To the Editor—Since the first occurrence of SARS-CoV-2, different vaccines have been produced, tested, and approved in record time. However, the exact vaccination effect has yet to be determined in the ever-changing landscape of SARS-CoV-2 variants.1,2
Breakthrough infections
As of June 15, 2021, the overall vaccination rate in Belgium was 62.8% having received 1 dose (adult population), of whom 35.2% had fully completed their vaccination scheme.3 Belgian healthcare workers (HCWs) have a high exposure to COVID-19: the estimated percentage of confirmed Belgian COVID-19 cases is significantly higher among those working in healthcare facilities than among employees in other industries as well as the national average.4 Because HCWs were prioritized in the vaccination strategy, studying this population provided early data with which to analyze its effect. Vaccination in Belgium is not mandatory.
Vaccination of HCWs in ZNA started on January 18, 2021. ZNA is a 2,500-bed, public, multiple-site, hospital network in the Antwerp region. It comprises 3 acute-care hospitals, a children’s hospital, and 5 chronic care facilities. Depending on their availability, 3 different vaccines were used: BNT162b2 (Comirnaty, BioNTech/Pfizer, Mainz, Germany), mRNA-1273 (COVID-19 Vaccine Moderna, Moderna, Cambridge, MA) and AZD1222 (Vaxzevria, Astra Zeneca, Cambridge, UK). The impact of vaccinations on the positive test ratio was evaluated from March 1 through April 30, 2021, a period with continuing and substantial viral circulation in the Belgian population.3 Tests were performed for contact tracing or COVID-like symptoms. Among 3,491 fully vaccinated ZNA HCWs, 9 (0.3%) tested positive for SARS-CoV-2 (RT-PCR, Cobas 6800, Roche). After excluding 1 case, following CDC guidelines on persistent shedding,5 22 (1.0%) of 2,215 unvaccinated HCWs (n = 584) or partially vaccinated HCWs (n = 1,631) tested positive. Partially vaccinated was defined as having received only 1 dose or the second dose <14 days prior.
There were no significant differences between gender and age distribution for either group (P = 0.6 for gender; P = 0.3 for age), with age ranging from 21 to 58 years. There were no known comorbidities or use of medication; among fully vaccinated HCWs, such factors might have explained a breakthrough infection.
Comparison of these proportions showed a significant difference between the 2 groups (odds ratio 3.9; 95% confidence interval, 1.8–8.4; P < .001). Of the 9 HCWs who were fully vaccinated, 5 HCWs were vaccinated with the Comirnaty vaccine and 4 were vaccinated with the Moderna vaccine. Because the second dose of Vaxzevria could only be administered after 12 weeks, no HCWs were fully vaccinated with the latter.
Of the 31 HCWs, 26 were asymptomatic and discovered through contact tracing. In addition, 5 HCWs were tested because of symptoms compatible with COVID-19. Of these 5 HCWs, 2 were fully vaccinated (mRNA-1273). The cases of 18 HCWs who tested positive were all independent of the other cases. The other 13 cases were partially clustered in 6 groups working in the same ward: 5 groups of 2 HCWs and 1 group with 3 linked cases.
Viral loads
Cycle threshold (Ct) values were available for 7 of the 9 fully vaccinated HCWs and for 17 of the partially or unvaccinated HCWs, allowing viral load comparison (Fig. 1). Interestingly, fully vaccinated HCWs had relatively high viral loads: Ct values of 25.1 and 25.7 in 2 HCWs, respectively, corresponding to 4.6 and 4.4 log copies/mL. No significant differences in Ct values were observed between the 2 groups. The assumption that vaccination not only prevents severe disease and hospitalization but also diminishes the viral load once exposed6 was not substantiated by our data.
Fig. 1.
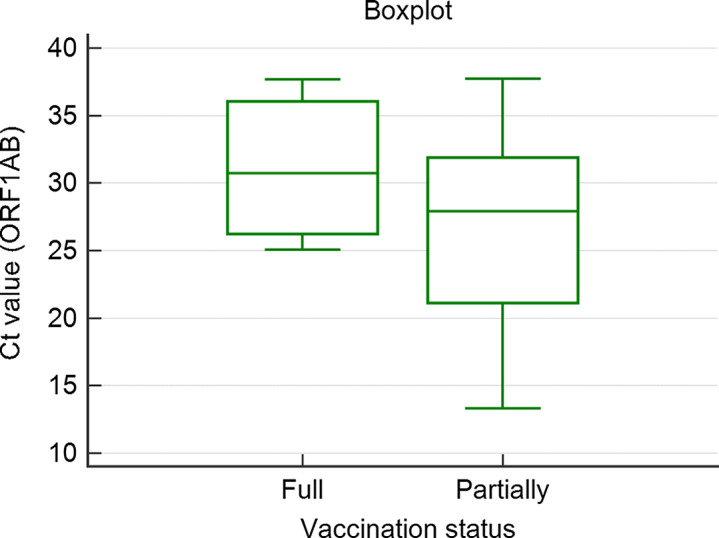
Boxplot showing the distribution of cycle threshold (Ct) values of the ORF1AB gene. ‘Partially’ on the right shows the data of the nonvaccinated and incompletely vaccinated group, ‘Full’ on the left shows data for the fully vaccinated group.
Sequencing
To gather more information about the influence of different strains, every breakthrough infection was genetically sequenced if the viral load was high enough to do so. Of the 7 fully vaccinated HCWs, 4 were eligible for whole-genome sequencing, and all 4 of these were infected with the B.1.1.7 strain. Of the unvaccinated and partially vaccinated HCWs, virus samples from 6 of these 17 were sequenced. Of these 6 SARS-COV-2–positive patients, 4 were infected with the B.1.1.7 strain as well, the other 2 had viral strains that originated from clade 20B, a variant first sequenced in Nigeria of unknown importance. During the investigation period, ˜71% of the sequenced Belgian strains consisted of the B.1.1.7 strain (>15,000 strains uploaded on Gisead).7
Our study has several limitations. One drawback of our investigation was the sample size. Although the study was performed in the largest hospital network in Belgium, our data are limited to Antwerp. We need larger, preferably international, studies with more statistical power to determine the true differences between these groups. Because this analysis was retrospective, we were unable to establish the baseline status of every employee before vaccination began. Invdividuals who shed the virus over an extended period (ie, “long-shedders”) were included in our analysis, which may have distorted the true difference between the groups. Information about the presence and titer values of antibodies to SARS-CoV-2 would have added value to our analysis as well.
In conclusion, vaccination led to a significant reduction in the incidence of SARS-CoV-2 infection rates in HCWs of an Antwerp-based multisite hospital. However, viral carriage was still present, and viral loads were not significantly lower than those of partially and unvaccinated HCWs. No information regarding an underlying immunodeficiency or relevant immunosuppressive medication was retained. The variants detected in the vaccinated HCWs reflected the current baseline epidemiology in the Antwerp region, where the dominant strain is B.1.1.7.
Not all patients have been vaccinated and substantial evidence suggests a lower efficacy in some immunosuppressed patients.8 Thus, we argue for maintaining strict contingency measures in the hospital setting.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
Prof. Dr. Van Damme reported that the University of Antwerp receives research grants from GSK Biologicals, Pfizer, SANOFI, Merck, Themis, Osivax, J&J and Abbott, The Bill & Melinda Gates Foundation, PATH, the Flemish government, and the European Union outside the submitted work. All other authors report no conflicts of interest relevant to this article.
References
- 1. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 2021;384:2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta RK. Will SARS-CoV-2 variants of concern affect the promise of vaccines? Nat Rev Immunol 2021;21:340–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19— Epidemiologisch bulletin van 17 juni 2021. Sciensano website. www.sciensano.be. Published 2021. Accessed July 13, 2021.
- 4.COVID-19 incidentie in de actieve beroepsbevolking per sector. Sciensano website. https://covid-19.sciensano.be/nl/covid-19-epidemiologische-situatie. Published 2021. Accessed July 13, 2021.
- 5.Interim guidance on ending isolation and precautions for adults with COVID-19. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Published 2021. Accessed July 13, 2021.
- 6. Levine-Tiefenbrun M, Yelin I, Katz R, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med 2021;27:790–792. [DOI] [PubMed] [Google Scholar]
- 7. Baele G, Cuypers L, Maes P, et al. Genomic surveillance of SARS-CoV-2 in Belgium, situation update 4th of May 2021. National Reference Laboratory UZ Leuven website. https://assets.uzleuven.be/files/2021-03/genomic_surveillance_update_210211.pdf. Published 2021. Accessed July 13, 2021.
- 8. Sonani B, Aslam F, Goyal A, Patel J, Bansal P. COVID-19 vaccination in immunocompromised patients. Clin Rheumatol 2021;40:797–798. [DOI] [PMC free article] [PubMed] [Google Scholar]


