Abstract
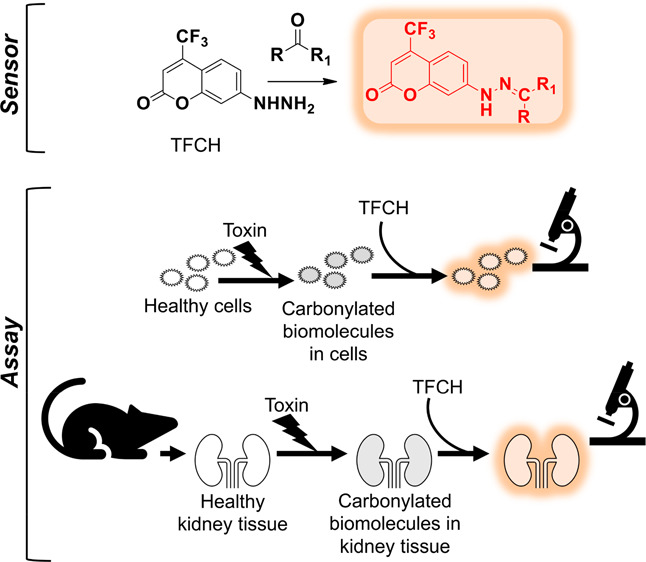
Drug-induced kidney injury frequently leads to aborted clinical trials and drug withdrawals. Sufficiently sensitive sensors capable of detecting mild signs of chemical insult in cell-based screening assays are critical to identifying and eliminating potential toxins in the preclinical stage. Oxidative stress is a common early manifestation of chemical toxicity, and biomolecule carbonylation is an irreversible repercussion of oxidative stress. Here, we present a novel fluorogenic assay using a sensor, TFCH, that responds to biomolecule carbonylation and efficiently detects modest forms of renal injury with much greater sensitivity than standard assays for nephrotoxins. We demonstrate that this sensor can be deployed in live kidney cells and in renal tissue. Our robust assay may help inform preclinical decisions to recall unsafe drug candidates. The application of this sensor in identifying and analyzing diverse pathologies is envisioned.
Keywords: live cells, kidney tissue, carbonylated biomolecules, oxidative stress, hydrazine, fluorogenic assay
Drug-induced kidney injury, or nephrotoxicity, is a critical limiting factor in the development of new therapeutics. Existing preclinical screening processes are often unable to predict nephrotoxicity in humans, which results in failed clinical trials.1,2 Currently used phenotypic preclinical screening assays measure alterations in cell viability, morphology, and mitochondrial function.2 However, the majority of these assays detect only severe forms of injury at high doses and/or after lengthy exposure to compounds. Therefore, these assays are insensitive to modest changes that can potentially generate a much greater magnitude of toxicity in vivo.1 Despite the recent progress,3−5 an unmet need for sensitive sensors and potent screening assays for detecting early signs of nephrotoxic insult continues to impede biomedical advancement and pose economic burden on the drug development process.
Unlike changes in cell viability and morphology, which require a more pronounced chemical insult, an early response to mild injury is oxidative stress (OS). OS is evidenced by an upsurge of oxidants, such as reactive oxygen species (ROS), and a depletion of reductants, such as reduced glutathione (GSH).6 While changes in the levels of ROS and GSH are transient, a cardinal irreversible consequence of OS is the carbonylation of biomolecules. Since these stable modified biomolecules are formed promptly after chemical injury, they can serve as an early reliable biomarker for identifying potential cytotoxins.
Carbonylation is commonly detected using alpha-effect amines as reporter molecules in biochemical assays.7,8 The conventional assays often require lengthy tedious downstream processing and harsh chemical components that can alter subcellular structures, thereby misrepresenting spatial distribution of carbonylated biomolecules.9 Moreover, end-point analyses of fixed cells or cell lysates were the only options until we demonstrated the first live cell compatible assay using synthetic probes, coumarin hydrazine (7-hydrazinyl-4-methyl-2H-chromen-2-one, CH; see Figure S1 for structures) and benzocoumarin hydrazine (7-hydrazinyl-4-methyl-2H-benzo[h]chromen-2-one, BzCH).10,11 Our approach was also validated by Vemula et al. using a commercially available probe, 7-(diethylamino)coumarin-3-carbohydrazide (DCCH)9 and very recently by others with a synthetic probe.12 Since crucial prerequisites for identifying mild phenotypes of chemical toxicity are high sensitivity of the probe and its applicability in the renal system, this work is aimed at achieving these objectives. Leveraging our experience in probe development for biomolecule carbonyls, we have developed a new sensor, 4-trifluoromethyl-7-hydrazinyl-2H-chromen-2-one (TFCH), that is particularly suited for detecting mild signs of nephrotoxin-induced carbonylation in live cells and living tissues.
Results and Discussion
TFCH Is a Fluorogenic Sensor for Oxidative-Stress Induced Carbonylation in Live Cells
We have previously shown that coumarin-based fluorophores have low inherent toxicity and can be readily internalized and washed out from the cells.10,11 It was therefore desirable to retain these features in the new fluorophore. Substitution of the methyl group of CH with a trifluoromethyl group was anticipated to have little effect on the physical properties of the probe while improving its photochemical properties.13,14 A trifluoromethyl substituent at C-4 is known to strongly red shift the absorption and emission envelopes of related aminocoumarins,15,16 which allows greater accessibility of the commonly used 405 nm laser line. Additionally, it is known that adding fluorine or trifluoromethyl groups to a fluorophore scaffold typically improves its photochemical stability.15,17,18 Therefore, a trifluoromethyl derivative of CH, TFCH (Figure S1), was designed to be an efficient live cell compatible probe that can detect low levels of biomolecule carbonylation resulting from mild nephrotoxicity.
TFCH’s ability to react with a model aliphatic aldehyde, propanal, in neutral aqueous solution was confirmed by absorption and fluorescence spectroscopy (Figure S2). Additionally, we prepared a hydrazone product of TFCH and propanal, TFCZ, as a model compound for carbonylation detection and assessed its optical properties (Figure 1). Hydrazone formation results in a bathochromic shift in the emission maximum and induces a substantial increase in the emission intensity relative to the unreacted probe (Figures 1B and S3 A–E). Such fluorogenicity is a desirable photochemical property especially for establishing one-step cell-based assays that do not necessitate a washing step.10 TFCZ also shows an exceptionally large Stokes shift of ∼145 nm, which eliminates the chance of self-quenching and is a generally useful feature for analytical assays11 (Figures S3).
Figure 1.
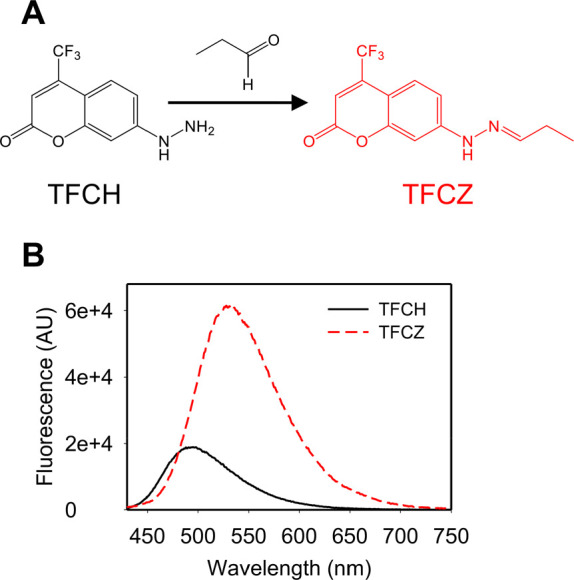
TFCH is a fluorogenic sensor for biomolecule carbonyls. Chemical structure of 4-trifluoromethyl-7-hydrazinyl-2H-chromen-2-one (TFCH) and its corresponding hydrazone with propanal (TFCZ) (A). Emission spectra (405 nm excitation) of 10 μM TFCH or TFCZ in phosphate buffer containing 0.5% (v/v) DMSO (B).
We confirmed the specificity of the hydrazine probe for carbonylated biomolecules using a model protein, oxidized bovine serum albumin (BSA). TFCH and its amine analogue, 4-methyl-7-hydrazinyl-2H-chromen-2-one (TFCA, Figure S4A), which is not expected to form a stable bond with carbonyls in aqueous solution, were examined as detection tools. As expected, only TFCH-oxidized BSA produced a bright fluorescent band in SDS-PAGE, while negligible fluorescence was associated with TFCA-oxidized BSA. Similarly, TFCH-unmodified BSA yielded a minimally fluorescent band (Figure S4).
Finally, the nature and environment of intracellular carbonyls are complex. Carbonylated biomolecules include proteins, lipids, and nucleic acids and are not completely described using simple model compounds. To assess the photochemical properties of TFCH-labeled cellular carbonyls, live A549 lung cancer cells (control or serum starved) were treated with CH or TFCH, washed, lysed, and the emission spectrum of each lysate was collected (Figure S5). The integrated emission intensity of the TFCH-treated cells was about 3- to 4-fold greater than that observed with CH.
TFCH was then employed to establish different live cell-based assay formats using serum-free media (SFM) as an OS-induction model in A549 cells. We demonstrated (1) a simple platereader-based assay appropriate for high-throughput screening; (2) a one-step (no-wash) high content screening compatible assay, which is particularly suitable for screening OS-inducing molecules that have a propensity to induce cell detachment;20 and (3) a two-step assay that is amenable to visualizing both live and fixed cells (Figure S6). Together, these data establish TFCH as a versatile tool for visualizing and quantifying biomolecule carbonyls in live cells using multiple assay formats.
A Sensitive Fluorescent Tool for Screening Chemical Toxin-Induced OS in Live Renal Cells
Two standard cellular models were selected for assessing small molecule-induced injury in renal cells: porcine kidney proximal tubule (LLC-PK1) cells and distal tubule-derived Madin-Darby Canine Kidney (MDCK) cells.21,22 Using MDCK cells, we first ensured that TFCH is not cytotoxic (Figure S7A,B). Next, to establish the utility of TFCH in these cell lines, three different stressors, SFM, menadione, or hydrogen peroxide, were used to model oxidative damage. TFCH detected a significant increase (∼220% to ∼770%) in carbonylation in both cell lines when subjected to the aforementioned OS-inducing agents (Figure S7C–E).
To directly validate our claim that the superior photochemical properties of TFCH deem it particularly suited for detecting mild oxidative damage in live cells, we compared the biomolecule-carbonyl sensing ability of our previously synthesized fluorophores CH and BzCH, the commercially available DCCH, and TFCH under the same experimental conditions. A brief exposure of MDCK cells to SFM was used to generate low levels of carbonylated biomolecules. Figure 2A shows that TFCH produced a strong signal in the SFM treated cells and a weak, albeit visible, signal in the control cells, which have an inherent but low level of oxidative stress.23 Under the same optical parameters, negligible fluorescence is observed from the other probes. The photomicrographs from Figure 2A were digitally enhanced in Figure 2B to demonstrate the presence of biomolecule carbonyls identified by CH, BzCH, and DCCH. While all four fluorophores were able to sense SFM-induced carbonylation, the signal generated by TFCH well surpassed that of the other fluorophores (Figure 2C). These data affirm TFCH’s superiority in detecting modest signs of cellular carbonylation and positively support the notion that TFCH may serve as a tool for detecting low levels of chemical toxicity in kidney cells.
Figure 2.
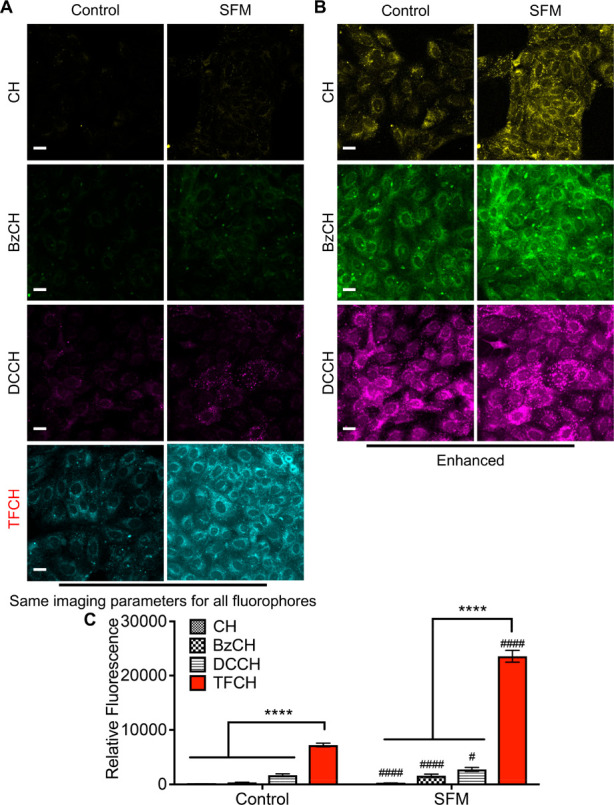
TFCH is better suited for sensing mild forms of oxidative injury in live cells. MDCK cells grown in standard media (control) or serum-free media (SFM) for 1.5 h were allowed to react with 20 μM fluorophore for 30 min, rinsed, fixed, and processed as described in the Methods. All the samples were imaged using the same imaging parameters (A). Images of the cells treated with CH, BzCH, and DCCH are enhanced for visual clarity (B). A pseudocolor was assigned to each fluorophore. Scale bar, 20 μm. Bar graphs showing quantification of cellular carbonyls detected by each fluorophore in control and serum-starved cells (C). Three independent experiments were performed, and fluorescence associated with >100 cells was quantified. An unpaired t test with Welch’s correction was performed to either compare the fluorescence signal generated by TFCH and the other fluorophores (****P < 0.0001) or to compare the fluorescence signal of each fluorophore in control and SFM treated cells (####P < 0.0001, #P < 0.05). Error bars represent SEM.
We used two drugs with known nephrotoxicity in humans, cisplatin (anticancer) and gentamicin (antibiotic), to further validate our tool and assay.24 The high expression level of copper transporters responsible for cisplatin endocytosis in proximal tubule cells makes these cells more vulnerable to cisplatin-induced injury.25,26 The same cells are also the primary site of injury for gentamicin.27 Loss of cell polarity of these renal epithelial cells and alteration in the actin cytoskeleton are prominent manifestations of nephrotoxicity.28−30 In addition, both drugs increase OS and initiate cell signaling pathways that ultimately lead to cell death and/or detachment.31,32 Change in cell morphology, OS status, and cell viability are thus the basis of conventional nephrotoxicity screenings. In order to validate our TFCH assay, we examined how it compares with other available assays.
Since our goal was to develop an assay that can detect modest injury, we first focused on determining experimental parameters that generate only sub-cytotoxic effects after 24 h of drug treatment. In particular, we chose drug concentrations that showed minimal toxicity (cell viability ≥65%) based on a resazurin assay (monitors cell metabolism) and a sulforhodamine B (SRB) assay (monitors cell number) (Figure 3). Using these conditions, we first examined structural changes, namely, the extent of loss of cell polarity and alterations in the actin cytoskeleton. These are two common phenotypes that form the basis of conventional nephrotoxicity assays.
Figure 3.
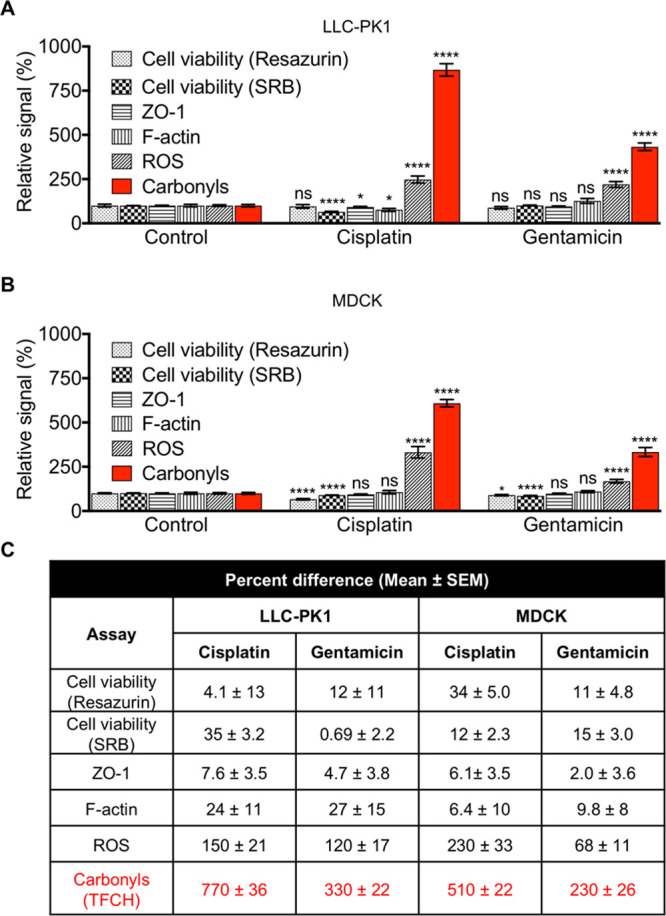
TFCH-mediated detection of carbonylation outperforms classical nephrotoxicity assays in renal epithelial cells. Bar graphs showing the effects of cisplatin (1.5 μg/mL) or gentamicin (0.58 mg/mL) on LLC-PK1 (A) or MDCK (B) cells after 24 h. Cell viability was assessed by a resazurin or SRB assay (independent experiments ≥ 2). Level of ZO-1 or actin stress fibers (F-actin) (independent experiments ≥ 2, number of cells quantified per condition, cell no. ≥ 60) was evaluated by immunocytochemistry; reactive oxygen species (ROS) or carbonylation (independent experiments ≥ 2, cell no. ≥ 200) level was assessed by CellROX Green or TFCH respectively; as described in theMethods. Error bars represent SEM. Percent difference of each treatment from the control (no drug treatment) recorded by each assay (C). An unpaired t test with Welch’s correction was performed. ****P < 0.0001, *P < 0.05, P > 0.05 was considered not significant (ns).
We performed immunocytochemistry to identify and quantify the intensity of zonula occludens-1 (ZO-1), a tight junction protein that defines cell polarity,33,34 and the level of intracellular actin stress fibers (represented by filamentous actin, F-actin). In LLC-PK1 cells, cisplatin induced tight junction disruption demonstrated by discontinuous ZO-1 staining pattern (inset in Figure S8A), and marginally significant decrease in ZO-1 intensity (Figure 3A). Additionally, the cells appeared rounded. F-actin reorganized to predominantly localize toward the cell periphery (inset in Figure S8A), while its intensity decreased in the central region (Figure 3A). In contrast, gentamicin did not affect LLC-PK1 cell polarity or the actin cytoskeleton (Figures 3A and S8A). In the case of MDCK cells, both cisplatin and gentamicin treatments had negligible effect on ZO-1 and F-actin level (Figures 3B and S8B). Together, relatively modest or lack of structural changes was observed. Overall, these assays were deemed not sufficiently sensitive to detect cellular damage under these conditions.
We next examined the effects of the drugs on the status of cellular OS by determining the level of ROS, a dominant precursor of carbonylated biomolecules. Although we established the assay conditions to generate mild cellular phenotypes, a significant increase (∼68–230%) in the level of ROS was observed in both cell lines treated with gentamicin and cisplatin (Figures 3 and S8). These data show that the ROS level exhibited the greatest magnitude of difference between injured and uninjured cells, suggesting that OS is the strongest phenotype tested thus far.
Finally, we tested TFCH’s ability to detect drug-induced cellular carbonyls, a stable downstream effector of ROS, under the same conditions. TFCH showed a strong response with both drugs in both cell lines (Figures 3 and S8). The injured cells were 230–770% more fluorescent than their uninjured counterpart when assayed with TFCH, whereas the best signal enhancement from the ROS assay was 230%. A side-by-side comparison of the changes in cell morphology or OS level generated by cisplatin and gentamicin clearly illustrates the superiority of the TFCH assay (Figure 3C).
Owing to the early induction of the biomarker and the desirable sensitivity of the fluorophore, we speculated that our assay may be able to detect injury after a brief exposure to the drugs, instead of 24 h. We treated the cells with the drugs for 3 h prior to adding TFCH. A significant increase in fluorescence of up to ∼190% in cisplatin- or gentamicin-injured cells was observed (Figure S9). The temporal sensitivity, along with the ease of performing the assay, enable data generation within hours. Together, these data show that the TFCH assay is the most sensitive assay tested herein and is capable of measuring mild signs of drug insult.
TFCH Is a Sensor for Detecting Carbonylation in Live Renal Tissue
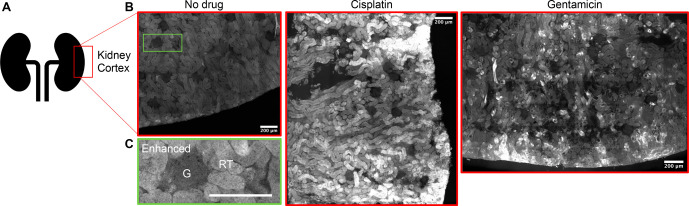
While monolayers of renal cells in culture serve as the current gold standard for screening nephrotoxins, the complexity of the renal system as a whole is not comprehensively represented by any single cell type in culture.1,35 More physiologically relevant screening platforms are critical for improving safety profile predictability. We thus tested the applicability of TFCH in detecting kidney tissue injury in live tissue slices. The kidney slices were maintained live during the experimental procedure using a pre-established protocol to support normal physiology of the tissue.36 Rat kidney slices subjected to cisplatin or gentamicin treatment followed by TFCH exhibited substantially higher levels of carbonyls compared to the uninjured control (Figures 4 and S10). The fluorescent labeling was mainly associated with the tubules and not the glomerulus (Figure S10B), which is in agreement with the existing paradigm that renal proximal tubules are the primary sites of drug-induced damage.26,27,37 Our data thus attest to the utility of this assay and fluorophore in a complex tissue system.
Figure 4.
TFCH detects drug-induced carbonylation in live rat kidney slices. Schematic representation showing the region of kidney used for imaging (A). Representative photomicrographs assembled (stitched) from multiple sections of the renal cortex (B). As indicated, rat kidney slices were exposed to cisplatin (150 μg/mL), gentamicin (4.6 mg/mL), or vehicle (buffer; no drug) for 1 h, followed by the addition of TFCH (2 μM) for 30 min. The tissue samples were washed, fixed, and processed as described in the Methods before imaging. An inset (enhanced) (C) of the control (no drug) slice is showing the location of renal tubule (RT) and glomerulus (G). Scale bar, 200 μm
In summary, we have developed a novel, rapid, and sensitive assay that probes for an early biomarker (OS-induced biomolecule-carbonyls) of drug-induced nephrotoxicity. Photochemical attributes of our fluorophore and the appropriateness of the biomarker allow for significantly improved sensitivity when compared to currently used assays. The ability to detect early signs of nephrotoxicity in kidney epithelial cells by using high-throughput/high-content-screening platforms is expected to facilitate facile preclinical drug safety screening.38 Expanding the utility of TFCH from the ex vivo setup demonstrated herein to in vivo investigation of drug-induced organ injury is envisioned.39−41
Oxidative damage is frequently associated with diverse forms of kidney injury, ranging from acute infection- or toxin-driven pathologies to chronic damage due to prolonged hyperglycemia.37,42,43 Given the commonality in the cellular response, fluorescent detection of carbonyls by TFCH can potentially be used to study a multitude of renal injury models. In conjunction with classical histology, this assay may serve as a reliable component of a composite scoring system accounting for both structural changes (classical histology) and chemical changes (carbonylation) associated with various tissue injury models. The prevalence of the biomarker and the adaptability of the assay to both cell monolayer and a tissue system support the notion that insult to other organs, not confined to the kidney, can be probed by TFCH.
Acknowledgments
The authors thank Dr. Anthony Sorrentino for his initial efforts in the synthesis of TFCH and its derivative, David Tuttle for his expert assistance in gel imaging and image processing, Prof. Ming An for the generous gift of A549 cells, Bradley Pedro for technical assistance with cell culture, immunocytochemistry, and image quantification, Prof. Richard Bouley for kindly providing the rat kidney slices and the essential facility to perform tissue-based experiments, and Dr. Anilkumar Nair for his expert assistance and training in tissue imaging.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssensors.1c00422.
Figures S1–S10, synthesis and characterization of TFCH and TFCZ, and details of experimental procedure (PDF)
Author Present Address
# Department of Pathology and Laboratory Medicine, Institute on Aging and Center for Neurodegenerative Disease Research, University of Pennsylvania School of Medicine, Philadelphia, PA 19104
Author Present Address
∥ AstaTech, Inc. Keystone Business Park 2525 Pearl Buck Road. Bristol PA, 19007
Author Contributions
KM and SB conceptualized the project. KM, SLB, and SS designed the research in consultation with DLS. KM wrote the paper in consultation with TIC. TIC, DLS, SLB, and SS edited the paper. KM, TIC, and HG designed and performed the experiments and analyzed the data.
This work was supported in part by the NIH (Grant R15 GM102867 and R15 CA227747 to SLB; R01 DK093773 and R01 DK087985 to SS), and in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (DLS). The Regional NMR Facility at Binghamton University is supported by the NSF (CHE-0922815). Some imaging was performed in the Microscopy Core of the Program in Membrane Biology, which is partially supported by the Centre for the Study of Inflammatory Bowel Disease Grant DK043351 and the Boston Area Diabetes and Endocrinology Research Center (BADERC) Award DK057521. One of the Zeiss confocal systems was purchased with an NIH shared instrumentation grant 1S10OD021577-01.
The authors declare the following competing financial interest(s): SS has pending or issued patents on novel kidney-protective therapies that have been out-licensed to Walden Biosciences in which she has financial interest. In addition, she stands to gain royalties from their commercialization. SLB and KM are inventors on pending patent application pertaining to the work presented here.
Notes
A preprint version of this work was deposited. Mukherjee, K.; Chio, T. I.; Gu, H.; Sackett, D. L.; Bane, S. L.; Sever, S. A Highly Sensitive Fluorogenic Assay for the Detection of Nephrotoxin-Induced Oxidative Stress in Live Cells and Renal Tissue. bioRxiv2020, 2020.06.01.121707.
Supplementary Material
References
- Soo J. Y.; Jansen J.; Masereeuw R.; Little M. H. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat. Rev. Nephrol. 2018, 14 (6), 378–393. 10.1038/s41581-018-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. X.; Blaskovich M. A.; Cooper M. A. Cell- and biomarker-based assays for predicting nephrotoxicity. Expert Opin. Drug Metab. Toxicol. 2014, 10 (12), 1621–35. 10.1517/17425255.2014.967681. [DOI] [PubMed] [Google Scholar]
- Vaidya V. S.; Ferguson M. A.; Bonventre J. V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–93. 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm S.; Adler M.; Vaidya V. S. A High-Throughput Screening Assay to Identify Kidney Toxic Compounds. Curr. Protoc Toxicol 2016, 69, 1–26. 10.1002/cptx.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M.; Ramm S.; Hafner M.; Muhlich J. L.; Gottwald E. M.; Weber E.; Jaklic A.; Ajay A. K.; Svoboda D.; Auerbach S.; Kelly E. J.; Himmelfarb J.; Vaidya V. S. A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro. J. Am. Soc. Nephrol. 2016, 27 (4), 1015–28. 10.1681/ASN.2015010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deavall D. G.; Martin E. A.; Horner J. M.; Roberts R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 1. 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmic M.; Javot H.; Bonzom J. M.; Lecomte-Pradines C.; Radman M.; Garnier-Laplace J.; Frelon S. In situ visualization of carbonylation and its co-localization with proteins, lipids, DNA and RNA in Caenorhabditis elegans. Free Radical Biol. Med. 2016, 101, 465–474. 10.1016/j.freeradbiomed.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Katerji M.; Filippova M.; Duerksen-Hughes P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell. Longevity 2019, 2019, 1. 10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula V.; Ni Z.; Fedorova M. Fluorescence labeling of carbonylated lipids and proteins in cells using coumarin-hydrazide. Redox Biol. 2015, 5, 195–204. 10.1016/j.redox.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K.; Chio T. I.; Sackett D. L.; Bane S. L. Detection of oxidative stress-induced carbonylation in live mammalian cells. Free Radical Biol. Med. 2015, 84, 11–21. 10.1016/j.freeradbiomed.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K.; Chio T. I.; Gu H.; Banerjee A.; Sorrentino A. M.; Sackett D. L.; Bane S. L. Benzocoumarin Hydrazine: A Large Stokes Shift Fluorogenic Sensor for Detecting Carbonyls in Isolated Biomolecules and in Live Cells. ACS Sensors 2017, 2 (1), 128–134. 10.1021/acssensors.6b00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkan H.; Telci D.; Dilek O. Design of Fluorescent Probes for Bioorthogonal Labeling of Carbonylation in Live Cells. Sci. Rep. 2020, 10 (1), 7668. 10.1038/s41598-020-64790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A. N.; Bliss D. E. Laser dye stability. Part 5. Appl. Phys. 1978, 16 (3), 289–295. 10.1007/BF00885124. [DOI] [Google Scholar]
- Fletcher A. N. Laser dye stability. Part 4. Appl. Phys. 1978, 16 (1), 93–97. 10.1007/BF00931428. [DOI] [Google Scholar]
- Schill H.; Nizamov S.; Bottanelli F.; Bierwagen J.; Belov V. N.; Hell S. W. 4-Trifluoromethyl-substituted coumarins with large Stokes shifts: synthesis, bioconjugates, and their use in super-resolution fluorescence microscopy. Chem. - Eur. J. 2013, 19 (49), 16556–65. 10.1002/chem.201302037. [DOI] [PubMed] [Google Scholar]
- Liu X.; Cole J. M.; Waddell P. G.; Lin T. C.; Radia J.; Zeidler A. Molecular origins of optoelectronic properties in coumarin dyes: toward designer solar cell and laser applications. J. Phys. Chem. A 2012, 116 (1), 727–37. 10.1021/jp209925y. [DOI] [PubMed] [Google Scholar]
- Sun W.-C.; Gee K. R.; Klaubert D. H.; Haugland R. P. Synthesis of Fluorinated Fluoresceins. J. Org. Chem. 1997, 62 (19), 6469–6475. 10.1021/jo9706178. [DOI] [Google Scholar]
- Casa S.; Henary M. Synthesis and Applications of Selected Fluorine-Containing Fluorophores. Molecules 2021, 26 (4), 1160. 10.3390/molecules26041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K.; Chio T. I.; Bane S. L. Visualization of oxidative stress-induced carbonylation in live mammalian cells. Methods Enzymol. 2020, 641, 165–181. 10.1016/bs.mie.2020.04.040. [DOI] [PubMed] [Google Scholar]
- Petejova N.; Martinek A.; Zadrazil J.; Teplan V. Acute toxic kidney injury. Renal Failure 2019, 41 (1), 576–594. 10.1080/0886022X.2019.1628780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K.; Virta H.. Growing Madin-Darby Canine Kidney Cells for Studying Epithelial Cell Biology. In Cell Biology, 3rd ed., Celis J. E., Ed.; 2006; pp 127–131. [Google Scholar]
- Halliwell B. Oxidative stress in cell culture: an under-appreciated problem?. FEBS Lett. 2003, 540 (1–3), 3–6. 10.1016/S0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- Tiong H. Y.; Huang P.; Xiong S.; Li Y.; Vathsala A.; Zink D. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol. Pharmaceutics 2014, 11 (7), 1933–48. 10.1021/mp400720w. [DOI] [PubMed] [Google Scholar]
- Ozkok A.; Edelstein C. L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 1. 10.1155/2014/967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. P.; Tadagavadi R. K.; Ramesh G.; Reeves W. B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2 (11), 2490–518. 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randjelovic P.; Veljkovic S.; Stojiljkovic N.; Sokolovic D.; Ilic I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017, 16, 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile D. P.; Anderson M. D.; Sutton T. A. Pathophysiology of acute kidney injury. Compr Physiol 2012, 2 (2), 1303–53. 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarit J.; de Hoogh A.; Obis E.; Alsina D.; Cabiscol E.; Ros J. Analysis of oxidative stress-induced protein carbonylation using fluorescent hydrazides. J. Proteomics 2012, 75 (12), 3778–88. 10.1016/j.jprot.2012.04.046. [DOI] [PubMed] [Google Scholar]
- Kruidering M.; van de Water B.; Zhan Y.; Baelde J. J.; Heer E.; Mulder G. J.; Stevens J. L.; Nagelkerke J. F. Cisplatin effects on F-actin and matrix proteins precede renal tubular cell detachment and apoptosis in vitro. Cell Death Differ. 1998, 5 (7), 601–14. 10.1038/sj.cdd.4400392. [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq M. P.; Tulkens P. M. Aminoglycosides: nephrotoxicity. Antimicrob. Agents Chemother. 1999, 43 (5), 1003–12. 10.1128/AAC.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsengeller Z. K.; Ellezian L.; Brown D.; Horvath B.; Mukhopadhyay P.; Kalyanaraman B.; Parikh S. M.; Karumanchi S. A.; Stillman I. E.; Pacher P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J. Histochem. Cytochem. 2012, 60 (7), 521–9. 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald M. A.; Choi W.; Kuo W. T.; Singh G.; Sailer A.; Wang Y.; Shen L.; Fanning A. S.; Turner J. R. The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J. Biol. Chem. 2018, 293 (45), 17317–17335. 10.1074/jbc.RA118.003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil E.; Capaldo C. T.; Macara I. G. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 2006, 17 (4), 1922–32. 10.1091/mbc.e05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E. J.; Wang Z.; Voellinger J. L.; Yeung C. K.; Shen D. D.; Thummel K. E.; Zheng Y.; Ligresti G.; Eaton D. L.; Muczynski K. A.; Duffield J. S.; Neumann T.; Tourovskaia A.; Fauver M.; Kramer G.; Asp E.; Himmelfarb J. Innovations in preclinical biology: ex vivo engineering of a human kidney tissue microperfusion system. Stem Cell Res. Ther. 2013, 4 (Suppl 1), S17. 10.1186/scrt378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouley R.; Breton S.; Sun T.; McLaughlin M.; Nsumu N. N.; Lin H. Y.; Ausiello D. A.; Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J. Clin. Invest. 2000, 106 (9), 1115–26. 10.1172/JCI9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres L. A.; da Cunha A. D. Jr. Acute nephrotoxicity of cisplatin: molecular mechanisms. J. Bras. Nefrol. 2013, 35 (4), 332–40. 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- Boutin M. E.; Voss T. C.; Titus S. A.; Cruz-Gutierrez K.; Michael S.; Ferrer M. A high-throughput imaging and nuclear segmentation analysis protocol for cleared 3D culture models. Sci. Rep. 2018, 8 (1), 11135. 10.1038/s41598-018-29169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Li J.; Lyu Y.; Miao Q.; Pu K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 2019, 18 (10), 1133–1143. 10.1038/s41563-019-0378-4. [DOI] [PubMed] [Google Scholar]
- Cheng P.; Chen W.; Li S.; He S.; Miao Q.; Pu K. Fluoro-Photoacoustic Polymeric Renal Reporter for Real-Time Dual Imaging of Acute Kidney Injury. Adv. Mater. 2020, 32 (17), e1908530 10.1002/adma.201908530. [DOI] [PubMed] [Google Scholar]
- Cheng P.; Miao Q.; Li J.; Huang J.; Xie C.; Pu K. Unimolecular Chemo-fluoro-luminescent Reporter for Crosstalk-Free Duplex Imaging of Hepatotoxicity. J. Am. Chem. Soc. 2019, 141 (27), 10581–10584. 10.1021/jacs.9b02580. [DOI] [PubMed] [Google Scholar]
- Kashihara N.; Haruna Y.; Kondeti V. K.; Kanwar Y. S. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010, 17 (34), 4256–69. 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenen K.; Andries A.; Mekahli D.; Van Schepdael A.; Jouret F.; Bammens B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34 (6), 975–991. 10.1007/s00467-018-4005-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.