quantitation of NTS by qPCR. Groups with no common letter are different by ANOVA and Duncan’s post hoc test, P < .05. Data are presented as mean + SEM. N = 4–5 monkeys/treatment group. B. NTS (16 kDa) detected as a single band in monkey granulosa cells (24 hours hCG) but not kidney (negative control). C-F. Ovaries collected from monkeys experiencing ovarian stimulation and either 0 (no hCG; C), 12 (D), 24 (E), or 36 (F) hours of hCG treatment were used for immunocytochemical detection of NTS. Omission of primary antibody resulted in lack of visible staining (D, inset). Immunodetection of NTS is brown; hematoxylin (blue) counterstain. Each image is oriented as shown in panel C, with ovarian stroma (st) lower left, granulosa cells (gc) central, and follicle antrum (an) in upper right. Images are at the same magnification and use bar in panel F = 100 μm. Representative of 4–5 monkeys/treatment group
NTS protein was also present in granulosa cells of ovulatory follicles. A protein consistent with expected size of NTS (16 kDa, 24) was detected in lysates of monkey granulosa cells (Figure 1B). NTS was localized primarily to granulosa cells of monkey ovulatory follicles by immunostaining (Figure 1C–F). The apparent increase in NTS detection in granulosa cells after hCG (Figure 1D) parallels the hCG-stimulated rise in granulosa cell NTS mRNA in the early ovulatory period (Figure 1A). When present, NTS immunostaining appeared to be located throughout the granulosa cell layer and not restricted to either the basal or antral portions of the granulosa cell layer.
Possible NTS immunodetection was also noted in the stroma outside the granulosa cell basement membrane. In order to identify candidate cell types for stromal NTS expression, cultures of monkey ovarian microvascular endothelial cells (mOMECs) and theca cells were examined. NTS mRNA was not consistently detected in mOMECs treated without or with either PGE2 or VEGFA, two LH-stimulated mediators of ovulatory angiogenesis.20,25 Similarly, NTS mRNA was low/non-detectable in cultured monkey theca cells; theca NTS mRNA was not consistently detected after cells were treated in vitro with hCG or forskolin, which activates the LH receptor-stimulated signaling cascade.26
3.2 |. Neutralization of follicular NTS impedes ovulation
To determine if intrafollicular NTS is necessary for ovulation, we utilized a well-established macaque model of controlled ovulation, coupled with intrafollicular administration of a NTS-neutralizing antibody.18 Dominant monkey follicles were injected with either a antibody against NTS or a control IgG. We anticipate that the NTS antibody binds to NTS in the follicle, preventing NTS interaction with its receptor(s). An ovulatory dose of hCG was administered immediately thereafter to initiate ovulatory changes. After 48 hours of exposure to hCG, each ovary was evaluated for ovulatory changes. At the time of ovary removal, control IgG-injected ovaries showed clear evidence of follicle rupture, with a prominent ovulatory stigmata visible at ovariectomy (Figure 2A, arrow) and also upon histological examination (Figure 2E). Histological examination also revealed the absence of oocytes within control IgG-injected follicles (Table 1).
FIGURE 2.
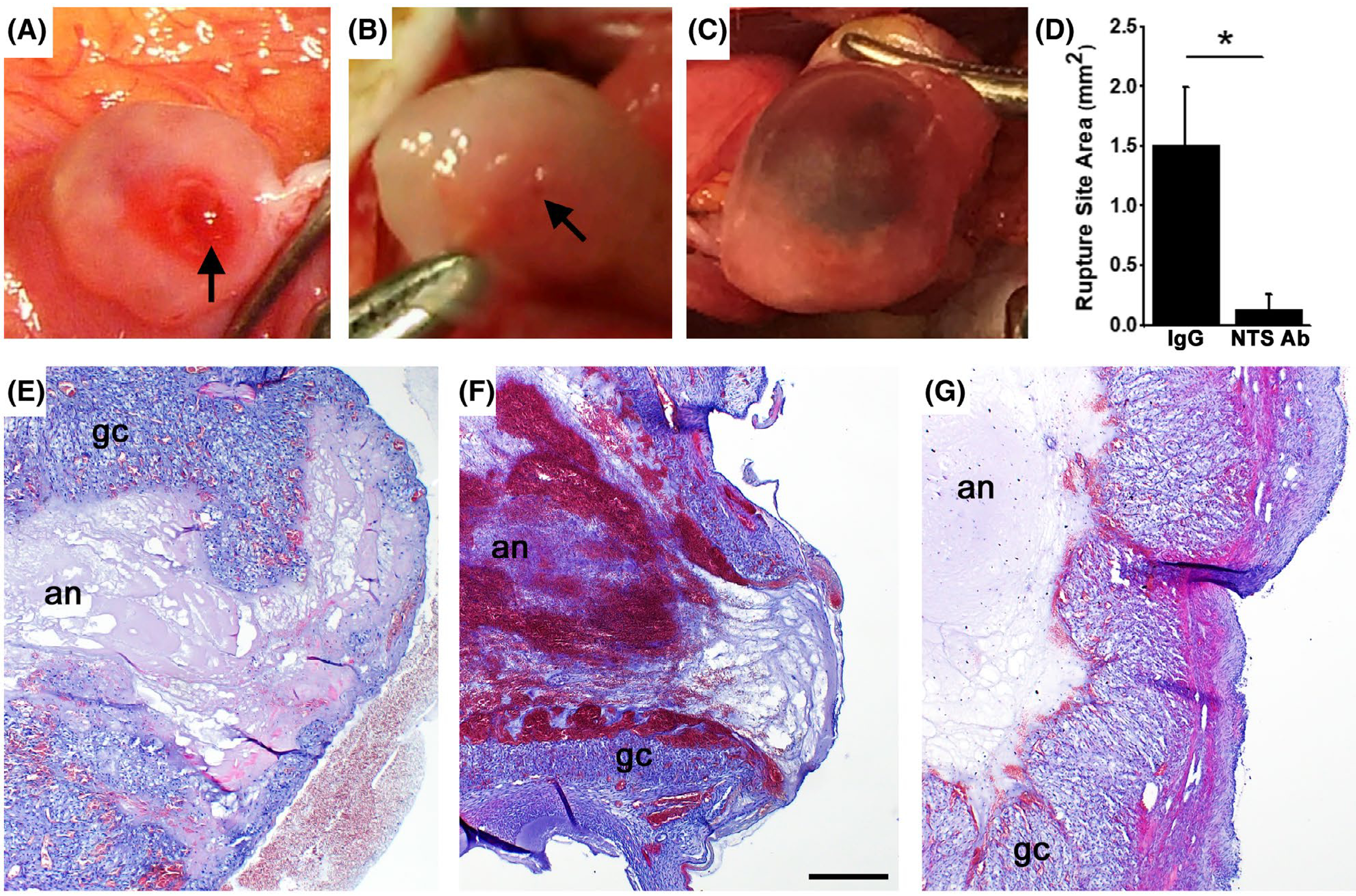
Follicle Rupture is compromised after Intrafollicular Injection with a NTS Antibody. Ovarian surface at ovariectomy after intrafollicular injection of control IgG (A) or NTS antibody (B,C). Rupture sites are indicated (arrows in A and B). Ovary shown in panel C did not rupture. Ovaries shown at similar magnification. D. Rupture site area for ovaries injected with control IgG or NTS antibody (NTS Ab) is expressed as mean + SEM; unpaired, two-tailed t test showed groups were different, P < .05, as indicated by the asterisk (*); n = 4 monkeys/treatment group. Histologic view of rupture sites for ovaries injected with control IgG (E) or NTS antibody (F). Histologic view of thinnest follicle apex of unruptured follicle after injection with NTS antibody (G). Tissues in panels E-G were stained with hematoxylin and eosin; granulosa cells (gc) and follicle antrum (an) are indicated. For panels E-G, all panels are at the same magnification and use bar in panel F = 300 μm. Images are representative of data from n = 4–5 monkeys/treatment group
TABLE 1.
Follicle rupture and oocyte retention after intrafollicular injection of a NTS antibody
| Control IgG | NTS Antibody | |
|---|---|---|
| Rupture site present | 4/4 | 1/4 |
| Oocyte retained in follicle | 0/4 | 2/4 |
NTS antibody injection resulted in formation of unruptured follicles with trapped oocytes. At ovariectomy, the majority of NTS antibody-injected follicles showed no evidence of rupture at ovariectomy and instead had the appearance of large, hemorrhagic cysts (Figure 2C). Upon histologic examination, the majority of NTS antibody-injected ovaries showed an intact luteinizing granulosa cell layer in the apical region and no evidence of follicle rupture (Figure 2G). Only one of four NTS antibody-injected follicles showed evidence of rupture, with a small red area of the ovarian surface (Figure 2B, arrow) and histological confirmation of a small rupture site (Figure 2F). Overall, the size of the rupture site was significantly reduced with NTS antibody injection when compared to control IgG injection (Figure 2D). Oocytes trapped in the antrum were identified in 50% of NTS antibody-injected follicles (Table 1). These retained oocytes were not in contact with the mural granulosa cell layer and had few or no associated cumulus cells (Figure 3A).
FIGURE 3.
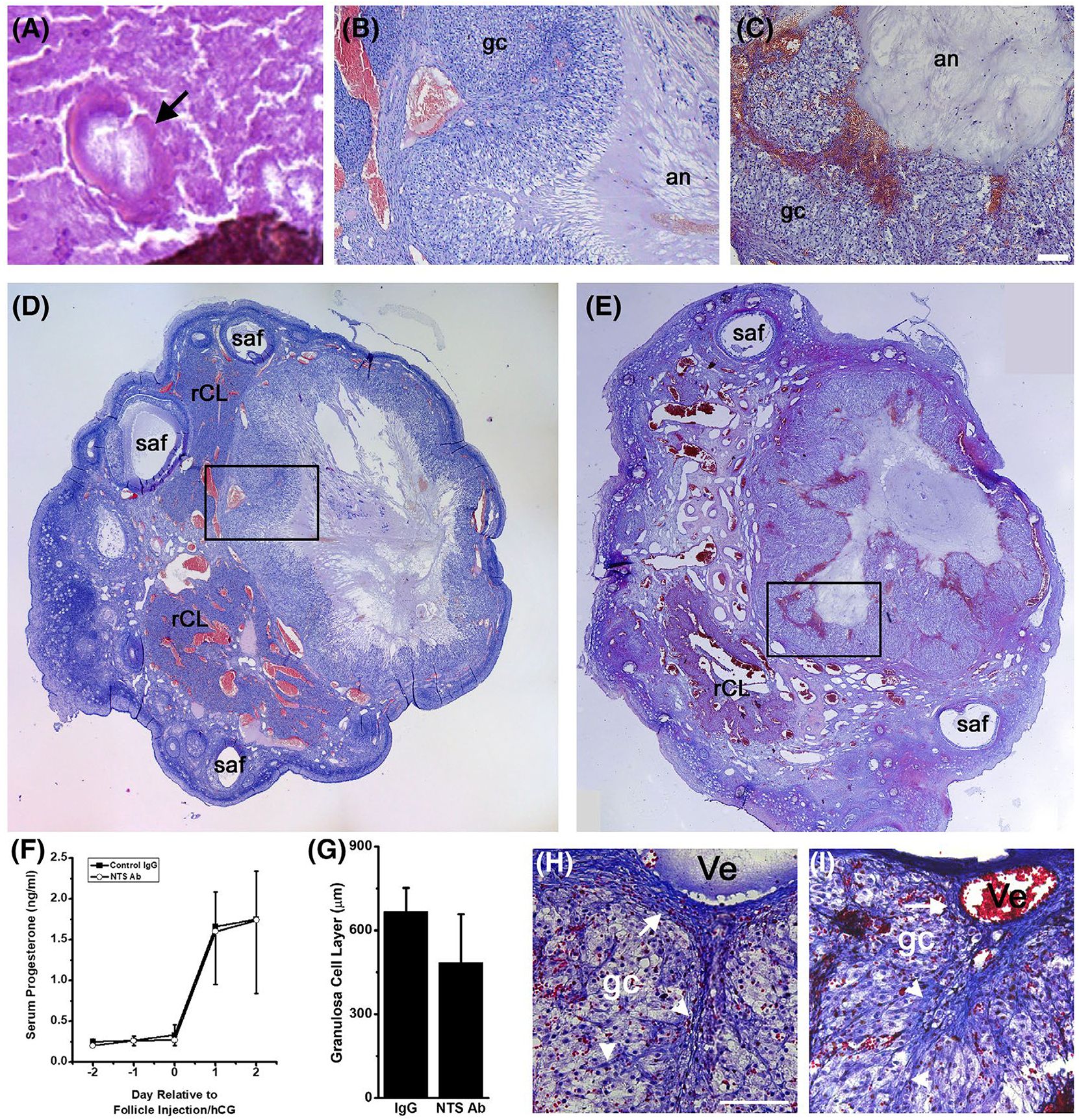
Oocyte Release and Luteinization after Intrafollicular Injection with a NTS Antibody. A. Oocyte (arrow) in the antrum of a follicle injected with NTS antibody; surrounding follicular fluid had fixed, cracked, stained, and folded (lower right). Note lack of cumulus cells surrounding the oocyte. Ovarian follicles injected with control IgG (B, D) or NTS antibody (C, E) show comparable luteinization. Boxes (D, E) show location of higher magnification images (B,C; bar = 100 μm). Location of luteinizing granulosa cells (gc) and follicle antrum (an) are indicated (B, C). Whole ovary images (D, E) show approximate largest diameter of the luteinizing follicle and are at similar magnification; small antral follicles (saf) and regressing corpora lutea from previous menstrual cycles (rCL) are noted. Tissues in panels A-E were stained with hematoxylin and eosin. F. Serum progesterone before and after follicle injection (Day 0) expressed as mean + SEM (Control IgG, black boxes) and mean-SEM (NTS Ab, white circles), n = 4 monkeys/treatment group. No differences were detected between groups by ANOVA with two repeated measures. G. Granulosa cell layer thickness was not different between control IgG and NTS antibody-injected follicles, n = 4 monkeys/treatment group. No differences were detected between groups by two-tailed unpaired t test. Tissue sections from ovaries injected with control IgG (H) and NTS antibody (I) were stained using the Ayoub-Shklar method to emphasize collagen (bright blue, white arrows) in the stroma surrounding the luteinizing follicle (upper portion of each image) and stromal invaginations (white arrowheads) into the luteinizing granulosa cells (gc, faint purple). Red blood cells (bright red) are present in stromal vessels (Ve) and among luteinizing granulosa cells. Images in panels H and I are at the same magnification and use bar in panel G = 50 μm. Images are representative of data from n = 4–5 monkeys/treatment group
NTS antibody injection resulted in well-luteinized, but highly hemorrhagic, follicles. Follicles injected with control IgG (Figure 3B,D) and NTS antibody (Figure 3C,E) showed hypertrophied granulosa cell layers (Figure 3G), with enlarged vessels in the surrounding stroma. NTS antibody-injected follicles contained pools of red blood cells near the luteinizing granulosa cell layer (Figure 3C,E) and throughout the follicle antrum (Figures 2F and 3E). This appearance contrasts with the limited accumulation of red blood cells in the antrum of control IgG-injected follicles (Figures 2E and 3B,D). Follicles injected with either control IgG or the NTS antibody showed evidence of structural luteinization, with stromal tissue integrating into the luteinizing granulosa cell layer near the location of large stromal vessels (Figure 3H,I). In addition, both treatments resulted in similar levels of serum progesterone after hCG administration, a measure of functional luteinization (Figure 3F).
3.3 |. The neurotensin response system in primate ovulatory follicles
To identify possible cellular targets of NTS action, each of the three NTS receptors was localized to cells of macaque ovulatory follicles. Ovaries were obtained after ovarian stimulation, either in the absence of hCG (0 hour) or 12, 24, or 36 hours after an ovulatory dose of hCG. SORT1 immunodetection was low to nondetectable in granulosa cells of ovulatory follicles before hCG (Figure 4A) and 12 hours after hCG administration (Figure 4B). Granulosa cell staining for SORT1 was present 24 hours after hCG (Figure 4C), with an apparent decline in SORT1 immunodetection observed by 36 hours after hCG (Figure 4D). Granulosa cell SORT1 mRNA showed a similar pattern, with low mRNA levels 0–12 hours after hCG, peak SORT1 mRNA levels 24 hours after hCG, and a return to low SORT1 mRNA levels 36 hours after hCG (Figure 5A). Ovarian tissues collected before and after hCG administration also showed possible SORT1 detection in ovarian stroma (eg, see Figure 4C), consistent with theca and vascular endothelial cells. For this reason, SORT1 mRNA levels were assessed in mOMECs and cultured theca cells. mOMECs expressed SORT1 with increased mRNA levels measured after 4 hour in vitro exposure to PGE2 (Figure 5C), a potent stimulus of follicular angiogenesis.11,20 Interestingly, in vitro treatment with VEGFA, also a potent stimulus of follicular angiogenesis,18,25 did not alter SORT1 mRNA levels (Figure 5C,D). Monkey theca expressed detectable SORT1 mRNA, but mRNA levels did not change after treatment with hCG or forskolin (Figure 5E,F). Detection of SORT1 in heart muscle by immunostaining (Figure 4E) served as a positive control.27 Furthermore, SORT1 protein was detected by western blot as a single band of about 100 kDa28 in many monkey tissues, including heart, granulosa cells, and mOMECs (Figure 4P) demonstrating the specificity of the SORT1 antibody.
FIGURE 4.
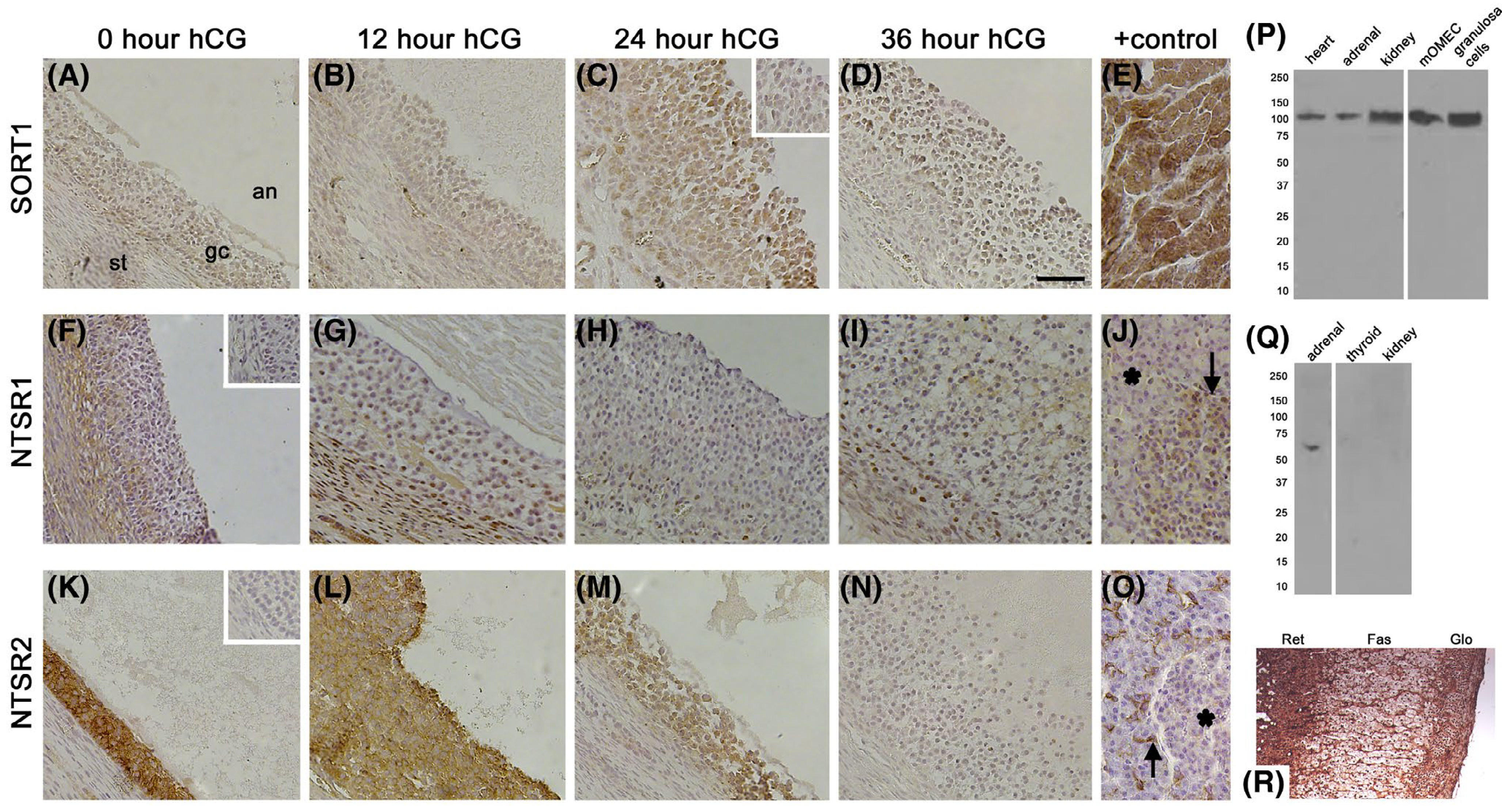
NTS Receptor Immunodetection in Monkey Ovulatory Follicles. Ovaries collected from monkeys experiencing ovarian stimulation and either 0 (no hCG; A, F, K), 12 (B, G, L), 24 (C, H, M), or 36 (D, I, N) hours of hCG treatment were used for immunocytochemical detection (brown) of SORT1 (A-E), NTSR1 (F-J), and NTSR2 (K-O); hematoxylin (blue) counterstain. Images are oriented as shown in panel A, with ovarian stroma (st) in lower left, granulosa cells (gc) central, and follicle antrum (an) in upper right. Positive (+) controls include heart (E), pancreatic islet (J, arrow), and intercalated duct cells of the exocrine pancreas (O, arrow). Absence of staining is noted for NTSR1 in the exocrine pancreas (J, asterisk) and for NTSR2 in the pancreatic islet (O, asterisk). Omission of primary antibody resulted in reduced staining in ovarian tissues (insets in panels C, F, and K). All images are at the same magnification and use bar in panel D = 100 μm. Representative of 3–4 ovaries/treatment group. P. SORT1 antibody detected a band of 100 kDa in monkey tissues, mOMECs, and granulosa cells (24 hour hCG) by western blot. Q. NTSR2 antibody detected a band of 65 kDa in monkey adrenal, but not thyroid or kidney, by western blot. In Panels P and Q, position of size standards (kDa) are shown at left; white lines indicate removal of a ladder lane. R. Immunodetection of NTSR2 (brown) in baboon adrenal; reticularis (Ret), fasciculata (Fas), and glomerulosa (Glo) zones of the adrenal cortex are indicated
FIGURE 5.
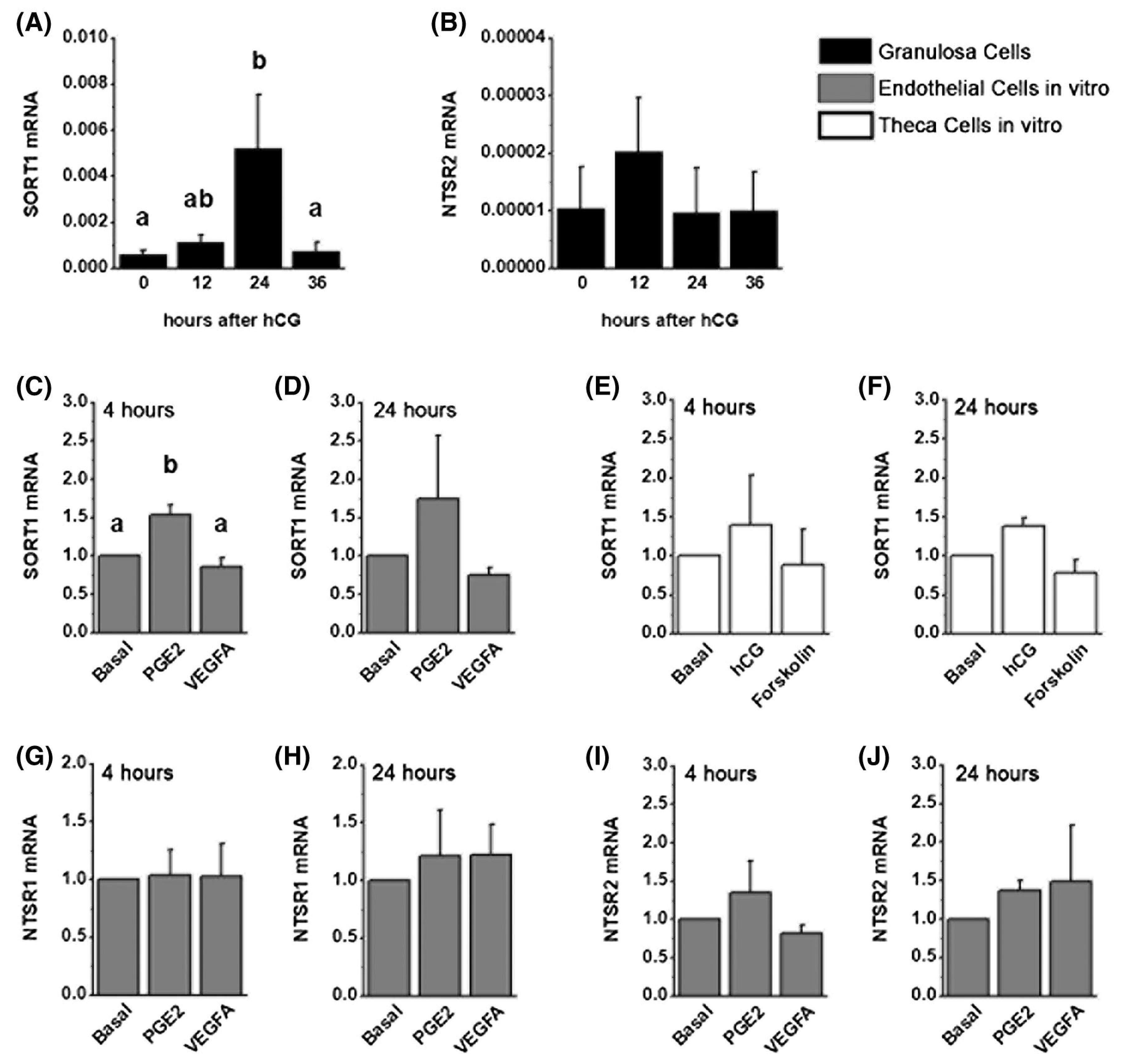
NTS Receptor mRNA in Ovarian Cells. Granulosa cells (black bars) collected from monkeys experiencing ovarian stimulation and either 0 (no hCG), 12, 24, or 36 hours of hCG were used for quantitation of SORT1 (A) and NTSR2 (B) by qPCR. N = 4–9 monkeys/treatment group. Monkey ovarian microvascular endothelial cells (gray bars) were cultured with PGE2 or VEGFA for either 4 hours (C, G, I) or 24 hours (D, H, J) before preparation of RNA and qPCR for quantitation of SORT1 (C,D), NTSR1 (G, H), and NTSR2 (I, J). Monkey theca cells (white bars) were cultured with hCG or forskolin for either 4 hours (E) or 24 hours (F) before preparation of RNA and qPCR for quantitation of SORT1 (E,F). N = 4 primary cell lines/treatment group. All data are presented as mean + SEM. Within each panel, data were assessed by ANOVA (A,B) or ANOVA with one repeated measure (C-J). Within each panel, treatment groups with no common letter were different by Duncan’s post hoc test, P < .05. No letters indicate no differences between treatment groups for that panel
NTSR1 immunodetection was minimal in ovulatory follicles obtained before and after hCG (Figure 4F–I). NTSR1 mRNA was undetectable in granulosa cells at all times examined. Stromal immunostaining indicated possible NTSR1 expression by theca cells and endothelial cells. NTSR1 mRNA was not detectable in cultured theca cells, and treatment with hCG or forskolin did not result in consistent detection of NTSR1 mRNA. NTSR1 mRNA was present in mOMECs, but NTSR1 levels did not change in response to PGE2 or VEGFA treatment (Figure 5G,H). NTSR1 mRNA was consistently detected in pancreas, and NTSR1 immunodetection was noted in pancreatic islets, which served as a positive control (Figure 4J).29,30 We have previously confirmed that this NTSR1 antibody detects NTSR1 in monkey testis.31
NTSR2 immunodetection in granulosa cells was strong before hCG administration, then was low to undetectable at 36 hour hCG, or just before ovulation (Figure 4K–N). Strong NTSR2 immunodetection was noted in the intercalated duct cells of the exocrine pancreas, which served as a positive control (Figure 4O).29,30 Interestingly, NTSR2 mRNA was detected in granulosa cells, but levels were low and variable throughout the ovulatory interval (Figure 5B). NTSR2 protein was not detected in the ovarian stroma by immunostaining. NTSR2 mRNA was detected at low levels in mOMECs (Figure 5I,J). NTSR2 mRNA was not detectable in cultured theca cells, and treatment with hCG or forskolin did not result in consistent detection of NTSR2 mRNA. Detection of NTSR2 in the adrenal gland by western blot (65 kDa band, consistent with manufacturer’s data, Figure 4Q) and immunostaining (Figure 4R) confirmed a previous report of NTSR2 in the adrenal32 and served as an additional positive control.
3.4 |. Neurotensin is pro-angiogenic in vitro
mOMECs were studied further to determine the effect of NTS on key events in ovulatory angiogenesis: endothelial cell migration, proliferation, and capillary sprout formation. NTS-stimulated mOMEC migration in a dose-dependent manner (Figure 6A) but did not alter proliferation (Figure 6B). In an in vitro model of capillary sprout formation, NTS increased the number of sprouts and length of sprouts in a dose-dependent manner after 2 days of culture (Figure 6C–G).
FIGURE 6.
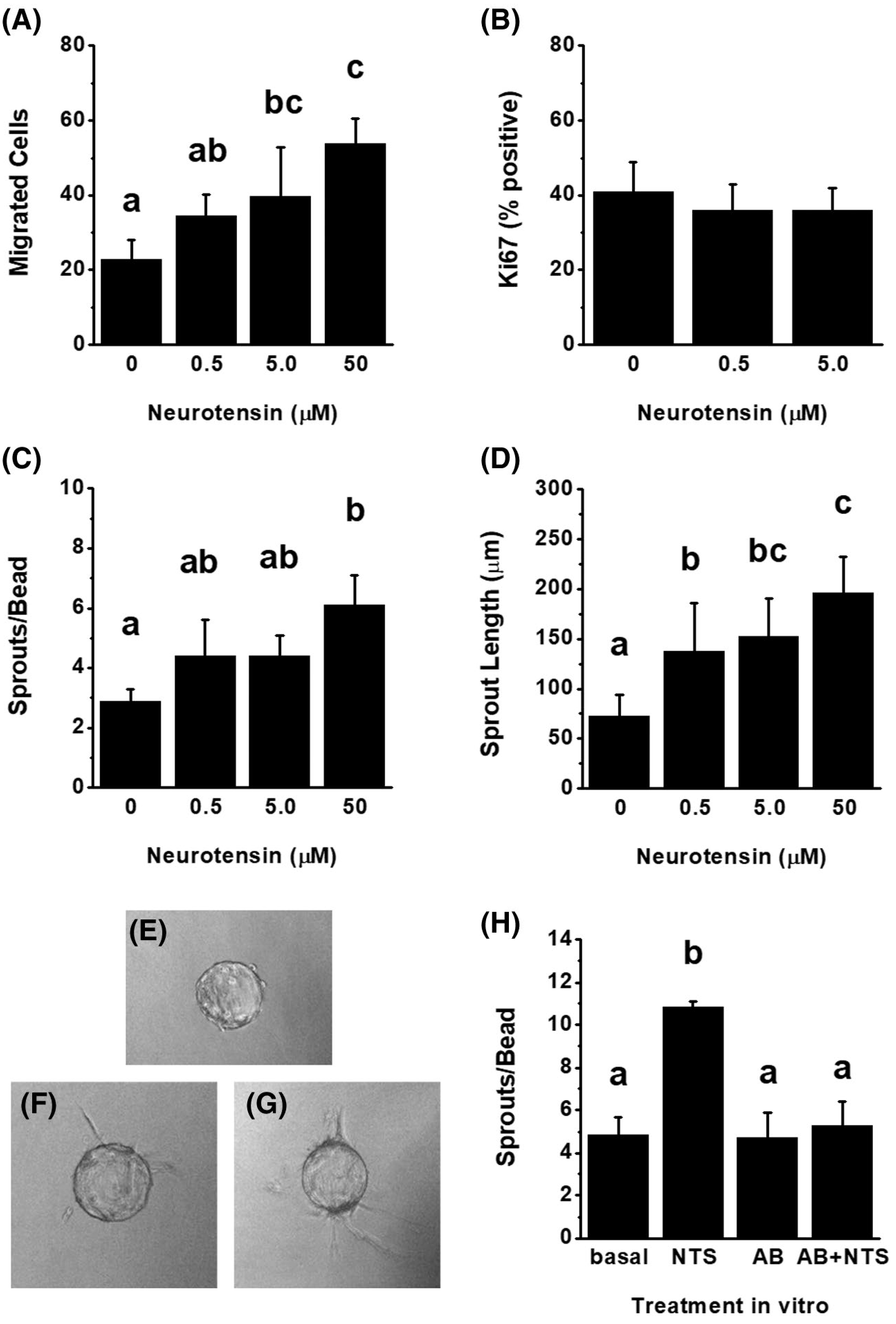
NTS is Pro-Angiogenic In Vitro. Monkey ovarian microvascular endothelial cells were treated with NTS (0.5–50 μM) and assessed after 24 hours for migration (A, n = 3) or Ki67 immunodetection (B, n = 3). Capillary-like sprout formation was assessed after treatment with NTS (0–50 μM) for 2 days (n = 4) and quantified for the number of sprouts/bead (C) and the length of sprouts (D). Representative mOMEC-overed beads at the start of culture (E, Day 0) and on Day 2 of culture without (F; 0 μM) and with NTS (G; 50 μM). H. Beads treated with basal media, NTS (5 μM), antibody alone (AB), or NTS preabsorbed with the NTS antibody (AB + NTS) were assessed after 1 day for sprout number (n = 3). Within each panel, data were assessed by ANOVA with one repeated measure; treatment groups with no common letter were different by Duncan’s post hoc test, P < .05. No letters indicate no differences between treatment groups for that panel
The sprout formation assay was used to confirm that the NTS antibody used for follicle injection can neutralize NTS activity. NTS preincubated with NTS antibody was unable to stimulate sprouting, similar to basal conditions and the antibody alone (Figure 6H).
4 |. DISCUSSION
Our studies demonstrate that NTS regulates key aspects of the ovulatory cascade, including follicle rupture and release of the oocyte. To date, many LH-regulated intrafollicular mediators of ovulation have been identified. These include the steroid hormone progesterone and the prostaglandin PGE2, each of which is required for primate ovulation.33–35 The ovulatory LH surge stimulates synthesis of protein growth factors, such as AREG and VEGFA, which are required for critical ovulatory changes including cumulus expansion and angiogenesis.36 Interactions between these LH-regulated, intrafollicular signaling pathways are well documented. For example, progesterone increases granulosa cell expression of key prostaglandin synthesis enzymes,37 and prostaglandins may be needed for optimal progesterone synthesis.38 This interconnected web of intrafollicular signals may support the rapid and temporally-coordinated changes necessary for successful ovulation.
NTS has been identified as a differentially expressed gene in granulosa cells of the ovulatory follicles of humans3 and mice,4 but we found no published studies examining NTS mRNA or protein in other cell types of the ovulatory follicle. Studies presented here confirm that macaque granulosa cell levels of NTS mRNA increase rapidly after the ovulatory gonadotropin surge. Granulosa cells appear to be the primary or exclusive source of NTS in the ovulatory follicle. Ovarian stroma appears to contain little NTS protein, and theca and ovarian endothelial cells expressed little or no NTS mRNA in vitro. Granulosa cell NTS expression increases rapidly after the ovulatory gonadotropin stimulus, consistent with a role for NTS as a regulator of the ovulatory cascade.
The essential ovulatory process of follicle rupture is disrupted by NTS neutralization. Only one of four ovaries injected with the NTS antibody showed evidence of a rupture site, with an ovulatory canal providing connectivity between the follicle antrum and the exterior of the ovary. The one rupture site formed in a NTS antibody-injected follicle was very small when compared to the size of rupture sites in ovaries injected with control IgG. In all other cases, NTS neutralization prevented follicle rupture. Rupture is an ovulatory function often observed to be compromised after blockage of a key intrafollicular signaling pathway. Disruption of progesterone, prostaglandins, VEGFA, PGF, and ANGPT2 have been show to decrease rates of follicle rupture.36 In particular, blockade of PGE2 and VEGFA synthesis/action in macaque follicles resulted in the formation of large, unruptured cysts, with granulosa cells removed from a very thin connective tissue covering at the apical area.11,18 In contrast, after NTS neutralization, the luteinizing granulosa cell layer was intact and in connection with a thicker connective tissue covering of the apical surface of unruptured follicles. NTS may be needed for both granulosa cell removal and follicle wall thinning associated with follicle rupture. The matrix metalloproteinase MMP9 is well established as a key proteinase for follicle rupture.39 NTS has been reported to increase expression and activity of MMP9 in normal and cancer cell lines.40,41 Establishing a causal link between NTS, MMPs, and proteolytic activity at the follicle apex will require further study.
Cumulus expansion may be regulated, at least in part, by NTS. During normal ovulation, cumulus expansion releases the oocyte from the mural granulosa cell layer.36 Aspects of cumulus expansion, such as withdrawal of transzonal processes and transfer of cAMP from cumulus cells to the oocyte,42 also facilitate resumption of oocyte meiosis. The oocyte retains large numbers of expanded cumulus cells at ovulation that aid in oocyte pickup by the fimbria, oviductal transport, and attraction of sperm for fertilization in the oviduct.43 Both oocytes found in NTS antibody-injected follicles were located in the antrums of unruptured follicles, consistent with cumulus expansion. However, these oocytes had few, if any, surrounding cumulus cells. In other systems, NTS increases expression and activity of the epidermal growth factor receptor (EGFR; 44–49). In the ovulatory follicle, EGFR mediates AREG action to trigger the essential process of cumulus expansion.36,42 Thus, neutralization of NTS may prevent proper expansion of the cumulus oocyte complex. It is not clear if NTS neutralization compromised resumption of oocyte meiosis. The retained oocytes did not appear to be germinal vesicle intact, nor were polar bodies noted. However, such observations are inconclusive when made using only histologic sections from a small number of ovaries.
Key aspects of structural and functional luteinization did not appear to be compromised in the absence of NTS action. Increased size of granulosa cells and subsequent thickening of the mural granulosa cell layer, with invading stroma and vessels were observed in the present study; these changes are hallmarks of structural luteinization.36 Serum progesterone increased after the ovulatory gonadotropin surge, a sign of enhanced follicular progesterone synthesis and a key measure of functional luteinization.50 By both of these criteria, NTS neutralization neither delayed nor diminished the early stages of luteinization after 2 days of exposure to an ovulatory gonadotropin stimulus. Early stages of luteal formation, while essential, do not ensure that the corpus luteum will become fully functional or undergo timely luteolysis, both of which must occur for maintenance of menstrual cyclicity.51
Vascular remodeling is a component of both ovulation and luteal formation. Increased ovarian blood flow, new capillary formation, and increased vascular permeability occur during the ovulatory cascade.36 Regulation of these processes is complex, mediated by LH-stimulation of granulosa cells to produce both vascular growth promoters and inhibitors.52 Blockade of NTS action appears to dysregulate key aspects of these ovulatory vascular changes. NTS neutralization with antibody injection yielded follicles that were hemorrhagic, with accumulation of red blood cells near the luteinizing granulosa cell layer and throughout the follicle antrum. Our in vitro studies showed that NTS stimulates endothelial cell migration and capillary sprout formation. These findings are consistent with previous reports, which demonstrated the ability of NTS to promote cell migration.41,53,54 In one intriguing study, NTS increased activity of urokinase plasminogen activator,55 a key protease secreted by migrating endothelial cells.56 Further studies will be needed to fully evaluate the role of NTS during the process of ovarian vascular changes that occur during the process of ovulation. In particular, accumulation of red blood cells within NTS-neutralized follicles may indicate a role for NTS to maintain vascular integrity or limit vascular permeability during ovulatory angiogenesis. Additionally, it remains to be determined if this angiogenesis is necessary for ovulation, a requirement for the formation of the corpus luteum, or both.
NTS acts at its target cells through a family of NTS receptors. NTSR1 and NTSR2 are seven transmembrane domain receptors.9 NTSR1 can couple to a number of G proteins to activate multiple signaling pathways.57 Specific G proteins have not been identified for mediating NTSR2 actions. A third NTS receptor, SORT1 (also called sortilin or NTSR3), is a multifunction protein with a single transmembrane domain.10 SORT1 functions as a NTS receptor; it also has intracellular sorting functions that are independent of NTS.58 The mechanism of SORT1 signal transduction in response to NTS binding is unknown. Finally, interaction between multiple NTS receptors can activate additional signaling pathways,10,59 adding yet another level of complexity to cellular responses to NTS.
Our studies demonstrate NTS receptors are dynamically regulated in the cells of the ovulatory follicle. Granulosa cells show increase SORT1 mRNA and protein after the ovulatory gonadotropin surge. While granulosa cells show an apparent decrease in NTSR2 protein after the ovulatory gonadotropin surge, NTSR2 mRNA copy number was low and unchanged in response to hCG. There was no evidence of NTSR1 mRNA or protein in granulosa cells. Immunodetection of NTS receptors in the follicular stroma led us to evaluate NTS receptor mRNA in cultures of monkey theca cells and monkey ovarian microvascular endothelial cells. Theca cells expressed detectable levels of SORT1 mRNA only. In contrast, all three receptors were expressed by endothelial cells, with SORT1 levels enhanced by PGE2, a key paracrine mediator of ovulatory angiogenesis.11,20 Overall, our findings focus interest on SORT1 as an important receptor for mediation of the ovulatory actions of NTS.
Information on NTS, its receptors, or NTS actions in reproductive function is very limited. NTS localization to specific brain regions suggested a link between NTS and the hypothalamic-anterior pituitary-ovarian axis.12,13 Well-controlled studies showed that hypothalamic NTS is not involved in regulation of pituitary gonadotropin release,12 but neural control of metabolism may directly or indirectly impact the reproductive endocrine axis.60 A few reports implicate NTS in aspects of ovarian function,61 oviductal transport,62,63 implantation,64 and sperm capacitation.4,65 While the number of replicate ovaries examined was small, ovulatory failure seen in the present study after neutralization of NTS in the primate follicle leads us to hypothesize that diminished NTS expression/action specifically in the ovary could cause a reduction or elimination of female fertility. Our studies did not examine effects of NTS on the oocyte and provide just an overview of NTS effects on other types of follicle cells. Further detailed studies will be required to fully evaluate the cellular changes initiated by NTS in each key cell type of the ovulatory follicle. Clinical interest in NTS has led to the development of NTS receptor selective agonists and antagonists.66–69 If future studies show that NTS is involved in regulation of human ovulation, then agonists may be useful for treatment of anovulatory causes of infertility, while antagonists hold promise as contraceptives to block ovulation while preserving luteal function and menstrual cyclicity.
Supplementary Material
ACKNOWLEDGMENTS
Recombinant human FSH and Ganirelix were generously provided by Merck & Co, Inc, Kenilworth, NJ. The Eunice Kennedy Shriver National Institutes of Child Health and Human Development provided Acyline and funding support (P01 HD071875 and R01 HD097675 to DMD and TEC).
Funding information
HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Grant/Award Number: P01 HD071875 and R01 HD097675
Abbreviations:
- ANOVA
analysis of variance
- cAMP
cyclic adenosine monophosphate
- FSH
follicle stimulating hormone
- GnRH
gonadotropin releasing hormone
- hCG
human chorionic gonadotropin
- IgG
immunoglobulin G
- LH
luteinizing hormone
- MMP
matrix metalloproteinase
- mOMEC
monkey ovarian microvascular endothelial cell
- NTSR3
neurotensin receptor 3, alternative name for SORT1
- PGE2
prostaglandin E2
Footnotes
CONFLICT OF INTEREST
The Authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- 1.Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. [DOI] [PubMed] [Google Scholar]
- 2.Yung Y, Aviel-Ronen S, Maman E, et al. Localization of luteinizing hormone receptor protein in the human ovary. Mol Hum Reprod. 2014;20:844–849. [DOI] [PubMed] [Google Scholar]
- 3.Wissing ML, Kristensen SG, Andersen CY, et al. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod. 2014;29:997–1010. [DOI] [PubMed] [Google Scholar]
- 4.Hiradate Y, Inoue H, Kobayashi N, et al. Neurotensin enhances sperm capacitation and acrosome reaction in mice. Biol Reprod. 2014;91(2):53. [DOI] [PubMed] [Google Scholar]
- 5.Boules M, Li Z, Smith K, Fredrickson P, Richelson E. Diverse roles of neurotensin agonists in the central nervous system. Front Endocrinol (Lausanne). 2013;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Song J, Zaytseva YY, et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533(7603): 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalafatakis K, Triantafyllou K. Contribution of neurotensin in the immune and neuroendocrine modulation of normal and abnormal enteric function. Regul Pept. 2011;170(1–3):7–17. [DOI] [PubMed] [Google Scholar]
- 8.Driessen TM, Zhao C, Whittlinger A, Williams H, Gammie SC. Endogenous CNS expression of neurotensin and neurotensin receptors is altered during the postpartum period in outbred mice. PLoS ONE. 2014;9(1):e83098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelaprat D. Interactions between neurotensin receptors and G proteins. Peptides. 2006;27(10):2476–2487. [DOI] [PubMed] [Google Scholar]
- 10.Mazella J, Beraud-Dufour S, Devader C, Massa F, Coppola T. Neurotensin and its receptors in the control of glucose homeostasis. Front Endocrinol (Lausanne). 2012;3:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SO, Harris SM, Duffy DM. Prostaglandin E2 (EP) receptors mediate PGE2-specific events in ovulation and luteinization within primate ovarian follicles. Endocrinology. 2014;155:1466–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dungan Lemko HM, Naderi R, Adjan V, et al. Interactions between neurotensin and GnRH neurons in the positive feedback control of GnRH/LH secretion in the mouse. Am J Physiol Endocrinol Metab. 2010;298(1):E80–E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248(19):6854–6861. [PubMed] [Google Scholar]
- 14.Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase and prostaglandin levels in periovulatory follicles: Implications for control of primate ovulation by PGE2. J Clin Endocrinol Metab. 2005;90:1021–1027. [DOI] [PubMed] [Google Scholar]
- 15.Davies WA, Berghorn KA, Albrecht ED, Pepe GJ. Developmental regulation of protein kinase-A and -C activities in the baboon fetal adrenal. Endocrinology. 1993;132(6):2491–2497. [DOI] [PubMed] [Google Scholar]
- 16.Seachord CL, Vande Voort CA, Duffy DM. Adipose-differentiation related protein: a gonadotropin-and prostaglandin-regulated protein in primate periovulatory follicles. Biol Reprod. 2005;72:1305–1314. [DOI] [PubMed] [Google Scholar]
- 17.Weick RF, Dierschke DJ, Karsch FJ, Butler WR, Hotchkiss J, Knobil E. Periovulatory time course of circulating gonadotropic and ovarian hormones in the rhesus monkey. Endocrinology. 1973;93:1140–1147. [DOI] [PubMed] [Google Scholar]
- 18.Bender HR, Trau HA, Duffy DM. Placental growth factor is required for ovulation, luteinization, and angiogenesis in primate ovulatory follicles. Endocrinology. 2018;159(2):710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bender HR, Campbell GE, Aytoda P, Mathiesen AH, Duffy DM. Thrombospondin 1 (THBS1) promotes follicular angiogenesis, luteinization, and ovulation in primates. Front Endocrinol (Lausanne) 2019;10:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trau HA, Davis JS, Duffy DM. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol Reprod. 2015;92:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy DM, Seachord CL, Dozier BL. Microsomal prostaglandin E synthase-1 (mPGES-1) is the primary form of PGES expressed by the primate periovulatory follicle. Hum Reprod. 2005;20:1485–1492. [DOI] [PubMed] [Google Scholar]
- 22.McAllister JM, Simpson ER. Human theca interna cells in long-term culture. Methods Toxicol. 10:329–339. PMID: 2676474. 10.1210/endo-125-4-1959 [DOI] [Google Scholar]
- 23.Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. New York, NY: McGraw Hill; 1968. [Google Scholar]
- 24.Bidard JN, de Nadai F, Rovere C, et al. Immunological and biochemical characterization of processing products from the neurotensin/neuromedin N precursor in the rat medullary thyroid carcinoma 6–23 cell line. Biochem J. 1993;291(Pt 1):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trau HA, Brannstrom M, Curry TEJ, Duffy DM. Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Hum Reprod. 2016;31:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAllister JM, Kerin JFP, Trant JM, et al. Regulation of cholesterol side-chain cleavage and 17α-hydroxylase/lyase activities in proliferating human theca interna cells in long term monolayer culture. Endocrinology. 1989;125:1959–1966. [DOI] [PubMed] [Google Scholar]
- 27.Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13(2):397406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghaemimanesh F, Ahmadian G, Talebi S, et al. The effect of sortilin silencing on ovarian carcinoma cells. Avicenna J Med Biotechnol. 2014;6(3):169–177. [PMC free article] [PubMed] [Google Scholar]
- 29.Coppola T, Beraud-Dufour S, Antoine A, Vincent J, Mazella J. Neurotensin protects pancreatic beta cells from apoptosis. Int J Biochem Cell Biol. 2008;40(10):2296–2302. [DOI] [PubMed] [Google Scholar]
- 30.Beraud-Dufour S, Coppola T, Massa F, Mazella J. Neurotensin receptor-2 and -3 are crucial for the anti-apoptotic effect of neurotensin on pancreatic beta-TC3 cells. Int J Biochem Cell Biol. 2009;41(12):2398–2402. [DOI] [PubMed] [Google Scholar]
- 31.Campbell GE, Jones EL, Comizzoli P, Duffy DM. Neurotensin stimulates sperm acrosome reaction and reduces percentages of fertilization in vitro. Fertil Steril Sci 2020;1:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicard F, Contesse V, Lefebvre Hervé, et al. The N-terminal neurotensin fragment, NT1–11, inhibits cortisol secretion by human adrenocortical cells. J Clin Endocrinol Metab. 2006;91(8):3131–3137. [DOI] [PubMed] [Google Scholar]
- 33.Bishop CV, Hennebold JD, Kahl CA, Stouffer RL. Knockdown of progesterone receptor (PGR) in macaque granulosa cells disrupts ovulation and progesterone production. Biol Reprod. 2016;94(5):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci U S A. 1996;93(5):1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Human Reproduction Update. 2015;21:652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: parallels with inflammatory processes. Endocr Rev. 2019;40(2):369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi Y, Wilson K, Hannon PR, et al. Coordinated regulation among progesterone, prostaglandins, and EGF-like factors in human ovulatory follicles. J Clin Endocrinol Metab. 2017;102(6):1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elvin JA, Yan C, Matzuk MM. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci U S A. 2000;97(18):10288–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curry TE Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. [DOI] [PubMed] [Google Scholar]
- 40.Souazé F, Dupouy S, Viardot-Foucault V, et al. Expression of neurotensin and NT1 receptor in human breast cancer: a potential role in tumor progression. Cancer Res. 2006;66(12):6243–6249. [DOI] [PubMed] [Google Scholar]
- 41.Akter H, Park M, Kwon OS, Song EJ, Park WS, Kang MJ. Activation of matrix metalloproteinase-9 (MMP-9) by neurotensin promotes cell invasion and migration through ERK pathway in gastric cancer. Tumour Biol. 2015;36(8):6053–6062. [DOI] [PubMed] [Google Scholar]
- 42.Robker RL, Hennebold JD, Russell DL. Coordination of ovulation and oocyte maturation: a good egg at the right time. Endocrinology. 2018;159(9):3209–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niringiyumukiza JD, Cai H, Xiang W. Prostaglandin E2 involvement in mammalian female fertility: ovulation, fertilization, embryo development and early implantation. Reprod Biol Endocrinol. 2018;16(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Florio A, Sancho V, Moreno P, Delle Fave G, Jensen RT. Gastrointestinal hormones stimulate growth of foregut neuroendocrine tumors by transactivating the EGF receptor. Biochim Biophys Acta. 2013;1833(3):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Younes M, Wu Z, Dupouy S, et al. Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget. 2014;5(18):8252–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mijatovic T, Gailly P, Mathieu V, et al. Neurotensin is a versatile modulator of in vitro human pancreatic ductal adenocarcinoma cell (PDAC) migration. Cell Oncol. 2007;29(4):315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amorino GP, Deeble PD, Parsons SJ. Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene. 2007;26(5):745–756. [DOI] [PubMed] [Google Scholar]
- 48.Su ZJ, Liu XY, Zhang JH, Ke SY, Fei HJ. Neurotensin promotes cholangiocarcinoma metastasis via the EGFR/AKT pathway. Gene. 2019;687:143–150. [DOI] [PubMed] [Google Scholar]
- 49.Müller KM, Tveteraas IH, Aasrum M, et al. Role of protein kinase C and epidermal growth factor receptor signalling in growth stimulation by neurotensin in colon carcinoma cells. BMC Cancer. 2011;11:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stouffer RL, Bishop CV, Bogan RL, Xu F, Hennebold JD. Endocrine and local control of the primate corpus luteum. Reprod Biol. 2013;13(4):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothchild I. The regulation of the mammalian corpus luteum. Recent Prog Horm Res. 1981;37:183–298. [DOI] [PubMed] [Google Scholar]
- 52.Xu F, Hazzard TM, Evans A, Charnock-Jones S, Smith S, Stouffer RL. Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception. 2005;71(4):239–248. [DOI] [PubMed] [Google Scholar]
- 53.Dupouy S, Doan VK, Wu Z, et al. Activation of EGFR, HER2 and HER3 by neurotensin/neurotensin receptor 1 renders breast tumors aggressive yet highly responsive to lapatinib and metformin in mice. Oncotarget. 2014;5(18):8235–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JT, Napier DL, Weiss HL, Lee EY, Townsend CM Jr, Evers BM. Neurotensin receptor 3/sortilin contributes to tumorigenesis of neuroendocrine tumors through augmentation of cell adhesion and migration. Neoplasia. 2018;20(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ushiro S, Mizoguchi K, Yoshida S, et al. Stimulation of cell-surface urokinase-type plasminogen activator activity and cell migration in vascular endothelial cells by a novel hexapeptide analogue of neurotensin. FEBS Lett. 1997;418(3):341–345. [DOI] [PubMed] [Google Scholar]
- 56.Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev. 2011;12:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Besserer-Offroy É, Brouillette RL, Lavenus S, et al. The signaling signature of the neurotensin type 1 receptor with endogenous ligands. Eur J Pharmacol. 2017;805:1–13. [DOI] [PubMed] [Google Scholar]
- 58.Nykjaer A, Willnow TE. Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci. 2012;35(4):261–270. [DOI] [PubMed] [Google Scholar]
- 59.Devader C, Moreno S, Roulot M, et al. Increased Brain Neurotensin and NTSR2 Lead to Weak Nociception in NTSR3/Sortilin Knockout Mice. Front Neurosci. 2016;10:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tena-Sempere M. Neuroendocrinology in 2016: neuroendocrine control of metabolism and reproduction. Nat Rev Endocrinol. 2017;13(2):67–68. [DOI] [PubMed] [Google Scholar]
- 61.Dissen GA, Hill DF, Costa ME, Les Dees CW, Lara HE, Ojeda SR. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology. 1996;137(1):198–209. [DOI] [PubMed] [Google Scholar]
- 62.Cerny KL, Garrett E, Walton AJ, Anderson LH, Bridges PJ. A transcriptomal analysis of bovine oviductal epithelial cells collected during the follicular phase versus the luteal phase of the estrous cycle. Reprod Biol Endocrinol. 2015;13:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reinecke M. Neurotensin in the human fallopian tube: immunohistochemical localization and effects of synthetic neurotensin on motor activity in vitro. Neurosci Lett. 1987;73(3):220–224. [DOI] [PubMed] [Google Scholar]
- 64.Sakumoto R, Hayashi KG, Saito S, Kanahara H, Kizaki K, Iga K. Comparison of the global gene expression profiles in the bovine endometrium between summer and autumn. J Reprod Dev. 2015;61(4):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umezu K, Hiradate Y, Oikawa T, et al. Exogenous neurotensin modulates sperm function in Japanese Black cattle. J Reprod Dev. 2016;62(4):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moha ou Maati H, Veyssiere J, Labbal F, et al. Spadin as a new antidepressant: absence of TREK-1-related side effects. Neuropharmacology. 2012;62(1):278–288. [DOI] [PubMed] [Google Scholar]
- 67.Luckmann M, Holst B, Schwartz TW, Frimurer TM. In silico investigation of the neurotensin receptor 1 binding site: overlapping binding modes for small molecule antagonists and the endogenous peptide agonist. Mol Inform. 2016;35(1):19–24. [DOI] [PubMed] [Google Scholar]
- 68.Richard F, Barroso S, Martinez J, Labbe-Jullie C, Kitabgi P. Agonism, inverse agonism, and neutral antagonism at the constitutively active human neurotensin receptor 2. Mol Pharmacol. 2001;60(6):1392–1398. [DOI] [PubMed] [Google Scholar]
- 69.Huber S, Casagrande F, Hug MN, et al. SPR-based fragment screening with neurotensin receptor 1 generates novel small molecule ligands. PLoS ONE. 2017;12(5):e0175842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


