In healthy people, a significant amount of nutrients is absorbed in the first 100–150 cm of the jejunum, but the absorption of water, sodium, and bile salts is most effective in the ileum and colon. In patients with short bowel syndrome (SBS), this function is disturbed.
SBS is a medical term defining metabolic disturbances occurring after extensive resection of the small intestine as a result of the reduction of the surface area for nutrient absorption. A vast majority of people with SBS demonstrate persistent diarrhoea, water–electrolyte balance disorders, weight loss, and deficiency of proteins, carbohydrates, fats, vitamins, bile salts and trace elements [1–5].
The most important aetiological SBS factors are: mesenteric ischaemia, Crohn’s disease, radiation enteropathy, malignant tumour, complications of surgical procedures, injures, etc. [1, 4].
There are 3 types of SBS: type I – end enterostomy, type II – jejunocolic anastomosis and type III – jejunoileocolic anastomosis [4].
Patients with SBS type I are provided parenteral nutrition (PN) if the remnant bowel is shorter than 115 cm. Patients with SBS type II with the remnant small intestine longer than 50 cm and the colon in continuity are largely independent of PN. Patients with SBS type III are PN dependent if the small intestine is shorter than 35 cm [1–9].
PN for many patients with short bowel syndrome is a life-saving procedure.
The study was conducted among 64 patients (45 women, 19 men), aged 29–92 years (mean: 61.75 ±14), divided into three groups, depending on the type of SBS. Each of the patients required long-term nutritional treatment provided by the Clinical Nutrition Department. The patients were intravenously administered individually selected compositions of a nutritional mixture, depending on the nutritional status, SBS type and laboratory results.
Over the period 2012–2017, every 3 months, selected laboratory parameters were evaluated in each patient during control medical visits. This procedure was conducted in accordance with the National Health Fund guidelines for patients receiving home parenteral nutrition (HPN).
Statistica (version 12, StatSoft Inc.) was used for the purpose of data management and statistical calculations. The following statistical methods were used in the study: the Kruskal-Wallis test in order to check whether there are statistically significant differences between the types of groups of patients with SBS in the (mean) concentration of the studied laboratory variables and the χ2 test in order to show a significant relationship between the type of SBS and the value of a measurable variable in laboratory tests, together with multiple comparisons. P-values < 0.05 were considered statistically significant.
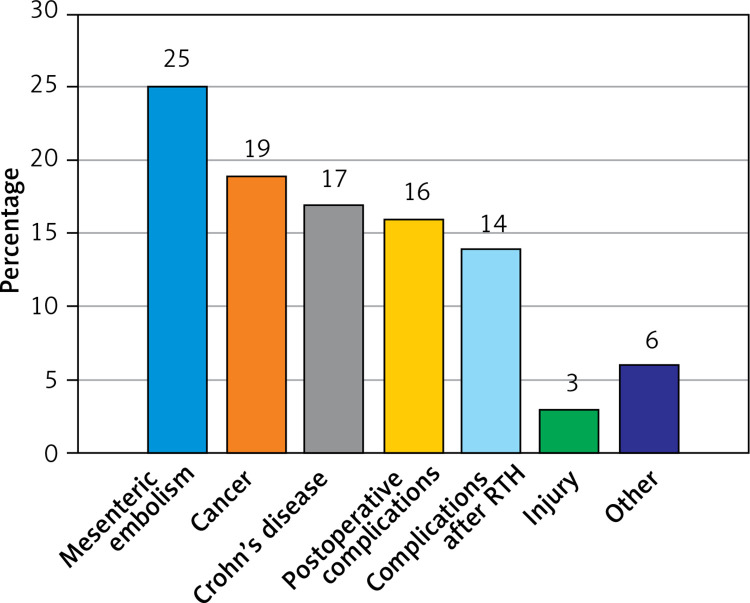
The most common causes of short bowel syndrome in the study group were: mesenteric embolism in 16 (25%) patients, cancer in 13 (19%) and Crohn’s disease in 11 (17%) patients (Figure 1).
Figure 1.
Etiology of SBS patients
The average length of the small intestine of patients with SBS type I was 95 ±72.29 cm; type II 75.57 ±50.43 cm and type III 96.53 ±40.28 cm. The average length of the large intestine of patients with type I was 60 ±56 cm, type II 88 ±32 and type III 104 ±25 cm (p < 0.05).
The average duration of parenteral nutrition was 51.38 ±46.01 months. The mean volume of intravenous nutrition was 2184.78 ±161.26 ml.
Composition of the PN: the average content of amino acids intake was 0.83 ±0.26 g/kg/day, glucose 4.16 ±1.37 g/kg/day and lipid 0.31 ±0.19 g/kg/day (Table I). The mean caloric content of PN in patients with SBS was 1336.81 ±167.13 kcal/day. The average body mass index (BMI) of our patients was 21.83 ±4 kg/m2, which indicates a proper nutritional status. Patients with all types of SBS were underweight as well as overweight. Twenty-two percent of patients with SBS type I, 9% with type II and 22% with type III were underweight (BMI < 18.5 kg/m2). A body composition analysis was not included in the study, because the study was retrospective, and according to the guidelines for patients receiving HPN measuring the body composition of patients is not required.
Table I.
Demographic and anatomical features and laboratory test results for the study population
| Characteristic | All patients (n = 64) | Group I (n = 23) | Group II (n = 23) | Group III (n = 18) | Kruskal-Wallis | χ2 |
|---|---|---|---|---|---|---|
| Patients demographics: | ||||||
| Age | 61.75 ±14 | 67.91 ±12.89 | 62.78 ±12.96 | 52.72 ±12.33 | NS | NS |
| BMI [kg/m2] | 21.83 ±4 | 22.90 ±4.83 | 21.83 ±3.46 | 20.41 ±3.15 | NS | NS |
| Anatomic features: | ||||||
| Remnant small intestine length [cm] | 88.75 ±56.21 | 95 ±72.29 | 75.57 ±50.43 | 96.53 ±40.28 | NS | NS |
| Remnant large intestine length [cm] | 77 ±47 | 60 ±56 | 88 ±32 | 104 ±25 | NS | < 0.05 |
| Parenteral nutrition: | ||||||
| Nutrition volume [ml] | 2184.78 ±161.26 | 2312.63 ±817.99 | 2238.10 ±490.81 | 2003.61 ±491.07 | < 0.05 | NS |
| Parenteral amino acid intake [g/kg/day] | 0.83 ±0.26 | 0.81 ±0.28 | 0.86 ±0.27 | 0.82 ±0.24 | NS | NS |
| Parenteral glucose intake [g/kg/day] | 4.16 ±1.37 | 4.17 ±1.27 | 4.49 ±1.63 | 3.82 ±1.23 | NS | NS |
| Parenteral lipid intake [g/kg/day] | 0.31 ±0.19 | 0.34 ±0.19 | 0.29 ±0.18 | 0.31 ±0.19 | NS | NS |
| Energy [kcal/db] [kcal/day] | 1336.81 ±167.13 | 1266.68 ±291.43 | 1527.58 ±1124.92 | 1216.18 ±262.67 | NS | NS |
| Parenteral infusion intake [days/week] | 6.11 ±0.97 | 6.04 ±1.07 | 6.30 ±0.93 | 5.96 ±0.92 | NS | NS |
| Laboratory test: | ||||||
| Sodium (136 – 145 mmol/l) | 139.37 ±2.55 | 138.88 ±2.63 | 139.71 ±1.67 | 139.56 ±3.34 | NS | NS |
| Potassium (3.5–5.1 mmol/l) | 4.71 ±1.23 | 4.96 ±1.3 | 4.35 ±0.68 | 4.88 ±1.60 | < 0,05 | < 0.05 |
| Calcium (8.4–10 mg/dl) | 9.33 ±0.44 | 9.32 ±0.35 | 9.36 ±0.54 | 9.30 ±0.42 | NS | NS |
| Magnesium (1.50–2.60 mg/dl) | 1.92 ±0.18 | 1.93 ±0.22 | 1.90 ±0.15 | 1.95 ±0.17 | NS | NS |
| Chloride (98–107 mmol/l) | 102.56 ±4.31 | 101.35 ±6.13 | 103.72 ±1.92 | 102.01 ±3.57 | NS | < 0,05 |
| Phosphates (2.5–4.5 mg/dl) | 3.59 ±0.42 | 3.57 ±0.36 | 3.56 ±0.57 | 3.67 ±0.27 | NS | NS |
| Urea (16.6–48.5 mg/dl) | 40.70 ±16.04 | 47.41 ±20.53 | 38.68 ±13.50 | 34.70 ±8.26 | < 0.05 | < 0.05 |
| Creatinine (0.5–0.9 mg/dl) | 1.09 ±0.47 | 1.28 ±0.56 | 0.96 ±0.41 | 1.04 ±0.37 | < 0.05 | < 0.05 |
| Triglycerides (≤ 150 mg/dl) | 135.24 ±57.4 | 141.46 ±60.94 | 124.97 ±44.84 | 140.44 ±67.74 | NS | NS |
| ALT (0–33 U/I) | 52.27 ±48.16 | 54.45 ±24.31 | 49.27 ±24.31 | 53.33 ±70.22 | NS | NS |
| AST (0–33 U/I) | 36.83 ±20.41 | 38.60 ±18.51 | 35.29 ±12.73 | 36.53 ±29.73 | NS | NS |
Nutritional recommendations and amounts of oral fluids were adjusted to the type of SBS. None of the patients received enteral nutrition. Each of the patients included in the study drank oral rehydration solutions (ORSs), which contained appropriate proportions of sodium, water and carbohydrates. Patients took commercial ORSs or prepared them at home, according to the given prescription. During hospitalization, patients are given 5% glucose solution and 0.9% NaCl in a 2 : 1 ratio to drink. We estimate that approximately 80% of patients follow the recommendations at home.
The average values of renal function parameters – urea and creatinine – in our patients on their first visit to the Clinical Nutrition Department were: SBS type I urea 50.57 ±17.47mg/dl, creatinine 1.19 ±0.60 mg/dl; for type II urea 37.41 ±19.05 mg/dl, creatinine 0.82 ±0.43 mg/dl and for type III urea 35.84 ±21.98 mg/dl, creatinine 1.06 ±0.62 mg/dl and gradually were normalized.
An analysis of the groups of SBS patients and studied variables showed that there were significant differences in the laboratory parameters. The patients with SBS type I demonstrated higher levels of blood alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, creatinine, and potassium concentration (p < 0.05) in comparison with patients with type II and III of SBS. The mean values of renal function parameters, calculated throughout the study period, were as follows for SBS type I patients: urea 47.41 ±20.53 mg/dl, creatinine 1.28 ±0.56 mg/dl. The lowest urea concentration was observed in patients with SBS type III, i.e. 34.70 ±8.26 mg/dl. The creatinine level observed in SBS type II patients was 0.96 ±0.41 mg/dl. Almost a quarter of the patients with end enterostomy demonstrated elevated urea concentration.
A vast majority of the studied patients demonstrated elevated liver enzymes. Other investigators have also observed liver dysfunction, which may be associated with long-term parenteral nutrition. Ławiński et al. noted high values of liver function parameters in patients with type I short bowel syndrome receiving parenteral nutrition. Nanji AA and Anderson FH observed elevated γ-glutamyltranspeptidase (GGTP) levels, which returned to normal after a complete withdrawal of parenteral nutrition [7, 8].
Long-term parenteral nutrition carries a risk of developing intestinal failure-associated liver disease (IFALD). According to guidelines of the European Society for Clinical Nutrition and Metabolism (ESPEN), appropriate oral or enteral nutrition as well as optimal composition of PN play a key role in prevention and treatment of this complication. It is particularly important to avoid excessive consumption of long chain fatty acids (LCFA) and polyunsaturated fatty acids and totally eliminate phytosterols [9–12].
In the Nutrition Treatment Centre, among patients with elevated liver function parameters their glucose content is individually adjusted in parenteral nutrition. Besides, when indicated, LCFA fat emulsion is replaced with medium chain triglycerides.
In a study by Guohao Wu, patients with IFALD received glucose in PN in the amount of 3.21 ±0.08 g/kg/day, which was lower than in our patients, whereas the fat emulsion content, i.e. 0.89 ±0.09 g/kg/day, was higher than in our patients [13].
Our analyses confirmed a significant relationship between the type of SBS and the concentrations of the renal function parameters serum urea and creatinine. The mean levels of urea and creatinine in the studied patients at their first visit to the Nutrition Treatment Centre were: in SBS type I: urea 50.57 mg/dl, creatinine 1.19 mg/dl; in SBS type II: urea 37.41 mg/dl, creatinine 0.82 mg/dl and in SBS type III: 35.84 mg/dl and 1.06 mg/dl, respectively. Electrolyte disturbances were compensated during the patient’s hospitalisation for the first 2–3 weeks. For the purpose of HPN, the nutrition team prepared the composition of the nutritional mixture, which was individually adjusted on the basis of results of tests performed during follow-up visits.
Delayed implementation of adequate nutritional treatment after major bowel resection was the most important cause of renal failure in the analysed patients. A large number of patients had spent several weeks at home without adequate treatment before they were referred to the Nutritional Treatment Centre. Failure of patients to follow the centre’s recommendations, i.e. to consume adequate oral fluids, especially ORS instead of water or sugary drinks, may be another cause of renal dysfunction.
A high-output stoma (HOS) that secretes more than 2,000 ml per day is a major challenge for both healthcare professionals and patients. A jejunum length < 100 cm is associated with greater fluid and electrolyte loss than patients can take orally [14].
Dehydration and renal failure in patients with end enterostomy can be a huge challenge for the treatment team. Patients who have undergone a small intestine resection should be under the care of medical professionals who are highly experienced in treating such patients. There are patients who will readily accept their disease and those who will fight it. An adequate choice of nutrients, electrolytes and water in parenteral nutrition, an individually adjusted diet, proper care of vascular access and constant monitoring of patients are the keys to proper functioning in SBS [15].
The study was conducted in accordance with the guidelines of the 1975 Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by the Bioethics Committee (Consent No. 3/2017).
In conclusion, patients with short bowel syndrome are at risk of exacerbations of liver and kidney functions and electrolyte imbalances. Patients with end enterostomy (SBS type I) are particularly at risk of renal failure. A proper composition of parenteral nutrition and a special diet enable patients to maintain proper hydration status, biochemical, metabolic and energy balance of the human body.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jonkers-Schuitema CF, Wanten G, Szczygieł B, Naber T, Kunecki M. Leczenie żywieniowe po rozległym wycięciu jelita (zespół krótkiego jelita) In: Sobotka L, editor. Podstawy Żywienia Klinicznego. 4th ed. Krakow: Scientifica; 2013. pp. 553–67. [Google Scholar]
- 2.Höllwarth ME. Short bowel syndrome: pathophysiological and clinical aspects. Pathophysiology. 1999;6:1–19. [Google Scholar]
- 3.Parrish CR. The clinician’s guide to short bowel syndrome. Pract Gastroenterol. 2005;8:67–105. [Google Scholar]
- 4.Jeppesen PB. Short bowel syndrome. Definition, classification, etiology, epidemiology, survival and costs. In: DiBaise JK, Parrish CR, Thompson JS, editors. Short Bowel Syndrome: Practical Approach to Management. CRC Press; 2016. pp. 1–13. [Google Scholar]
- 5.Nightingale JM. Short bowel syndrome. Anatomical and physiological considerations. In: DiBaise JK, Parrish CR, Thompson JS, editors. Short Bowel Syndrome: Practical Approach to Management. CRC Press; 2016. pp. 29–42. [Google Scholar]
- 6.Konrad D, Gallant B. Clinical and nutritional assessment in the patient with short bowel syndrome. In: DiBaise JK, Parrish CR, Thompson JS, editors. Short Bowel Syndrome: Practical Approach to Management. CRC Press; 2016. pp. 115–27. [Google Scholar]
- 7.Nanji AA, Anderson FH. Sensitivity and specificity of liver function tests in the detection of parenteral nutrition-associated cholestasis. JPEN J Parenter Enteral Nutr. 1985;9:307–8. doi: 10.1177/0148607185009003307. [DOI] [PubMed] [Google Scholar]
- 8.Ławiński M, Bzikowska A, Omidi M, Majewska K, Zielińska-Borkowska U. Liver disease in patients qualified for home parenteral nutrition – a consequence of a failure to adjust rtu bags in the primary centre? Pol Przeg Chir. 2014;86:279–84. doi: 10.2478/pjs-2014-0049. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Lau KS, Ramanathan V, et al. Ileostomy creation in colorectal cancer surgery: risk of acute kidney injury and chronic kidney disease. J Surg Res. 2017;210:204–12. doi: 10.1016/j.jss.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Plauth M, Bernal W, Dasarathy S, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreesen M, Foulon V, Vanhaecht K, Pourcq LD, Hiele M, Willems L. Guidelines recommendations on care of adult patients receiving home parenteral nutrition: a systematic review of global practisec. Clin Nutr. 2012;31:602–8. doi: 10.1016/j.clnu.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Pironi L, Boeykens K, Bozzetti F, et al. ESPEN guideline on home parenteral nutrition. Clin Nutr. 2020;39:1645–66. doi: 10.1016/j.clnu.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Wu G, Jiang Y, Zhu X, et al. Prevalence and risk factors for complications in adult patients with short bowel syndrome receiving long-term home parenteral nutrition. Asia Pac J Clin Nutr. 2017;26:591–7. doi: 10.6133/apjcn.082016.08. [DOI] [PubMed] [Google Scholar]
- 14.Nagle D, Pare T, Kennan E, Marcet K, Tizio S, Poylin V. Ileostomy pathway virtually eliminates readmisiions for dehydration in new ostomates. Dis Colon Rectum. 2012;55:1266–72. doi: 10.1097/DCR.0b013e31827080c1. [DOI] [PubMed] [Google Scholar]
- 15.Beck-Kaltenbach N, Voigt K, Rumstadt B. Renal impairment caused by temporary loop ileostomy. Int J Colorectal Dis. 2011;26:626. doi: 10.1007/s00384-010-1086-3. [DOI] [PubMed] [Google Scholar]