Abstract
Circulating concentration and activity of secretory phospholipase A2 (sPLA2) and lipoprotein-associated phospholipase A2 (Lp-PLA2) have been proven as biomarkers of increased risk of atherosclerosis-related cardiovascular disease (ASCVD). Lp-PLA2 might be part of the atherosclerotic process and may contribute to plaque destabilisation through inflammatory activity within atherosclerotic lesions. However, all attempts to translate the inhibition of phospholipase into clinically beneficial ASCVD risk reduction, including in randomised studies, by either non-specific inhibition of sPLA2 (by varespladib) or specific Lp-PLA2 inhibition by darapladib, unexpectedly failed. This gives us a strong imperative to continue research aimed at a better understanding of how Lp-PLA2 and sPLA2 regulate vascular inflammation and atherosclerotic plaque development. From the clinical viewpoint there is a need to establish and validate the existing and emerging novel anti-inflammatory therapeutic strategies to fight against ASCVD development, by using potentially better animal models and differently designed clinical trials in humans.
Keywords: atherogenesis, phospholipases, biomarker, secretory phospholipases A2, lipoprotein-associated phospholipase A2 (Lp-PLA2), prognosis, anti-inflammatory agents
Introduction – the A2 group phospholipases (PLA2s)
The phospholipases are enzymes that hydrolyse phospholipids. They are classified into different groups by their molecular weight, their catalytic residues, and their dependence (or lack thereof) on calcium [1]. The A2 group of phospholipases specifically hydrolyse the ester bond of the fatty acid at the sn-2 position of the glycerophospholipids and, by doing so, release both fatty acids and lysophospholipids [1, 2].
Secretory PLA2 (sPLA2) are calcium-dependent, low-molecular-weight enzymes that include different groups, named I-III, V, and IX-XIV. Also cytosolic PLA2 (cPLA2, GIV) are calcium dependent. On the other hand, calcium independent groups are GV PLA2 (iPLA2) and lipoprotein-associated phospholipase A2 (Lp-PLA2 or, as it is also called, platelet-activating factor acetylhydrolases; PAF-AH, GVII/GVIII). We also know of lysosomal PLA2 (GXV) and adipose-specific phospholipase A2 (AdPLA2, GXVI) [1, 3–6].
sPLA2 family includes 12 isoforms, and despite sharing some common features they are functionally distinct proteins with specific tissue distributions [7–9] and enzymatic properties [9]. They hydrolyse phospholipids from the surface of cell membranes, native lipoproteins, and oxidatively-modified lipoproteins to produce many different bioactive lipids that include arachidonic acid (and consequently also eicosanoids – prostaglandins, thromboxanes, leukotrienes), non-esterified fatty acids, lysophospholipids, lyso-platelet acting factor, and oxidised non-esterified fatty acids [3, 8]. In contrast, Lp-PLA2 requires oxidised phospholipids as a substrate (platelet-activating factor (PAF), PAF-like substances and oxidised phospholipids) [3, 10].
Circulating concentration and enzymatic activity of secretory phospholipase A2 (sPLA2) and lipoprotein-associated phospholipase A2 (Lp-PLA2) have been evaluated as biomarkers of cardiovascular risk in populations of apparently healthy individuals, as well as in patients with established coronary heart disease (CHD) [1, 3–6].
On the role of PLA2s in atherogenesis
Secretory phospholipase A2 (sPLA2) and atherosclerosis
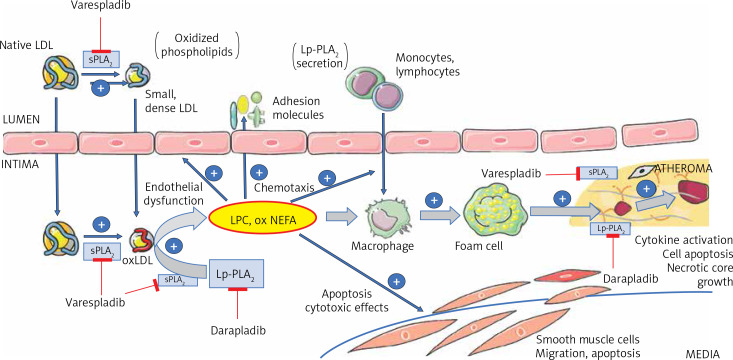
Six isoforms of the sPLA2 family are described to be present in atherosclerotic lesions: IIA, IID, IIE, III, V, and X, and they have been reported to have a potential causal role in atherogenesis [3, 5] (Figure 1). Phosphatidylcholine hydrolysis by sPLA2 results in very-low density lipoprotein (VLDL) and low density lipoprotein (LDL) particles with altered conformation of apolipoprotein B (apoB). These processes result in smaller, denser, and more electronegative lipoprotein particles [1, 3, 5] that are less avidly internalised by the hepatic apoB/E (LDL) receptor [3, 11], with a prolonged residence time in the circulation and further susceptibility to oxidation [3]. GV and GX sPLA2 enzymes hydrolyse phosphatidylcholine on the surface of VLDL and LDL at least 20-fold more efficiently than GIIA sPLA2 and as such have a potential to act extracellularly [1, 3, 12–14]. On the other hand, GIIA sPLA2 shows enhanced ability to hydrolyse oxidised LDL and acts within intima and macrophages [1, 3]. Hydrolysis of phospholipids on high-density lipoprotein (HDL) results in impaired cholesterol-efflux capacity – the ability of HDL to accept cholesterol from macrophages [3, 5, 15]. The conformational changes in apoB also increase intimal proteoglycan binding [1, 3, 16–18] and therefore promote retention of these atherogenic lipoproteins and cholesterol crystal precipitation [19]. sPLA2-mediated phospholipid hydrolysis further increases its vasoactive, chemotactic, and proinflammatory role [3, 5] because it increases oxidative stress through generation of arachidonic acid (including eicosanoids), lysophospholipids, and non-esterified fatty acids [3, 5, 20].
Figure 1.
Schematic presentation of the roles of sPLA2 and Lp-PLA2 in atherogenesis, as well as the potential sites for therapeutic inhibition, by using either sPLA2 non-specific (e.g. varespladib), or a specific Lp-PLA2 inhibitor (darapladib)
sPLA2 – secretory phospholipase A2, Lp-PLA2 – lipoprotein-associated phospholipase A2, LPC – lysophosphatidylcholine, oxNEFA – oxidised non-esterified fatty acids, oxLDL – oxidised low-density lipoprotein particle.
Lipoprotein-associated phospholipase A2 and atherosclerosis
Lp-PLA2 is secreted primarily by macrophages and by some other inflammatory and non-inflammatory cells involved in atherogenesis [21] (Figure 1). In plasma, Lp-PLA2 circulates in active form as a complex with LDL (80–85%), HDL (15–20%), and, to a lesser extent, Lp(a) [10, 21].
Endothelial dysfunction is a well-established vascular response to cardiovascular risk factors, which precedes the development of atherosclerosis and is involved in the promotion of both the early and late mechanisms of progression [22]. It is characterised by the expression of more adhesion molecules and increased endothelial permeability; hence, LDL particles can transmigrate more easily to arterial intima [10, 22]. Because of the reduced content of antioxidants in arterial intima LDL particles are exposed to further oxidation. Consequently, the Lp-PLA2 is activated by an abundance of oxidised phospholipids present in OxLDL [10]. As such, Lp-PLA2-driven hydrolysis of the oxidized phospholipids to some extent confines the modifications of OxLDL and may be interpreted as a protective function [2, 10]. Some studies have provided evidence that loss-of-function (V279F) mutations possibly increase the incidence and severity of cardiovascular conditions compared with non-carriers [2, 4]. However, most of the studies support the concept that Lp-PLA2 contributes to the development and progression of atherosclerosis. The underlying explanation is that, following the process of Lp-PLA2-enhanced phospholipid hydrolysis, high contents of oxidised non-esterified fatty acids (OxNEFA) and lysophospholipids are produced, which promote expression of adhesion molecules, stimulate cytokines production (TNF-α, IL-6), and attract macrophages to the arterial intima [3, 10]. A deleterious feed-forward mechanism may also be associated because recruitment of additional inflammatory cells in activated plaques may result in further Lp-PLA2 production and activity [2]. Activated macrophages and foam cells produce even more Lp-PLA2 [23]. The presence of OxLDL, as well as lysophospholipids and OxNEFA produced by Lp-PLA2, stimulate the growth of the plaque and eventually lead to the formation of a necrotic core and can be decisive regarding plaque rupture susceptibility that can culminate in a cardiovascular event [10]. High levels of Lp-PLA2 and lysophospholipids are found in thin-cap fibroatheromas and ruptured plaques but are almost absent in stable lesions [2, 24].
Circulating Lp-PLA2 determination
To detect Lp-PLA2 in blood we can measure either its mass concentration (ng/ml) or its enzymatic activity (nmol/min/ml). Lp-PLA2 mass concentration measured by the standard ELISA immunoassays has been proven to be less accurate than spectrophotometric assay of enzymatic activity assessment for risk stratification, presumably because it only detects a reduced percentage of total Lp-PLA2 that is not in interaction with lipoprotein [25, 26]. The Food and Drug Administration (FDA) approved the PLAC® Test for measuring the Lp-PLA2 mass concentration (2003) and PLAC® Test Activity (2014) for enzymatic activity in order to improve diagnostics and prediction of ASCVD in clinical practice [26, 27]. The performance of the PLAC® Test is superior to other alternative commercially available tests [27]. It is a standard indirect ELISA immunoassay that uses two monoclonal antibodies: a primary antibody to bind to Lp-PLA2 enzyme from the blood sample and an enzyme-conjugated secondary antibody to detect it. The PLAC® Test Activity uses the Lp-PLA2 to hydrolyse the sn-2 position of the substrate, 1-myristoyl-2-(4-nitrophenylsuccinyl) phosphatidylcholine, producing a coloured reaction product 4-nitrophenol. The rate of 4-nitrophenol production is monitored spectrophotometrically, and the Lp-PLA2 activity is calculated by the rate of change of absorbance.
To be clinically useful, a biomarker must have a precisely established decision value. AACE/ACE Guidelines for Management of Dyslipidaemia and Prevention of Cardiovascular Disease (2017) state that an Lp-PLA2 mass concentration less than 200 ng/ml is normal, ≥ 200 and < 223 ng/ml is intermediate, and ≥ 223 ng/ml is high [28]. Lp-PLA2 mass concentration of 400 ng/ml does not seem to impart much more risk than 250 ng/ml [23]. The manufacturer of the PLAC® Test Activity declares an enzyme activity cut-off point at 225 nmol/min/ml that identifies high cardiovascular risk patients.
Lp-PLA2 and cardiovascular risk
Epidemiological studies
Lp-PLA2 has continuously been confirmed as a cardiovascular risk marker independent of and additive to traditional risk factors [28–30]. The presence and activity of Lp-PLA2 within a plaque was associated with vulnerable, rupture-prone plaques [31–33], and it appears that Lp-PLA2 is released from these plaques into the circulation [34, 35]. Contrary to hs-CRP, Lp-PLA2 is a specific vascular inflammation marker [35, 36] that potentially fills an important vacancy of having a non-invasive marker indicating the existence of atherosclerotic plaques prone to rupture. When both Lp-PLA2 and hs-CRP are increased together, they provide an even greater predictive capability [28, 37–39].
When adjusting for cardiac, inflammatory, and renal function biomarkers, the independent associations between Lp-PLA2 activity in stable patients at high risk for, or with established CHD remained a significant marker of increased risk for MI, ischaemic stroke, and other composite cardiovascular events [21, 23, 40], hospitalisation for heart failure, and cardiovascular and total mortality, whereas the associations with recurrent ischaemic events such as MI and stroke were attenuated [21, 23, 40]. Similarly, there was no association between Lp-PLA2 activity and cardiovascular outcomes in patients with acute coronary syndromes, in which the majority have elevated cardiac and inflammatory markers [21, 23, 40, 41]. Many studies support Lp-PLA2 activity as a risk indicator of adverse outcomes both in patients with stable CHD and in the general population [6, 40, 42–51]. Although most of the studies confirm that Lp-PLA2 activity is significant in ASCVD risk assessment in low-risk populations, the magnitude of this improvement is relatively modest in comparison to improvement in the moderate or higher-risk population [23].
Lp-PLA2 should be considered as a clinically useful cardiovascular risk marker that in adjunction to other major risk factors and markers improves the identification of individuals whose ASCVD risk is greater than clinically apparent. Therefore, we are potentially able to adjust absolute ASCVD risk status and modify the intensity of risk-reducing interventions [23]. The low biologic fluctuation and high vascular specificity of Lp-PLA2 makes it possible to use a single measurement in clinical decision making, and it also permits clinicians to follow the Lp-PLA2 marker serially [23].
Genetic studies
The Lp-PLA2 mass concentration or activity are heritable traits [52]. The gene encoding Lp-PLA2 (PLA2G7), (6p12-21.1) is organised in 12 exons and encodes 441 amino acids [2]. Over the years several polymorphisms of PLA2G7 have been identified. On the foundation that elevated Lp-PLA2 levels were correlated with certain diseases, including atherosclerosis, most studies so far have focused on gene variants that weaken Lp-PLA2 mass concentration or activity (loss-of-function alleles) [2].
Some of the most commonly researched polymorphisms of PLA2G7 gene are Val279Phe mutation (rs16874954/rs76863441), Val379Ala mutation (rs1051931), Arg92His mutation (rs1805017), and Ile198Thr mutation (rs1805018) [2, 53, 54]. A recent study also identified significant association of rs13218408 mutation with the level of Lp-PLA2 mass concentration and activity, which also has a significant joint effect with widely validated coding polymorphism rs16874954 on the level of Lp-PLA2 [54]. Huang et al. reported that the heterozygous carriers of the rs16874954 minor allele had a significant reduction of 32.4% in Lp-PLA2 activity and 34.4% in mass concentration, and almost no detectable enzyme activity and mass were found in the homozygous carriers [54]. Similarly, Gregson et al. showed the reduction of Lp-PLA2 activity by 45% for every rs16874954 minor allele inherited [53].
The Genome-Wide Association Study of Lp-PLA2 Activity and Mass in the Framingham Heart Study identified four distinct gene regions showing highly significant associations with Lp-PLA2 activity, all of which are known to include genes involved in cholesterol metabolism – APOE/APOC1 region on chromosome 19, CELSR2/PSRC1 on chromosome 1, SCARB1 on chromosome 12, and ZNF259/BUD13 in the APOA5/APOA1 gene region on chromosome 11 [52]. All of these loci remained significantly associated with Lp-PLA2 activity after accounting for their association with serum lipid and lipoprotein levels [52].
Many genetic studies have established that PLA2G7 variants that reduce Lp-PLA2 activity to levels comparable to darapladib have no effect on the risk of CHD and outcomes. These results oppose, to a certain extent, a causal role of Lp-PLA2 during atherosclerotic progression [2, 53, 55, 56].
Reflections on evidence-based ASCVD prevention guidelines
Different studies have shown that circulating Lp-PLA2 activity levels could be an index of systemic inflammation and serve as an independent risk factor for CAD [57, 58]. A meta-analysis that included all prospective studies conducted on Lp-PLA2 showed a relationship between Lp-PLA2 activity and mass and incidence of CAD, stroke, and cardiovascular mortality [6]. This evidence means that nearly a decade ago the guidelines of major international societies, including the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the American Society of Endocrinology, included Lp-PLA2 activity measurement among the biomarkers deemed useful for ASCVD risk stratification of asymptomatic adult patients (in most cases by Class IIb recommendation). The use of this marker was declared to be particularly advantageous as part of a refined risk assessment in patients at moderate cardiovascular risk (> 2 risk factors) and in those at high-risk of a recurrent acute atherothrombotic event in whom an increase of Lp-PLA2 activity levels was supposed to guide the lipid-lowering treatment to reach LDL-cholesterol levels lower than the primarily recommended target [59].
The updated, contemporary guidelines abandoned such recommendations because there is a lack of studies that examine the degree to which Lp-PLA2 improves on existing traditional risk prediction models in terms of clinically important magnitudes of reclassification, which can then be translated into differences in treatment approach that would potentially improve patient outcomes [60–63]. This could in principle be demonstrated with clinical trials, but the expected difference in outcomes would probably be so small that the sample size of the trial would be impractically large. Decision modelling could be used as another approach to estimate differences in patient outcomes due to improved reclassification of risk [64, 65]. A robust validated model using Lp-PLA2 levels to predict CHD outcomes is necessary in order to use the test to manage patients. No studies identified have evaluated whether a testing strategy that uses Lp-PLA2 levels improves health outcomes. Although Lp-PLA2 is associated with ASCVD risk, changes in patient management that would occur as a result of obtaining its levels in practice are not well defined. Lp-PLA2 decreased substantially after treatment with different lipid lowering medications, including statins, ezetimibe, fibrates, and omega-3-fatty acids [66–70]. However, in treated patients, Lp-PLA2 levels may no longer be associated with risk of CAD, and thus may not be useful as a measure of treatment response.
Therapeutic modalities specifically for reduction of sLPA2/Lp-PLA2 that have been tested
In addition to numerous laboratory observations as well as animal studies, which confirmed the role of sPLA2 in the inflammatory milieu of atherosclerosis, clinical research also supported its value as a prognostic marker among patients with (both stable and unstable) ASCVD. Significantly higher sPLA2 levels were observed among patients immediately after undergoing PCI, and elevated sPLA2 was shown as an independent predictor of recurrent events in the longer term [71]. Increased sPLA2 was highly predictive of recurrent CV events, revascularisation, as well as death also in patients with acute coronary syndromes (unstable AP, NSTEMI, and STEMI) [72, 73].
On this basis, sPLA2 was identified as an attractive therapeutic target to improve cardiovascular outcomes (Figure 1). By using varespladib methyl, a non-specific inhibitor of sPLA2 activity (inhibiting sPLA2-GIIA, GV and GX), in phase 2 testing trials, the sPLA2 levels were decreased by approximately 80% in a dose-dependent manner, in patients with stable CHD, as well as in those with acute coronary syndrome (ACS) (Table I), while there were no differences in rates of major adverse CV events between the actively treated and placebo groups [74–76]. An interventional study on patients with ACS followed (VISTA-16 (Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 weeks)), in which, despite lowering plasma levels of sPLA2-IIA by 78%, as well as LDL-C and hs-CRP, varespladib surprisingly failed to express its clinical efficacy; even more, the composite primary outcome (CV mortality, nonfatal MI and stroke, and unstable AP) increased by 25% [77]. At least partly this failure can be explained from the perspective of the so-called sPLA2 pan-inhibition, which influences not only its atherogenic, but also its atheroprotective roles (e.g. of the sPLA2-GX) [78].
Table I.
Summary presentation of clinical studies (phases II and III) using either non-specific sPLA2 inhibitor varespladib or specific Lp-PLA2 inhibitor darapladib, in various clinical settings
| Variable | Patients | Duration | Effect(s) | Reference |
|---|---|---|---|---|
| sPLA2 inhibition with varespladib methyl: | ||||
| Phase II clinical trials: | ||||
| PLASMA | N = 396; stable CHD | 8 weeks | ↓ of sPLA2-GIIA by 69–96% | Rosenson RS, et al. (2009, 2011) [74] |
| PLASMA II | N = 135; stable CHD | 8 weeks | ↓ of sPLA2-GIIA by 73–84% | Rosenson RS, et al. (2011) [75] |
| FRANCIS | N = 625; up to 96 hrs after the ACS | 24 weeks | ↓ of sPLA2-GIIA by 82.4%; ↓ of hs-CRP and LDL-C; ↓ of major adverse CVD events (NS) |
Rosenson RS, et al. (2010) [76] |
| Rosenson RS, et al. | N = 624; pts with ACS, comparison of pts with and without DM | 8 weeks | ↓ of sPLA2-GIIA by 83.6% in DM pts, and by 82.4% in nonDM | Rosenson RS, et al. (2011) [86] |
| SPIDER-PCI | N = 144; stable CHD, before & after PCI | 3–5 days before & 5 days after PCI | ↓ of sPLA2-GIIA by up to 95% | Dzavik V, et al. (2010) [87] |
| Phase III clinical trials: | ||||
| VISTA-16 | N = 5,145; recent ACS | 16 weeks, survival at 6 months | ↑ of 1° outcome (CV death, non-fatal MI, UAP) – HR 1.25; ↓ of sPLA2 by 78%, ↓ of hs-CRP and LDL-C |
Nicholls SJ, et al. (2014) [77] |
| Lp-PLA2 inhibition with darapladib: | ||||
| Phase II clinical trials: | ||||
| Johnson A, et al. | N = 59; pts before elect.endarterectomy | 2 weeks | ↓ of Lp-PLA2 by 80% | Johnson A, et al. (2004) [88] |
| Mohler ER, et al. | N = 959; stable CHD | 12 weeks | ↓ of Lp-PLA2 by 43–66%; no significant effect on plasma lipids or hsCRP; ↓ of IL-6 | Mohler ER, et al. (2008) [89] |
| IBIS-2 | N = 330; pts with documented coronary disease, after PCI | 12 months | ↓ of Lp-PLA2 by 59%, ↓ plaque necrotic core volume; no change of atheroma deformability |
Serruys PW, et al. (2008) [79] |
| Phase III clinical trials: | ||||
| STABILITY | N = 15,828; stable CHD | 3.7 years | No difference in 1° outcome (CV death, MI, stroke) – HR 0.94; ↓ of total and major coronary events |
White HD, et al. (2014) [81] |
| SOLID-TIMI 52 | N = 13,026; up to 30 days after MI | 2.5 years | No difference in 1° outcome (CHD death, MI, urg.revasc.) – HR 0.99; ↓ of Lp-PLA2 by 65% | O’Donoghue ML, et al. (2014) [41] |
CHD – coronary heart disease, ACS – acute coronary syndrome, UAP – unstable angina pectoris, MI – myocardial infarction, PCI – percutaneous coronary intervention, LDL-C – low-density lipoprotein cholesterol, hs-CRP – high-sensitivity C-reactive protein, DM – diabetes mellitus.
As described above, Lp-PLA2 also mediates the formation of some important bioactive mediators (lysophosphatidylcholine and oxNEFA), known to be proinflammatory and directly implicated into the ASCVD process. Lysophosphatidylcholine serves as a potent chemoattractant for monocytes, resulting in foam cell accumulation within the arterial wall, and Lp-PLA2 was shown to be highly expressed in the necrotic cores as well as thin-cap fibroatheromas and ruptured plaques. These data suggest that inhibition of Lp-PLA2 may stabilise atherosclerotic plaque and is potentially beneficial in patients with both stable and unstable ASCVD. Using darapladib, a direct selective inhibitor of Lp-PLA2, its activity can be reduced by over 60% [79] (Table I, Figure 1). In preclinical studies on animal models it was indeed shown that besides decreasing Lp-PLA2 in plasma, darapladib also inhibits the development of atherosclerotic lesions and reduces the macrophage content and the necrotic core area in plaques [80]. In an early clinical study, IBIS-2 (Integrated Biomarker and Imaging Study 2), a significant difference was found in the reduction of the atheroma necrotic core volume in comparison to the group treated with placebo [79]. Highly surprisingly and unexpectedly, in the clinical intervention trials which followed, both in the STABILITY (Stabilisation of Atherosclerotic Plaque by Initiation of Darapladib Therapy), studying patients with chronic stable CAD, and SOLID-TIMI 52 (Stabilisation Of pLaques usIng Darapladib-Thrombolysis In Myocardial Infarction 52), encompassing patients with either NSTEMI or STEMI within 30 days after the event, no significant beneficial clinical effects (on incidence rates of death, MI, stroke, or urgent revascularisation) were demonstrated by using selective inhibition of Lp-PLA2 with darapladib [41, 81]. The effect of Lp-PLA2 inhibition on arterial wall inflammation in carotid arterial disease was studied using rilapladib. Analyses performed on the basis of MRI/PET imaging of the carotid plaques failed to demonstrate a significant effect of short-term rilapladib (compared with that of placebo) in individuals with stable atherosclerotic disease concurrently on statin therapy [82]. It is worth noting that in plaque-based analysis, the nominal reduction from baseline seen in the rilapladib group (ca. 3% to 6%) approaches that typically observed with low-dose statins [83].
The failure of translation of specific (in case of Lp-PLA2) or nonspecific (in case of sPLA2) inhibition into the clinically effective therapeutic modality can be discussed using several possible explanations. First of all, and probably the most reliable one, is that it is in principle not necessary that there is a true causal relationship between the biomolecule identified as a biomarker of increased ASCVD risk and the clinical endpoints. Because it is not necessary for the targeted biomarker to actually be lying in the pathway from disease to clinical endpoint, the elevated plasma levels of phospholipases may not represent the optimal surrogates for clinical events despite being proven as clearly correlated with advanced atherosclerotic lesions [84]. Because there are multiple pathways involved in the inflammatory atherosclerotic disease process, affecting only one of them could not be sufficient to translate clinically. In addition, in both the STABILITY and SOLID-TIMI 52 trials the great majority of patients (95% and 94%, respectively; despite only 6.2–7.4% of patients being on high-intensity statins) were already treated with statins, already shown as potent multifaceted anti-inflammatory agents. Second, the estimation of the effect of the treatment could be over- or underestimated due to the fact that out of many existing atherogenesis-related inflammatory molecules and pathways, targeting only one surrogate cannot exclude the possibility that the others remain active in their influence on the clinical outcome of interest. If the targeted surrogate lies in several pathways, and its treatment may affect them differentially, it may be beneficial on one, but with undesirable or harmful (or unpredicted) consequences on the other. Third, medications targeted at one pathway or molecule may also have substantial off-target effects, e.g. as in the case of statins, which besides their potent LDL-C lowering effects may also have multiple beneficial effects on inflammation (by reducing oxidised LDL, macrophages, and T cells in atherosclerotic plaques as well as levels of hs-CRP and adhesion molecules, such as E-selectin, P-selectin, and ICAM-1) [85]. Direct targeting of any of these pathways individually would probably have been less successful.
Concluding remarks – in summary
Circulating concentrations and enzymatic activities of both sPLA2 and Lp-PLA2 have been proven as biomarkers of increased risk of ASCVD, independently and in addition to traditional risk factors. In the recent past, many international guidelines have supported the measurement of Lp-PLA2 in order to refine the overall cardiovascular risk assessment in subjects classified as being at intermediate or high ASCVD risk. Such recommendations were recently abandoned, due to the presumed limited supplementary value in absolute ASCVD risk determination, not unrelated also to the almost unknown cost-effectiveness.
Bearing in mind the abundance of data demonstrating the predictive value of Lp-PLA2, as well as with contemporary orientation towards a more personalised approach, it could still be of use as one of the valid prognostic markers playing a role in everyday clinical practice. Probably the most appropriate groups of patients in whom its measurement is warranted, and for whom inclusion into an overall risk assessment/stratification could prove itself to be beneficial, are patients with metabolic syndrome, diabetes mellitus, and those with already manifested ASCVD. As for the latter, the results of one of the earliest statin trials, LIPID (Long-term Intervention with Pravastatin in Ischaemic Disease), have to be highlighted, which showed not only the persistence of the remarkably high prognostic value of reduced Lp-PLA2 during treatment even after adjustment for more than 20 risk factors at inclusion, but a predictive value at least equal to or higher than the decrease in LDL-C [50].
From the clinical viewpoint there is a need to establish and validate the existing and emerging novel anti-inflammatory therapeutic strategies to treat ASCVD. This can be achieved not only by the use of improved animal models in preclinical research, which would better resemble the whole complexity of the phospholipase involvement in the ASCVD pathogenesis, but also with somewhat differently designed future clinical trials in humans.
Failure of translation of the inhibition of phospholipases into clinically beneficial risk reduction in some of the randomised clinical studies so far somehow challenged also the pathogenetic role of sPLA2/Lp-PLA2 in ASCVD development and plaque destabilisation. However, it gives us a strong imperative to continue research aimed at better understanding how Lp-PLA2 and sPLA2 regulate vascular inflammation and atherosclerotic plaque development. It seems plausible to point towards some of the major intrinsic limitations of the completed phase III clinical trials. It is important to note that in all of them almost the entire study population were taking statins, which are known to decrease Lp-PLA2 by up to 35%, and to point out the relatively high reported rate of drug discontinuation (up to 20% in both SOLID TIMI 52 and STABILITY trials) [41, 81]. Besides the true potential for the improvement of the overall compliance with the existing drugs calling at repeatedly, but without significant success, it could be that there is a room for another, possibly better tolerated Lp-PLA2 inhibitor.
The five most important challenges for further research related to sPLA2/Lp-PLA2
Better understanding of the complex role of sPLA2/Lp-PLA2 in atherogenesis
Improved animal models to be used in preclinical phase studies
More precise quantification of the additive value of sPLA2/Lp-PLA2 in absolute ASCVD risk assessment
Cost-effectiveness studies related to the use of sPLA2/Lp-PLA2 both as a prognostic biomarker and/or as a therapeutic target
Better designed clinical trials with the use of either existing or newly developed sPLA2/Lp-PLA2 inhibitors
Conflict of interest
ZF reports grants, has given talks, acted as a consultant of Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Krka Pharma, Novo Nordisk, Pfizer, Sanofi, and Servier, all outside the submitted work. JT – none. MB has received research grant(s)/support from Amgen, Mylan, Sanofi and Valeant, and has served as a consultant for Akcea, Amgen, Daiichi-Sankyo, Freia Pharmaceuticals, KRKA, MSD, Mylan, Novartis, Polfarmex, Polpharma, Sanofi-Aventis, Servier, Esperion, and Resverlogix.
References
- 1.Talmud PJ, Holmes MV. Deciphering the causal role of sPLA2s and Lp-PLA2 in coronary heart disease. Arterioscl Thromb Vasc Biol. 2015;35:2281–9. doi: 10.1161/ATVBAHA.115.305234. [DOI] [PubMed] [Google Scholar]
- 2.Huang F, Wang K, Shen J. Lipoprotein-associated phospholipase A2: the story continues. Med Res Rev. 2020;40:79–134. doi: 10.1002/med.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J. 2012;33:2899–909. doi: 10.1093/eurheartj/ehs148. [DOI] [PubMed] [Google Scholar]
- 4.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–85. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A2 research: from cells to animals to humans. Progr Lipid Res. 2011;50:152–92. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–44. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Ann Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 8.Rosenson RS, Gelb MH. Secretory phospholipase A 2: a multifaceted family of proatherogenic enzymes. Curr Cardiol Rep. 2009;11:445. doi: 10.1007/s11886-009-0064-2. [DOI] [PubMed] [Google Scholar]
- 9.Jönsson-Rylander AC, Lundin S, Rosengren B, Pettersson C, Hurt-Camejo E. Role of secretory phospholipases in atherogenesis. Curr Atheroscl Rep. 2008;10:252–9. doi: 10.1007/s11883-008-0039-6. [DOI] [PubMed] [Google Scholar]
- 10.Silva IT, Mello APQ, Damasceno NRT. Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A2 (Lp-PLA2): a review. Lipids Health Dis. 2011;10:170. doi: 10.1186/1476-511X-10-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinman Y, Krul ES, Burnes M, Aronson W, Pfleger B, Schonfeld G. Lipolysis of LDL with phospholipase A2 alters the expression of selected apoB-100 epitopes and the interaction of LDL with cells. J Lipid Res. 1988;29:729–43. [PubMed] [Google Scholar]
- 12.Gorshkova IN, Menschikowski M, Jaross W. Alterations in the physicochemical characteristics of low and high density lipoproteins after lipolysis with phospholipase A2. A spin-label study. Biochim Biophys Acta. 1996;1300:103–13. doi: 10.1016/0005-2760(95)00237-5. [DOI] [PubMed] [Google Scholar]
- 13.Gesquiere L, Cho W, Subbaiah PV. Role of group IIa and group V secretory phospholipases A2 in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry. 2002;41:4911–20. doi: 10.1021/bi015757x. [DOI] [PubMed] [Google Scholar]
- 14.Karabina SA, Brochériou I, Le Naour G, et al. Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J. 2006;20:2547–9. doi: 10.1096/fj.06-6018fje. [DOI] [PubMed] [Google Scholar]
- 15.Qiu C, Zhao X, Zhou Q, Zhang Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: a systematic review and meta-analysis. Lipids Health Dis. 2017;16:212. doi: 10.1186/s12944-017-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakala JK, Öörni K, Pentikäinen MO, Hurt-Camejo E, Kovanen PT. Lipolysis of LDL by human secretory phospholipase A2 induces particle fusion and enhances the retention of LDL to human aortic proteoglycans. Arterioscl Thromb Vasc Biol. 2001;21:1053–8. doi: 10.1161/01.atv.21.6.1053. [DOI] [PubMed] [Google Scholar]
- 17.Sartipy P, Camejo G, Svensson L, Hurt-Camejo E. Phospholipase A2 modification of low density lipoproteins forms small high density particles with increased affinity for proteoglycans and glycosaminoglycans. J Biol Chem. 1999;274:25913–20. doi: 10.1074/jbc.274.36.25913. [DOI] [PubMed] [Google Scholar]
- 18.Flood C, Gustafsson M, Pitas RE, Arnaboldi L, Walzem RL, Borén J. Molecular mechanism for changes in proteoglycan binding on compositional changes of the core and the surface of low-density lipoprotein-containing human apolipoprotein B100. Arterioscl Thromb Vasc Biol. 2004;24:564–70. doi: 10.1161/01.ATV.0000117174.19078.85. [DOI] [PubMed] [Google Scholar]
- 19.Lange Y, Ye J, Steck TL. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Nat Acad Sci. 2004;101:11664–7. doi: 10.1073/pnas.0404766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tietge UJF, Pratico D, Ding T, et al. Macrophage-specific expression of group IIA sPLA2 results in accelerated atherogenesis by increasing oxidative stress. J Lipid Res. 2005;46:1604–14. doi: 10.1194/jlr.M400469-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res. 2012;53:1767–82. doi: 10.1194/jlr.R024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khafaji H, Carr C, Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–98. [PMC free article] [PubMed] [Google Scholar]
- 23.Corson MA, Jones PH, Davidson MH. Review of the evidence for the clinical utility of lipoprotein-associated phospholipase A2 as a cardiovascular risk marker. Am J Cardiol. 2008;101:S41–50. doi: 10.1016/j.amjcard.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Münzel T, Gori T. Lipoprotein-associated phospholipase A2, a marker of vascular inflammation and systemic vulnerability. Eur Heart J. 2009;30:2829–31. doi: 10.1093/eurheartj/ehp311. [DOI] [PubMed] [Google Scholar]
- 25.De Stefano A, Mannucci L, Tamburi F, et al. Lp-PLA2, a new biomarker of vascular disorders in metabolic diseases. Int J Immunopathol Pharmacol. 2019;33:2058738419827154. doi: 10.1177/2058738419827154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topbas C, Swick A, Razavi M, Anderson NL, Pearson TW, Bystrom C. Measurement of lipoprotein-associated phospholipase A2 by use of 3 different methods: exploration of discordance between ELISA and activity assays. Clin Chem. 2018;64:697–704. doi: 10.1373/clinchem.2017.279752. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Guo X, Hou L, et al. Measuring lipoprotein-associated phospholipase A2 activity in China: Protocol comparison and recalibration. J Clin Lab Anal. 2019;33:e22628. doi: 10.1002/jcla.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 29.Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and Cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82:159–65. doi: 10.4065/82.2.159. [DOI] [PubMed] [Google Scholar]
- 30.Kotani K, Serban MC, Penson P, Lippi G, Banach M. Evidence-based assessment of lipoprotein(a) as a risk biomarker for cardiovascular diseases – some answers and still many questions. Crit Rev Clin Lab Sci. 2016;53:370–8. doi: 10.1080/10408363.2016.1188055. [DOI] [PubMed] [Google Scholar]
- 31.Weintraub HS. Identifying the vulnerable patient with rupture-prone plaque. Am J Cardiol. 2008;101:S3–S10. doi: 10.1016/j.amjcard.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Kolodgie F, Burke A, Skorija K, Kutys R, Makuria A, Virmani R. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscl Thromb Vasc Biol. 2006;26:2523–9. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 33.Lavi S, McConnell JP, Rihal Charanjit S, Prasad A, et al. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation. Circulation. 2007;115:2715–21. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 34.Ferretti G, Bacchetti T, Johnston TP, Banach M, Pirro M, Sahebkar A. Lipoprotein(a): a missing culprit in the management of athero-thrombosis? J Cell Physiol. 2018;233:2966–81. doi: 10.1002/jcp.26050. [DOI] [PubMed] [Google Scholar]
- 35.Garg PK, McClelland RL, Jenny NS, et al. Lipoprotein-associated phospholipase A2 and risk of incident cardiovascular disease in a multi-ethnic cohort: the Multi Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;241:176–82. doi: 10.1016/j.atherosclerosis.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler K, Winkelmann BR, Scharnagl H, et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors. Circulation. 2005;111:980–7. doi: 10.1161/01.CIR.0000156457.35971.C8. [DOI] [PubMed] [Google Scholar]
- 37.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-Associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2004;109:837–42. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 38.Koenig W, Khuseyinova N, Löwel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population. Circulation. 2004;110:1903–8. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 39.May H, Horne B, Anderson J, et al. Lipoprotein-associated phospholipase A(2) independently predicts the angiographic diagnosis of coronary artery disease and coronary death. Am Heart J. 2006;152:997–1003. doi: 10.1016/j.ahj.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Wallentin L, Held C, Armstrong PW, et al. Lipoprotein-associated phospholipase A2 activity is a marker of risk but not a useful target for treatment in patients with stable coronary heart disease. J Am Heart Assoc. 2016;5:e003407. doi: 10.1161/JAHA.116.003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–15. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 42.Kleber ME, Siekmeier R, Delgado G, et al. C-reactive protein and lipoprotein-associated phospholipase A2 in smokers and nonsmokers of the ludwigshafen risk and cardiovascular health study. In: Pokorski M, editor. Oxidative Stress and Cardiorespiratory Function. Springer, Cham; 2015. pp. 15–23. [DOI] [PubMed] [Google Scholar]
- 43.The Emerging Risk Factors C Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307:2499–506. doi: 10.1001/jama.2012.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleber ME, Wolfert RL, De Moissl GD, et al. Lipoprotein associated phospholipase A2 concentration predicts total and cardiovascular mortality independently of established risk factors (The Ludwigshafen Risk and Cardiovascular Health Study) Clin Lab. 2011;57:659–67. [PubMed] [Google Scholar]
- 45.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscl Thromb Vasc Biol. 2006;26:1586–93. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 46.Corsetti JP, Rainwater DL, Moss AJ, Zareba W, Sparks CE. High lipoprotein-associated phospholipase A2 is a risk factor for recurrent coronary events in postinfarction patients. Clin Chem. 2006;52:1331–8. doi: 10.1373/clinchem.2006.066845. [DOI] [PubMed] [Google Scholar]
- 47.Sabatine MS, Morrow DA, O’Donoghue M, et al. Prognostic utility of lipoprotein-associated phospholipase A2 for cardiovascular outcomes in patients with stable coronary artery disease. Arterioscl Thromb Vasc Biol. 2007;27:2463–9. doi: 10.1161/ATVBAHA.107.151670. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, MacFadyen JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A2 mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin Chem. 2012;58:877–86. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]
- 49.O’Donoghue M, Morrow DA, Sabatine MS, et al. Lipoprotein-Associated Phospholipase A2 and Its Association With Cardiovascular Outcomes in Patients With Acute Coronary Syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy–Thrombolysis In Myocardial Infarction) Trial. Circulation. 2006;113:1745–52. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 50.White HD, Simes J, Stewart RAH, et al. Changes in lipoprotein-associated phospholipase A2 activity predict coronary events and partly account for the treatment effect of pravastatIn: results from the long-term intervention with pravastatin in ischemic disease study. J Am Heart Assoc. 2013;2:e000360. doi: 10.1161/JAHA.113.000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quispe R, Hendrani AD, Baradaran-Noveiry B, et al. Characterization of lipoprotein profiles in patients with hypertriglyceridemic Fredrickson-Levy and Lees dyslipidemia phenotypes: the Very Large Database of Lipids Studies 6 and 7. Arch Med Sci. 2019;15:1195–202. doi: 10.5114/aoms.2019.87207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suchindran S, Rivedal D, Guyton J, et al. Genome-wide association study of Lp-PLA2 activity and mass in the framingham heart study. PLoS Genetics. 2010;6:e1000928. doi: 10.1371/journal.pgen.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gregson JM, Freitag DF, Surendran P, et al. Genetic invalidation of Lp-PLA2 as a therapeutic target: large-scale study of five functional Lp-PLA2-lowering alleles. Eur J Prev Cardiol. 2016;24:492–504. doi: 10.1177/2047487316682186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qi Y, Zhao D, Jia Z, et al. A previously unreported impact of a PLA2G7 gene polymorphism on the plasma levels of lipoprotein-associated phospholipase A2 activity and mass. Sci Rep. 2016;6:37465. doi: 10.1038/srep37465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casas JP, Ninio E, Panayiotou A, et al. PLA2G7 genotype, lipoprotein-associated phospholipase A2 activity, and coronary heart disease risk in 10 494 cases and 15 624 controls of European Ancestry. Circulation. 2010;121:2284–93. doi: 10.1161/CIRCULATIONAHA.109.923383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polfus LM, Gibbs RA, Boerwinkle E. Coronary heart disease and genetic variants with low phospholipase A2 activity. N Engl J Med. 2015;372:295–6. doi: 10.1056/NEJMc1409673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkler K, Winkelmann BR, Scharnagl H, et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: the Ludwigshafen Risk and Cardiovascular Health Study. Circulation. 2005;111:980–7. doi: 10.1161/01.CIR.0000156457.35971.C8. [DOI] [PubMed] [Google Scholar]
- 58.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111:570–5. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 59.Davidson MH, Corson MA, Alberts MJ, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol. 2008;101:51F–7F. doi: 10.1016/j.amjcard.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 60.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts), Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–81. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilstrap LG, Wang TJ. Biomarkers and cardiovascular risk assessment for primary prevention: an update. Clin Chem. 2012;58:72–82. doi: 10.1373/clinchem.2011.165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahebkar A, Simental-Mendía LE, Watts GF, Serban MC, Banach M, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group Comparison of the effects of fibrates versus statins on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of head-to-head randomized controlled trials. BMC Med. 2017;15:22. doi: 10.1186/s12916-017-0787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ioannidis JP, Tzoulaki I. Minimal and null predictive effects for the most popular blood biomarkers of cardiovascular disease. Circ Res. 2012;110:658–62. doi: 10.1161/RES.0b013e31824da8ad. [DOI] [PubMed] [Google Scholar]
- 65.Tzoulaki I, Siontis KC, Evangelou E, Ioannidis JP. Bias in associations of emerging biomarkers with cardiovascular disease. JAMA Intern Med. 2013;173:664–71. doi: 10.1001/jamainternmed.2013.3018. [DOI] [PubMed] [Google Scholar]
- 66.Saougos VG, Tambaki AP, Kalogirou M, et al. Differential effect of hypolipidemic drugs on lipoprotein-associated phospholipase A2. Arterioscler Thromb Vasc Biol. 2007;27:2236–43. doi: 10.1161/ATVBAHA.107.147280. [DOI] [PubMed] [Google Scholar]
- 67.Ryu SK, Mallat Z, Benessiano J, et al. Phospholipase A2 enzymes, high-dose atorvastatin, and prediction of ischemic events after acute coronary syndromes. Circulation. 2012;125:757–66. doi: 10.1161/CIRCULATIONAHA.111.063487. [DOI] [PubMed] [Google Scholar]
- 68.Filippatos TD, Gazi IF, Liberopoulos EN, et al. The effect of orlistat and fenofibrate, alone or in combination, on small dense LDL and lipoprotein-associated phospholipase A2 in obese patients with metabolic syndrome. Atherosclerosis. 2007;193:428–37. doi: 10.1016/j.atherosclerosis.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Agouridis AP, Tsimihodimos V, Filippatos TD, et al. The effects of rosuvastatin alone or in combination with fenofibrate or omega 3 fatty acids on inflammation and oxidative stress in patients with mixed dyslipidemia. Expert Opin Pharmacother. 2011;12:2605–11. doi: 10.1517/14656566.2011.591383. [DOI] [PubMed] [Google Scholar]
- 70.Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M, Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group effect of ezetimibe monotherapy on plasma lipoprotein(a) concentrations in patients with primary hypercholesterolemia: a systematic review and meta-analysis of randomized controlled trials. Drugs. 2018;78:453–62. doi: 10.1007/s40265-018-0870-1. [DOI] [PubMed] [Google Scholar]
- 71.Liu PY, Li YH, Wei-Chuan T, et al. Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur Heart J. 2003;24:1824–32. doi: 10.1016/j.ehj.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Kugiyama K, Ota Y, Sugiyama S, et al. Prognostic value of plasma levels of secretory type II phospholipase A2 in patients with unstable angina pectoris. Am J Cardiol. 2000;86:718–22. doi: 10.1016/s0002-9149(00)01069-9. [DOI] [PubMed] [Google Scholar]
- 73.Mallat Z, Steg G, Benessiano J, et al. Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. J Am Coll Cardiol. 2005;46:1249–57. doi: 10.1016/j.jacc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 74.Rosenson RS, Hislop C, McConnell D, et al. Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet. 2009;373:649–58. doi: 10.1016/S0140-6736(09)60403-7. Erratum in: Lancet 2011; 377: 1494. [DOI] [PubMed] [Google Scholar]
- 75.Rosenson RS, Elliott M, Stasiv Y, et al. Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease. Eur Heart J. 2011;32:999–1005. doi: 10.1093/eurheartj/ehq374. [DOI] [PubMed] [Google Scholar]
- 76.Rosenson RS, Hislop C, Elliott M, et al. Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J Am Coll Cardiol. 2010;56:1079–88. doi: 10.1016/j.jacc.2010.06.015. Erratum in: J Am Coll Cardiol 2011; 57: 1501. [DOI] [PubMed] [Google Scholar]
- 77.Nicholls SJ, Kastelein J, Schwartz GG, et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–62. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 78.Ait-Oufella H, Herbin O, Lahoute C, et al. Group X secreted phospholipase A2 limits the development of atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2013;33:466–73. doi: 10.1161/ATVBAHA.112.300309. [DOI] [PubMed] [Google Scholar]
- 79.Serruys PW, Garcia-Garcia HM, Buszman P, et al. Effects of the direct-lipoprotein associated phospholipase A2 inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–82. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 80.Wilensky RL, Shi Y, Mohler ER, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nature Med. 2008;14:1059–66. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White HD, Held C, Stewart R, et al. Darapladib for preventing ischemic events in stable coronary disease. N Eng J Med. 2014;370:1702–11. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 82.Tawakol A, Singh P, Rudd JHF, et al. Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 Inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. JACC. 2014;63:86–91. doi: 10.1016/j.jacc.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 83.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multi-center FDG-PET/CT feasibility study. J Am Coll Cardiol. 2013;62:909–17. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 84.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–13. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 85.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 86.Rosenson RS, Fraser H, Gould M, Hislop C. Anti-inflammatory effects of vares pladib methyl in diabetic patients with acute coronary syndrome. Cardiovasc Drugs Ther. 2011;25:539–44. doi: 10.1007/s10557-011-6344-2. [DOI] [PubMed] [Google Scholar]
- 87.Dzavik V, Lavi S, Thorpe K, et al. The sPLA2 inhibition to decrease enzyme release after percutaneous coronary intervention (SPIDER-PCI) trial. Circulation. 2010;122:2411–8. doi: 10.1161/CIRCULATIONAHA.110.950733. [DOI] [PubMed] [Google Scholar]
- 88.Johnson A, Zalewski A, Janmohamed S, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) activity, an emerging CV risk marker, can be inhibited in atherosclerotic lesions and plasma by novel pharmacologic intervention: the results of a multicenter clinical study. Circulation. 2004;110:III–590. [Google Scholar]
- 89.Mohler ER, 3rd, Ballantyne CM, Davidson MH, et al. Darapladib Investigators The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–41. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]