Abstract
Introduction
We aimed to investigate the efficacy and side effects of bendamustine in relapsed/refractory lymphoma patients in Turkey.
Material and methods
In this retrospective study, we included relapsed/refractory Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) patients who underwent multiple lines of chemotherapy. The primary endpoint was to determine the objective response and toxicity.
Results
Ninety-nine patients with a median age of 59.8 years were included in the study. Eighty-one patients had NHL (follicular lymphoma: 10, diffuse large B-cell lymphoma: 27, mantle-cell lymphoma: 18, marginal zone lymphoma: 9, small lymphocytic lymphoma/chronic lymphocytic leukemia: 17) and 18 patients had HL. The patients had previously received a median of three lines of chemotherapy (range: 2–8) except autologous stem cell transplantation (ASCT); 19 patients (HL: 11, NHL: 8) had undergone ASCT. The objective response rate (ORR) was 74.3%, the complete response rate was 57% (= 53), and the partial response rate was 16.6% ( = 19). The overall survival (OS) rate at 1 year was 74.6%. The progression-free survival (PFS) rate at 1 year was 62.5%. The most common side effects were lymphopenia, anemia and neutropenia. Side effects which were observed as grade 3 and higher levels were lymphopenia (14.1%), neutropenia (10.1%) and fatigue (7.1%).
Conclusions
Objective response rate of bendamustine was found to be 74.3% in relapsed/refractory HL and NHL patients. It appears to be an effective option as a salvage treatment for patients who have previously received multiple lines of therapy.
Keywords: bendamustine, lymphoma, Hodgkin lymphoma
Introduction
Non-Hodgkin lymphoma (NHL) consists of malignant neoplasms of the lymphoid tissues derived from progenitor or mature B, T, and NK cells [1]. The other malign neoplasms derived from lymphoid tissue are chronic lymphocytic leukemia (CLL) and Hodgkin lymphoma (HL). Most patients with these neoplasms attain complete remission after an initial treatment and achieve long-term disease control. However, relapse may eventually occur in some of the patients. Moreover, some patients can have refractory disease that either does not respond to initial therapy or progresses after an initial response. Treatment options for lymphomas consist of chemotherapy, immunotherapy, radiation therapy, or a combination of these [2]. A subset of patients, who are relapsed or refractory, is treated with high-dose chemotherapy followed by stem cell transplantation. Currently, there is no standard salvage chemotherapy regimen for relapsed/refractory NHL, HL, and CLL, and clinical practice at this point is highly variable [3]. Toxicity of conventional salvage chemotherapy regimens are substantial, and patient comorbidities may influence the selection of a particular chemotherapeutic regimen for each case.
Bendamustine is a cytotoxic compound which was synthesized as a hybrid molecule intended to combine the activities of the purine antimetabolite benzimidazole [4]. Most importantly, bendamustine exhibits only partial cross resistance with other alkylators, making it a treatment of choice for relapsed/refractory patients [5]. Bendamustine is indicated for the treatment of CLL and B cell NHL progressing within 6 months of treatment with rituximab monotherapy or a rituximab-containing regimen [6, 7].
In order to assess the efficacy of bendamustine outside clinical trials, we conducted a retrospective study in patients with relapsed/refractory lymphoid malignancies treated with bendamustine. Although the efficacy and safety of bendamustine had been examined in several clinical trials, this was the first study to evaluate its efficacy and safety in a Turkish cohort.
Material and methods
Data were retrospectively collected from medical records of patients treated in nine oncology centers in Turkey. All patients treated with at least one cycle of bendamustine alone or in combination with rituximab for histologically confirmed recurrent or refractory lymphoma between 1st January 2011 and 1st January 2017 were included. Eligible histological diagnoses were diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and Hodgkin lymphoma (HL). Response assessments were performed based on radiological imaging results and physical examination records. Physician notes, hospital admissions and results of laboratory test that were performed before every treatment cycle were reviewed for adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. This study was approved by the institutional ethics committee of Gulhane School of Medicine.
Statistical analysis
Considering the clinical heterogeneity of histological subtypes, efficacy results were reported separately for each histological subtype. Continuous variables were summarized as median and interquartile range (quartiles 1 to 3), and categorical variables were summarized as counts and percentages. Progression-free survival (PFS) and overall survival (OS) estimates were calculated with the Kaplan-Meier method. The PFS was defined as the interval between the initiation of bendamustine and recurrence or death from any cause. The OS was measured from the initiation of bendamustine to death from any cause. Statistical analyses were performed with the SPSS software (PASW Statistics for Windows, version 18.0, Chicago, SPSS Inc.).
Results
Patient characteristics
A total of 99 patients who received bendamustine for relapsed or refractory lymphoma were included. Of those, 27 patients had DLBCL, 18 patients had MCL, 18 patients had HL, 17 patients had CLL/SLL, 10 patients had FL, and 9 patients had MZL. Baseline characteristics of the patients are shown in Table I. Median age was 28.2 years in patients with HL and 71.4 years in patients with DLBCL. Patients generally received bendamustine as third-line treatment in DLBCL, FL, and CLL/SLL groups; as second-line treatment in MCL and MZL patients; and as fifth-line treatment in patients with HL. Patient demographics and disease characteristics are summarized in Table I.
Table I.
Patient characteristics
| Parameter | DLBCL | FL | MCL | MZL | CLL/SLL | HL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total, n (%) | 27 | (100) | 10 | (100) | 18 | (100) | 9 | (100) | 17 | (100) | 18 | (100) |
| Age [years]: | ||||||||||||
| Median | 71.4 | 66.9 | 65.6 | 62.7 | 64 | 28.2 | ||||||
| Range | (27–89) | (49–82) | (49–79) | (45–78) | (37–85) | (16–72) | ||||||
| Gender, n (%): | ||||||||||||
| Male | 15 | (55.6) | 6 | (60) | 15 | (83.3) | 3 | (33.3) | 13 | (76.5) | 13 | (72.2) |
| Female | 12 | (44.4) | 4 | (40) | 3 | (16.7) | 6 | (66.7) | 4 | (23.5) | 5 | (27.8) |
| Stage at diagnosis: | ||||||||||||
| I or II | 8 | (29.6) | 0 | (0) | 2 | (11.1) | 3 | (33.3) | 8 | (47.1) | 5 | (27.8) |
| III or IV | 19 | (70.3) | 10 | (100) | 16 | (88.9) | 6 | (66.7) | 9 | (52.9) | 13 | (72.2) |
| Bulky disease at diagnosis: | ||||||||||||
| Present | 9 | (33.3) | 1 | (10) | 4 | (22.2) | 2 | (22.2) | 5 | (29.4) | 5 | (27.8) |
| Extranodal disease: | ||||||||||||
| Present | 9 | (33.3) | 6 | (60) | 10 | (55.6) | 7 | (77.8) | 4 | (23.5) | 6 | (33.3) |
| ECOG status, n (%): | ||||||||||||
| 0 or 1 | 14 | (51.9) | 7 | (70) | 11 | (61.1) | 7 | (77.8) | 13 | (76.5) | 16 | (88.9) |
| 2 or 3 | 13 | (48.1) | 3 | (30) | 7 | (38.9) | 2 | (22.2) | 4 | (23.5) | 2 | (11.1) |
| B symptoms, n (%): | ||||||||||||
| Present | 7 | (25.9) | 3 | (30) | 4 | (22.2) | 4 | (44.4) | 7 | (41.2) | 13 | (68.5) |
| Refractory to previous chemotherapy: | ||||||||||||
| Yes | 15 | (55.6) | 2 | (22.2) | 9 | (50) | 2 | (22.2) | 3 | (18.8) | 12 | (66.7) |
| Disease duration* [months]: | ||||||||||||
| Median | 14.5 | 39.2 | 32.8 | 22.2 | 34.2 | 36.6 | ||||||
| Range | (2–140) | (16–148) | (4–67) | (8–158) | (15–114) | (18–114) | ||||||
| Previous treatments, n (%): | ||||||||||||
| Rituximab | 27 | (100) | 9 | (90) | 14 | (77.8) | 8 | (88.9) | 14 | (82.4) | 0 | (0) |
| ABVD | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 17 | (94.4) |
| CHOP | 25 | (92.6) | 7 | (70) | 12 | (66.7) | 3 | (33.3) | 6 | (35.3) | 0 | (0) |
| FC | 1 | (3.7) | 2 | (20) | 0 | (0) | 0 | (0) | 11 | (64.7) | 0 | (0) |
| CVP | 4 | (14.8) | 3 | (30) | 3 | (16.7) | 3 | (33.3) | 6 | (35.3) | 1 | (5.6) |
| DHAP | 0 | (0) | 0 | (0) | 3 | (16.7) | 0 | (0) | 0 | (0) | 10 | (55.6) |
| ICE | 8 | (29.6) | 1 | (10) | 3 | (16.7) | 0 | (0) | 1 | (5.9) | 9 | (50) |
| Other | 13 | (48.1) | 1 | (10) | 8 | (44.4) | 0 | (0) | 2 | (11.8) | 12 | (66.7) |
| Radiotherapy | 11 | (40.7) | 4 | (40) | 3 | (16.7) | 1 | (11.1) | 0 | (0) | 6 | (33.3) |
| Autologous SCT | 1 | (3.7) | 2 | (20) | 5 | (27.8) | 0 | (0) | 0 | (0) | 11 | (61.1) |
| Treatment setting, n (%): | ||||||||||||
| 2nd-line | 10 | (37) | 4 | (40) | 9 | (53) | 8 | (80) | 8 | (47.1) | 1 | (5.6) |
| 3rd-line | 12 | (44.4) | 2 | (20) | 5 | (29.4) | 1 | (10) | 7 | (41.2) | 1 | (5.6) |
| Beyond 3rd-line | 5 | (18.5) | 4 | (40) | 3 | (17.6) | 1 | (10) | 2 | (11.8) | 16 | (88.8) |
| Median | 3 | 3 | 2 | 2 | 3 | 5 | ||||||
| Range | (2–6) | (2–5) | (2–5) | (2–7) | (2–5) | (2–8) | ||||||
The percentages in parentheses are column percentages.
Disease duration refers to the time from initial diagnosis of lymphoma to the initiation of study treatment.
ABVD – Adriamycin, bleomycin, vinblastine, dacarbazine, CHOP – cyclophosphamide, doxorubicin, vincristine, prednisone, CLL/SLL – chronic lymphocytic lymphoma/small lymphocytic lymphoma, CVP – cyclophosphamide, vincristine, prednisone, DHAP – dexamethasone, cytarabine, cisplatin, DLBCL – diffuse large B-cell lymphoma, ECOG – Eastern Cooperative Oncology Group, FC – fludarabine, cyclophosphamide, FL – follicular lymphoma, HL – Hodgkin lymphoma, ICE – ifosfamide, carboplatin, etoposide, MCL – mantle-cell lymphoma, MZL – marginal zone lymphoma, SCT – stem cell transplantation.
Treatment exposure and efficacy
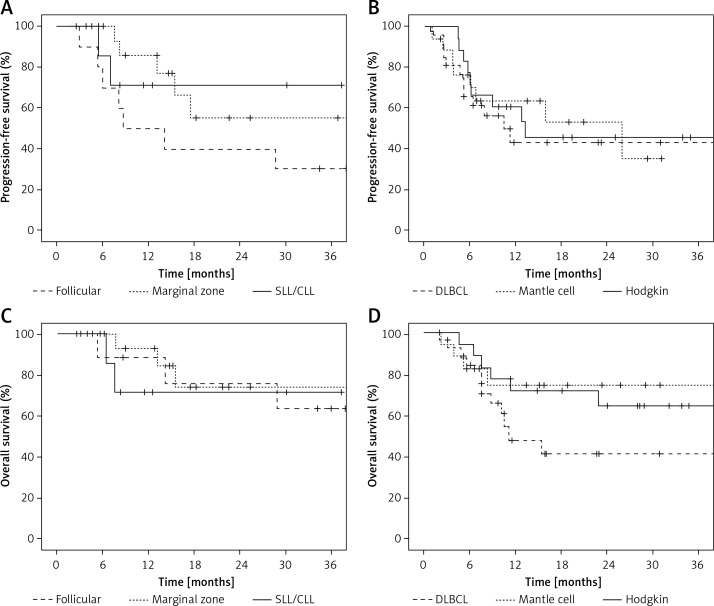
Patients received a median of 4 cycles of bendamustine in the DLBCL group and 6 cycles in other groups (Table II). A total of 538 cycles of bendamustine were administered. The median duration of bendamustine treatment ranged from 4.5 (in the DLBCL group) to 5.6 months (in the MCL group). The most commonly preferred schedule was 90 mg/m2 administered every three weeks. Objective response rates were 55.6% in DLBCL, 61.1% in HL, 72.2% in MCL, 80% in FL, 88.2% in CLL/SLL, and 88.9% in MZL patients (Table III). The majority of the responses were complete responses ranging from 38.8% in HL to 88.9% in MZL patients (Table III). At a median follow-up of 22 months, the estimates of 1-year PFS were 43% in DLBCL, 50% in FL, 61.1% in HL, 64.6% in MCL, 71.4% in MZL, and 85.7% in CLL/SLL groups; and the estimates of 1-year OS were 48.2% in DLBCL, 71.4% in MZL, 71.8% in HL, 74.9% in MCL, 88.9% in FL, and 92.9% in CLL/SLL (Table III, Figure 1).
Table II.
Treatment exposure
| Parameter | DLBCL (n = 27) | FL (n = 10) | MCL (n = 18) | MZL (n = 9) | CLL/SLL (n = 17) | HL (n = 18) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment duration [months]: | ||||||||||||
| Median | 4.5 | 5.3 | 5.6 | 4.9 | 5.3 | 5.3 | ||||||
| Range | (0.7–37.3) | (1.7–7.6) | (1.7–28.7) | (1.6–6.4) | (3.6–9) | (0.3–22) | ||||||
| No. of cycles: | ||||||||||||
| Median | 4 | 6 | 6 | 6 | 6 | 6 | ||||||
| Range | (1–8) | (2–9) | (2–38) | (2–8) | (4–6) | (1–10) | ||||||
| Bendamustine dosage, n (%): | ||||||||||||
| 90 mg/m2 | 17 | (63) | 8 | (80) | 16 | (88.9) | 9 | (100) | 17 | (100) | 14 | (77.8) |
| 100 mg/m2 | 9 | (33.3) | 2 | (20) | 2 | (11.1) | 0 | (0) | 0 | (0) | 1 | (5.6) |
| 120 mg/m2 | 1 | (3.7) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 3 | (16.7) |
Note: The percentages in parentheses are column percentages. CLL/SLL – chronic lymphocytic lymphoma/small lymphocytic lymphoma, DLBCL – diffuse large B-cell lymphoma, FL – follicular lymphoma, HL – Hodgkin lymphoma, MCL – mantle cell lymphoma, MZL – marginal zone lymphoma.
Table III.
Efficacy results
| Parameter | DLBCL (n = 27) | FL (n = 10) | MCL (n = 18) | MZL (n = 9) | CLL/SLL (n = 17) | HL (n = 18) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Best response, n (%): | ||||||||||||
| Complete response | 12 | (44.4) | 6 | (60) | 11 | (61.1) | 8 | (88.9) | 9 | (52.9) | 7 | (38.8) |
| Partial response | 3 | (11.1) | 2 | (20) | 2 | (11.1) | 0 | (0) | 6 | (35.3) | 4 | (22.2) |
| Stable disease | 3 | (11.1) | 0 | (0) | 2 | (11.1) | 0 | (0) | 1 | (5.9) | 5 | (27.8) |
| Progressive disease | 8 | (29.6) | 2 | (20) | 2 | (11.1) | 1 | (11.1) | 0 | (0) | 1 | (5.6) |
| Unknown | 1 | (3.7) | 0 | (0) | 1 | (5.6) | 0 | (0) | 1 | (5.9) | 1 | (5.6) |
| ORR, % (95% CI) | 55.6 (35.3–74.5) | 80 (44.4–97.5) | 72.2 (46.5–90.3) | 88.9 (51.8–99.7) | 88.2 (63.6–98.5) | 61.1 (38.6–79.7) | ||||||
| Progression-free survival: | ||||||||||||
| No. of events | 13 | 7 | 8 | 2 | 5 | 9 | ||||||
| 1-year PFS, % (95% CI) | 43 (21.1–63.3) | 50 (18.4–75.3) | 63.9 (36.5–82) | 71.4 (25.8–92) | 85.7 (53.9–96.2) | 61.1 (35.3–79.2) | ||||||
| 2-year PFS, % (95% CI) | 43 (21.1–63.3) | 40 (12.3–67) | 53.3 (24.3–75.6) | 71.4 (25.8–92) | 55.1 (22–79) | 45.8 (20.9–67.8) | ||||||
| Median PFS [months] (95% CI) | 11.1 (5.5–16.7) | 8.6 (0–17.9) | 25.9 (3.1–48.6) | NE | NE | 13.2 (NE) | ||||||
| Overall survival: | ||||||||||||
| No. of events | 12 | 3 | 5 | 2 | 3 | 6 | ||||||
| 1-year OS, % (95% CI) | 48.2 (25.3–67.9) | 88.9 (43.3–98.4) | 74.9 (45.6–89.9) | 71.4 (25.8–92) | 92.9 (59.1–99) | 71.8 (47.2–87.9) | ||||||
| 2-year OS, % (95% CI) | 41.3 (25.3–67.9) | 76.2 (33.2–93.5) | 74.9 (45.6–89.9) | 71.4 (25.8–92) | 73.9 (37.9–91) | 64.6 (37–82.5) | ||||||
Note: The percentages in parentheses are column percentages. CI – confidence interval, CLL/SLL – chronic lymphocytic lymphoma/small lymphocytic lymphoma, DLBCL – diffuse large B-cell lymphoma, FL – follicular lymphoma, HL – Hodgkin lymphoma, MCL – mantle cell lymphoma, MZL – marginal zone lymphoma, NE – not estimable, ORR – objective response rate, OS – overall survival, PFS – progression-free survival.
Figure 1.
Progression-free (A, B) and overall survival (C, D) curves with bendamustine treatment in histological subtypes of relapsed/refractory lymphoma
DLBCL– diffuse large B-cell lymphoma, SLL/CLL – small lymphocytic lymphoma/chronic lymphocytic leukemia.
After bendamustine treatment, stem cell transplantation (SCT) was performed in 8% of patients who had at least a partial response. Autologous SCT was performed in 6 patients and two of them had CR (one DLBCL and one HL). Allogeneic SCT was performed in two HL patients who achieved CR with bendamustine. Details of bendamustine efficacy in bridging to SCT and rates of SCT are shown in Table IV.
Table IV.
Post-bendamustine stem cell transplantation rates
| SCT | Lymphoma type | N | % | Bendamustine dose [mg/m2] and number of cycles | Post-bendamustine response |
|---|---|---|---|---|---|
| Autologous | Total | 6 | |||
| HL | 3 | 90–90–120/4–9–4 | 1 CR and 2 PR | ||
| FL | 1 | 90/2 | PR | ||
| DLBCL | 1 | 100/8 | CR | ||
| MCL | 1 | 90/4 | PR | ||
| Allogeneic | Total | 2 | 1 | ||
| HL | 2 | 100–90/4 | CR |
DLBCL – CR – complete response, diffuse large B-cell lymphoma, FL – follicular lymphoma, HL – Hodgkin lymphoma, MCL – mantle-cell lymphoma, PR – partial response, SCT – stem cell transplantation.
Safety and adverse events
Hematological adverse events associated with bendamustine were common but usually low grade; the incidence of lymphopenia, anemia, neutropenia and thrombocytopenia was 74.7%, 64.6%, 61.6%, and 45.5%, respectively (Table V). Nausea and diarrhea were observed in 41.4% and 27.3% of the patients. Other toxicities were elevated alanine aminotransferase (25.2%), peripheral edema (22.2%), elevated aspartate aminotransferase (21.2%), rash (17.2), and alopecia (15.1%). At least one dose delay or dose reduction was required in 25.3% and 11.1% of patients, respectively. Adverse events did not necessitate treatment discontinuation in any patients. No deaths were attributed to bendamustine treatment. At a median follow-up duration of 22 months no secondary malignancies have been recorded.
Table V.
Adverse events
| Adverse event* | Any grade | Grade 3 or 4 (%) (n = 99) | ||
|---|---|---|---|---|
| All (%) (n = 99) | Among responders (%) (n = 70) | Among non-responders (%) (n = 29) | ||
| Lymphopenia | 74 (74.7) | 53 (75.7) | 21 (72.4) | 14 (14.1) |
| Anemia | 64 (64.6) | 43 (61.4) | 21 (72.4) | 5 (5.1) |
| Neutropenia | 61 (61.6) | 47 (67.1) | 14 (48.3) | 10 (10.1) |
| Fatigue | 50 (50.5) | 39 (55.7) | 11 (37.9) | 7 (7.1) |
| Thrombocytopenia | 45 (45.5) | 31 (44.3) | 14 (48.3) | 5 (5.1) |
| Nausea | 41 (41.4) | 29 (41.4) | 12 (41.4) | 2 (2) |
| Diarrhea | 27 (27.3) | 19 (27.1) | 8 (27.6) | 1 (1) |
| Elevated ALT | 25 (25.2) | 16 (22.9) | 9 (31) | 1 (1) |
| Peripheral edema | 22 (22.2) | 16 (22.9) | 6 (20.7) | 0 (0) |
| Elevated AST | 21 (21.2) | 14 (20) | 7 (24.1) | 0 (0) |
| Rash | 17 (17.2) | 12 (17.1) | 5 (17.2) | 0 (0) |
| Alopecia | 15 (15.1) | 13 (18.6) | 2 (6.9) | NA |
Adverse events observed in 15% or more of the patients, and grade 3 or 4 adverse events observed in 2% or more of the patients are listed.
ALT – alanine aminotransferase, AST – aspartate aminotransferase.
Discussion
Bendamustine is a bimodal chemotherapeutic agent having both alkylating and antimetabolite properties. The combined cytotoxic activity of bendamustine has resulted in high response rates and durable responses in patients with heavily pretreated indolent and aggressive lymphomas. This study presents the longitudinal multicenter results of bendamustine in heavily pretreated indolent B-NHL, DLBCL and HL patients. In our study, there were 99 patients with diverse histologies and defined by their primary oncologist/hematologist as refractory patients. Hence, the results are important in terms of a possible treatment option in a patient group with dismal prognosis.
The number of cycles of administered bendamustine, treatment duration, dose intensity and the median number of previous chemotherapy regimens are the important parameters in terms of bendamustine efficacy. Median administered number of cycles [8–11] and median duration of treatment (4.5–5.6 months) were comparable with the accepted dose intensity [9–11]. The bendamustine dose was 90 to 120 mg/m2 per cycle and more than 80% of indolent lymphoma patients received 90 mg/m2 as compared to 37% of DLBCL patients who received at least 100 mg/m2. None of our patients was scheduled for lower doses of bendamustine (such as 60 mg/m2). The median number of prior regimens administered was two for indolent B-NHL, three for DLBCL, and five for HL. These results were found to be similar to the results of Ghesquieres et al. and Ohmachi et al. [11, 12]. However, our HL patient population was more heavily pre-treated than the others. None of the patients required discontinuation of treatment due to adverse events.
In this trial, ORR was found as 55.6% in patients with heavily pretreated DLBCL with the combination of bendamustine and rituximab. The estimated 1-year PFS and OS were found to be 43% and 48.2%. These results are comparable with the results of more aggressive R-ESHAP and R-ICE regimens. Although our patient population was refractory, the ORR, PFS, and OS data were found to be similar with the results of previous reports covering non-refractory cases [13, 14]. In a small study including relapsed but not refractory cases with DLBCL, ORR was reported as 57% [15]. Horn et al. reported a 55% response rate in cases similar to our population composed of 75% DLBCL [16]. The highest success rate with bendamustine in refractory DLBCL patients has been reported in a phase II trial and the response rate was 63% [17].
Authors from Memorial Sloan Kettering Cancer Center reported an 53% ORR in cases with relapsed HL [18]. ORR with bendamustine as a single agent has been reported between 50 and 53% in cases with refractory HL [10, 11, 19]. Although the duration of response was short with a median PFS of 5 months, favorable treatment results were achieved in HL. In our study ORR was 61.1% with an estimated 1-year survival of 61.1% and 1-year estimated OS was 71.8%. AutoSCT and alloSCT were performed in 2 patients who were heavily pretreated (median 5 lines) and these 2 cases were more refractory than the previously reported cases.
Besides the efficacy of bendamustine in high grade DLBCL and refractory HL, bendamustine is a salutary treatment agent in low grade lymphomas and MCL. Earlier studies and German group trials showed favorable single agent bendamustine activity in CLL [20, 21]. Later on, Fischer reported 45% and 60% response rates in 78 cases with CLL in fludarabine refractory and naive patients, respectively [16]. The median event-free survival observed by the German group was 14.7 months. In our study group, bendamustine was used as a median fourth line agent in CLL patients, ORR was 88.2%, and estimated 1-year PFS and OS were 85.7 and 92.9%, respectively. These results show the success of bendamustine treatment in refractory CLL/SLL patients.
The efficacy of bendamustine has also been analyzed in rituximab pre-treated or refractory patients with indolent B-cell lymphomas and FL and ORR has been found between 71% and 86% [12, 14, 22–25]. In our study ORR was 72.2% in MCL, 80% in FL and 88.9% in MZL patients. A complete response was detected in 88.9% of MZL patients. At a median follow-up of 22 months, 1-year PFS was 50% in FL, 64.6% in MCL and 71.4% in MZL; and 1-year OS was 71.4% in MZL, 74.9% in MCL, and 88.9% in FL. Although our patient population is heterogeneous regarding its characteristics, the main shared feature is the refractoriness and relapse rates, which are important for determining the importance of ORR and event-free survival rates.
The major toxicities observed with bendamustine were reversible myelosuppression, infections and gastrointestinal adverse events [9, 26]. Reported infections were recurring herpes zoster infections and cytomegalovirus infections [9, 22, 25, 27]. Bendamustine was well tolerated in our patients and was not directly connected to life-threatening adverse events. Although adverse events were frequent, grade III–IV toxicity was low; namely 1% diarrhea and 14% lymphopenia. Adverse events did not cause treatment to be stopped; a one-dose delay or dose reduction was required in 25.3% and 11.1% of patients, respectively. Due to the retrospective and multicentric nature of our study, data regarding herpes zoster and CMV infections were not satisfactory. No deaths were attributed to bendamustine treatment in the median follow-up of 22 months.
In conclusion, the present study suggests that bendamustine is a promising agent in lymphoma, both in patients with low and high-grade lymphomas as well HL who have failed multiple lines of treatments. Bendamustine may represent a valuable palliative option providing high PFS rates and a reasonable option for bridging to SCT. It is important to note that OS rates were higher than PFS rates, which is also a success of bendamustine treatment since it allows further treatment beyond bendamustine, and as a bridge therapy to other treatments and SCT. However, due to its heterogeneity and retrospective nature, the power of the present study may be limited, which further clarifies the need for prospective studies.
Acknowledgments
We thank our patients and their families who participated in the research helpfully and devotedly without expecting material compensation.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Anderson T, Chabner BA, Young RC, et al. Malignant lymphoma. 1. The histology and staging of 473 patients at the National Cancer Institute. Cancer. 1982;50:2699–707. doi: 10.1002/1097-0142(19821215)50:12<2699::aid-cncr2820501202>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 4.Tageja N. Bendamustine: safety and efficacy in the management of indolent non-Hodgkins lymphoma. Clin Med Insights Oncol. 2011;5:145–56. doi: 10.4137/CMO.S6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leoni LM. Bendamustine: rescue of an effective antineoplastic agent from the mid-twentieth century. Semin Hematol. 2011;48(Suppl 1):S4–11. doi: 10.1053/j.seminhematol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 7.Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4473–9. doi: 10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- 8.Vacirca JL, Acs PI, Tabbara IA, et al. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B cell lymphoma. Ann Hematol. 2014;93:403–9. doi: 10.1007/s00277-013-1879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penne M, Sarraf Yazdy M, Nair KS, Cheson BD. Extended follow-up of patients treated with bendamustine for lymphoid malignancies. Clin Lymphoma Myeloma Leuk. 2017;17:637–44. doi: 10.1016/j.clml.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Corazzelli G, Angrilli F, D’Arco A, et al. Efficacy and safety of bendamustine for the treatment of patients with recurring Hodgkin lymphoma. Br J Haematol. 2013;160:207–15. doi: 10.1111/bjh.12120. [DOI] [PubMed] [Google Scholar]
- 11.Ghesquieres H, Stamatoullas A, Casasnovas O, et al. Clinical experience of bendamustine in relapsed or refractory Hodgkin lymphoma: a retrospective analysis of the French compassionate use program in 28 patients. Leuk Lymphoma. 2013;54:2399–404. doi: 10.3109/10428194.2013.776165. [DOI] [PubMed] [Google Scholar]
- 12.Ohmachi K, Ando K, Ogura M, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2059–64. doi: 10.1111/j.1349-7006.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidmann E, Kim SZ, Rost A, et al. Bendamustine is effective in relapsed or refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1285–9. doi: 10.1093/annonc/mdf189. [DOI] [PubMed] [Google Scholar]
- 14.Rigacci L, Puccini B, Cortelazzo S, et al. Bendamustine with or without rituximab for the treatment of heavily pretreated non-Hodgkin’s lymphoma patients: a multicenter retrospective study on behalf of the Italian Lymphoma Foundation (FIL) Ann Hematol. 2012;91:1013–22. doi: 10.1007/s00277-012-1422-5. [DOI] [PubMed] [Google Scholar]
- 15.Walter E, Schmitt T, Dietrich S, et al. Rituximab and bendamustine in patients with CD20+ diffuse large B-cell lymphoma not eligible for cyclophosphamide, doxorubicin, vincristine and prednisone-like chemotherapy. Leuk Lymphoma. 2012;53:2290–2. doi: 10.3109/10428194.2012.682311. [DOI] [PubMed] [Google Scholar]
- 16.Horn J, Kleber M, Hieke S, et al. Treatment option of bendamustine in combination with rituximab in elderly and frail patients with aggressive B-non-Hodgkin lymphoma: rational, efficacy, and tolerance. Ann Hematol. 2012;91:1579–86. doi: 10.1007/s00277-012-1503-5. [DOI] [PubMed] [Google Scholar]
- 17.Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013;31:2103–9. doi: 10.1200/JCO.2012.46.5203. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31:456–60. doi: 10.1200/JCO.2012.45.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kharfan-Dabaja MA, Hamadani M, Sibai H, Savani BN. Managing Hodgkin lymphoma relapsing after autologous hematopoietic cell transplantation: a not-so-good cancer after all! Bone Marrow Transplant. 2014;49:599–606. doi: 10.1038/bmt.2013.226. [DOI] [PubMed] [Google Scholar]
- 20.Lissitchkov T, Arnaudov G, Peytchev D, Merkle K. Phase-I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre-treated patients with B-chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy. J Cancer Res Clin Oncol. 2006;132:99–104. doi: 10.1007/s00432-005-0050-z. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann MA, Goebeler ME, Herold M, et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica. 2005;90:1357–64. [PubMed] [Google Scholar]
- 22.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–14. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Jagt R. Bendamustine for indolent non-Hodgkin lymphoma in the front-line or relapsed setting: a review of pharmacokinetics and clinical trial outcomes. Expert Rev Hematol. 2013;6:525–537. doi: 10.1586/17474086.2013.841538. [DOI] [PubMed] [Google Scholar]
- 24.Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol. 2016;17:57–66. doi: 10.1016/S1470-2045(15)00447-7. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K, Takayama N, Aisa Y, et al. A phase II study of bendamustine plus rituximab in Japanese patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma previously treated with rituximab: BRB study. Int J Hematol. 2015;101:554–62. doi: 10.1007/s12185-015-1767-3. [DOI] [PubMed] [Google Scholar]
- 26.Salar A, Domingo-Domenech E, Panizo C, et al. Long-term results of a phase II study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma. Blood. 2017;130:1772–4. doi: 10.1182/blood-2017-07-795302. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Friedberg JW, Kahl BS, et al. Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10:452–7. doi: 10.3816/CLML.2010.n.079. [DOI] [PubMed] [Google Scholar]