Abstract
Study Objectives:
The review aimed to identify the factors influencing adherence to oral appliance therapy in adults with obstructive sleep apnea.
Methods:
The protocol was initially registered with the International Register of Systematic Reviews (Prospero: CRD42019122615) prior to undertaking a comprehensive electronic search of databases and references without language and date restrictions. Quality assessment was undertaken using the Cochrane Collaboration’s risk of bias tool and Quality in Prognosis Studies (QUIPS) tool.
Results:
Studies exhibited low or unclear risk of bias for the domains assessed by the respective quality assessment tools. The influence of independent variables such as disease characteristics, patient characteristics, appliance features, and psychological and social factors on adherence levels was also assessed. There was a total of 31 included studies, which consisted of 8 randomized controlled trials, 2 controlled clinical trial, 7 prospective cohorts, 11 retrospective cohorts, and the remaining 3 studies were a case-series, case-control, and a mixed-methods. All 31 included studies were subject to qualitative analysis, with only 4 studies included in the quantitative analysis. Results of the meta-analysis demonstrated increased adherence with custom-made appliances, with a pooled mean difference of −1.34 (−2.02 to −0.66) and low levels of heterogeneity (I2 = 0%).
Conclusions:
A weak relationship was observed between objective adherence and patient and disease characteristics, such as age, sex, obesity, apnea-hypopnea index, and daytime sleepiness, to oral appliance therapy. Nonadherent patients reported more side effects with oral appliance therapy than users and tended to discontinue the treatment within the first 3 months. Custom-made oral appliances were preferred and increased adherence reported in comparison to ready-made appliances. Further research is imperative to examine the relationship between psychosocial factors and adherence to oral appliance therapy.
Citation:
Tallamaraju H, Newton JT, Fleming PS, Johal A. Factors influencing adherence to oral appliance therapy in adults with obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(7):1485–1498.
Keywords: obstructive sleep apnea, patient adherence, oral appliance therapy, dental sleep medicine
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by the repeated episodic collapse of the upper airway during sleep, with resultant sleep deprivation.1 Severe long-term effects of this disease include excessive daytime sleepiness, cognitive dysfunction, hypertension, impaired quality of life, and increased cardiovascular morbidity and mortality.2
Based on the severity of OSA there are 2 main treatment modalities, continuous positive airway pressure (CPAP) and oral appliances (OA)3. Both treatments are lifelong, with sustained adherence to treatment of paramount importance. Successful treatment may lead to improvements in quality of life, considerable cost saving to the health provider, and a reduction in the risk of motor vehicle collisions and cardiovascular disease.4
CPAP is mainly used for those with moderate to severe OSA and highly effective and regarded as the gold standard of treatment.5 However, side effects such as pressure sores, mask dislodgement, claustrophobia, air leakage, and nasal congestion have made it unpopular and intolerable among patients. According to a recent update of the American Academy of Sleep Medicine guidelines, OA therapy (OAT) can be prescribed to those with mild to moderate OSA, particularly if they express it as a preference. OAT remains the second-line treatment of choice for patients who refuse or are unable to tolerate CPAP therapy.6
OAT reduces daytime sleepiness and improves the apnea-hypopnea index (AHI) by posturing the mandible and maintaining an open pharyngeal airway.7 However, studies have consistently demonstrated that CPAP is more effective than OAT at reducing sleep-disordered breathing and achieving complete control of OSA.8 Despite the greater effect of CPAP on objective polysomnographic parameters, it does not appear to be more effective at achieving better health outcomes. It seems that the higher efficacy of CPAP is offset by greater OAT adherence. Adherence with CPAP is reportedly over 1 h/night lower than with OA.9 This discrepancy may explain why, despite the superior efficacy of CPAP, as determined by the AHI, no significant differences were observed in terms of quality of life and cognitive and functional outcomes.10
The short-term efficacy of OAT has been studied in many randomized controlled trials (RCTs), with encouraging results in all age groups.11–15 However, long-term studies report an unchanged or only minor decrease in the efficacy of OAT.16–20 Rose et al19 observed an increase in the mean AHI, from 4.2 to 8.3 events/h after 2 years of OAT. Deterioration in OSA severity and a loss of OA efficacy were found in a small sample of patients (n = 9) treated continuously for more than 15 years.21 However, OAT was reported to be effective in two-thirds of patients (n = 279) after 3 years of treatment.22 Despite the limited number of long-term studies, no significant changes appear to have been detected in the efficacy of OAT.17,18,20 Notwithstanding this, a decrease in blood pressure is reported from OAT compared with a placebo and equivalent to that of CPAP in the relatively small samples studied.24–27
Adherence with OAT has until recently been limited to self-reported data, with the inherent risks of overreporting. Based on this subjective reporting, adherence with OA therapy appears to decline over time, Hoffstein et al reported a wide range of adherence (4–76%) in the first year of appliance use23. In a further study, adherence after 1 year was 83%24 declining to 62–64% after 4 to 6 years.25,26 The ability to assess adherence objectively provides a more valid measure of a treatment modality’s effectiveness. With CPAP therapy, the presence of an inbuilt adherence monitor has provided valuable insight into the limitations of self-reported use, with patients overestimating by up to 1 hour.27 More recently, Vanderveken et al28 and Johal et al29 reported on the safety and feasibility, at 3 and 18 months, respectively, of objective measurement techniques with OAT in the same cohort of patients who demonstrated a range of sleep-disordered breathing from snoring to OSA.
Thus, the current review aims to assess the factors influencing adherence to OAT in adults with obstructive sleep apnea and the potential effectiveness of interventions to promote improved adherence.
METHODS
Following the registration of the protocol with the International Register of Systematic Review (Prospero: CRD42019122615), a systematic review of the literature was undertaken to identify studies exploring the factors influencing adherence to oral appliance therapy in patients. The search strategy was designed to access both published and unpublished materials and comprised three stages:
A search of MEDLINE Ovid and Embase to identify relevant keywords contained in the title, abstract, and subject descriptors.
Terms identified in this way and the synonyms used by respective databases were then used in an extensive search of the literature.
Reference lists and bibliographies of the articles collected from those identified in stage 2 were searched.
The initial search terms were “obstructive sleep apnea”, “oral appliance”, and “patient adherence” and “compliance.” Articles indexed in the following database with no restrictions in relation to the date of publication and language of the article were searched: Ovid, Embase, Scopus, Cochrane Library, and Web of Science. Primary authors and experts in the field of sleep and respiratory medicine were contacted. The additional literature search included Google Scholar to identify any other relevant published work. An example of the search strategy used is shown in Table S1 (92.6KB, pdf) in the supplemental material.
The title and abstracts of the studies identified were assessed independently by 2 reviewers (H.T., A.J.) and were included or excluded based on the following PEO criteria:
Population: Adults with OSA receiving oral appliance therapy
Exposure of interest: Disease characteristics, patient characteristics, appliance features, and psychological and social factors
Outcome: Adherence
Study design: Prognostic studies both retrospective or prospective observational in nature and randomized or nonrandomized controlled trials
Exclusions: Studies comparing CPAP or surgical intervention with oral appliance therapy were excluded
The first 2 reviewers (H.T., A.J.) obtained full-text reports of studies meeting the selection criteria for screening, and any disagreement was resolved by consulting a third reviewer to reach a consensus (T.N.).
Risk of bias and quality assessment in individual studies
Two authors independently assessed the risk of bias of the included studies (H.T., A.J.; Figure 1, and any disagreements were resolved by further discussion and consensus. Due to the diversity in the design of the included studies, 2 different tools were used to assess their quality. RCTs were assessed using the Cochrane Collaboration’s risk of bias tool.30 The following 5 domains were considered: random sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data, and selective reporting. The domain blinding of participants and personnel was not considered due to the nature of the questions addressed by this review. Observational studies were critically appraised using the Quality in Prognosis Studies (QUIPS) tool.31 This tool assesses the risk of bias in studies of prognostic factors and comprises 6 domains: study participation, study attrition, prognostic factor management, outcome measurement, study confounding, and statistical analysis and reporting.
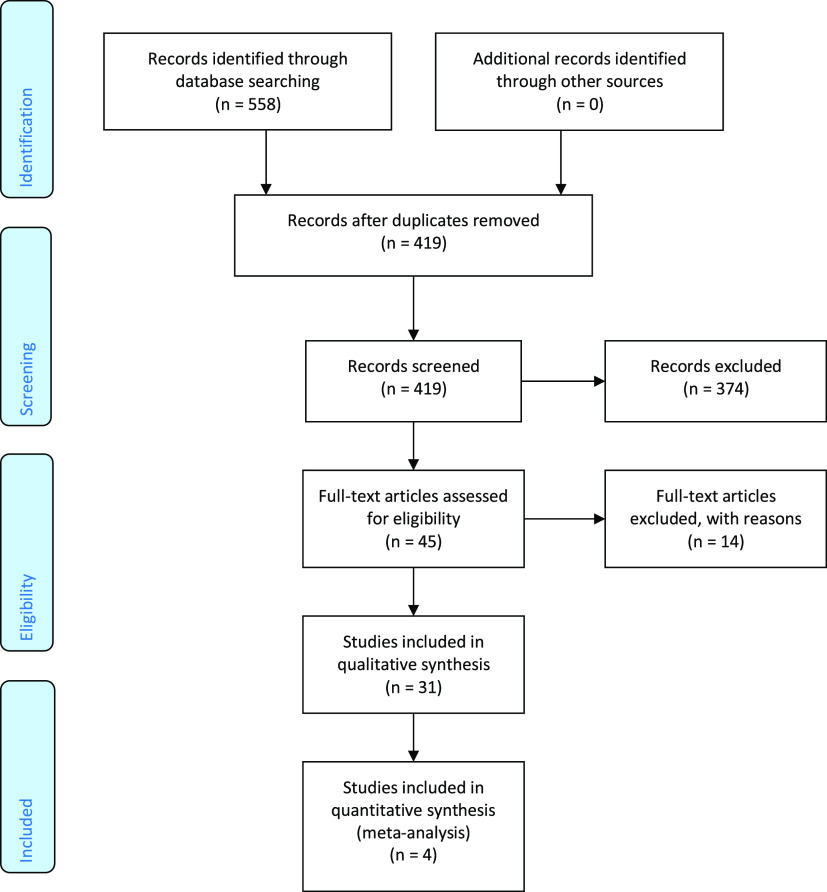
Figure 1. A PRISMA flow diagram shows the number of articles identified at each stage of the search.
Data items and collection
The influence of independent variables such as disease characteristics, patient characteristics, appliance features, and psychological and social factors on the outcome, ie, adherence, reported in the included studies was recorded and categorized based on these factors. The findings of the studies were synthesized in a narrative manner. Information regarding study design, sample size, participants and settings, type of oral appliance used, strategies or interventions employed to increase adherence, and method of adherence measurement (objective or self-reported) were recorded (Table 1 and Table 2).
Table 1.
Characteristics and principal outcomes of the included studies.
| Sr. No. | Study | Study Design | Participants & Settings | Exposure (Patient or Disease Characteristics, Type of Appliance, Psychological or Social Factor) |
Outcome (Increased/Decreased or No effect on Adherence) |
Appliance | Measurement of Adherence | Intervention for Adherence |
|---|---|---|---|---|---|---|---|---|
| 1 | Clark et al, 200057 | Retrospective observational study | Orofacial Pain & Oral medicine, University of California (n = 53, M/F: 46/7, Mean age: 55.7 y, Mean AHI < 30 events/h) | Side effects | Decreased adherence | Herbst Appliance | Self-reported | Nil |
| 2 | McGown et al, 200152 | Retrospective observational study | Middlesex Hospital, RNTNE Hospital, RLH (n = 126, Mean AHI < 30 events/h) | Patient Characteristics | No association with adherence | Modified Adjustable Silensor and Herbst Device | Self-reported | Nil |
| Side effects | Decreased adherence | |||||||
| Psychological (Self-perceived changes) and Social factors | Increased adherence | |||||||
| 3 | Rose et al, 200253 | Retrospective observational study | Respiratory Care, University Hospital of Frieburg, Germany (n = 188, M/F: 168/23, Mean age: 54.4 y) | Patient & Disease Characteristics | No association with adherence | Custom-made OA (Esmarch IPG) | Self-reported | Nil |
| Side effects | Decreased adherence | |||||||
| Psychological (Self-perceived Changes) | Decreased adherence | |||||||
| 4 | De Almeida et al, 200526 | Retrospective observational study | University of British Columbia, Canada (n = 544, M/F: 202/49, Mean age: 49.9 y, Mean AHI: 30.25 events/h) | Patient & Disease characteristics | No association with adherence | Oral Appliance | Self-reported | Nil |
| Side effects | Decreased adherence | |||||||
| Social factors (Bed partners satisfaction)* | Increased adherence | |||||||
| 5 | Izci et al, 200551 | Retrospective observational study | Department of Sleep Medicine, Edinburgh University (n = 144, M/F: 114/30, Mean age: 51 y, Mean AHI: 24 events/h) | Patient characteristics | No association with adherence | Mandibular Repositioning Splint | Self-reported | Nil |
| Psychological factors (Marital Satisfaction)** | Increased adherence | |||||||
| Side effects | Decreased adherence | |||||||
| 6 | Bates et al, 200656 | Prospective observational study | Department of Orthodontics, Victoria Hospital (n = 121, M/F: 83/38, Mean age: 49.55 y, Mean AHI: 18.21 events/h) | Side effects | Decreased adherence | Mandibular Repositioning Splint | Self-reported | Nil |
| 7 | Vanderveken et al, 200841 | Randomized Control trial | University of Antwerp, Belgium (n = 35, M/F: 29/6, Mean age: 49 y, Mean AHI: 14 events/h) | Appliance fabrication and titration procedure (Ready-made OA vs Custom-made OA) | Increased adherence with Custom-made OA | Ready-made OA (SnoreGuard Plus) and Custom-made OA | Self-reported | Nil |
| 8 | Ghazal et al, 200920 | Randomized Control trial | Respiratory Care, University Hospital of Freinburg, Germany (n = 103, M/F: 48/55, Mean age: 50.5 y, Mean AHI: 34.5 events/h) | Patient & Disease characteristics | No association with adherence | IST and Thornton Anterior Positioner (TAP) | Self-reported | Nil |
| Appliance Fabrication (IST vs TAP) | Increased adherence with IST | |||||||
| 9 | Tsuda et al, 201049 | Prospective observational study | Kyushu Dental University, Japan (n = 47, M/F: 40/7, Mean age: 53.1 y, Mean AHI: 21.3 events/h) | Patient & Disease Characteristics (BMI and ESS) | Decreased adherence in association with higher ESS and BMI | Boil- Bite Appliance (TheraSnore) | Self-reported | Nil |
| Side effects | Decreased adherence | |||||||
| 10 | Cunali et al, 201132 | Randomized Control trial | Federal University of Sao Paulo, Brazil (n = 29, M/F: 10/19, Mean age: 48.5 y, Mean AHI: 17 events/h) | Intervention- Support Therapy | Increased adherence | OA (Brazilian Repositioning device BRD) | Self-reported | Support Therapy (Mandibular Exercises) |
| 11 | Brette et al, 201246 | Prospective observational study | Antoine-Beclere & Argenteuil Hospitals (n = 140, M/F: 108/32, Mean age: 62 y, Mean AHI: 27 events/h) | Patient & Disease Characteristics | Decreased adherence | Custom-made adjustable device (OPM4 J device) | Self-reported | Nil |
| Social Support | Decreased adherence | |||||||
| Appliance characteristics | Decreased adherence | |||||||
| 12 | Freidman et al, 201255 | Case series | Advanced Centre for Specialty Care, Chicago (n = 180, M/F: 130/50, Mean age: 61.5 y, Mean AHI: 33.9 events/h) | Side effects | Decreased adherence | Ready-made OA (SomnoGuard AP) and Custom-made OA (Thornton Adjustable Positioner TAP 3) | Self-reported | Nil |
| Appliance Fabrication (Ready-made OA vs Custom-made OA) | Increased adherence with Custom-made OA | |||||||
| 13 | Zhou et al, 201239 | Randomized Control trial | Department of Orthodontics, Tongji University (n = 16, M/F: 13/3, Mean age: 45.23 y, Mean AHI: 38 events/h) | Appliance fabrication and titration procedure (Monobloc OA vs two-piece OA) | Increased adherence with Monobloc OA | Monobloc OA (Activator) and Bibloc OA (Silent Nite) | Self-reported | Nil |
| 14 | Dieltjens et al, 201354 | Case-control study | University of Antwerp, Belgium (n = 82, M/F: 56/26, Mean age: 49.5 y, Mean AHI: 18 events/h) | Psychological factors (Type D personality) | Decreased adherence | Custom-made Mono Bloc OA and Custom-made Bibloc titratable OA (RespiDent Butterfly) | Self-reported | Nil |
| 15 | Ingman et al, 201348 | Retrospective observational study | Department of Oral & Maxillofacial Diseases, Helsinki University Hospital (n = 96, M/F: 68/28, Mean age: 50.5 y, Mean AHI: 18.4 events/h) | Patient characteristics (length of the maxilla, mandible and soft palate, oropharyngeal space, crepitation at TMJ) | Increased adherence with shorter mesio-distal length of the maxilla and mandible, and crepitation at right TMJ | Mandibular Advancement Splint | Self-reported | Nil |
| 16 | Lee at al, 201335 | Nonrandomized control trial | Department of Otorhinolaryngology, Seoul National University (n = 153, M/F: 138/15, Mean age: 51.2 y, Mean AHI: 32.8 events/h) | Appliance fabrication and titration procedure (Monobloc OA vs Bibloc OA) | Increased adherence with Bibloc OA | Monobloc and Bibloc OA | Self-reported | Nil |
| 17 | Quinnell et al, 201437 | Randomized Control trial | Papworth Hospital Sleep Centre, (n = 90, M/F: 72/81, Mean age: 50.9 y, Mean AHI: 13.8 events/h) | Appliance fabrication (Boil- Bite vs Semibespoke vs Be-spoke) | Increased adherence with the Be-spoke oral appliance | Boil-bite OA (Sleep pro 1), Semibespoke OA (Sleep pro 2), and Bespoke OA | Self-reported | Nil |
| 18 | Wang et al, 201438 | Randomized Control trial | Dept. of Otorhinology, Hospital of Anhui Medical University (n = 22, M/F: 22/0, Mean age: 51.9 y, Mean AHI: 48.16 events/h) | Appliance Type (Adjustable OA vs Nonadjustable OA) | Increased adherence with the adjustable OA | Rod Type OA (Erkodent Silensor) and Controllable appliance (Twin Bloc) | Self-reported | Nil |
| 19 | Dieltjens et al, 201533 | Prospective observational study | Antwerp University Hospital, Belgium (n = 51, M/F: 38/13, Mean age: 49.3 y, Mean AHI: 14.9/h, Mean AHI: 18.4 events/h) | Patient (Anthropometric) & Disease characteristics (Polysomnographic measure) | No association with adherence | Custom-made titratable OA (RespiDent Butterfly) | Objective (Theramon Sensors) | Nil |
| Side effects | Decreased adherence | |||||||
| 20 | Prescinotto et al, 201544 | Retrospective observational study | Federal University of Sao Paulo, Brazil (n = 28, M/F: 9/19, Mean age: 48.8 y, Mean AHI; 17.5 events/h) | Patient characteristics (upper airway abnormalities) | No association with adherence | Custom-made OA | Self-reported | Nil |
| 21 | Attali et al, 201622 | Prospective observational study | Pitié-Salpétrière, France (n = 279, M/F: 98/81, Mean age: 58 y, Mean AHI: 26 events/h) | Appliance factors | Decreased adherence | Ready-made OA (Naval Resmed) and Custom-made OA (Somnodent SomnoMed) | Self-reported | Nil |
| Side effects | Decreased adherence | |||||||
| Psychological factors | Decreased adherence | |||||||
| 22 | Carballo et al, 201642 | Retrospective observational study | Veterans Affairs Medical Centre, Brazil (n = 33, M/F: 32/1, Mean age: 71.4 y) | Psychological and social factors | No association with Adherence | Oral Appliance | Self-reported | Nil |
| 23 | Makihara et al, 201658 | Retrospective observational study | Kyushu Dental University, Japan (n = 48, M/F: 35/13, Mean age: 64.9 y) | Side effects | Decreased adherence | Boil- Bite Appliance (TheraSnore) | Self-reported | Nil |
| Psychological factors | Decreased adherence | |||||||
| 24 | Nerfeldt et al, 201643 | Prospective intervention study | Department of Clinical Science, Karolinska Institute Stockholm, Sweden (n = 66, M/F: 37/35, Mean Age: 48.5 y, Mean AHI: 16 events/h) | Disease Characteristics (Arousers vs Desaturaters) | Increased adherence in arousers | Monobloc titratable OA | Self-reported | Nil |
| 25 | Vecchierini et al, 201650 | Prospective intervention study | Multicenter (n = 369, M/F: 273/96, Mean age: 52.6 y, Mean AHI: 29.5 events/h) | Side effects | Decreased adherence in the early stages of the treatment | Custom-made OA (Narval) | Self-reported | Nil |
| 26 | Al-Dharrab et al, 201736 | Randomized Control trial | Faculty of Dentistry, King Abdul-Aziz University (n = 12, M/F: 2/10, Mean age: 46 y, Mean AHI: 26 events/h) | Appliance fabrication and titration procedure (Titratable vs Nontitratable) | Increased adherence with Titratable appliance | Custom-made titratable OA (Foresta Dent, Bite Jumping screw) and nontitratable OA | Self-reported | Nil |
| 27 | Gagnadoux et al, 201734 | Nonrandomized control trial | University of Angers and Saint-Antoine Hospital, France (n = 158, M/F: 104/54, Mean age: 54 y, Mean AHI: 27.7 events/h) | Appliance fabrication and titration procedure (Ready-made OA vs Custom-made OA) | Increased adherence with Custom-made OA | Ready-made OA (BluePro) and Custom-made OA (Somnodent and Amo Device) | Self-reported | Nil |
| 28 | Haviv et al, 201759 | Mixed-methods | Department of Oral Medicine, Hebrew University (n = 52, M/F: 48/4, Mean age: 56.75 y, Mean AHI ≤ 40 events/h) | Side effects | Decreased adherence | Herbst Device | Self-reported | Nil |
| Psychological factors | Decreased adherence | |||||||
| 29 | Johal et al, 201740 | Randomized Control trial | Royal London Dental Hospital, Queen Mary University of London, (n = 35, M/F: 21/14, Mean age: 44.9 y, Mean AHI: 13.3 events/h) | Appliance fabrication and titration procedure (Ready-made OA vs Custom-made OA) | Increased adherence with Custom-made OA | Ready-made OA (Snoreshield) and Custom-made OA | Self-reported | Nil |
| 30 | Nishigawa et al, 201747 | Retrospective observational study | Department of General Dentistry, Tokushima University Hospital Japan (n = 40, M/F: 28/12, Mean age: 57.8 y) | Side effects | Decreased adherence | Herbst Appliance | Self-reported | Nil |
| Psychological factors | Decreased adherence | |||||||
| 31 | Saglam-Aydinatay et al, 201845 | Retrospective observational study | Department of Orthodontics, Hacettepe University, Ankara, Turkey (n = 69, M/F: 52/17, Mean age: 54.4 y, Mean AHI < 30 events/h) | Patient & Disease Characteristics | No association with adherence | Monobloc OA and Twin-bloc OA | Self-reported | Nil |
| Side effects | Decreased adherence | |||||||
| Psychological (Self-perceived changes) and social factors | Increased adherence |
*Improvement reported by the partner in the patient's snoring. ** Marital quality and bed sharing. AHI = apnea-hypopnea index, BMI = body mass index, F = female, M = male, OA = oral appliance, TAP = Thornton anterior positioner.
Table 2.
Factors of influence on oral appliance adherence.
| Factors | Decreased Adherence | Increased Adherence | No Significant Association with Adherence | Caveat |
|---|---|---|---|---|
| Patient and disease characteristics | Anthropometric characteristics (age, sex, obesity) | |||
| Disease severity | ||||
| Baseline sleepiness | ||||
| Polysomnographic parameters | ||||
| Anatomical characteristics (length of the maxilla, mandible and soft palate, oropharyngeal space, crepitation at TMJ) | ||||
| Upper airway or facial skeletal abnormalities | ||||
| Desaturaters (patients with oxygen desaturations) | Arousers (patients with respiratory arousals) | Significant improvement in the ESS among the arousers | ||
| OA therapy as the first line of treatment | Strong predictor for treatment continuation | |||
| Complete symptom resolution | Contributes to the perception of OSA but not a strong predictor alone | |||
| Appliance fabrication and titration | Monobloc OA | Bi-Bloc OA | Relatively free mandibular movement | |
| Ready-made (Nontitratable) OA | Custom-made (Titratable) OA | More reported side-effects with ready-made as compared to custom-made | ||
| Patients not using the OA for > 2 years | More likely to discontinue the treatment | |||
| Regular dental follow-up | Helps in minimizing early side-effects which lead to early discontinuation of the treatment | |||
| Psychological and social factors | Lack of perceived benefits | Leads to early discontinuation of the treatment, consistent factor | ||
| Support from their bed partners | Improved sleep quality of the bed partner with OA use is associated with increased adherence |
ESS = Epworth Sleepiness Scale, OA = oral appliance, TMJ = temporomandibular joint.
Meta-analysis
A meta-analysis was performed using Review Manager (RevMan; Version 5.3. Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for studies with low and/or unclear risk of bias, similar study design, and comparing 2 types (ready-made vs custom-made) oral appliances prescribed for patients with OSA in regards to patient adherence. Results were analyzed using forest plots with weighted mean differences between ready-made vs custom-made appliances in relation to patient adherence, ie, mean nightly (hours) use of the appliance. The studies were weighted using the inverse variance method and tested for heterogeneity using the Chi square test to assess the significance of heterogeneity and I2 statistics to measure the diversity between studies. Pooled studies with I2 < 25% were regarded as homogenous, and those with I2 > 75% were considered to demonstrate high heterogeneity. A fixed-effects model was used and a P value of less than .05 was considered statistically significant, reported along with the 95% confidence interval.
RESULTS
Following the removal of duplicates, 419 articles were considered eligible for screening of the title and abstract. The abstracts were assessed against the selection criteria, with 45 articles considered eligible for full-text screening. Subsequently, fourteen studies were excluded (Table S2 (92.6KB, pdf) ), with a total of 31 studies included in the review, which consisted of 8 RCTs, 2 controlled clinical trials, 7 prospective cohorts, 11 retrospective cohorts, while the remaining 3 studies were a case-series, case-control, and a mixed-methods study (Figure 2). All 31 included studies were subject to qualitative analysis, with 4 studies subject to a meta-analysis. All included studies were undertaken in academic medical centers or sleep centers (Table S3 (92.6KB, pdf) ).
Figure 2. Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
The majority of the included studies investigated the influence of side effects (45%), disease and patient characteristics (41%), and appliance characteristics (32%) on patient adherence. The efficacy of strategies or interventions to increase patient adherence to OAT in adult patients with OSA was assessed in only a single study.32 While a self-reported measure of adherence was used in the majority of included studies, objective monitoring of adherence was reported in just 1 study.33 Studies that considered psychological and social factors (38%) focused on the impact of constructs, such as bed-partner satisfaction levels (improvement reported in the patient’s snoring by their partner), self-perceived changes, and type D personality (a combination personality type of negative affectivity and social inhibition) on oral appliance adherence.
Risk of bias within studies
The risk of bias (Figure 1) assessment for random sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data, and selective reporting was assessed for the included RCTs (n = 8) and controlled clinical trials (n = 2). The majority of these studies demonstrated a low or unclear risk of bias for the above domains. Only 2 studies34,35 demonstrated a high risk of bias for random sequence generation, otherwise a low risk of bias was assessed in relation to selective reporting for all included studies. In terms of allocation concealment, 5 studies20,36–38 demonstrated an unclear risk of bias, 3 studies32,40,41 indicated a low risk of bias, and a high risk of bias was observed in 2 studies.34,35 Due to no clear description concerning blinding of outcome assessors, 7 studies20,32,35–39 exhibited an unclear risk of bias and a low risk of bias was observed in the rest of the 3 studies.34,40,41 High risk of bias for incomplete outcome data was observed in only 1 study,34 whereas the remaining 9 studies20,32,35–41 exhibited a low risk of bias. The findings along with the comments for the judgement are summarized in Table S4 (92.6KB, pdf) .
Similarly, observational studies (n = 21) were found to demonstrate a low or moderate risk of bias concerning study participation, study attrition, prognostic factor management, outcome management, study confounding, and statistical analysis and reporting. Four studies42–45 exhibited a moderate risk of bias for study participation, whereas the remaining studies (n = 17) indicated a low risk of bias. All studies demonstrated a low risk of bias for the domains-outcome measurement and statistical analysis and reporting. In terms of study attrition, 3 studies43,46,47 exhibited a moderate risk of bias, while a low risk of bias was observed in the remaining studies. Furthermore, 8 studies33,43–46,48–50 demonstrated a low risk of bias for prognostic factor measurement, and remaining studies exhibited a moderate risk of bias. The majority of the studies indicated a moderate risk of bias for study confounding, while a low risk of bias was observed in 2 studies33,51 (Table S5 (92.6KB, pdf) ).
Qualitative study analysis
Patient and disease characteristics
The current review identified 13 studies exploring the influence of patient and disease characteristics, which reported neither supine-dependent OSA, age, obesity, sex, or sleepiness to be related to OAT tolerability.20,26,33,43–46,48,49,51–54 There were no significant sex differences detected in relation to the cessation of appliance use. Neither disease severity or baseline sleepiness was found to be a predictor of OAT adherence.26 While the above-reported studies relied upon self-reported adherence, an additional single study found no correlation between objective adherence and anthropometric characteristics, polysomnographic parameters, or excessive daytime sleepiness.33 Furthermore, among the 13 studies included, 1 study found no significant association between adherence and the following patient anatomical characteristics: upper airway or facial skeletal abnormalities, such as pharyngeal alterations (P = .62), retrognathia (P = .34), Class II dental occlusion (P = .64), craniofacial alterations (P = .44), or nasal alterations (P = .38)44. Although the findings are not statistically significant, these should be viewed carefully as the authors relied upon self-reported adherence, rather than objective adherence.
Appliance fabrication and titration
In terms of appliance factors, 11 studies examined the influence of appliance fabrication and titration on OAT adherence.20,34–41,54,55 One study compared the modified Herbst appliance (IST) with the Thornton anterior positioner (TAP), which differed in their ability to open the mouth during sleep in a protrusive position.20 Although the TAP was more effective in treating OSA, its long-term acceptance was less than that of the IST.20
Three studies comparing Mono-Bloc OA with Bi-Bloc OA, with regards to their adherence, have reported rather conflicting results.35,39,54 Zhou and Lou37 suggested that monobloc appliance should be considered, as almost half of the patients preferred the appliance to the bibloc device. However, the findings were based on a very small sample size (n = 16). On the contrary, a large prospective single-center study, with a sample size of 153 patients, observed an adherence rate of 83.3% with the Bi-Bloc OA and 68.8% with the Mono-Bloc OA, at 1 year (P = .04). The authors concluded that the relatively free mandibular movement may explain the difference in adherence rates.35 Similarly, Dieltjens et al,54 while examining the association between Type D personality (a combination personality type of negative affectivity and social inhibition) and OAT adherence, observed a higher discontinuation rate with monobloc OAT in comparison to bi- or duo-bloc appliance (95% confidence interval, 1.77–47.09; P = .008) when adjusted for Type D personality, age, sex, and decrease in AHI. However, the findings of the above studies should again be interpreted with caution as they failed to assess adherence using an objective measure and the marked differences in study designs.
Seven studies evaluating the impact of ready-made (nonadjustable/nontitratable) and custom-made OAT observed an overwhelming patient-reported preference for custom-made OAT in comparison to ready-made devices.34,36–38,40,41,55 The adherence was higher with the custom-made OAT despite more reported dental discomfort (P = .03).34 In an additional RCT with a crossover design, Johal et al reported a response rate of only 24% with a ready-made OAT vs the 64% in the custom-made OAT39. It has to be acknowledged that adherence was assessed from self-reports and can be at risk of bias. More recently, the addition of objective adherence monitors has served to confirm the reported levels of self-reported adherence with OAT.28,29
Side effects
Side effects, such as dental pain, muscular pain, and excessive salivation associated with OAT may prevent early acceptance of the device and contribute to nonadherence.56 Moreover, side effects arising from long-term OAT use, such as bite change, may also lead to poor patient adherence.22,33,52,57 The current review identified 14 studies examining the influence of early and long-term side effects on OAT adherence.22,26,33,47,49–53,55,56–59
The most common self-reported reason for discontinuing the treatment was a lack of treatment effect or discomfort or pain on OAT use, consistent with other reported studies.22,45,51,56,57 Furthermore, early discontinuation (< 2 years) of treatment was observed due to side effects, discomfort, and inefficacy. In contrast, patients discontinued treatment due to no specific reasons after 2 or more years.22 Additionally, the higher rates of treatment discontinuation with ready-made OAT was found to be associated with higher reported side effects in comparison to the custom-made OAT.34,37,40,41
Psychological and social factors
Among the 31 included studies, 12 studies22,26,42 examined the influence of the psychological and social factors on OAT adherence. One study reported low rates of perceived effectiveness, self-efficacy, and social support for OAT as a cohort (n = 39) of older patients had low expectation for positive outcomes.42 However, given that other included studies identified psychological factors, such as a lack of perceived benefits by the patients and their bed partner, and cognitive perceptions such as complete symptom resolution as influential on OAT adherence, the above findings are highly contentious.22,26,47,51,53,59 Likewise, 2 studies identified that social factors, such as poor marital satisfaction (marital quality and bed sharing frequency) (P < .04), support from their partner, and shame caused by the disease symptoms to be associated with continued usage of OAT.45,51 Nevertheless, the above findings should be viewed carefully due to marked differences in study designs and lack of objective assessment of adherence.
Quantitative analysis
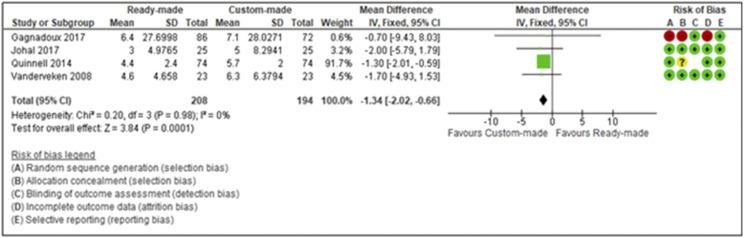
A meta-analysis was undertaken in relation to the use of ready-made OAT vs custom-made OAT with regards to patient adherence (Figure 3). Based on these studies,34,37,40,41 increased adherence was observed with custom-made appliances, with a pooled mean difference of −1.34 (−2.02 to −0.66), with low levels of heterogeneity (I2 = 0%).
Figure 3. Forest plot of patient-reported adherence for custom-made oral appliance and ready-made oral appliance.
The forest plot demonstrates 4 studies that indicate increased patient adherence with custom-made appliances in comparison to ready-made appliances. The squares represent the point estimate of the corresponding studies. The area of each square is proportional to the study’s weight in the meta-analysis, and the lateral points (horizontal line) indicate the confidence intervals of the respective study. The overall effect or the summary estimate is plotted as a diamond, and the lateral points demonstrate the confidence intervals of the estimate. A mean difference of zero (vertical line) indicates no effect; studies with confidence intervals crossing the vertical line are inconclusive. Powerful studies have narrower confidence intervals. In the graph, the Quinnell study and the overall effect estimate have narrow confidence intervals that do not cross zero, indicating that the meta-analysis could be considered as statistically significant.
DISCUSSION
Given that oral appliances are removable and have to be used indefinitely, adherence to treatment is of utmost importance for achieving successful therapy.6 However, adherence to OAT for OSA is highly variable.14 The current review observed that the relationship between OAT adherence and patient and disease characteristics such as age, sex, obesity, AHI, and daytime sleepiness is relatively weak. Furthermore, no association was observed between objective adherence and anthropometric characteristics, polysomnographic parameters, and excessive daytime sleepiness. It also appears that disease severity and sleepiness may not be associated with OAT adherence. The majority of the included studies exploring the impact of patient and disease characteristics, were retrospective in nature and highly heterogeneous in terms of study participants.
Dieltjens et al conducted a prospective clinical trial to identify the determinants of objective adherence to OAT in patients with OSA32. Previous studies on OAT adherence have relied upon patient-reported adherence, which is subject to overestimation.26,60 Moreover, objective compliance monitors with OAT have only been introduced recently.28 The trial (n = 51) observed no correlation between objective adherence and anthropometric characteristics, polysomnographic parameters, and excessive daytime sleepiness. Nevertheless, the authors did emphasize the influence of socially disturbing snoring, reporting objective adherence correlated significantly with a decrease in socially disturbing snoring, as reported by the partner compared with baseline visual analog scale scores for snoring without the appliance.35 Nerfeldt and Friberg43 investigated the difference between “arousers” (patients with respiratory arousals) and “desaturaters” (patients with oxygen desaturations) in terms of adherence rates. The authors observed that patients with greater numbers of arousal showed higher adherence (85%) than among the “desaturaters” (55%; P = .034). It was reasoned that the above difference in adherence rate was due to a significant improvement in the Epworth Sleepiness Score among the arousers (Epworth Sleepiness Score ≥ 10), which was not seen among the desaturaters. Furthermore, OAT as a first-line treatment was reported to be a strong predictor (odds ratio 1.77, 95% confidence interval 1.03–3.03; P = .0375) for treatment continuation.22 Similarly, complete symptom resolution (odd ratio 1.78, 95% confidence interval 1.03–3.03, P = .0384) was also a strong predictor for OAT adherence.22 These findings support an important role for disease chronicity in terms of patient adherence, which was similar to those reported for other chronic diseases.61 They also reinforce the link between disease chronicity and long-term treatment persistence, while indicating that patients intolerant of or nonadherent with CPAP are more likely to discontinue OAT.22 However, Izci et al51 in a large sample (n = 144) of patients with OSA demonstrated that usage of OAT was not significantly affected by whether a patient was CPAP nonadherent or a refuser (P > .3). Nonetheless, the findings of the above studies should be interpreted with caution, as the studies failed to provide an objective measure of adherence and also due to the marked differences in study design and participant settings with regards to race and ethnicity. However, Johal et al demonstrated excellent long-term objective adherence with OAT in a sample of 42 patients with OSA, who were CPAP intolerant28.
Nonetheless, it is interesting to evaluate these findings in the context of CPAP adherence. A weak association between patient and disease characteristics, such as disease severity, AHI, oxygen desaturation, and Epworth Sleepiness Score on CPAP adherence has been observed.62–64 Although nasal resistance influences initial CPAP acceptance, nasal anatomy, not necessarily patient-reported nasal complaints, may be influential on CPAP adherence.64–67 Furthermore, initial CPAP adherence appears to be closely associated with higher neighborhood socioeconomic factors, independent of individual demographic and clinical factors.68 These findings suggest that socioenvironmental factors are important in terms of patient adherence among patients with OSA. Studies have also examined race as influential on CPAP adherence, all of which have reported lower CPAP adherence in African Americans compared with Caucasian users.69,70 Factors such as race and ethnicity-based differences in OAT adherence were not examined, as no studies have been published exploring such factors. Similarly, a low socioeconomic index is only considered a barrier to accessing OAT, as its influence on treatment adherence is yet to be explored.71 Thus, additional studies are needed to understand and help characterize the individual considerations needed for initiating and managing OA treatment within diverse patient groups.
In terms of appliance characteristics, both patient-reported adherence and preference favored the use of custom-made appliances. The preference was not only reflected in the higher number of nights per week but also the number of hours per night that the appliance was used.40,41 The findings are consistent with a recent systematic review and meta-analysis.72 Moreover, as OAT for OSA is entirely dependent on patient behavior, patient preference or acceptance cannot be disregarded. However, the majority of the studies were limited to self-reported use and lacked an objective adherence measurement. This reflects the relatively recent introduction of objective adherence monitors.28 Notwithstanding this, a lack of retention with the ready-made OAT was the most frequently cited reason for discomfort and nonadherence.37,40,41,49,55
In relation to side effects, nonusers experienced 1 or more adverse effects and tend to discontinue the treatment earlier, ie, within the first 3 months, whereas those who use the device for longer periods experienced milder problems.52,58 In a questionnaire-based retrospective study, nonusers reported a higher average number of side effects than users.52 Similarly, Makihara et al58 reported that one-third of the nonusers discontinued the OAT within the first month and 40% within in the next 3 months. The most common reasons for discontinuation of treatment were discomfort or lack of treatment effects.26,52,57 Specifically, pain originating from the masticatory muscles or the temporomandibular joints may be one of the main reasons for poor adherence or abandonment.32 Consequently, Cunali et al32 randomized 29 OSA adult patients with temporomandibular disorders into 2 groups, the exercise support therapy group and placebo therapy (PT) group, and they were evaluated prior to and 120 days after OAT. The authors observed higher treatment adherence in the support therapy group (P < .05) compared to the placebo therapy group, as there was a significant reduction of pain intensity in the former group (P < .05) but not in the latter. Long-term occlusal changes may occur with OAT,26 as such, dental follow-up may be useful in encouraging adherence while limiting possible side effects and the risk of cessation of treatment in long-term OAT users. In terms of the influence of sex, in a retrospective study (n = 251), women experienced and reported more side effects and seemed to have a greater tendency to abandon treatment than men, as 46.8% of the women who answered a questionnaire based-survey had discontinued the use of OAT compared to the 32.8% of men.26 However, given that the study was retrospective, with data collection from patients at different time intervals, the findings should be interpreted with caution.26
Psychological and social factors, such as mood and perception of treatment benefits, and bed partner satisfaction levels were significantly correlated with OAT use.51 Dieltjens et al identified that self-reported adherence to OAT was significantly lower for adults with OSA and Type D personality, a combination personality type of negative affectivity and social inhibition, compared to patients with OSA without the said personality type53. These findings are in agreement with similar observations reported by Brostrom et al73 in regards to lower CPAP adherence with type D personality. Objective adherence was found to be significantly correlated with a more pronounced decrease in socially disturbing snoring.33 Research shows that adoption of new health behavior, like a new physical activity routine or adhering to a prescribed medication regimen, is a challenging endeavor, involving a variety of social, emotional, and cognitive factors.74 However, evidence in terms of psychological and social factors with regards to OAT adherence is highly underrepresented, which contrasts with the volume of literature concerning CPAP adherence. Efforts to enhance patient education ranging from telephone support to home visits, motivational enhancement, or augmented support,75,76 have been shown to improve CPAP adherence when compared to standard care. It has also been suggested in a recent Cochrane review that educational, supportive, and behavioral interventions may increase CPAP usage to varying degrees.77 However, no studies evaluating the efficacy of the above-mentioned interventions in relation to OAT adherence were identified in this review. Evidence concerning the impact of psychological factors, such as patient’s perceptions, self-efficacy, and social support on OAT adherence is highly underrepresented in the field of sleep medicine in comparison to various sleep apnea treatments. Therefore, further research is imperative for the development of tailor-made interventions to enhance adherence in patients with low mood and/or psychological disorders.
Strengths and limitations
This is the first systematic review to assess the factors influencing adherence or nonadherence in adult patients with OSA on OAT. To limit publication bias, comprehensive search strategies were implemented along with the use of Covidence, a core component of Cochrane’s review toolkit. The review followed the PRISMA reporting guidelines, and the Cochrane Handbook of systematic review was used for risk of bias assessment for the included RCTs.
In terms of limitations, the search yield was limited to 8 RCTs demonstrating low or unclear risk of bias. Furthermore, the application of a meta-analysis in nonrandomized controls trials leads to bias arising from methodological issues and marked differences in study designs. Another possible limitation is the limited evidence identified concerning the impact of psychological and social factors and the effect of strategies or interventions to improve OA adherence.
CONCLUSIONS
A weak relationship was observed between objective OAT adherence and patient and disease characteristics such as age, sex, obesity, AHI, and daytime sleepiness. Nonadherent patients reported more side effects than users and tended to discontinue treatment within the first 3 months. Increased patient adherence was identified with custom-made OAT in comparison to ready-made OA. The review identified limited evidence concerning the influence of psychological and social factors on OAT adherence. Given that majority of the studies relied upon patient-reported adherence, the review observed a considerable lack of objective adherence monitoring.
Further research would be beneficial to describe the determinants of adherence, such as risk perception, self-efficacy, and outcome expectancy and to facilitate patient education and development of tailor-made interventions to enhance adherence to OAT. Similarly, the lack of objective adherence monitoring necessitates the need for future studies that assess adherence objectively.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Department of Oral Bioengineering, Institute of Dentistry, Queen Mary University of London, London, UK. The systematic review is part of a PhD program that is university-sponsored. The authors Prof. Ama Johal and Prof. Padhraig S. Fleming are salaried employees of the Centre for Oral Bioengineering, Queen Mary University of London, and Prof Tim Newton of the Department of Population and Patient Health, King’s College London. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
Author contributions: All authors were responsible for data collection, analysis, and interpretation as well as final manuscript preparation and approval.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- IST
Herbst appliance
- OA
oral appliance
- OAT
oral appliance therapy
- OSA
obstructive sleep apnea
- RCT
randomized controlled trial
- TAP
Thornton anterior positioner
REFERENCES
- 1.Banno K, Kryger MH. Sleep apnea: clinical investigations in humans. Sleep Med. 2007;8(4):400–426. 10.1016/j.sleep.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Deng T, Wang Y, Sun M, Chen B. Stage-matched intervention for adherence to CPAP in patients with obstructive sleep apnea: a randomized controlled trial. Sleep Breath. 2013;17(2):791–801. 10.1007/s11325-012-0766-3 [DOI] [PubMed] [Google Scholar]
- 3.SIGN . Management of Obstructive Sleep Apnoea/Hypopnoea Syndrome in Adults: A National Clinical Guideline. Edinburgh, UK: Scottish Intercollegiate Guidelines Network; 2003. [Google Scholar]
- 4.Rejon-Parrilla JC, Garau M, Sussex J. Obstructive Sleep Apnoea: Health Economics Report. London: Office of Health Economics; 2014. [Google Scholar]
- 5.Zozula R, Rosen R. Compliance with continuous positive airway pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7(6):391–398. 10.1097/00063198-200111000-00005 [DOI] [PubMed] [Google Scholar]
- 6.Ramar K, Dort LC, Katz SG, Lettieri CJ, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: An update for 2015. J Clin Sleep Med. 2015;11(7):773–827. 10.5664/jcsm.4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2004:CD004435. [DOI] [PubMed] [Google Scholar]
- 8.Sharples LD, Clutterbuck-James AL, Glover MJ, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev. 2016;27:108–124. 10.1016/j.smrv.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. 10.1164/rccm.201212-2223OC [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):555–568. 10.1007/s11325-017-1590-6 [DOI] [PubMed] [Google Scholar]
- 11.Marklund M, Franklin KA. Treatment of elderly patients with snoring and obstructive sleep apnea using a mandibular advancement device. Sleep Breath. 2015;19(1):403–405. 10.1007/s11325-014-0987-8 [DOI] [PubMed] [Google Scholar]
- 12.Dal-Fabbro C, Garbuio S, D’Almeida V, Cintra FD, Tufik S, Bittencourt L. Mandibular advancement device and CPAP upon cardiovascular parameters in OSA. Sleep Breath. 2014;18(4):749–759. 10.1007/s11325-014-0937-5 [DOI] [PubMed] [Google Scholar]
- 13.Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J. 2012;39(5):1241–1247. 10.1183/09031936.00144711 [DOI] [PubMed] [Google Scholar]
- 14.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10(2):215–227. 10.5664/jcsm.3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marklund M, Carlberg B, Forsgren L, Olsson T, Stenlund H, Franklin KA. Oral appliance therapy in patients with daytime sleepiness and snoring or mild to moderate sleep apnea: a randomized clinical trial. JAMA Intern Med. 2015;175(8):1278–1285. 10.1001/jamainternmed.2015.2051 [DOI] [PubMed] [Google Scholar]
- 16.Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 2011;82(2):162–168. 10.1159/000324580 [DOI] [PubMed] [Google Scholar]
- 17.Gauthier L, Laberge L, Beaudry M, Laforte M, Rompré PH, Lavigne GJ. Mandibular advancement appliances remain effective in lowering respiratory disturbance index for 2.5-4.5 years. Sleep Med. 2011;12(9):844–849. 10.1016/j.sleep.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Marklund M, Sahlin C, Stenlund H, Persson M, Franklin KA. Mandibular advancement device in patients with obstructive sleep apnea: long-term effects on apnea and sleep. Chest. 2001;120(1):162–169. 10.1378/chest.120.1.162 [DOI] [PubMed] [Google Scholar]
- 19.Rose EC, Barthlen GM, Staats R, Jonas IE. Therapeutic efficacy of an oral appliance in the treatment of obstructive sleep apnea: a 2-year follow-up. Am J Orthod Dentofacial Orthop. 2002;121(3):273–279. 10.1067/mod.2002.121006 [DOI] [PubMed] [Google Scholar]
- 20.Ghazal A, Sorichter S, Jonas I, Rose EC. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J Sleep Res. 2009;18(3):321–328. 10.1111/j.1365-2869.2009.00738.x [DOI] [PubMed] [Google Scholar]
- 21.Marklund M. Long-term efficacy of an oral appliance in early treated patients with obstructive sleep apnea. Sleep Breath. 2016;20(2):689–694. 10.1007/s11325-015-1280-1 [DOI] [PubMed] [Google Scholar]
- 22.Attali V, Chaumereuil C, Arnulf I, et al. Predictors of long-term effectiveness to mandibular repositioning device treatment in obstructive sleep apnea patients after 1000 days. Sleep Med. 2016;27-28:107–114. 10.1016/j.sleep.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Medicine. 2007; 11(1): 1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieltjens M, Braem MJ, Vroegop AVMT, et al. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144(5):1495–1502. 10.1378/chest.13-0613 [DOI] [PubMed] [Google Scholar]
- 25.Walker-Engström ML, Tegelberg A, Wilhelmsson B, Ringqvist I. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study. Chest. 2002;121(3):739–746. 10.1378/chest.121.3.739 [DOI] [PubMed] [Google Scholar]
- 26.de Almeida FR, Lowe AA, Tsuiki S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med. 2005;1(2):143–152. 10.5664/jcsm.8978 [DOI] [PubMed] [Google Scholar]
- 27.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. 10.1164/ajrccm/147.4.887 [DOI] [PubMed] [Google Scholar]
- 28.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. 10.1136/thoraxjnl-2012-201900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johal A, Jauhar P, Alqattan F, Kassim S, Mc Cloughlin K. The efficacy of mandibular advancement appliances as a treatment alternative to continuous positive airway pressure in moderate OSAHS. J Sleep Disord Manage. 2016;2(2). 10.23937/2572-4053.1510013 [DOI] [Google Scholar]
- 30.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: Wiley; 2019. 10.1002/9781119536604 [DOI] [Google Scholar]
- 31.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 32.Cunali PA, Almeida FR, Santos CD, et al. Mandibular exercises improve mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath. 2011;15(4):717–727. 10.1007/s11325-010-0428-2 [DOI] [PubMed] [Google Scholar]
- 33.Dieltjens M, Verbruggen AE, Braem MJ, et al. Determinants of objective compliance during oral appliance therapy in patients with sleep-disordered breathing: A prospective clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(10):894–900. 10.1001/jamaoto.2015.1756 [DOI] [PubMed] [Google Scholar]
- 34.Gagnadoux F, Nguyen XL, Le Vaillant M, et al. Comparison of titrable thermoplastic versus custom-made mandibular advancement device for the treatment of obstructive sleep apnoea. Respir Med. 2017;131:35–42. 10.1016/j.rmed.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 35.Lee WH, Wee JH, Lee CH, et al. Comparison between mono-bloc and bi-bloc mandibular advancement devices for obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2013;270(11):2909–2913. 10.1007/s00405-013-2417-0 [DOI] [PubMed] [Google Scholar]
- 36.Al-Dharrab A. A randomized cross over study comparing the efficacy of two mandibular advancement appliances in the treatment of mild-moderate obstructive sleep apnea. Cranio. 2017;35(6):379–384. 10.1080/08869634.2016.1252563 [DOI] [PubMed] [Google Scholar]
- 37.Quinnell TG, Bennett M, Jordan J, et al. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO). Thorax. 2014;69(10):938–945. 10.1136/thoraxjnl-2014-205464 [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Liu Y-h. [Comparison of the efficacy of 2 types of mandibular advancement device in severe obstructive sleep apnea hypopnea syndrome]. Shanghai Kou Qiang Yi Xu. 2014;23(6):713–717. [PubMed] [Google Scholar]
- 39.Zhou J, Liu YH. A randomised titrated crossover study comparing two oral appliances in the treatment for mild to moderate obstructive sleep apnoea/hypopnoea syndrome. J Oral Rehabil. 2012;39(12):914–922. 10.1111/joor.12006 [DOI] [PubMed] [Google Scholar]
- 40.Johal A, Haria P, Manek S, Joury E, Riha R. Ready-made versus custom-made mandibular repositioning devices in sleep apnea: A randomized clinical trial. J Clin Sleep Med. 2017;13(2):175–182. 10.5664/jcsm.6440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178(2):197–202. 10.1164/rccm.200701-114OC [DOI] [PubMed] [Google Scholar]
- 42.Carballo NJ, Alessi CA, Martin JL, et al. Perceived effectiveness, self-efficacy, and social support for oral appliance therapy among older veterans with obstructive sleep apnea. Clin Ther. 2016;38(11):2407–2415. 10.1016/j.clinthera.2016.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nerfeldt P, Friberg D. Effectiveness of oral appliances in obstructive sleep apnea with respiratory arousals. J Clin Sleep Med. 2016;12(8):1159–1165. 10.5664/jcsm.6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prescinotto R, Haddad FLM, Fukuchi I, et al. Impact of upper airway abnormalities on the success and adherence to mandibular advancement device treatment in patients with obstructive sleep apnea syndrome. Braz J Otorhinolaryngol. 2015;81(6):663–670. 10.1016/j.bjorl.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saglam-Aydinatay B, Taner T. Oral appliance therapy in obstructive sleep apnea: Long-term adherence and patients experiences. Med Oral Patol Oral Cir Bucal. 2018;23(1):e72–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brette C, Ramanantsoa H, Renouardiere J, Renouardiere R, Roisman G, Escourrou P. A mandibular advancement device for the treatment of obstructive sleep apnea: long-term use and tolerance. Int Orthod. 2012;10(4):363–376. 10.1016/j.ortho.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 47.Nishigawa K, Hayama R, Matsuka Y. Complications causing patients to discontinue using oral appliances for treatment of obstructive sleep apnea. J Prosthodont Res. 2017;61(2):133–138. 10.1016/j.jpor.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 48.Ingman T, Arte S, Bachour A, Bäck L, Mäkitie A. Predicting compliance for mandible advancement splint therapy in 96 obstructive sleep apnea patients. Eur J Orthod. 2013;35(6):752–757. 10.1093/ejo/cjs092 [DOI] [PubMed] [Google Scholar]
- 49.Tsuda H, Almeida FR, Masumi S, Lowe AA. Side effects of boil and bite type oral appliance therapy in sleep apnea patients. Sleep Breath. 2010;14(3):227–232. 10.1007/s11325-009-0304-0 [DOI] [PubMed] [Google Scholar]
- 50.Vecchierini M-F, Attali V, Collet J-M, et al.ORCADES investigators . A custom-made mandibular repositioning device for obstructive sleep apnoea-hypopnoea syndrome: the ORCADES study. Sleep Med. 2016;19:131–140. 10.1016/j.sleep.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 51.Izci B, McDonald JP, Coleman EL, Mackay TW, Douglas NJ, Engleman HM. Clinical audit of subjects with snoring & sleep apnoea/hypopnoea syndrome fitted with mandibular repositioning splint. Respir Med. 2005;99(3):337–346. 10.1016/j.rmed.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 52.McGown AD, Makker HK, Battagel JM, L’Estrange PR, Grant HR, Spiro SG. Long-term use of mandibular advancement splints for snoring and obstructive sleep apnoea: a questionnaire survey. Eur Respir J. 2001;17(3):462–466. 10.1183/09031936.01.17304620 [DOI] [PubMed] [Google Scholar]
- 53.Rose E, Staats R, Schulte-Mönting J, Ridder GJ, Jonas IE. [Long term compliance with an oral protrusive appliance in patients with obstructive sleep apnoea]. Dtsch Med Wochenschr. 2002;127(23):1245–1249. 10.1055/s-2002-32102 [DOI] [PubMed] [Google Scholar]
- 54.Dieltjens M, Vanderveken OM, Van den Bosch D, et al. Impact of type D personality on adherence to oral appliance therapy for sleep-disordered breathing. Sleep Breath. 2013;17(3):985–991. 10.1007/s11325-012-0788-x [DOI] [PubMed] [Google Scholar]
- 55.Friedman M, Hamilton C, Samuelson CG, et al. Compliance and efficacy of titratable thermoplastic versus custom mandibular advancement devices. Otolaryngol Head Neck Surg. 2012;147(2):379–386. 10.1177/0194599812439683 [DOI] [PubMed] [Google Scholar]
- 56.Bates CJ, McDonald JP. Patients’ and sleeping partners’ experience of treatment for sleep-related breathing disorders with a mandibular repositioning splint. Br Dent J. 2006;200(2):95–101. 10.1038/sj.bdj.4813149 [DOI] [PubMed] [Google Scholar]
- 57.Clark GT, Sohn JW, Hong CN. Treating obstructive sleep apnea and snoring: assessment of an anterior mandibular positioning device. J Am Dent Assoc. 2000;131(6):765–771. 10.14219/jada.archive.2000.0275 [DOI] [PubMed] [Google Scholar]
- 58.Makihara E, Kawano T, Miyajima R, Masumi SI, Enciso R, Clark GT. Assessment of oral appliance for obstructive sleep apnea patients. Clin Exp Dent Res. 2016;2(2):155–161. 10.1002/cre2.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haviv Y, Zini A, Almoznino G, Keshet N, Sharav Y, Aframian DJ. Assessment of interfering factors in non-adherence to oral appliance therapy in severe sleep apnea. Oral Dis. 2017;23(5):629–635. 10.1111/odi.12633 [DOI] [PubMed] [Google Scholar]
- 60.Marklund M, Franklin KA. Long-term effects of mandibular repositioning appliances on symptoms of sleep apnoea. J Sleep Res. 2007;16(4):414–420. 10.1111/j.1365-2869.2007.00615.x [DOI] [PubMed] [Google Scholar]
- 61.Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31(7):1283–1296. 10.1185/03007995.2015.1053048 [DOI] [PubMed] [Google Scholar]
- 62.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572–575. 10.1016/S0140-6736(94)91522-9 [DOI] [PubMed] [Google Scholar]
- 63.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149(1):149–154. 10.1164/ajrccm.149.1.8111574 [DOI] [PubMed] [Google Scholar]
- 64.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430–435. 10.1378/chest.121.2.430 [DOI] [PubMed] [Google Scholar]
- 65.Li H-Y, Engleman H, Hsu C-Y, et al. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep. 2005;28(12):1554–1559. 10.1093/sleep/28.12.1554 [DOI] [PubMed] [Google Scholar]
- 66.Morris LG, Setlur J, Burschtin OE, Steward DL, Jacobs JB, Lee KC. Acoustic rhinometry predicts tolerance of nasal continuous positive airway pressure: a pilot study. Am J Rhinol. 2006;20(2):133–137. 10.1177/194589240602000202 [DOI] [PubMed] [Google Scholar]
- 67.Sugiura T, Noda A, Nakata S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration. 2007;74(1):56–60. 10.1159/000089836 [DOI] [PubMed] [Google Scholar]
- 68.Platt AB, Field SH, Asch DA, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32(6):799–806. 10.1093/sleep/32.6.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scharf SM, Seiden L, DeMore J, Carter-Pokras O. Racial differences in clinical presentation of patients with sleep-disordered breathing. Sleep Breath. 2004;8(4):173–183. 10.1055/s-2004-860894 [DOI] [PubMed] [Google Scholar]
- 70.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 71.Fleury M, Le Vaillant M, Pelletier-Fleury N; IRSR sleep cohort group . Socio-economic status: A barrier to access to mandibular advancement device therapy for patients with obstructive sleep apnea syndrome in France. PLoS One. 2015;10(9):e0138689. 10.1371/journal.pone.0138689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johal A, Agha B. Ready-made versus custom-made mandibular advancement appliances in obstructive sleep apnea: A systematic review and meta-analysis. J Sleep Res. 2018;27(6):e12660. 10.1111/jsr.12660 [DOI] [PubMed] [Google Scholar]
- 73.Broström A, Strömberg A, Mårtensson J, Ulander M, Harder L, Svanborg E. Association of Type D personality to perceived side effects and adherence in CPAP-treated patients with OSAS. J Sleep Res. 2007;16(4):439–447. 10.1111/j.1365-2869.2007.00620.x [DOI] [PubMed] [Google Scholar]
- 74.Schwarzer R, Luszczynska A. How to overcome health-compromising behaviors: The health action process approach. Eur Psychologist. 2008;13(2):141–151. 10.1027/1016-9040.13.2.141 [DOI] [Google Scholar]
- 75.Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep. 1997;20(4):284–289. 10.1093/sleep/20.4.284 [DOI] [PubMed] [Google Scholar]
- 76.Hui DS, Chan JK, Choy DK, et al. Effects of augmented continuous positive airway pressure education and support on compliance and outcome in a Chinese population. Chest. 2000;117(5):1410–1416. 10.1378/chest.117.5.1410 [DOI] [PubMed] [Google Scholar]
- 77.Askland K, Wright L, Wozniak DR, Emmanuel T, Caston J, Smith I. Educational, supportive and behavioural intreventions to improve usage of continuous positive airway pressure machines in adults with obstuctive sleep apnoea. Cochrane Database of Systematic Reviews. 2020 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.