Abstract
Introduction
Hypertension may cause target organ damage leading to hypertensive heart disease (HHD). The burden caused by HHD in Poland has not been studied systematically. The purpose of this study was to describe the burden of HHD in Poland in terms of prevalence, mortality, disability-adjusted life years lost (DALY) and key risk factors.
Material and methods
Data were obtained from the Global Burden of Diseases, Injuries and Risk Factors (GBD) Study database. The GBD uses a wide range of data sources and complex statistical methods to estimate disease burden for all countries by age, sex, and year. HHD was defined by ICD-9 codes 402-402.91 and ICD-10 codes I11-I11.9. From the GBD 2016 estimates, we extracted data for Poland between 1990 and 2016.
Results
Hypertensive heart disease is the fourth most important cause of cardio- and cerebrovascular death, after ischemic heart disease, stroke and cardiomyopathy. In 2016, there were about 180 000 people diagnosed with HHD in Poland and close to 5000 HHD-related deaths. HHD prevalence increased from 0.29% in 1990 to 0.47% in 2016 and was higher in women, while mortality increased from 11.2 to 12.7 per 100 000, largely due to population aging. Age-standardized death and DALY rates declined between 1990 and 2016 and were lower than in Central Europe but higher than in Western Europe.
Conclusions
Our data suggest a need for national initiatives to improve the diagnosis and treatment of hypertension, slow the progression of HHD, and reduce the related risks and premature deaths.
Keywords: hypertensive heart disease, Global Burden of Disease Study, prevalence, mortality, disability-adjusted life years lost
Introduction
Approximately 32% of the adult Polish population, or 10.5 million people, have hypertension and in only 26% of them is blood pressure adequately controlled [1]. Hypertension, especially long-lasting and poorly controlled, is the leading risk factor for premature death and disability [2–5]. These adverse outcomes are mainly driven by acute and late consequences of high blood pressure that lead to damage of the target organs, such as arterial blood vessels, heart, and kidneys [6–8].
The prevalence of cardiac damage caused by hypertension varies across age, gender, and ethnicity [6, 7]. Cardiac complications caused by hypertension include a wide range of asymptomatic and symptomatic disease stages. A frequent sign of early cardiac damage is left ventricular hypertrophy (LVH), which is commonly diagnosed either by conventional electrocardiography or echocardiography-derived, sex-specific indices of left ventricular mass. Advanced cardiac damage is often associated with overt heart failure, ischemic heart disease, and atrial fibrillation.
One of the potential consequences of increased blood pressure is hypertensive heart disease (HHD). To date, information on the burden of HHD in the population is scarce. The purpose of this study was to provide data on the prevalence of HHD as well as mortality and disability-adjusted life years lost (DALY) due to HHD in Poland in the last 25 years. To this end, we extracted and analyzed data from the Global Burden of Disease, Injuries and Risk Factors (GBD) Study 2016 [9].
Material and methods
The GBD study is the largest systematic approach to describe the global distribution and causes of a wide spectrum of major diseases, injuries, and health risk factors for 195 countries and territories [9]. It uses health data collected from vital records registries, censuses, health surveys, demographic surveillance, administrative reports, scientific research, and other sources. The data are then fed into algorithm that generate disease burden estimates. The results are regularly updated with new data to improve the accuracy of past estimates [10, 11]. The GBD database provides access to a complete set of age, national and subnational, and gender-specific estimates of burden across a wide range of causes.
In the GBD 2016 study, ICD-10 and ICD-9 codes were used in the extraction of vital registration, hospital and physician visit data and mapped to the 2016 GBD causes of death and non-fatal disease sequelae. Hypertensive heart disease was mapped to the following codes: I11-I11.9 (ICD-10) and 402-402.91 (ICD-9) [10, 11]. Death rates by age, gender, year, and location were estimated using complex statistical procedures known as ensemble modeling, described in detail elsewhere [11, 12]. Prevalence estimates were based on the frequency of heart failure and the estimated attribution of heart failure to six different etiologies, including HHD, obtained from the literature and physician claims data in the US [10]. Prevalence was modeled with DisMod-MR 2.1, a Bayesian meta-regression tool developed specifically for the GBD study that ensures consistency of epidemiological parameters for the conditions studied [10, 12]. A range of additional analytical techniques were applied to correct for biases and inconsistencies in the data, as described in the GBD publications [6–12]. DALYs were calculated as the sum of years of life lost due to premature mortality and years lived with disability (a measure of non-fatal health loss); hence DALY, as a measure of overall disease burden, expresses lost years of healthy life [12].
From the GBD 2016 estimates, we extracted data for Poland and obtained the prevalence, mortality, and DALY rates between 1990 and 2016. To assess the impact of aging on disease burden, we present both all-age (non-standardized) rates and age-standardized rates based on the GBD standard world population. We display the trends over time, describe the rates according to age and gender, and provide comparative data for Central Europe as a whole (including Poland) and Western Europe. Conventional confidence intervals are not available for GBD estimates; instead, 95% uncertainty intervals (UI) were generated through computer simulation by taking 1000 draws from the posterior distribution of each estimate, with upper and lower bounds determined by the 2.5th and 97.5th values of the draws [10–12].
The study utilized existing data from the GBD 2016 study and does not require ethical approval. The GBD study complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations [13].
Results
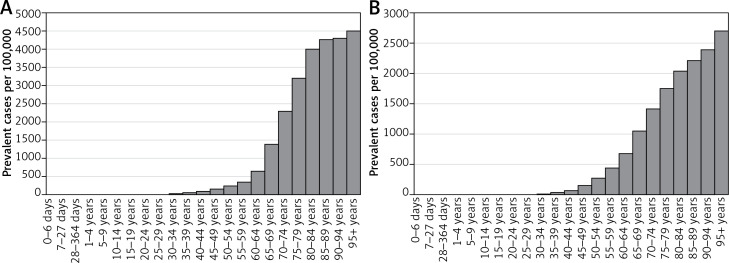
In 2016, HHD was ranked 6th out of 9 cardio- and cerebrovascular conditions evaluated in the GBD in terms of disease prevalence, and 4th (out of 9) with respect to cardio- and cerebrovascular death rates, after ischemic heart disease, stroke, and cardiomyopathy (Table I). There were about 181 000 prevalent cases of HHD in Poland, equivalent to a prevalence rate of 469 per 100 000 (95% UI: 389–557) or 0.47% (Table II). Prevalence in women (0.62%) was more than double that in men (0.30%). The higher rate in women was partly explained by older age, although age-standardized rates were still substantially higher in women. Hypertensive heart disease was strongly related to age in both sexes. This relationship appeared stronger in women, especially for ages 60–64 to 80–84 (Figure 1).
Table I.
Ranking of major cardio- and cerebrovascular diseases according to prevalence and mortality in Poland, 2016
| Disease | Prevalence | Mortality | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Ischemic heart disease | 1 | 1 | 1 | 1 |
| Stroke | 3 | 3 | 2 | 2 |
| Cardiomyopathy | 5 | 5 | 3 | 3 |
| Hypertensive heart disease | 6 | 6 | 4 | 4 |
| Atrial fibrillation | 4 | 4 | 7 | 5 |
| Peripheral artery disease | 2 | 2 | 6 | 6 |
| Aortic aneurysm | NA | NA | 5 | 7 |
| Rheumatic heart disease | 7 | 7 | 8 | 8 |
| Endocarditis | 8 | 8 | 9 | 9 |
NA – not available.
Table II.
Prevalence, mortality and DALY rates per 100 000 for hypertensive heart disease in Poland in 1990 and 2016 (95% uncertainty intervals)
| Parameter | All ages | Age-standardized | ||
|---|---|---|---|---|
| 1990 | 2016 | 1990 | 2016 | |
| Prevalence: | ||||
| Men | 188.8 (156.9–228.4) | 304.4 (251.7–369.5) | 228.7 (189.8–273.5) | 231.5 (190.7–277.9) |
| Women | 289.7 (315.7–463.8) | 623.3 (508.9–749.2) | 321.1 (265.1–385.7) | 335.0 (275.3–400.8) |
| All | 289.7 (239.7–345.3) | 469.0 (389.3–556.5) | 289.6 (242.0–343.9) | 297.7 (246.3–352.0) |
| Mortality: | ||||
| Men | 8.2 (6.2–13.7) | 9.5 (7.0–17.9) | 10.6 (8.3–18.8) | 7.7 (5.6–14.5) |
| Women | 14.0 (10.0–25.5) | 15.6 (10.7–35.2) | 12.0 (8.5–22.0) | 7.3 (5.0–16.6) |
| All | 11.2 (9.0–18.1) | 12.7 (9.7–22.6) | 11.8 (9.4–19.4) | 7.7 (5.9–13.6) |
| DALY: | ||||
| Men | 187.8 (131.0–277.2) | 191.1 (136.3–324.5) | 212.1 (154.3–320.8) | 143.8 (104.2–244.5) |
| Women | 241.3 (160.7–377.3) | 217.7 (157.2–448.0) | 202.8 (134.4–313.7) | 114.7 (82.5–236.9) |
| All | 215.2 (168.8–293.7) | 204.8 (163.7–324.3) | 211.5 (166.7–292.9) | 130.6 (104.3–204.6) |
DALY – disability-adjusted life years.
Figure 1.
Prevalence of hypertensive heart disease by age and sex in Poland, 2016: A – females, B – males
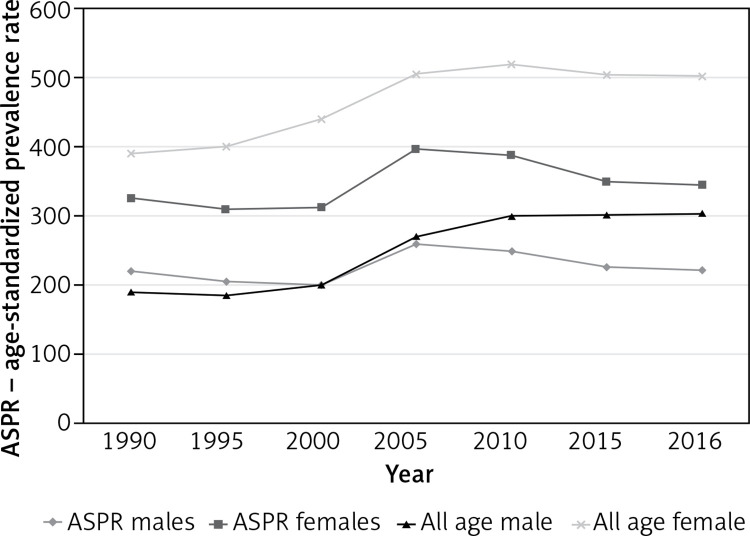
Between 1990 and 2016, prevalence of HHD increased by about 60% in both men and women (Table II). This increase was almost entirely explained by population aging, as age-standardized rates remained virtually unchanged (Figure 2). In both sexes, the trend was relatively flat between 1990 and 2000, then showed some increase until 2005, followed by a gradual decline.
Figure 2.
All-age and age-standardized prevalence of hypertensive heart disease in Poland, 1990-2016
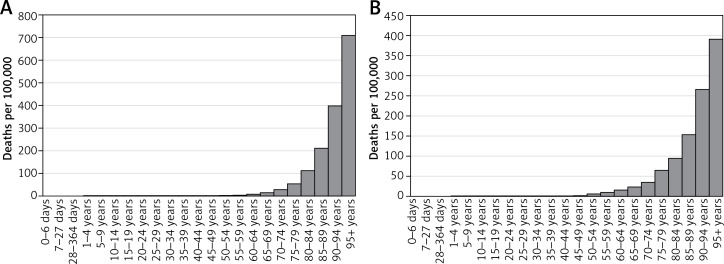
An estimated 4891 persons died from HHD in Poland in 2016, 1783 men and 3108 women. All-age death rates were 9.5 (7.0–17.9) per 100 000 in men and 15.6 (10.7–35.2) in women (Table II) and were exponentially related to age in both sexes (Figure 3). Age-standardized death rates were 7.7 (5.6–14.5) in men and 7.3 (5.0–16.6) in women. In both sexes there was a decline in age-standardized death rates between 1990 and 2016; however, the decline was faster in women (39% reduction) than in men (27%).
Figure 3.
Mortality due to hypertensive heart disease by age and sex in Poland, 2016: A – females, B – males
Age-standardized DALY rates showed a decreasing trend similar to mortality, with a steeper slope for women. As a result, whereas DALYs were similar in men and women in 1990, more DALYs were attributed to HHD in men (143.8, 104.2–244.5 per 100 000) than in women (114.7, 82.5–236.9) in 2016 (Table II).
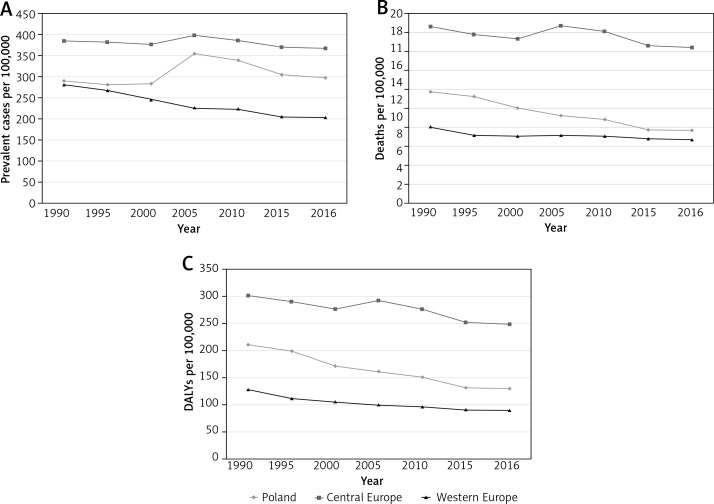
Poland was ranked 8th (from lowest to highest) among Central European countries in terms of age-standardized HHD prevalence and 5th in terms of age-standardized death rates (after Montenegro, Albania, Bosnia and the Czech Republic, data not shown). With respect to prevalence, Poland (0.30%, 0.25–0.35) was positioned about mid-way between Central Europe (0.37%, 0.31–0.43) and Western Europe (0.20%, 0.17–0.24) (Figure 4). Death rates in Poland were slightly higher than in Western Europe (7.7, 5.9–13.6 vs. 6.8, 3.8–8.1) but declining faster, and substantially lower than in Central Europe (16.4, 10.1–20.9).
Figure 4.
Age-standardized prevalence (A), mortality (B), and DALYs (C), for hypertensive heart disease in Poland, Central Europe, and Western Europe (both sexes)
By definition, HHD can occur only in persons with hypertension (100% risk factor attribution). However, only a small fraction of persons with hypertension develop overt HHD. The risk of HHD is affected by other factors (Table III). The most important among those is high body mass index, with a risk factor attribution of 54% for DALYs. Other factors that increase the risk of HHD are alcohol consumption (30% attribution), high-sodium diet (23%), and smoking (19%).
Table III.
Attribution of DALYs due to hypertensive heart disease to risk factors in Poland, 2016
| Parameter | Attributable DALYs per 100,000 (95% UI) | Risk factor attribution percent (95% UI) |
|---|---|---|
| High blood pressure | 204.8 (163.7–324.3) | 100.0 |
| High body mass index | 110.6 (64.5–189.2) | 54.1 (34.9–74.2) |
| Alcohol consumption | 61.2 (41.0–96.5) | 30.1 (21.6–39.0) |
| Diet high in sodium | 45.2 (8.5–103.7) | 22.5 (4.0–53.9) |
| Smoking | 39.2 (26.8–60.5) | 19.3 (13.7–23.9) |
DALY – disability-adjusted life years, UI – uncertainty intervals.
Discussion
This is the first report evaluating the epidemiology and trends in HHD in Poland over the past 25 years. In 2016, HHD was the fourth most important cardio- and cerebrovascular cause of death in Poland, after ischemic heart disease, stroke and cardiomyopathy. Due to population aging, the prevalence of HHD is going up. We found prevalence to be higher in women, even after adjusting for age. This is interesting, given that the prevalence of hypertension is similar in both sexes. At the same time, age-adjusted HHD mortality has been dropping faster, and is now lower, in women. The burden of HHD in Poland is lower than in Central Europe as a whole, but higher than in Western Europe. The fact that Poland’s rank among Central European countries is better for mortality than prevalence may suggest more effective treatment of HHD or a tendency to report milder cases of the condition.
In our study, as expected, the prevalence of HHD increased steeply with age. A similar age-dependent increase was also seen for HHD-related deaths and DALYs. The latter can be thought of as an assessment of the gap between current health status and an ideal health situation, in which the entire population remains healthy to an advanced age. We found that between 1990 and 2016 the age-standardized rates of HHD-related DALYs in Poland decreased, but remained higher than DALYs for Western Europe, whereas all-age DALYs remained relatively stable.
In this report, we extracted and analyzed data from the GBD study. In contrast to pooled analyses of the results from epidemiological studies, the GBD study uses available data from many sources, and provides trends in measures of disease burden over time based on state-of-the-art modeling methods. Such an approach may be especially useful in quantifying the sequelae of a broad spectrum of conditions, such as hypertension-induced HHD, the rates of which are difficult to estimate in cross-sectional studies.
Compared to other studies, the GBD estimates of the proportion of persons with hypertension who are diagnosed with HHD are relatively low. HHD encompasses conditions that range in severity from asymptomatic LVH to severe heart failure (HF). In commonly used ICD-10 coding categories, HHD is composed of two subcategories: hypertension with HF and hypertension without HF. In clinical practice, the latter code can be assigned not only to LVH, cardiomegaly or left ventricular diastolic dysfunction, but also to comorbid ischemic heart disease, atrial fibrillation, or other arrhythmias. Among hypertensive patients without LVH, as many as 33% have evidence of asymptomatic left diastolic dysfunction [11]. In GBD, however, HHD prevalence was based on the estimated fraction of HF attributed exclusively to hypertension. This may be one source of the differences in HHD prevalence between GBD and other studies. Another source of variation across studies is differences in methods of diagnosing HHD. For example, prevalence of LVH based on electrocardiographic findings was estimated at approximately 3% for men and 1.5% for women; however, the rate based on echocardiographic findings increased to 15–20% [14].
Other studies have found large differences in the proportion of LVH among hypertensive patients across age, gender, duration of hypertension, and geographic location, with rates ranging from 2% in Ethiopia [6], 23% in Spain [15] and 28% in Nigeria [16] to as high as 33% in Greece [7]. Similarly, the rates of hypertension-related HF vary considerably across populations. In the Framingham Study [17] and the Olmsted County Study [18], hypertension accounted for about 25% of HF cases. In the elderly population, 68% of HF cases were attributed to hypertension [19, 20]. On the other hand, Fox et al. [21] reported hypertension to be the primary causal factor for HF in only 4.4% of the incident cases of HF examined prospectively. Relatively low rates of HF were found in cross-sectional studies in newly diagnosed hypertensive patients in Nigeria (8.8%) [16] and Greece (7%) [7].
While HHD is by definition caused by high blood pressure, several additional factors contribute to the HHD burden in people with hypertension [22]. Of those, smoking has been declining in the past decade in Poland, sodium-high diet remained relatively unchanged, while obesity and alcohol consumption have increased. Given these trends, the observed decline in age-standardized deaths due to HHD is likely due to improved control of high blood pressure. Nonetheless, further improvements are possible, as only about one fourth of persons with hypertension have their blood pressure under control [1]. Although better diagnosis and treatment of hypertension are critical for reducing the burden of HHD, additional improvements could be achieved through reductions in obesity, smoking, sodium, and alcohol consumption.
In conclusion, our data support the findings from previous studies that emphasize the need for intensive screening for early stages of hypertension-related cardiac damage to prevent symptomatic HHD in the elderly, where most cases occur [23–28], and suggest a need for national initiatives that will slow the progression of HHD in patients with hypertension and reduce the related risk of disability and premature death.
Acknowledgments
The GBD Study is funded by the Bill & Melinda Gates Foundation. This work was supported by the Pomeranian Medical University (Grant for Young Researchers No. MB-315-201/16).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Polish Society of Hypertension Guidelines for the management of hypertension. Arterial Hypertens. 2015;19:53–83. [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A, et al. International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard G, Banach M, Cushman M, et al. Is blood pressure control for stroke prevention the correct goal? The lost opportunity of preventing hypertension. Stroke. 2015;46:1595–600. doi: 10.1161/STROKEAHA.115.009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malyszko J, Muntner P, Rysz J, Banach M. Blood pressure levels and stroke: J-curve phenomenon? Curr Hypertens Rep. 2013;15:575–81. doi: 10.1007/s11906-013-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abegaz TM, Tefera YG, Befekadu Abebe T. Target organ damage and the long term effect of nonadherence to clinical practice guidelines in patients with hypertension: a retrospective cohort study. Int J Hypertens. 2017;2017:2637051. doi: 10.1155/2017/2637051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papazafiropoulou A, Skliros E, Sotiropoulos A, et al. Prevalence of target organ damage in hypertensive subjects attending primary care: C.V.P.C. study (epidemiological cardio-vascular study in primary care) BMC Fam Pract. 2011;12:75. doi: 10.1186/1471-2296-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasek-Bal A, Gąsior Z. Cardiovascular diseases in patients 65 years and younger with non-cardiogenic stroke. Arch Med Sci. 2016;12:556–62. doi: 10.5114/aoms.2016.59929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute for Health Metrics and Evaluation (IHME) Rethinking development and health: Findings from the Global Burden of Disease Study. Seattle, WA: IHME; 2016. [Google Scholar]
- 10.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–6. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 13.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 14.Riaz K, Ahmed A. Hypertensive heart disease. Medscape. 2014. p. 162449. Access at: http://emedicine.medscape.com/article/162449.
- 15.Cea-Calvo L, Conthe P, Gómez-Fernández P, de Alvaro F, Fernandez-Perez C. RICARHD Investigators Target organ damage and cardiovascular complications in patients with hypertension and type 2 diabetes in Spain: a cross-sectional study. Cardiovasc Diabetol. 2006;5:23. doi: 10.1186/1475-2840-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayodele OE, Alebiosu CO, Akinwusi PO, Mejiuni A. Target organ damage and associated clinical conditions in newly diagnosed hypertensives attending a tertiary health facility. Niger J Clin Pract. 2007;10:319–25. [PubMed] [Google Scholar]
- 17.Kannel WB, Cobb J. Left ventricular hypertrophy and mortality – results from the Framingham Study. Cardiology. 1992;81:291–8. doi: 10.1159/000175819. [DOI] [PubMed] [Google Scholar]
- 18.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122:1023–8. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roger VL. Epidemiology of heart failure. Circulation Res. 2013;113:646–59. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamasaki N, Kitaoka H, Matsumura Y, Furuno T, Nishinaga M, Doi Y. Heart failure in the elderly. Intern Med. 2003;42:383–8. doi: 10.2169/internalmedicine.42.383. [DOI] [PubMed] [Google Scholar]
- 21.Fox KF, Cowie MR, Wood DA, et al. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–36. doi: 10.1053/euhj.2000.2289. [DOI] [PubMed] [Google Scholar]
- 22.GBD 2016 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–65. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 24.Twiner MJ, Marinica AL, Kuper K, et al. Screening and treatment for subclinical hypertensive heart disease in emergency department patients with uncontrolled blood pressure: a cost-effectiveness analysis. Acad Emerg Med. 2017;24:168–76. doi: 10.1111/acem.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy PD, Flack JM. Should African-Americans with elevated blood pressure be routinely screened for hypertensive heart disease? Exp Rev Cardiovasc Ther. 2012;10:1201–4. doi: 10.1586/erc.12.130. [DOI] [PubMed] [Google Scholar]
- 26.Wouters OJ, O’Donoghue DJ, Ritchie J, Kanavos PG, Narva AS. Early chronic kidney 24: diagnosis, management and models of care. Nat Rev Nephrol. 2015;11:491–502. doi: 10.1038/nrneph.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, et al. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980-2014. JAMA. 2017;317:1762–92. doi: 10.1001/jama.2017.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banach M, Bromfield S, Howard G, et al. Association of systolic blood pressure levels with cardiovascular events and all-cause mortality among older adults taking antihypertensive medication. Int J Cardiol. 2014;176:219–26. doi: 10.1016/j.ijcard.2014.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]