Abstract
Background:
Sleep disturbances and insufficient sleep are highly prevalent. Both clinical sleep disorders and multiple forms of experimental sleep loss predict heightened inflammation. As such, it is necessary to investigate potential protective factors. Given that trait positive affect (PA) is associated with reduced inflammation, and buffers the proinflammatory effects of stress, it is possible that high trait positive affect might protect individuals from an inflammatory response to sleep disruption. The present study tested this hypothesis in an experimental sleep disruption paradigm with assessment of cellular inflammation.
Methods:
Data were drawn from good sleeping adults (n=79) who participated in a randomized, within-subjects crossover experiment comparing the effects of two nights of sleep disruption versus two nights of uninterrupted sleep. Stimulated monocytic production of intracellular proinflammatory cytokines tumor necrosis factor (TNF) and interleukin-6 (IL-6) were assayed using flow cytometric methods and indexed as the percentage of monocytes expressing TNF, IL-6, or co-expressing both. Hypotheses were evaluated using linear mixed effects models.
Results:
Controlling for negative affect, body mass index, age, and sex, PA significantly moderated the associations between sleep condition and stimulated monocyte production of IL-6 (b = −1.03, t = −2.02, p = .048) and its co-expression with TNF (b = −.93, t = −2.00, p = .049), such that inflammatory responses were blunted among those high in PA with increases principally among those low in PA. The effect on TNF was similar in terms of effect size, but marginally significant.
Conclusions:
Activation of cellular inflammation in response to sleep disruption is buffered by PA independent of negative affect. Interventions that promote PA might protect persons from the inflammatory activation following sleep loss, with the potential to mitigate the adverse health consequences of sleep disturbance.
Keywords: positive affect, experimental sleep disruption, interleukin-6, tumor necrosis factor, stimulated monocyte production
1. Introduction
Insomnia is an extensive public health problem, with approximately 25% of U.S. adults reporting insomnia complaints (i.e., difficulty initiating or maintaining sleep, waking up too early) and 10% meeting diagnostic criteria for chronic insomnia (LeBlanc et al., 2009). Although insomnia is diagnosed based on subjective complaints, approximately 50% demonstrate objective sleep curtailment defined as nightly sleep duration <6 hours (Vgontzas et al., 2009). Epidemiological data suggest that insomnia predicts chronic disease risk and all-cause mortality (Finan et al., 2013; Kripke et al., 2002; Sofi et al., 2014), and point towards increased inflammation following sleep disruption as one underlying biological mechanism (Irwin, 2015, 2019). Insomnia is associated with increased inflammation (as reviewed by Irwin, 2019), and several studies implicate curtailed sleep as a major factor underlying this link (Fernandez-Mendoza et al., 2017; Floam et al., 2015; Syauqy et al., 2019), leading to investigations into the effects of experimental sleep deprivation on proinflammatory cytokine function. Partial sleep deprivation has been shown in human experimental models to activate the cellular expression of interleukin-6 (IL-6) and tumor necrosis factor (TNF), as well as upregulate inflammatory gene expression (Irwin, 2006). In addition, prolonged experimental sleep restriction promotes increased cellular IL-6 production, which fails to normalize following recovery sleep (Simpson et al., 2016). Further, several experimental studies have reported elevations in circulating levels of TNF, IL-6 and C-reactive protein (CRP) following sleep deprivation (Irwin et al., 2004; Meier-Ewert et al., 2004; Shearer et al., 2001). Although null findings have also been reported (e.g., Abedelmalek et al., 2013; Schmid et al., 2011; Stamatakis and Punjabi, 2010), there is strong experimental evidence corroborated by naturalistic data (Erten et al., 2005; Friedman et al., 2005) suggesting an influence of sleep disruption on inflammatory mechanisms (Irwin, 2019). Given the high prevalence and health risks conferred by insomnia, particularly in the context of objectively shortened sleep, it is important to identify protective factors that may buffer the detrimental effects of sleep disruption with partial sleep loss on inflammation.
Trait positive affect (PA), defined as the tendency to experience pleasurable emotions such as joy, enthusiasm, and contentment (Pressman & Cohen, 2005), has been the subject of emerging research exploring its broad health-protecting influence (see Pressman & Cohen, 2005) and favorable impact on inflammatory processes (Stellar et al., 2015; A Steptoe et al., 2005; Andrew Steptoe et al., 2007), which extends the well-documented detrimental effects of negative affect (NA; defined as the tendency to experience distressing emotions like anger and sadness) on inflammation (Slavich & Irwin, 2014). Importantly, PA has been characterized as partially independent from negative affect (Keyes, 2005; Ryff et al., 2006) and not merely its opposite (Tellegen et al., 1999). Moreover, trait PA can be reliably increased through psychosocial intervention (Fredrickson et al., 2008; Lyubomirsky et al., 2005, 2011) underscoring its clinical relevance.
Individuals higher in PA show more favorable inflammatory profiles relative to those lower in PA. Specifically, higher PA is associated with lower IL-6 and CRP among healthy adults (Prather et al., 2007; Stellar et al., 2015; Andrew Steptoe et al., 2008; Sturgeon et al., 2016), and with lower levels of other multiple inflammatory biomarkers in clinical samples (Brouwers et al., 2013). These data accord with theory linking PA and health. The “main effects model” (Pressman and Cohen, 2005, Pressman et al., 2019) characterizes PA as a factor exerting direct effects on health by reducing tonic levels of health-degrading biological processes (e.g., inflammation). It is not known if trait PA protects against the proinflammatory effects of sleep disturbance; however, research suggests that it can buffer the inflammatory consequences of other pathophysiological perturbations (e.g., stress; Steptoe et al., 2005; Morozink et al., 2010).
1.1. The present study
In line with theory and empirical data suggesting a protective role of PA, the purpose of the present study was to test the degree to which PA might moderate (i.e., buffer) the effect of experimental sleep disruption on cellular markers of inflammation, after adjusting for NA and relevant demographic characteristics. Data were drawn from a racially diverse sample of healthy, good sleeping adults who participated in a randomized, within-subjects crossover experiment comparing the effects of two nights of sleep continuity disruption (i.e., a forced awakening [FA] protocol versus two nights of normal, usual sleep [US]). The FA protocol is designed to acutely model the type of partial sleep loss profile commonly observed in severe insomnia, i.e., multiple and sometimes prolonged awakenings distributed throughout the night (Smith et al., 2019). In this investigation, we sought to evaluate whether PA moderates the effects on cellular inflammation. Specifically, we evaluated TNF and IL-6 using flow cytometric methods, reporting percentage of monocytes expressing TNF or IL-6, or co-expression of TNF and IL-6. We examined monocytes because these cells are the principal cells in peripheral circulation that respond to an inflammatory insult with early production of pro inflammatory cytokines. We hypothesized that individuals lower in PA would demonstrate greater increases in stimulated intracellular production of all three markers following FA relative to those high in PA.
2. Methods
Hypotheses were evaluated using data drawn from a larger parent study (NCT01794689) investigating inflammation as a potential mechanism underlying alterations in morphine analgesia in response to experimental pain after sleep continuity disruption. The parent study was designed to investigate intracellular cytokine expression, rather than basal levels, due to systematic review findings indicating that brief experimental sleep disruption alters intracellular cytokine production but not circulating levels (Irwin et al., 2016). It appears that multiple nights of sleep loss are needed to induce reliable increases in systemic markers of inflammation (Irwin 2019). The present study is distinct from the parent project in that it does not explore pain-related phenomena, but rather investigates the degree to which PA moderates the direct effect of sleep continuity disruption on inflammation. The methods pertaining to the present analysis are described here; the full parent project methodology has been described elsewhere (Smith et al., under review). All methods were approved by the University of California, Los Angeles and Johns Hopkins University Institutional Review Boards. Participants provided written informed consent prior to participation.
2.1. Participants
Data were drawn from healthy, good sleeping adults (N = 79). Of the 100 participants that were randomized in the parent project, 81 contributed valid data on stimulated monocytic production of inflammatory markers in response to sleep manipulation (see Study Design). Of these, two cases were removed due to invalid trait affect data, yielding the 79 included cases. Participants were demographically diverse (see Table 1). Eligibility procedures and inclusion/exclusion criteria are presented in Supplemental Material.
Table 1.
Sample Demographic Characteristics
| Total n | 79 | |
| Age, mean (SD) | 27.8 (6.4) | |
| Female, n (%) | 43 | 54.4% |
| Race, n (%) | ||
| BMI, mean (SD) | 25.7(3.8) | |
| African American | 26 | 32.9% |
| Asian | 11 | 13.9% |
| White | 36 | 45.6% |
| Multiracial | 3 | 3.8% |
| Other | 2 | 2.5% |
| Unknown | 1 | 1.3% |
| Highest Degree Completed, n (%) | ||
| Graduate | 16 | 20.3% |
| Undergraduate | 37 | 46.8% |
| Technical School | 1 | 1.3% |
| High School | 24 | 30.4% |
| Unknown | 1 | 1.3% |
| Employment Status, n (%) | ||
| Homemaker | 1 | 1.3% |
| Student | 31 | 39.2% |
| Unemployed | 10 | 12.7% |
| Working for pay, full-time | 18 | 22.8% |
| Working for pay, part-time | 18 | 22.8% |
| Unknown | 1 | 1.3% |
Note. Includes participants providing blood samples at X2.
2.2. Study Design
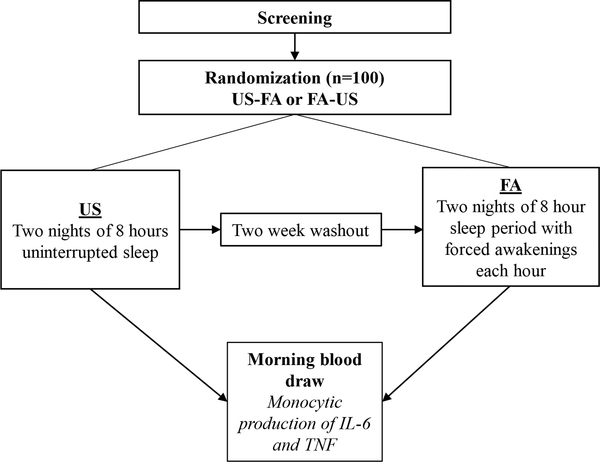
The study employed a within-subjects crossover design. Participants were randomly assigned to the order of sleep condition (i.e., US first or FA first) using a randomization scheme stratified on sex, body mass index (25> vs. ≤25), and age (18–32 vs. 33–48) given their impact on inflammation. Trait PA and NA scores were obtained during the screening phase prior to randomization. Participants were admitted to the Clinical Research Unit (CRU) with the first night serving to rule out occult sleep disorders and as an adaption to polysomnographic monitoring, and then completed either two consecutive nights of FA or US. Seven blood samples were drawn on the morning following the second sleep manipulation night as part of an extended procedure involving experimental pain induction, opioid administration, and quantitative sensory testing, which was employed pursuant to the parent study aims. In this study we analyzed markers obtained from the first and second of the 7 blood draws, which indicate baseline cellular inflammatory responses to sleep manipulation (as opposed to responses to sleep manipulation coupled with induced pain and/or opioids), henceforth termed X1 and X2. These baseline draws were respectively completed at approximately 07:30 and 08:30. Two baseline draws were obtained to establish a stable baseline consistent with recommended practices (Irwin et al., 2006; Korn et al., 1984). Analyses presented below were conducted separately for X1 and X2 samples.
After a minimum two-week washout period, participants returned to the CRU to complete the other sleep condition, which did not include an adaptation night prior to the two nights of sleep manipulation (i.e., FA vs. US). The flow of study procedures is shown visually in Figure 1.
Figure 1.
Participant flow through the protocol.
2.3. Sleep Procedures
Participants were required to remain in the CRU during sleep conditions to protect their safety and maximize the integrity of experimental conditions. Participants were prohibited from napping outside designated sleep periods, which occurred between 23:00 to 7:00, and also from using caffeine, nicotine and alcohol. Heart-healthy food options were provided (i.e., low in fat and sodium, not fried), and lunch was usually served around 12 pm. Polysomnography (PSG) monitoring was maintained throughout both sleep conditions to assess sleep architecture.
2.3.1. US Procedures
Participants were given an opportunity to sleep undisturbed for an 8-hour period.
2.3.2. FA Procedures
Participants experienced partial sleep deprivation via a standardized FA protocol that our group developed previously (Smith et al., 2007) which is designed to be an experimental model of the type of sleep disruption experienced by patients with insomnia (i.e., multiple awakenings throughout the night and a loss of total sleep time). Specifically, 7 of the 8 sleeping hours were divided into thirds, and one 20-minute interval from each hour was randomly selected as a FA period, during which nurses awakened participants and ensured they stayed awake during the interval. Further, one of the 8 hours was randomly selected as a 60-minute FA period, during which participants were kept awake throughout. Participants were encouraged to sit upright in bed and the lights were kept on to minimize the likelihood of microsleep. The maximum possible total sleep time was 280 minutes.
2.4. Blood Draw Procedures
Approximately 30 minutes after participants awoke after two nights of sleep manipulation (at approximately 07:30), a CRU nurse inserted a single port lumen peripheral catheter in an upper extremity vein and obtained the first blood sample. The second sample was collected at approximately 830h. A continuous saline infusion at 250cc/h was maintained that was flushed before and after all blood draws. Samples were drawn and immediately transported for preparation by a research technician.
2.5. Measures
2.5.1. Positive and Negative Affect
Trait affect was quantified using the positive and negative affect scale scores obtained with the Positive and Negative Affect Schedule-Expanded Form (PANAS-X; Watson & Clark, 1999). The PA and NA scales are each comprised of 10 adjectives describing various emotions. Respondents are asked to indicate the degree to which they experienced each emotion within the past several weeks on Likert-type scales (1 = “very slightly or not at all”; 5 = “extremely”). The construct validity of the PANAS-X PA and NA scales has been well-established in community samples, while items on each scale have shown strong internal consistency (Watson & Clark, 1999).
2.5.2. Inflammation
Lipopolysaccharide stimulated monocyte production of intracellular inflammatory cytokines TNF and IL-6 was evaluated using flow cytometric methods, and the percentage of monocytes expressing TNF or IL-6, or co-expression of TNF and IL-6 was calculated, as previously described (Irwin et al., 2006, 2016). We also provide details in Supplemental Material.
2.5.3. Demographics
Participants self-reported their sex, race, ethnicity, employment status and education level during screening. BMI was calculated using in-person measurements of height and weight.
2.6. Statistical Approach
Relationships between PA, sleep condition (FA vs. US) and stimulated monocyte expression of TNF, IL-6, and the co-expression TNF and IL-6 were explored using linear mixed models allowing for random intercepts in accord with recommended procedures in longitudinal analysis (Singer & Willett, 2009). Random slopes were tested as well, but models did not converge, and so random slopes were not included in final analyses. PA, sleep condition and their interaction were included as fixed predictors, while NA, sex and BMI were included as fixed covariates. Given that hypotheses were tested with interaction terms involving a level 2 variable (PA) and a level 1 variable (sleep condition), to adequately control for negative affect, we similarly entered it as an interaction term with sleep condition. Models were specified using the lme4 and lmerTest packages in R (Bates et al., 2015; Kuznetsova et al., 2017) using restricted maximum likelihood estimation. Significance values were computed using Satterthwaite’s method (see Kuznetsova et al., 2017). For each model we report the marginal R2 (a pseudo R2 reflecting the proportion of outcome variance explained by the fixed effects), as well as the conditional R2, which indicates the amount explained by both fixed and random effects (Nakagawa et al., 2017). Significant interactions were probed using the Johnson-Neyman method with the interactions R package (Long, 2019). The Johnson-Neyman technique presents the range of moderator values in which the slope of the focal predictor (in this case, sleep condition) is and is not significant at a specified alpha level (Bauer et al., 2005). We estimated the magnitude (i.e., effect size) of the interaction effect in each model by examining the percent reduction in level-1 variance with the addition of the PA by Sleep interaction term, relative to an identical model but with PA entered as a main effect.
3. Results
3.1. Sleep Manipulation Check
Averaged across the two nights, FA predicted fewer minutes of total sleep time, Stage N1, N2, N3, and REM compared with US (p’s < .001), indicating that the experimental manipulation effectively reduced total sleep time and altered sleep architecture. Full results of this comparison are shown in Table S1.
3.2. Cytokine Analyses
Distributions on all outcomes approximated normality and contained no outliers. Variances on all outcomes were equal across groups (US and FA) as suggested by Barlett’s test (p’s > .05). Primary analyses were conducted separately for the X1 and X2 samples. Time (i.e., X1 vs X2) did not interact with sleep condition to predict any inflammatory marker (p’s > .05), and so we examined the effect of Time separately within each sleep condition. Following US, monocytic production of all markers increased from X1 to X2 (p’s = .05 for IL-6, <.001 for TNF as well as its co-expression with IL-6). Following FA, there were no significant changes in monocytic production of cytokines from X1 to X2 (full statistics available in Supplemental Tables 2 and 3). Overall, primary models described below produced findings that were similar in direction at X1 and X2, but effect sizes were consistently more robust at X2 across markers. Below, we detail the X2 findings, and summarize the sleep condition x PA results at X1 with full statistics available in Supplemental Material (Supplemental Tables 4–6).
3.2.1. Interleukin-6
At the first resting blood draw (X1), there was a trend toward increased monocytic IL-6 production following FA relative to US (p = .06). PA did not significantly moderate the effect of Sleep Condition in those models. Full statistics are available in Supplementary Table 4.
At the second resting blood draw (X2), monocytic production of IL-6 did not significantly increase on average following FA relative to US (see Table 2, Model 1; p = .19). However, there was a significant PA X Sleep Condition interaction effect, indicating that monocytic production of IL-6 increased from US to FA selectively among those low in PA, after adjusting for NA, age, sex and BMI (p = .048; Table 2, Model 4) (see Figure 2A). Specifically, the Johnson-Neyman method indicated that when PA was less than 31.6 (observed PA values ranged from 14 to 50), sleep disruption induced an increase in monocytic production of IL-6 (p < .05). To illustrate (see Figure 2A), at one standard deviation below the mean for PA (PA = 27.2, which falls within the significant Johnson-Neyman interval), sleep disruption was associated with increased IL-6 (b = 11.57, t = 2.38, p = .02), whereas at one standard deviation above the mean for PA (PA = 41.4), there was no association between sleep disruption and monocytic production of IL-6 (b = −2.66, t = −0.55, p = .58). For perspective, a mean trait PA score of 32 (SD = 7.2) has been reported as normative in young adult men and women living in the United States (Watson et al., 1988); thus, PA values in this sample were consistent with population norms. In the final model, the fixed effects explained 8% of the variance in IL-6, and the PA by Sleep interaction contributed to a 3% reduction in the level-1 variance component (as shown by comparing Model 3 with Model 4). Of note, there was no association between PA and IL-6 production under control (i.e., usual sleep) conditions (b = 0.40, t = 0.97, p = .33) adjusting for NA, sex, age and BMI, suggesting that IL-6 production was not already higher in a tonic sense among those low in PA.
Table 2.
IL-6 Expression
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | CI (95%) | P-Value | Estimates | CI (95%) | P-Value | Estimates | CI (95%) | P-Value | Estimates | CI (95%) | P-Value |
| Intercept | 46.03 | 42.22 – 49.85 | <0.001 | 43.84 | 38.85 – 48.82 | <0.001 | 65.00 | 23.82 – 106.17 | 0.003 | 42.91 | −3.22 – 89.05 | 0.071 |
| Sleep (US v. FA) | 4.43 | −2.08 – 10.93 | 0.187 | −0.15 | −22.30 – 22.01 | 0.990 | 43.27 | −4.33 – 90.87 | 0.079 | |||
| Sex | 1.55 | −6.50 – 9.60 | 0.707 | 1.31 | −6.68 – 9.29 | 0.749 | ||||||
| Age | 0.35 | −0.29 – 0.99 | 0.290 | 0.39 | −0.25 – 1.02 | 0.236 | ||||||
| BMI | −1.08 | −2.11 – −0.04 | 0.046 | −1.04 | −2.07 – −0.02 | 0.050 | ||||||
| Negative Affect | 0.04 | −1.23 – 1.31 | 0.948 | 0.34 | −0.94 – 1.63 | 0.603 | ||||||
| Positive Affect | −0.13 | −0.74 – 0.48 | 0.686 | 0.35 | −0.41 – 1.11 | 0.371 | ||||||
| Sleep by Negative Affect | 0.33 | −1.23 – 1.89 | 0.676 | −0.28 | −1.93 – 1.37 | 0.738 | ||||||
| Sleep by Positive Affect | −1.03 | −2.03 – −0.03 | 0.048 | |||||||||
| Random Effects | ||||||||||||
| σ2 | 362.00 | 360.07 | 361.42 | 350.26 | ||||||||
| τ00 | 82.87 id | 82.20 id | 82.10 id | 83.39 id | ||||||||
| ICC | 0.19 | |||||||||||
| N | 79 id | 79 id | 78 id | 78 id | ||||||||
| Observations | 135 | 135 | 134 | 134 | ||||||||
| Marginal R2 / Conditional R2 | 0.000 / 0.186 | 0.011 / 0.195 | 0.057 / 0.231 | 0.080 / 0.257 | ||||||||
Notes. σ2 = level 1 variance; τ00 = between subject, random intercept variance; ICC = intraclass correlation coefficient
Figure 2.
Effect Modification by Positive Affect
3.2.2. Tumor Necrosis Factor
At the first resting blood draw (X1), there was a significant, positive effect of sleep condition (FA) on monocytic production of TNF (p = .02). PA did not significantly moderate the effect of Sleep Condition in those models. Full statistics are available in Supplementary Table 3.
At the second resting blood draw (X2), there was no main effect of sleep condition (p = .43; see Table 3, Model 2). The PA X Sleep Condition interaction failed to reach statistical significance after adjusting for NA, age, sex and BMI (p = .075; see Table 3, Model 4), but the pattern was similar to that of IL-6 such that monocytic production of TNF trended upwards following FA among those low in PA. To illustrate (see Figure 2B), at one standard deviation below the PA mean, sleep disruption marginally predicted increased monocytic production of TNF (b = 8.16, t = 1.97, p = .049), whereas sleep disruption and monocytic production of TNF were unassociated at one standard deviation above (b = −2.74, t = −0.67, p = .51). The fixed effects explained 11% of the variance in monocytic production of TNF. As shown by comparing Model 3 with Model 4, the PA by Sleep interaction was associated with a 2% reduction in level 1 variance. There was no association between PA and TNF production under control (i.e., usual sleep) conditions (b = −0.04, t = −0.11, p = .91) adjusting for NA, sex, age and BMI, suggesting that TNF production was not already higher in a tonic sense among those low in PA.
Table 3.
TNF Expression
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimate | CI (95%) | P-Value | Estimate | CI (95%) | P-Value | Estimate | CI (95%) | P-Value | Estimate | CI (95%) | P-Value |
| Intercept | 49.15 | 45.47 – 52.82 | <0.001 | 48.04 | 43.47 – 52.60 | <0.001 | 71.26 | 32.97 – 109.54 | 0.001 | 54.28 | 12.10 – 96.46 | 0.013 |
| Sleep (US v. FA) | 2.25 | −3.25 – 7.74 | 0.426 | −3.96 | −22.65 – 14.73 | 0.680 | 29.37 | −11.17 – 69.90 | 0.161 | |||
| Sex | 6.65 | −0.86 – 14.16 | 0.087 | 6.46 | −0.99 – 13.90 | 0.094 | ||||||
| Age | 0.25 | −0.34 – 0.85 | 0.405 | 0.29 | −0.30 – 0.88 | 0.345 | ||||||
| BMI | −0.78 | −1.74 – 0.19 | 0.118 | −0.75 | −1.71 – 0.20 | 0.128 | ||||||
| Negative Affect | 0.04 | −1.10 – 1.18 | 0.946 | 0.27 | −0.89 – 1.43 | 0.648 | ||||||
| Positive Affect | −0.41 | −0.98 – 0.16 | 0.159 | −0.05 | −0.74 – 0.64 | 0.884 | ||||||
| Sleep by Negative Affect | 0.49 | −0.83 – 1.80 | 0.472 | 0.01 | −1.39 – 1.41 | 0.990 | ||||||
| Sleep by Positive Affect | −0.79 | −1.64 – 0.06 | 0.075 | |||||||||
| Random Effects | ||||||||||||
| σ2 | 247.45 | 250.21 | 254.30 | 249.34 | ||||||||
| τ00 | 127.20 id | 124.59 id | 105.77 id | 104.50 id | ||||||||
| ICC | 0.34 | |||||||||||
| N | 79 id | 79 id | 78 id | 78 id | ||||||||
| Observations | 135 | 135 | 134 | 134 | ||||||||
| Marginal R2 / Conditional R2 | 0.000 / 0.340 | 0.003 / 0.335 | 0.094 / 0.360 | 0.110 / 0.373 | ||||||||
Notes. σ2 = level 1 variance; τ00 = between subject, random intercept variance; ICC = intraclass correlation coefficient
3.2.3. Tumor Necrosis Factor and Interleukin-6 Co-Expression
At the first resting blood draw (X1), there was a significant effect of sleep condition on co-expression of TNF and IL-6 (p = .04). PA did not significantly moderate the effect of Sleep Condition in those models. Full statistics are available in Supplementary Table 4.
At the second resting blood draw (X2), there was no main effect of sleep condition on the co-expression of TNF and IL-6 (p = .25; see Table 4, Model 2). However, there was a significant PA X Sleep Condition interaction, after adjusting for NA, sex, age and BMI (p = .04, see Table 4, Model 4). Compared with US, FA induced an increase in TNF and IL-6 co-expression among those low in PA. Specifically, the Johnson-Neyman method revealed that when PA was less than 30.7, there was a positive relationship between sleep disruption and the co-expression of TNF and IL-6 (p < .05). To illustrate (see Figure 2C), at one standard deviation below the mean (PA = 27.2, which falls within the significant Johnson-Neyman interval), sleep disruption predicted increased TNF and IL-6 co-expression (b = 10.21, t = 2.31, p = .02), whereas at one standard deviation above the mean (PA = 41.4), sleep disruption was unassociated with the co-expression of TNF and IL-6 (b = −2.66, t = −0.61, p = .55). The fixed effects explained 11% of the outcome variance. As shown by comparing Model 3 and Model 4, the PA by Sleep interaction contributed to a 3% reduction in the level 1 variance component. There was no association between PA and the co-expression of TNF and IL-6 under control (i.e., usual sleep) conditions (b = 0.15, t = 0.41, p = .68) adjusting for NA, sex, age and BMI, suggesting that TNF and IL-6 coproduction was not already higher in a tonic sense among those low in PA.
Table 4.
TNF and IL-6 Co-Expression
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Estimate | CI (95%) | P-Value | Estimate | CI (95%) | P-Value | Estimate | CI (95%) | P-Value | Estimate | CI (95%) | P-Value |
| Intercept | 33.54 | 30.08 – 37.00 | <0.001 | 31.79 | 27.26 – 36.32 | <0.001 | 64.80 | 28.36 – 101.24 | 0.001 | 44.88 | 3.82 – 85.94 | 0.035 |
| Sleep (US v. FA) | 3.54 | −2.39 – 9.46 | 0.246 | −4.78 | −24.92 – 15.35 | 0.643 | 34.41 | −8.82 – 77.65 | 0.124 | |||
| Sex | 4.09 | −3.03 – 11.21 | 0.264 | 3.87 | −3.18 – 10.93 | 0.286 | ||||||
| Age | 0.29 | −0.27 – 0.86 | 0.310 | 0.33 | −0.23 – 0.89 | 0.254 | ||||||
| BMI | −1.07 | −1.99 – −0.15 | 0.025 | −1.04 | −1.95 – −0.13 | 0.028 | ||||||
| Negative Affect | −0.35 | −1.48 – 0.78 | 0.546 | −0.08 | −1.23 – 1.07 | 0.892 | ||||||
| Positive Affect | −0.32 | −0.86 – 0.22 | 0.252 | 0.11 | −0.57 – 0.79 | 0.747 | ||||||
| Sleep by Negative Affect | 0.63 | −0.79 – 2.04 | 0.390 | 0.07 | −1.43 – 1.57 | 0.926 | ||||||
| Sleep by Positive Affect | −0.93 | −1.84 – −0.02 | 0.049 | |||||||||
| Random Effects | ||||||||||||
| σ2 | 297.75 | 298.47 | 299.59 | 290.61 | ||||||||
| τ00 | 68.29 id | 66.27 id | 54.54 id | 55.65 id | ||||||||
| ICC | 0.19 | 0.18 | 0.15 | 0.16 | ||||||||
| N | 79 id | 79 id | 78 id | 78 id | ||||||||
| Observations | 135 | 135 | 134 | 134 | ||||||||
| Marginal R2 / Conditional R2 | 0.000 / 0.187 | 0.009 / 0.189 | 0.085 / 0.226 | 0.108 / 0.251 | ||||||||
Notes. σ2 = level 1 variance; τ00 = between subject, random intercept variance; ICC = intraclass correlation coefficient
4. Discussion
Consistent with hypotheses, results suggested that trait PA buffers the cellular inflammatory response to sleep disruption, independent of NA. Specifically, we observed that two nights of sleep continuity disruption led to increased cellular inflammatory responses selectively among individuals low in trait PA, even after accounting for NA, sex, age and BMI. This pattern was observed in all three markers tested (i.e., IL-6, TNF, TNF their co-expression). Effect sizes across markers were similar, despite marginal statistical significance in TNF (p = .075). Empirical data suggest that people higher in PA tend to live longer (Ostir et al., 2000; see Cohen & Pressman, 2006) and have better overall physical health than those lower in PA (see Cohen & Pressman, 2006). These findings add to that broad body of work, raising the possibility that PA may confer resilience to the inflammatory consequences of disrupted sleep.
The present findings also add to a growing body of evidence supporting the unique role of PA (i.e., beyond NA) among individuals experiencing sleep disruption. Experimental evidence from our laboratory suggests that acute sleep continuity disruption selectively attenuates next-day positive but not negative affective functioning (Finan et al., 2017) which is corroborated by naturalistic studies documenting detrimental effects of sleep disruption on next-day PA and not NA (Bower et al., 2010). Relatedly, use of the emotion regulation strategy positive reappraisal, which commonly leads to increased PA (Folkman & Moskowitz, 2000), buffered the effect of short total sleep time on cellular IL-6 response to acute marital conflict (Wilson et al., 2017). The present findings provide another perspective on the importance of improving PA in the context of sleep difficulties: sleep disruption can lead not only to reduced daytime PA, but also to increased inflammation among those lacking positive affective resources. There has already been an interest in applying positive psychological interventions to insomnia on the basis that patients with insomnia are often anhedonic (Harvey et al., 2009), and initial studies investigating the efficacy of mindfulness-based interventions among patients with insomnia support their ability to enhance PA (Ong et al., 2018), as well as reduce inflammation (Black et al., 2015; Irwin et al., 2015). Of note, negative affect did not modify the effect of sleep disruption on cellular inflammation in our study, underscoring the unique relevance of PA.
The present findings encourage investigations on whether psychological interventions with the capacity to enhance PA can ameliorate the inflammatory consequences of sleep disruption among those initially lacking in PA. Further, although this study was conducted in healthy adults, these findings may have implications for individuals with insomnia. For instance, it may be important to evaluate PA in the context of insomnia treatment, as those low in PA may be disproportionately likely to experience heightened inflammation and therefore experience more health complications from disrupted sleep. It is also possible that those low in PA may be most likely to benefit from psychological interventions that enhance PA.
5. Limitations and Conclusions
Some nuances and design limitations of the present study deserve attention. First, the sleep disruption manipulation yielded significant increases in cellular inflammatory responses at the first available resting blood draw (X1) that were attenuated at the second resting blood draw (X2), which was obtained approximately 60 minutes later. This suggests that the effect of sleep disruption on cellular inflammatory response may begin to dissipate/normalize over the course of the morning hours. Indeed, prior work has shown that morning inflammatory elevations following sleep loss are relatively transient, resolving completely after a single night of normal sleep (Irwin et al., 2008). Our study was not designed to evaluate methodological questions regarding the time-variant nature of resting inflammatory states, but given the fact that the primary interaction effects (PA X Sleep Condition) were consistently observed at X2, but not X1, we speculate that the salutary benefit of trait PA may dissipate at higher levels of inflammatory insult. Future work should be designed a priori to investigate this possibility.
It is worth noting that PA and physical exercise frequency are positively associated (Cameron et al., 2017; Garcia et al., 2012), likely in a bi-directional manner (Mandolesi et al., 2018). As such, it is possible that the moderating effect of PA on the association between sleep disruption and cellular inflammation was partially due to physical activity engagement and/or physical fitness. Future studies might look more directly at the role of physical fitness and exercise in this context.
The present study was conducted with healthy adults, and it therefore remains unclear to what degree these findings may generalize to those with insomnia or other individuals with sleep disorders. Furthermore, we did not investigate the role of cortisol or stress in this or the parent study, precluding our capacity to evaluate their role in these findings. Further, although strong in internal validity, the ecological validity of the present findings needs to be established through naturalistic studies. Finally, the biological mechanisms underlying the protective effect of PA observed in this study could not be ascertained. One way PA is thought to favorably impact the immune system is by dampening sympathetic nervous system activation (Marsland et al., 2007), which escalates when sleep is disrupted (see Irwin, 2015). Further research on this topic is needed.
A strength of the present study is its identification of a modifiable vulnerability factor (i.e., low PA) in the context of a rigorous experimental sleep disruption design. Findings point towards potential clinical implications of PA interventions to improve health outcomes in the context of disrupted sleep. Findings encourage further investigation on whether a buffering effect on the inflammatory consequences of sleep disruption is a pathway through which PA supports better health over time.
Supplementary Material
Highlights.
Cellular inflammation increased after experimental sleep disruption only among people low in positive affect
Positive affect is protective independent of negative affect and demographic factors
Negative affect did not modify the effect of sleep disruption on cellular inflammation
Acknowledgments
Source(s) of Financial Support for the Project: NIH K23 DA035915 (PHF)
NIH R01 DA0329922 (MTS, MRI); NIH T32 5T32NS070201-15 (CAH), F32 DA04939302 (CJM)
Footnotes
Conflict of Interests
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance Eur. J. Appl. Physiol, 113 (2013), pp. 241–248, 10.1007/s00421-012-2432-7 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–47. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bauer DJ, Curran PJ, & Thurstone LL (2005). Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research, 40(3), 373–400. 10.1207/s15327906mbr4003_5 [DOI] [PubMed] [Google Scholar]
- Black DS, O’Reilly GA, Olmstead R, Breen EC, & Irwin MR (2015). Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbance: A randomized clinical trial. JAMA Internal Medicine, 175(4), 494–501. 10.1001/jamainternmed.2014.8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower B, Bylsma LM, Morris BH, & Rottenberg J (2010). Poor reported sleep quality predicts low positive affect in daily life among healthy and mood-disordered persons: Sleep quality and positive affect. Journal of Sleep Research, 19(2), 323–332. 10.1111/j.1365-2869.2009.00816.x [DOI] [PubMed] [Google Scholar]
- Brouwers C, Mommersteeg PMC, Nyklíček I, Pelle AJ, Westerhuis BLWJJM, Szabó BM, & Denollet J (2013). Positive affect dimensions and their association with inflammatory biomarkers in patients with chronic heart failure. Biological Psychology, 92(2), 220–226. 10.1016/j.biopsycho.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Cameron DS, Bertenshaw EJ, & Sheeran P (2017). Positive affect and physical activity: Testing effects on goal setting, activation, prioritisation, and attainment. 10.1080/08870446.2017.1314477 [DOI] [PubMed] [Google Scholar]
- Erten Y, Kokturk O, Yuksel A, Elbeg S, Ciftci TU, Pasaoglu H, Ozkan S, Bali M, Arinsoi T, & Sindel S (2005). Relationship between sleep complaints and proinflammatory cytokines in haemodialysis patients. Nephrology, 10(4), 330–335. 10.1111/j.1440-1797.2005.00418.x [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, & Bixler EO (2017). Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain, Behavior, and Immunity. 10.1016/j.bbi.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, & Smith MT (2013). The association of sleep and pain: An update and a path forward. In Journal of Pain (Vol. 14, Issue 12, pp. 1539–1552). Churchill Livingstone. 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Quartana PJ, Remeniuk B, Garland EL, Rhudy JL, Hand M, Irwin MR, & Smith MT (2017). Partial sleep deprivation attenuates the positive affective system: effects across multiple measurement modalities. Sleep, 40(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floam S, Simpson N, Nemeth E, Scott-Sutherland J, Gautam S, & Haack M (2015). Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. Journal of Sleep Research, 24(3), 296–304. 10.1111/jsr.12259 [DOI] [PubMed] [Google Scholar]
- Folkman S, & Moskowitz JT (2000). Stress, positive emotion, and coping. Current Directions in Psychological Science 10.1111/1467-8721.00073 [DOI] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, & Finkel SM (2008). Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. Journal of Personality and Social Psychology, 95(5), 1045. 10.1037/a0013262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, & Ryff CD (2005). Social relationships, sleep quality, and interleukin-6 in aging women. PNAS, 102, 18757–18762. www.pnas.orgcgidoi10.1073pnas.0509281102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Archer T, Moradi S, & Andersson-Arntén A-C (2012). Exercise Frequency, High Activation Positive Affect, and Psychological Well-Being: Beyond Age, Gender, and Occupation. Psychology, 03(04), 328–336. 10.4236/psych.2012.34047 [DOI] [Google Scholar]
- Harvey AG, McGlinchey E, & Gruber J (2009). Toward an affective science of insomnia treatments. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment, 427–446. [Google Scholar]
- Irwin MR (2015). Why Sleep Is Important for Health: A Psychoneuroimmunology Perspective. Annual Review of Psychology, 66(1), 143–172. 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR (2019). Sleep and inflammation: partners in sickness and in health. In Nature Reviews Immunology. 10.1038/s41577-019-0190-z [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, Arevalo JMG, Ma J, Nicassio P, Bootzin R, & Cole S (2015). Cognitive Behavioral Therapy and Tai Chi Reverse Cellular and Genomic Markers of Inflammation in Late-Life Insomnia: A Randomized Controlled Trial. Biological Psychiatry, 78(10), 721–729. 10.1016/j.biopsych.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry, 80(1), 40–52. 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Rinetti G, Redwine L, Motivala S, Dang J, & Ehlers C (2004). Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain, Behavior, and Immunity, 18(4), 349–360. 10.1016/j.bbi.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, & Cole S (2006). Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Archives of Internal Medicine, 166(16), 1756–1762. 10.1001/archinte.166.16.1756 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, & Cole S (2008). Sleep Loss Activates Cellular Inflammatory Signaling. Biological Psychiatry, 64(6), 538–540. 10.1016/j.biopsych.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes CLM (2005). Mental illness and/or mental health? Investigating axioms of the complete state model of health. Journal of Consulting and Clinical Psychology, 73(3), 539–548. 10.1037/0022-006X.73.3.539 [DOI] [PubMed] [Google Scholar]
- Korn EL, Dorey F, Spina CA, & Fahey JL (1984). The Use of Three Baseline Values in Intervention Studies: Application to Evaluation of Immune Modulation Therapies. Immunobiology, 167(5), 431–436. 10.1016/S0171-2985(84)80075-3 [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, & Marler MR (2002). Mortality associated with sleep duration and insomnia. Archives of General Psychiatry, 59(2), 131–136. 10.1001/archpsyc.59.2.131 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software, 82(13). 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- LeBlanc M, Mérette C, Savard J, Ivers H, Baillargeon L, & Morin CM (2009). Incidence and risk factors of insomnia in a population-based sample. Sleep, 32(8), 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA (2019). interactions: Comprehensive, User-Friendly Toolkit for Probing Interactions. https://cran.rproject.org/package=interactions [Google Scholar]
- Lyubomirsky S, Dickerhoof R, Boehm JK, & Sheldon KM (2011). Becoming happier takes both a will and a proper way: An experimental longitudinal intervention to boost well-being. Emotion, 11(2), 391–402. 10.1037/a0022575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, Sheldon KM, & Schkade D (2005). Pursuing Happiness: The Architecture of Sustainable Change. 10.1037/1089-2680.9.2.111 [DOI] [Google Scholar]
- Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, & Sorrentino G (2018). Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. In Frontiers in Psychology (Vol. 9, Issue APR, p. 509). Frontiers Media S.A. 10.3389/fpsyg.2018.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Pressman S, & Cohen S (2007). Positive affect and immune function. In Psychoneuroimmunology, Two-Volume Set. 10.1016/B978-012088576-3/50042-3 [DOI]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, & Mullington JM (2004). Effect of sleep loss on C-Reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology, 43(4), 678–683. 10.1016/j.jacc.2003.07.050 [DOI] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample Heal. Psychol, 29 (2010), pp. 626–635, 10.1037/a0021360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Johnson PCD, & Schielzeth H (2017). The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of the Royal Society Interface, 14(134). 10.1098/rsif.2017.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JC, Xia Y, Smith-Mason CE, & Manber R (2018). A Randomized Controlled Trial of Mindfulness Meditation for Chronic Insomnia: Effects on Daytime Symptoms and Cognitive-Emotional Arousal. Mindfulness, 9(6), 1702–1712. 10.1007/s12671-018-0911-6 [DOI] [Google Scholar]
- Ostir GV, Markides KS, Black SA, & Goodwin JS (2000). Emotional Well-Being Predicts Subsequent Functional Independence and Survival. Journal of the American Geriatrics Society, 48(5), 473–478. 10.1111/j.1532-5415.2000.tb04991.x [DOI] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Muldoon MF, & Manuck SB (2007). Positive affective style covaries with stimulated IL-6 and IL-10 production in a middle-aged community sample. Brain, Behavior, and Immunity, 21(8), 1033–1037. 10.1016/j.bbi.2007.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, & Cohen S (2005). Does positive affect influence health? Psychological Bulletin, 131(6), 925. 10.1037/0033-2909.131.6.925 [DOI] [PubMed] [Google Scholar]
- Pressman SD, Jenkins BN, Moskowitz JT Positive Affect and Health: What Do We Know and Where Next Should We Go? Annu. Rev. Psychol, 70 (2019), pp. 627–650, 10.1146/annurev-psych-010418-102955 [DOI] [PubMed] [Google Scholar]
- Ryff CD, Dienberg Love G, Urry HL, Muller D, Rosenkranz MA, Friedman EM, Davidson RJ, & Singer B (2006). Psychological well-being and ill-being: do they have distinct or mirrored biological correlates? Psychotherapy and Psychosomatics, 75(2), 85–95. https://doi.org/90892 [pii] [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, O’brian Smith E, Szuba MP, Van Dongen HPA, & Dinges DF (2001). Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. Journal of Allergy and Clinical Immunology, 107(1), 165–170. 10.1067/mai.2001.112270 [DOI] [PubMed] [Google Scholar]
- Simpson NS, Diolombi M, Scott-Sutherland J, Yang H, Bhatt V, Gautam S, Mullington J, & Haack M (2016). Repeating patterns of sleep restriction and recovery: Do we get used to it? Brain, Behavior, and Immunity, 58, 142–151. 10.1016/j.bbi.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, & Willett JB (2009). Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. In Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. 10.1093/acprof:oso/9780195152968.001.0001 [DOI] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Remeniuk B, Finan PH, Speed TJ, Tompkins DA, Robinson M, Gonzalez K, Bjurstrom MF, & Irwin MR (2019). Sex differences in measures of central sensitization and pain sensitivity to experimental sleep disruption: Implications for sex differences in chronic pain. Sleep, 42(2), 1–15. 10.1093/sleep/zsy209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Cesari F, Casini A, Macchi C, Abbate R, & Gensini GF (2014). Insomnia and risk of cardiovascular disease: A meta-analysis. European Journal of Preventive Cardiology, 21(1), 57–64. 10.1177/2047487312460020 [DOI] [PubMed] [Google Scholar]
- Stamatakis KA, Punjabi NM Effects of sleep fragmentation on glucose metabolism in normal subjects Chest, 137 (1) (2010), pp. 95–101, 10.1378/chest.09-0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar JE, John-Henderson N, Anderson CL, Gordon AM, McNeil GD, & Keltner D (2015). Positive affect and markers of inflammation: Discrete positive emotions predict lower levels of inflammatory cytokines. Emotion, 15(2), 129. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, & Marmot M (2005). Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences of the United States of America, 102(18), 6508–6512. https://doi.org/0409174102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe Andrew, Leigh Gibson, E., Hamer M, & Wardle J (2007). Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology, 32(1), 56–64. 10.1016/j.psyneuen.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Steptoe Andrew, O’Donnell K, Badrick E, Kumari M, & Marmot M (2008). Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: The Whitehall II study. American Journal of Epidemiology, 167(1), 96–102. 10.1093/aje/kwm252 [DOI] [PubMed] [Google Scholar]
- Sturgeon JA, Arewasikporn A, Okun MA, Davis MC, Ong AD, & Zautra AJ (2016). The psychosocial context of financial stress: Implications for inflammation and psychological health. Psychosomatic Medicine, 78(2), 134–143. 10.1097/PSY.0000000000000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syauqy A, Chien-Yeh H, Hsiao-Hsien R, Lukas Kurniawan A, & Chao JC-J (2019). Association of sleep duration and insomnia symptoms with components of metabolic syndrome and inflammation in middle-aged and older adults with metabolic syndrome in Taiwan. Nutrients, 11(8). 10.3390/nu11081848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A, Watson D, & Clark LA (1999). On the dimensional and hierarchical structure of affect. Psychological Science, 10(4), 297–303. [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, & Vela-Bueno A (2009). Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 10.1093/sleep/32.4.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1999). The PANAS-X: Manual for the Positive and Negative Affect Schedule - Expanded Form. The University of Iowa. 10.17077/48vt-m4t2 [DOI] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Jaremka LM, Fagundes CP, Andridge R, Peng J, Malarkey WB, Habash D, Belury MA, & Kiecolt-Glaser JK (2017). Shortened sleep fuels inflammatory responses to marital conflict: Emotion regulation matters. Psychoneuroendocrinology. 10.1016/j.psyneuen.2017.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.