Abstract
Study Objectives:
To evaluate home sleep apnea testing (HSAT) using a type 3 portable monitor to help diagnose sleep-disordered breathing (SDB) and identify respiratory events including obstructive sleep apnea, central sleep apnea, and Cheyne-Stokes respiration in adults with stable chronic heart failure.
Methods:
Eighty-four adults with chronic heart failure (86.9% males, age [mean ± standard deviation] 58.7 ± 16.3 years, body mass index 29.4 ± 13.0 kg/m2, left ventricular ejection fraction 40.3% ± 11.5%) performed unattended HSAT followed by an in-laboratory polysomnography (PSG) with simultaneous portable monitor recording.
Results:
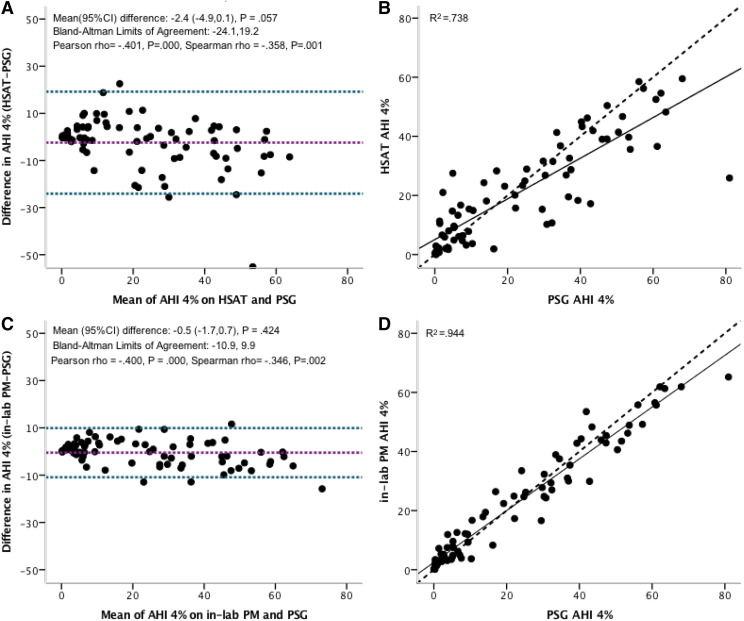
The apnea-hypopnea index was 22.0 ± 17.0 events/h according to HSAT, 26.8 ± 20.5 events/h on an in-laboratory portable monitor, and 23.8 ± 21.3 events/h using PSG (P = .373). A Bland-Altman analysis of the apnea-hypopnea index using HSAT vs PSG showed a mean difference (95% confidence interval) of −2.4 (−4.9 to 0.1) events/h and limits of agreement (±2 standard deviations) of −24.1 to 19.2 events/h. HSAT underestimated the apnea-hypopnea index to a greater extent at a higher apnea-hypopnea index (rho = –.358; P < .001). Similar levels of agreement from HSAT vs PSG were observed when comparing the obstructive apnea index, central apnea index, and percentage of time in a Cheyne-Stokes respiration pattern. When we used an apnea-hypopnea index ≥ 5 events/h to diagnose SDB, HSAT had 86.7% sensitivity, 76.5% specificity, 92.9% positive predictive value, and 61.9% negative predictive value compared to PSG. Detection of Cheyne-Stokes respiration using HSAT showed 94.6% sensitivity, 91.1% specificity, 88.6% positive predictive value, and 97.6% negative predictive value compared to PSG.
Conclusions:
HSAT with a type 3 portable monitor can help diagnose SDB and identify obstructive sleep apnea, central sleep apnea, and Cheyne-Stokes respiration events in adults with chronic heart failure.
Citation:
Li S, Xu L, Dong X, et al. Home sleep apnea testing of adults with chronic heart failure. J Clin Sleep Med. 2021;17(7):1453–1463.
Keywords: polysomnogram, home sleep apnea testing, apnea-hypopnea index, chronic heart failure
BRIEF SUMMARY
Current Knowledge/Study Rationale: Current clinical practice guidelines recommend in-laboratory polysomnography to diagnose sleep-disordered breathing in adults with major comorbidities because of a lack of evidence supporting the use of home portable monitor testing. This study evaluated the performance of a portable monitor (Nox-T3) used for home sleep apnea testing to diagnose sleep-disordered breathing and identify different types of respiratory events in adults with chronic heart failure.
Study Impact: Home sleep apnea testing had good agreement with in-laboratory polysomnography to help diagnose sleep-disordered breathing and identify respiratory events including obstructive sleep apnea, central sleep apnea, and Cheyne-Stokes respiration. The ability to use home sleep apnea testing in adults with chronic heart failure will increase their access to diagnosis and thereby treatment for sleep-disordered breathing.
INTRODUCTION
Chronic heart failure (CHF) is a life-threatening disease with decreased long-term quality of life. Sleep-disordered breathing (SDB), which occurs in approximately 46%–81% of adults with CHF,1–3 is associated with exacerbation and worse prognosis of CHF.4,5 Positive airway pressure treatment of SDB in patients with heart failure may improve the apnea-hypopnea index (AHI), cardiac function, quality of life, and heart transplant–free survival.6–9 Symptoms and characteristics of SDB, such as daytime sleepiness and obesity, are often absent in adults with CHF.10 Therefore, performing sleep testing to diagnose SDB is important for CHF management.
Most previous trials validating HSAT to diagnose SDB excluded adults with cardiorespiratory disorders.11–13 As a result, current clinical practice guidelines recommend that polysomnography (PSG) be performed to diagnose SDB in adults with cardiorespiratory conditions such as CHF and chronic obstructive pulmonary disease (COPD).14 The relatively high cost and limited availability of in-laboratory PSG restricts the access of adults with cardiorespiratory disorders to diagnostic sleep testing. The coronavirus pandemic has further increased the need for HSAT in adults with cardiorespiratory disorders. These individuals are more vulnerable to the serious adverse consequences of COVID-19 infection.15 The ability to perform HSAT instead of PSG in adults with CHF would reduce their risk of virus exposure.
Recent studies have reported the ability of portable monitor (PM) testing to diagnose SDB in adults with comorbidities. We reported moderate agreement of HSAT using a type 3 PM vs PSG to diagnose obstructive sleep apnea (OSA) in adults with COPD.16 Aurora et al17 validated a type 3 PM recording vs simultaneous PSG performed at bedside in hospitalized patients with CHF. There is no comprehensive analysis of HSAT to identify OSA, central sleep apnea (CSA), and Cheyne-Stokes respiration (CSR) in adults with medically stable CHF.
The main goal of the study was to validate the use of an unattended type 3 PM, self-applied at home to diagnose SDB, and the secondary aim was to evaluate its ability to identify different types of respiratory events including OSA, CSA, and CSR in adults with stable CHF.
METHODS
Protocol
Ninety-three consecutive patients with CHF referred to sleep testing between January 2016 and January 2019 were recruited into the study. The inclusion criteria were (1) New York Heart Association Class II–IV symptoms, (2) chronic treatment with loop diuretic, and (3) ejection fraction < 50% or chronic diastolic dysfunction based on standard guidelines.18 Exclusion criteria included a history of diagnosis or treatment of SDB, inability or unwillingness to provide informed consent, hospitalization within the preceding 3 months, a change in medication in the previous month, a new medical diagnosis in the previous 2 months (eg, myocardial infarction, active infection, thyroid disease, depression or psychosis, cirrhosis, surgery, or cancer), previous sleep testing, prior diagnosis of a sleep disorder or irregular work schedule in the previous 3 months, and supplemental oxygen therapy. The institutional review boards at Peking University People’s Hospital in Beijing, China (2015PHB1878) and the Corporal Michael J. Crescenz Veterans Affairs Medical Center in Philadelphia, Pennsylvania (identification number: 01603; Prom number: 0034) approved the project, and all participants provided written informed consent.
The study consisted of 3 monitoring procedures: PSG, as a gold standard to diagnose SDB; HSAT, with a PM used outside of the sleep laboratory; and in-laboratory PM, simultaneous monitoring with PSG. Participants initially performed an overnight HSAT using the Nox-T3 PM at home (Nox-T3, Nox Medical Inc., Reykjavik, Iceland), followed within 1 week by an in-laboratory PSG (Alice6, Philips Respironics, Inc., Murrysville, PA, conducted at Peking University People’s Hospital; Sandman, Natus Medical Inc., Pleasanton, CA, conducted at Crescenz Veterans Affairs Medical Center) with a simultaneous Nox-T3 PM recording. The order of home and in-laboratory testing was fixed to evaluate whether individuals without experience with sleep testing could perform the HSAT successfully. Participants were instructed to sleep in whatever positions were comfortable for them and to take their regular medications.
PM and PSG recordings
The following signals were recorded during the PM recordings: nasal pressure, rib cage and abdominal movement by respiratory inductance plethysmography, snoring, body position, activity, and heart rate and oxygen saturation by pulse oximetry (Nonin pulse oximeter, Nonin Medical, Plymouth, MN). For the HSAT, a trained sleep technologist showed participants how to apply the sensors at the sleep center. A successful HSAT required at least 3 hours of recording containing the oxygen saturation and at least 1 of the respiratory signals (nasal pressure, rib cage movement, abdominal movement). The ability of participants to perform HSAT was assessed as the percentage of individuals with a successful initial HSAT. If the initial HSAT was unsuccessful, then the participant performed another HSAT at home after the PSG. If the second attempt was unsuccessful, then the HSAT was not repeated.
The in-laboratory PSGs were performed according to the recommendations of the American Academy of Sleep Medicine.19 The following signals were recorded: electroencephalogram (F3M2, F4M1, C3M2, C4M1, O1M2, O2M1), bilateral electrooculogram, chin muscle electromyogram, oronasal thermistor, nasal pressure, rib cage and abdominal movement, electrocardiogram (lead 1), snoring, body position, bilateral anterior tibialis electromyograms, and heart rate and oxygen saturation by pulse oximetry (the Alice 6 system uses a Masimo pulse oximeter, Masimo Corp., Irvine, CA; the Sandman uses a Nellcor pulse oximeter, Medtronic, Minneapolis, MN). During the PSG, the sleep technologist performed a simultaneous recording with the PM. Separate sensors were used for the PM and PSG.
PM and PSG scoring
Experienced technologists scored the PM and PSG recordings in a blinded fashion. Analysis of start time and stop time on the PM recordings was determined based on the participant’s responses on the poststudy questionnaire and the activity signal on the recording. The PM recordings were initially scored automatically using Noxturnal software and then manually edited. The PSGs were scored manually with the aid of computer software. Using the American Academy of Sleep Medicine 2012 criteria for scoring PSGs and HSATs,20 apneas were defined as a ≥ 90% reduction in airflow from baseline for at least 10 seconds. Apneas were scored using the oronasal thermistor signal on PSG and the nasal pressure signal on the PM. Obstructive apneas were defined as an apnea associated with respiratory effort, and central apneas were defined as an apnea during which respiratory effort was absent. Mixed apneas were defined as an apnea during which respiratory effort was initially absent but appeared in the latter part of the event. Hypopneas were defined as a ≥ 30% reduction in a respiratory signal for ≥ 10 seconds associated with either a ≥ 4% reduction in oxygen saturation or a ≥ 3% reduction in oxygen saturation on PM and/or an arousal on PSG. CSR was scored when there were at least 3 consecutive central apneas and/or central hypopneas separated by a crescendo and decrescendo change in breathing amplitude with a cycle length of ≥ 40 seconds. When the PM nasal pressure signal was absent or could not be scored, respiratory events were scored using the flow signal derived from the respiratory inductance plethysmography signals. The AHI (average number of apneas and hypopneas per hour), obstructive apnea index (OAI; average number of obstructive apneas per hour), central apnea index (CAI; average number of central apneas per hour), and other sleep test measures were calculated based on total analysis time on the PM recordings and total sleep time on PSG. The diagnosis of OSA vs CSA was dependent on whether the majority of apneas were obstructive vs central, respectively.
Statistical analysis
Continuous variables were summarized using means and standard deviations, and categorical variables were summarized using counts and percentages. Comparisons of respiratory parameters across the 3 monitoring methods were compared using 1-way analysis of variance, accounting for multiple observations per patient. Pairwise contrasts among methods were performed if there was at least suggestive evidence (P < .05) to reject the global null hypothesis of no difference between the monitoring methods. The statistical significance of these comparisons was defined using domain-specific Bonferroni corrected P values (equal to 0.05 divided by the number of measures within each domain).
To assess the level of agreement between the monitoring methods, we utilized paired tests and methods described by Bland and Altman.21,22 Specifically, for a given metric (eg, AHI), we first calculated the patient-specific difference for each pair of methods and tested whether this was significantly different from zero using paired t tests. Next, for each pair of monitoring methods, we examined the relationship between the patient-specific difference and the average value using the 2 techniques. This relationship was evaluated graphically and statistically for bias, including examining the average difference and limits of agreement (mean difference ± 2 standard deviations) and testing for significant correlation between the difference and the mean. Primary agreement analysis compared the AHI based on hypopneas associated with ≥ 4% oxygen desaturation (AHI of 4%) and the OAI, CAI, and percentage of time in CSR (CSR%) using HSAT and the in-laboratory PM to the respective measures obtained using PSG. Secondary analyses repeated this comparison using the ≥ 3% rule for scoring hypopneas.
To understand the ability of HSAT to accurately diagnose SDB when compared to PSG in adults with CHF, we examined the diagnostic characteristics of HSAT and the in-laboratory PM. We calculated the sensitivity, specificity, positive predictive value, and negative predictive value at AHI thresholds of ≥ 5 events/h, ≥ 10 events/h, ≥ 15 events/h, and ≥ 30 events/h for each PM recording compared to PSG. In addition, we calculated the percentage agreement and kappa coefficients among the 3 testing methods for these binary cut points and a summary statistic of all 4 AHI categories (< 5 events/h, 5–15 events/h, 15–30 events/h, and ≥ 30 events/h).
RESULTS
Sample characteristics
Of the 93 enrolled participants, 3 participants withdrew because of loss of interest and 6 participants were withdrawn when they refused to repeat the HSAT after the first attempt failed. The remaining 84 participants included in the final analysis had a mean age of 58.7 ± 16.3 years, a mean left ventricular ejection fraction of 40.3 ± 11.5%, and a body mass index of 29.4 ± 13.0 kg/m2, and 86.9% were male (Table 1). When compared to the U.S. participants (n = 17), the Chinese participants (n = 67) were younger in age (56.6 ± 17.2 vs. 69.9 ± 7.7 P = .038) and had a lower BMI (P = .012). In addition, Chinese participants had a longer total sleep time (P < .001), a greater percentage of stage N1 (P < .011) and stage N3/N4 (P < .011) sleep, and a lower percentage of stage N2 sleep (P < .001) according to PSG compared to the U.S. participants.
Table 1.
Participant characteristics overall and by site.
| Measures | Total | PKU | Penn VA | P* |
|---|---|---|---|---|
| General | ||||
| n | 84 | 67 | 17 | |
| Age, y | 58.7 ± 16.3 | 56.6 ± 17.2 | 66.9 ± 7.7 | .038 |
| Sex, n (%) | ||||
| Male | 73 (86.9) | 56 (83.6) | 17 (100) | .073 |
| Female | 11 (13.1) | 11 (16.4) | 0 | |
| BMI, kg/m2 | 29.4 ± 13.0 | 27.3 ± 5.2 | 37.4 ± 26.1 | .012 |
| LVEF, % | 40.3 ± 11.5 | 39.5 ± 11.2 | 43.5 ± 12.5 | .100 |
| LVEF < 50% (% of participants) | 71 (84.5) | 59 (88.1) | 12 (70.6) | .075 |
| Epworth Sleepiness Scale score | 8.6 ± 4.2 | 8.7 ± 3.9 | 8.5 ± 4.6 | .811 |
| COPD, n (%) | 11 (13.1) | 2 (3.0) | 9 (52.9) | < .001 |
| PSG results | ||||
| n | 79 | 65 | 14 | |
| Total sleep time, min | 354.4 ± 65.5 | 367.9 ± 61.7 | 291.6 ± 42.8 | < .001 |
| Sleep efficiency, % | 74.6 ± 11.9 | 74.5 ± 12.2 | 75.0 ± 10.4 | .964 |
| Sleep stages, % | ||||
| N1 | 17.6 ± 17.0 | 18.4 ± 15.1 | 13.6 ± 24.2 | .011 |
| N2 | 51.1 ± 16.9 | 47.4 ± 13.3 | 67.9 ± 21.7 | < .001 |
| N3/N4 | 9.1 ± 9.1 | 10.2 ± 9.0 | 4.2 ± 8.5 | .003 |
| REM sleep | 15.5 ± 7.5 | 15.8 ± 6.9 | 14.3 ± 9.8 | .449 |
| AHI 4%, events/h† | 23.8 ± 21.3 | 24.2 ± 20.6 | 22.0 ± 24.9 | .590 |
| OAI, events/h | 6.9 ± 10.8 | 7.7 ± 11.6 | 2.8 ± 3.9 | .007 |
| CAI, events/h | 7.3 ± 10.9 | 7.8 ± 10.9 | 4.8 ± 11.0 | .410 |
| Total number of OSA | 41.4 ± 70.0 | 75.4 ± 9.4 | 20.0 ± 5.3 | .003 |
| Total number of CSA | 44.1 ± 70.0 | 72.5 ± 9.0 | 55.4 ± 14.8 | .240 |
| Mean SpO2 during sleep, % | 94.1 ± 3.2 | 94.4 ± 3.0 | 92.5 ± 3.4 | .028 |
| Minimum SpO2 during sleep, % | 79.9 ± 11.8 | 80.1 ± 11.5 | 78.9 ± 13.8 | .920 |
Values are presented as mean ± standard deviation for continuous variables or n (%) for categorical variables. *P values from Mann-Whitney U test or Fisher’s exact test comparing patients from PKU and Penn VA. †Scoring of hypopneas required ≥ 4% oxygen desaturation according to HSAT and ≥ 4% oxygen desaturation and/or an arousal according to PSG. AHI = apnea-hypopnea index, BMI = body mass index, CAI = central apnea index, COPD = chronic obstructive pulmonary disease, CSA = central sleep apnea, HSAT = home sleep apnea test, LVEF = left ventricular ejection fraction, OAI = obstructive apnea index, OSA = obstructive sleep apnea, Penn VA = Crescenz Veterans Affairs Medical Center, PKU = Peking University People’s Hospital, PSG = polysomnography, REM = rapid eye movement, SpO2 = oxygen saturation.
Success rate of testing
The initial HSAT was unsuccessful in 10 of the 93 participants (10.8%), including the 6 participants who were withdrawn from the study. Four individuals performed the HSAT successfully on the second attempt, decreasing the failure rate to 6.5%.
Comparison of respiratory parameters
Figure 1 illustrates the percentage of participants with no SDB and mild, moderate, and severe SDB based on the AHI from PSG, the in-laboratory PM, and HSAT. Using a threshold of an AHI > 5 events/h, 73.4%, 76.9%, and 78.7% of participants were diagnosed with SDB according to PSG, the in-laboratory PM, and HSAT, respectively. There were no significant differences in the mean percentage of specific types of events across the 3 testing methods (P = .803; Figure 2). Compared to HSAT and the in-laboratory PM, PSG showed a lower number of hypopneas associated with ≥ 4% oxygen desaturation (P = .001), but the hypopnea index did not differ across the 3 sleep tests (Table S1 (311KB, pdf) in the supplemental material). Compared to PSG, mean oxygen saturation was significantly lower on both the HSAT (P < .001) and in-laboratory PM (P = .002). A separate comparison between the in-laboratory PM and PSG in the 2 centers indicated that mean SpO2 was significantly lower according to both the HSAT (P < .001) and in-laboratory PM (P = .003) in the Peking University People’s Hospital center. In the University of Pennsylvania center, with a small patient sample, mean SpO2 tended to be lower according to both the HSAT and in-laboratory PM, but it did not reach statistical significance (Table S2 (311KB, pdf) in the supplemental material). However, there was no significant difference in the oxygen desaturation index across the 3 types of tests (Table S1 (311KB, pdf) ).
Figure 1. Percentage of participants with and without sleep apnea.
Percentage of participants without sleep apnea (AHI < 5 events/h) and mild (5 ≤ AHI < 15 events/h), moderate (15 ≤ AHI < 30 events/h), and severe (AHI ≥ 30 events/h) sleep apnea according to PSG, the in-laboratory PM, and HSAT. Scoring of hypopneas in all 3 recordings required a ≥ 4% oxygen desaturation event. AHI = apnea-hypopnea index, HSAT = home sleep apnea test, PM = portable monitor, PSG = polysomnography, SDB = sleep-disordered breathing.
Figure 2. Percentage of different types of events diagnosed from PSG, the in-laboratory PM, and HSAT.
The mean ± SD percentage of different types of events (obstructive apnea, central apnea, mixed apnea and hypopnea) according to PSG, the in-laboratory PM, and HSAT. Scoring of hypopneas in all 3 recordings required a ≥ 4% oxygen desaturation event. HSAT = home sleep apnea test, PM = portable monitor, PSG = polysomnography, SD = standard deviation.
Agreement of AHI between monitoring methods
Bland-Altman and identity plots in Figure 3 compared an AHI of 4% according to PSG, HSAT, and the in-laboratory PM. The Bland-Altman analysis of an AHI of 4% using HSAT vs PSG showed a mean difference of –2.4 events/h (95% confidence interval [CI], –4.9 to –0.1; P = .057), with limits of agreement ranging from –24.1 to 19.2 events/h. The mean difference in the Bland-Altman plot of AHI using the in-laboratory PM vs PSG was only –0.5 events/h (95% CI, –1.7 to –0.7; P = .424) with narrower limits of agreement of –10.9 to 9.9 events/h. In both situations, there was evidence for a significant negative correlation between the difference and the mean, suggesting that the PM underestimates the AHI at higher AHI values. The squared correlation coefficient (R2) for AHI using PSG vs HSAT was 0.738 and 0.944, respectively, when comparing the PSG vs the in-laboratory PM, suggesting a high amount of shared variability. The closer relationship between PSG and the simultaneous in-laboratory PM supports the importance of differences in environment and night-to-night variability on sleep test results. Similar results were observed when the scoring of hypopneas on the PM required a ≥ 3% oxygen desaturation event and the scoring of hypopneas using PSG required a ≥ 3% oxygen desaturation event and/or an arousal (Figure S1 (311KB, pdf) in the supplemental material).
Figure 3. AHI 4% measured by HSAT and the in-laboratory PM compared to PSG.
Bland-Altman (A, C) and identity (B, D) plots of manually edited AHI 4% using PSG compared to manually edited HSAT (A, B) and the in-laboratory PM (C, D). Scoring of hypopneas in all 3 recordings required a ≥ 4% oxygen desaturation event. The solid line represents regression and the dashed line is the line of identity (B, D). AHI = apnea-hypopnea index, CI = confidence interval, HSAT = home sleep apnea test, PM = portable monitor, PSG = polysomnography.
Table 2 compares the diagnostic characteristics for the different cutoffs of the manually edited AHI 4% from the HSAT and in-laboratory PM compared to PSG. Using a threshold of AHI ≥ 5 events/h, we found that the HSAT had an 86.7% sensitivity, 76.5% specificity, 92.9% positive predictive value, and 61.9% negative predictive value. There was 84.4% agreement between the 2 methods, with a high kappa coefficient of 0.582 (Table 3). Similar results for HSAT were observed at AHI cutoffs of ≥ 10 and ≥ 15 events/h. The simultaneous in-laboratory PM showed similar or higher predictive results. Moreover, even when dividing the AHI 4% into 4 clinically meaningful groups (< 5 events/h, 5–15 events/h, 15–30 events/h, and ≥ 30 events/h), we continued to observe moderate to high reliability, with kappa coefficients of 0.540 for PSG vs HSAT, 0.624 for PSG vs the in-laboratory PM, 0.563 for HSAT vs the in-laboratory PM, and percentage agreements ranging from 64.9%–72.0% (Table 3). Similar results were observed when hypopneas were scored when associated with a ≥ 3% oxygen desaturation event according to the in-laboratory PM recording and a ≥ 3% oxygen desaturation event and/or an arousal according to PSG (Table S3 (311KB, pdf) and Table S4 (311KB, pdf) in the supplemental material).
Table 2.
Values for different cutoffs of manually edited AHI 4% measured by HSAT and the in-laboratory PM vs PSG.
| AHI 4%, Events/h | Prevalence | Sensitivity | Exact 95% CI | Specificity | Exact 95% CI | PPV | NPV | |||
|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | |||||||
| HSAT vs PSG | ≥ 5 | 77.9 | 86.7 | 74.9 | 93.7 | 76.5 | 49.8 | 92.2 | 92.9 | 61.9 |
| ≥ 10 | 58.4 | 95.6 | 83.6 | 99.2 | 75.0 | 56.2 | 87.9 | 84.3 | 92.3 | |
| ≥ 15 | 53.2 | 92.7 | 79.0 | 98.1 | 83.3 | 66.5 | 93.0 | 86.4 | 90.9 | |
| ≥ 30 | 40.3 | 71.0 | 51.8 | 85.1 | 97.8 | 87.0 | 99.9 | 95.7 | 83.3 | |
| In-laboratory PM vs PSG | ≥ 5 | 76.0 | 91.2 | 80.0 | 96.7 | 83.3 | 57.7 | 95.6 | 94.5 | 75.0 |
| ≥ 10 | 58.7 | 95.4 | 83.3 | 99.2 | 87.1 | 69.2 | 95.8 | 91.3 | 93.1 | |
| ≥ 15 | 53.3 | 97.5 | 85.3 | 99.9 | 91.4 | 75.8 | 97.8 | 92.9 | 97.0 | |
| ≥ 30 | 40.0 | 83.3 | 64.5 | 93.7 | 97.8 | 86.8 | 99.9 | 96.2 | 89.8 | |
Prevalence, sensitivity, specificity, PPV, and NPV for different cutoffs of manually edited AHI 4% from HSAT and the in-laboratory PM vs the PSG. Scoring of hypopneas required ≥ 4% oxygen desaturation according to HSAT and the in-laboratory PM and ≥ 4% oxygen desaturation and/or an arousal according to PSG. AHI = apnea-hypopnea index, CI = confidence interval, HSAT = home sleep apnea test, LB = lower bound, NPV = negative predictive value, PM = portable monitor, PPV = positive predictive value, PSG = polysomnography, UB = upper bound.
Table 3.
Comparison of categorized data from manually edited AHI derived from PSG, the in-laboratory PM, and HSAT in the kappa coefficient of agreement.
| HSAT vs PSG | In-Laboratory PM vs PSG | HSAT vs In-Laboratory PM | ||||
|---|---|---|---|---|---|---|
| Classification | Percentage Agreement | Kappa | Percentage Agreement | Kappa | Percentage Agreement | Kappa |
| AHI 4%* | ||||||
| Summary of all 4 AHI categories† | 64.9 | 0.540 | 72.0 | 0.624 | 66.7 | .563 |
| ≥ 5 events/h | 84.4 | 0.582 | 89.3 | 0.718 | 89.3 | .696 |
| ≥ 10 events/h | 87.0 | 0.725 | 92.0 | 0.833 | 88.0 | .738 |
| ≥ 15 events/h | 88.3 | 0.764 | 94.7 | 0.892 | 90.7 | .810 |
| ≥ 30 events/h | 87.0 | 0.718 | 92.0 | 0.830 | 88.0 | .728 |
*Scoring of hypopneas required ≥ 4% oxygen desaturation according to HSAT and the in-laboratory PM and ≥ 4% oxygen desaturation and/or an arousal according to PSG. †Patients classified into 4 AHI categories: AHI < 5 events/h, 5 ≤ AHI < 15 events/h, 15 ≤ AHI < 30 events/h, and ≥ 30 events/h. AHI = apnea-hypopnea index, HSAT = home sleep apnea test, PM = portable monitor, PSG = polysomnography.
Agreement of OAI, CAI, and CSR between monitoring methods
Good agreement was also found when comparing OAI and CAI across the 3 types of testing (Figure 4 and Figure 5). The Bland-Altman analysis of OAI and CAI using HSAT vs PSG showed a mean difference of –0.3 events/h (95% CI, –1.6 to –1.0; P = .646) and –1.1 events/h (95% CI, –2.5 to –0.3; P = .113), respectively, with limits of agreement ranging from –11.2 to 10.6 events/h and –13.2 to 10.9 events/h. The mean difference in the Bland-Altman plot of OAI and CAI on the in-laboratory PM vs PSG was 0.1 events/h (95% CI, –0.7 to –1.0; P = .774) and –1.0 events/h (95% CI, –2.0 to –0.1; P = .031), respectively, with limits of agreement of –7.2 to 7.4 events/h and –9.1 to 7.0 events/h. In both situations, there was a significant negative correlation between the difference and mean, suggesting that at higher OAI and CAI values the PM results in larger underestimates of OAI and CAI. The squared correlation coefficients (R2) for OAI and CAI were .750 and .689, respectively, comparing the PSG vs HSAT and .885 and .866, respectively, when comparing the PSG vs the in-laboratory PM, suggesting a high amount of shared variability.
Figure 4. OAI measured by HSAT and the in-laboratory PM compared to PSG.
Bland-Altman (A, C) and identity (B, D) plots of manually edited OAI according to PSG compared to manually edited HSAT (A, B) and the in-laboratory PM (C, D). The solid line represents regression and the dashed line is the line of identity (B, D). AHI = apnea-hypopnea index, HSAT = home sleep apnea test, OAI = obstructive apnea index, PM = portable monitor, PSG = polysomnography.
Figure 5. CAI measured by HSAT and the in-laboratory PM compared to PSG.
Bland-Altman (A, C) and identity (B, D) plots of manually edited CAI according to PSG compared to manually edited HSAT (A, B) and the in-laboratory PM (C, D). The solid line represents regression and the dashed line is the line of identity (B, D). AHI = apnea-hypopnea index, CAI = central apnea index, HSAT = home sleep apnea test, PM = portable monitor, PSG = polysomnography.
An example of the simultaneous in-laboratory PM and PSG recordings of CSR is shown in Figure S2 (311KB, pdf) in the supplemental material. The CSR pattern was present in 40.5% of participants according to PSG for an average of 20.9 ± 21.3% of total sleep time (range, 1.1%–82.6%). The Bland-Altman analysis of CSR% using HSAT vs PSG showed a mean difference of –0.9 (95% CI, –2.9 to –1.2; P = .405), with limits of agreement ranging from –18.4 to 16.7 (Figure S3 (311KB, pdf) in the supplemental material). The mean difference in the Bland-Altman plot of CSR% using the in-laboratory PM vs PSG was –0.1 events/h (95% CI, –1.9 to –2.1; P = .928) with limits of agreement of –17.0 to 17.2 events/h. Compared to PSG, the HSAT had a 96.9% sensitivity, 91.9% specificity, 88.6% positive predictive value, and 97.6% negative predictive value. The in-laboratory PM testing vs PSG showed similar results.
DISCUSSION
This is the first study to comprehensively validate HSAT using a type 3 PM to diagnose OSA, CSA, and CSR in adults with stable CHF. We found that HSAT had high sensitivity, specificity, and predictive values in diagnosing SDB when compared to PSG. Even closer agreement was present when the PM recording was obtained simultaneously with the PSG, thereby controlling for differences because of night-to-night variability and environment. In addition, we found that the PM had good agreement with PSG to identify OSA, CSA, and CSR. The ability to use HSAT to diagnose SDB can increase access to care for adults with CHF. Adults with CHF have increased physical disabilities, which may limit their ability to travel to a sleep laboratory for overnight testing. The need to travel away from home has become of particular concern during the COVID-19 pandemic. PMs can be mailed to and from these individuals, eliminating the need to travel to the sleep center and thereby reducing their risk of virus exposure.
The failure rate of 10.8% on initial HSAT in our participants with CHF was higher than in 2 earlier studies of adults without major comorbidities (6.3%) and with COPD (5.6%),11,16 but it was within the range of results reported by previous studies using a variety of PMs for HSAT in adults with comorbidities.23–25 The HSAT failure rate of the participants with CHF fell to 6.5% with repeat testing. Even accounting for the additional cost of repeat testing with either HSAT or in-laboratory PSG, these failure rates would more likely than not reduce the overall cost of testing compared to just using PSG and improve access to testing for adults with CHF. The good agreement of HSAT results with in-laboratory PSG and the acceptable failure rate of initial HSAT showed the ability to use the Nox-T3 portable monitor for HSAT to diagnose SDB and identify different types of SDB, improving access to diagnosis and treatment for patients with CHF. However, the participants in our study had a fairly young average age; it is necessary to replicate findings in older patients with CHF. Differences were observed between Chinese and U.S. participants. Chinese participants were less obese but had similar AHI to U.S. participants, which may be explained by that Chinese patients with OSA had more craniofacial abnormalities. Furthermore, the small sample size of U.S. participants may contribute to the differences in age and prevalence of COPD between two centers.
Although PM is currently being used clinically to evaluate SDB in patients hospitalized with CHF,24,26–28 there are a limited number of studies evaluating the accuracy of PM to diagnose SDB in adults with stable and unstable CHF.17,25,29 Aurora et al17 reported robust agreement of AHI between an attended type 3 PM recording and PSG performed simultaneously at bedside before discharge in adults admitted to the hospital with decompensated CHF. Khayat, Jarjoura, Patt, et al25 reported good agreement in AHI between attended in-laboratory type 3 PM recording in adults hospitalized with decompensated CHF and PSG obtained 6 to 8 weeks after discharge. Agreement of AHI between HSAT and PSG in the study of Smith et al29 was poor because of technical problems, low sleep efficiency, positional differences, and small sample size. Our study confirms and extends the findings of Aurora et al17 and Khayat, Jarjoura, Patt, et al25 by validating self-applied HSAT in adults with medically stable CHF. The sample size was larger, and the participants with CHF were all stable on optimized treatment in our study.
Adults with CHF are more likely to have CSA and CSR.30,31 It is therefore important that HSAT be capable of identifying both of these conditions. Aurora et al17 reported good agreement in CAI and a similar proportion of CSR between a simultaneous attended PM and PSG. Another study evaluated the ability of the nasal cannula pressure signal to detect CSR.32 Compared to simultaneously recorded PSG, the nasal cannula results had 87.1% sensitivity and 94.9% specificity. Our study evaluated the diagnostic value of a PM for identifying CSA and CSR at home and in the laboratory. Bland-Altman analysis of CAI using HSAT recording vs PSG revealed a mean difference of –1.1 (95% CI, –2.5 to 0.3) events/h with limits of agreement of –13.2 to 10.9 events/h in our study. We obtained a sensitivity of 96.9% and a specificity of 91.1% according to HSAT vs PSG. Together with the findings from a previous study on OSA without major comorbidity and with fewer CSAs, data in the current study indicated that the Nox-T3 PM with both nasal flow and a respiratory inductance plethysmography system may have an advantage to identify both OSA and CSA-CSR.11 This could be attributed to the technique of respiratory inductance plethysmography used by the Nox system to detect respiratory movement. However, this hypothesis remains to be investigated in the future.
Adults with CHF have relatively low baseline oxygen saturation compared to individuals without comorbidities. Although the AHI was not significantly different between the PM recordings and PSG in our study, a greater number of hypopneas were identified from HSAT than from PSG. This finding may have resulted from lower mean oxygen saturation on the PM compared to PSG. Notably, however, there was no significant difference in the oxygen desaturation index between the PM and PSG. These findings were similar to previous results in adults without comorbidities and in adults with COPD.11,16 Differences in the oxygen saturation between the PM recordings and PSG were likely found because of differences among pulse oximeters. As noted in patients with overlap syndrome (COPD and OSA),16 standardized pulse oximetry calibration across manufacturers is also needed to help clinical decision of oxygen therapy in SDB patients with CHF.
The strength of our study is its evaluation of HSAT using a type 3 PM to help diagnose SDB and identify different types of SDB including OSA, CSA, and CSR in adults with stable CHF. The ability to accurately distinguish OSA and CSA in adults with CHF is of particular importance given the relatively high prevalence of CSA in adults with CHF and the different management approaches to CSA and OSA. Our study also had some limitations. First, our study did not assess night-to-night variability, which could have been evaluated if 2 PSGs and 2 HSATs had been performed. However, the comparison of the simultaneous in-laboratory PM and PSG helped address this issue. Second, the participants were recruited from a group of adults with stable CHF referred to a sleep center who likely had a high pretest probability of SDB. The results might differ in a community-based population of individuals with stable CHF with a lower probability of SDB. Furthermore, larger studies are needed that build on these findings to assess how to select patients who are unlikely to be able to perform HSAT successfully.
In summary, our study validates the use of HSAT to help diagnose SDB and identify OSA, CSA, and CSR events in adults with stable CHF. AHI had good agreement between PSG and HSAT, and even closer agreement was observed between the simultaneous in-laboratory PM and PSG. Adults with CHF are at increased risk of having CSA and CSR. The HSAT showed good agreement with PSG in being able to differentiate OSA vs CSA and identify the presence of CSR.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript for publication. Work for this study was performed at the Peking University People’s Hospital and the University of Pennsylvania. S.T.K was supported by the National Institutes of Health (HL094307) and receives grant support from Philips Respironics. F.H. was supported by research grants from the Beijing Municipal Science and Technology Commission (Z161100002616012). L.X. was supported by the Young Elite Scientists Sponsorship Program of the China Association for Science and Technology. Potential conflicts of interest: Dr. Pack is the John L. Miclot Professor of Medicine at the University of Pennsylvania; funds for this endowment were provided by the Philips Respironics Foundation. The remaining authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
Author contributions: S.L., L.X., A.I.P., F.H., and S.T.K. contributed to the study design. S.L., L.X., Y.C., X.Z., T.B., B.Z., Y.Y., and S.T.K. contributed to the data collection. S.L., L.X., B.T.K., and S.T.K. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S.L., L.X., F.H., A.I.P., B.T.K., X.D., and S.T.K. drafted and revised the article.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CAI
central apnea index
- CHF
chronic heart failure
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CSA
central sleep apnea
- CSR
Cheyne-Stokes respiration
- HSAT
home sleep apnea testing
- OAI
obstructive apnea index
- OSA
obstructive sleep apnea
- PM
portable monitor
- PSG
polysomnography
- SDB
sleep-disordered breathing
REFERENCES
- 1.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–2159. 10.1161/01.CIR.97.21.2154 [DOI] [PubMed] [Google Scholar]
- 2.Herrscher TE, Akre H, Øverland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17(5):420–425. 10.1016/j.cardfail.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Arzt M, Woehrle H, Oldenburg O, et al. ; SchlaHF Investigators . Prevalence and predictors of sleep-disordered breathing in patients with stable chronic heart failure: the SchlaHF registry. JACC Heart Fail. 2016;4(2):116–125. 10.1016/j.jchf.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625–1631. 10.1016/j.jacc.2006.12.046 [DOI] [PubMed] [Google Scholar]
- 5.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. 10.1161/01.CIR.99.11.1435 [DOI] [PubMed] [Google Scholar]
- 6.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–366. 10.1164/rccm.200306-752OC [DOI] [PubMed] [Google Scholar]
- 7.Bradley TD, Logan AG, Kimoff RJ, et al. ; CANPAP Investigators . Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025–2033. 10.1056/NEJMoa051001 [DOI] [PubMed] [Google Scholar]
- 8.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. 10.1056/NEJMoa022479 [DOI] [PubMed] [Google Scholar]
- 9.Arzt M, Floras JS, Logan AG, et al. ; CANPAP Investigators . Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation. 2007;115(25):3173–3180. 10.1161/CIRCULATIONAHA.106.683482 [DOI] [PubMed] [Google Scholar]
- 10.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716–1722. 10.1001/archinte.166.16.1716 [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Han F, Keenan BT, et al. Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in Chinese adults. J Clin Sleep Med. 2017;13(5):675–683. 10.5664/jcsm.6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittle AT, Finch SP, Mortimore IL, MacKay TW, Douglas NJ. Use of home sleep studies for diagnosis of the sleep apnoea/hypopnoea syndrome. Thorax. 1997;52(12):1068–1073. 10.1136/thx.52.12.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portier F, Portmann A, Czernichow P, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162(3 Pt 1):814–818. 10.1164/ajrccm.162.3.9908002 [DOI] [PubMed] [Google Scholar]
- 14.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasoni D, Italia L, Adamo M, et al. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22(6):957–966. 10.1002/ejhf.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Y, Xu L, Han F, et al. Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. J Clin Sleep Med. 2019;15(4):587–596. 10.5664/jcsm.7720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aurora RN, Patil SP, Punjabi NM. Portable sleep monitoring for diagnosing sleep apnea in hospitalized patients with heart failure. Chest. 2018;154(1):91–98. 10.1016/j.chest.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–2550. 10.1093/eurheartj/ehm037 [DOI] [PubMed] [Google Scholar]
- 19.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. 10.1093/sleep/28.4.499 [DOI] [PubMed] [Google Scholar]
- 20.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346(8982):1085–1087. 10.1016/S0140-6736(95)91748-9 [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. 10.1016/S0140-6736(86)90837-8 [DOI] [PubMed] [Google Scholar]
- 23.Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463–1469. 10.1093/eurheartj/ehu522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kauta SR, Keenan BT, Goldberg L, Schwab RJ. Diagnosis and treatment of sleep disordered breathing in hospitalized cardiac patients: a reduction in 30-day hospital readmission rates. J Clin Sleep Med. 2014;10(10):1051–1059. 10.5664/jcsm.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15(9):739–746. 10.1016/j.cardfail.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khayat RN, Javaheri S, Porter K, et al. In-hospital management of sleep apnea during heart failure hospitalization—a randomized controlled trial. J Card Fail. 2020;26(8):705–712. 10.1016/j.cardfail.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khayat RN, Abraham WT, Patt B, Pu M, Jarjoura D. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009;136(4):991–997. 10.1378/chest.09-0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18(7):534–540. 10.1016/j.cardfail.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith LA, Chong DW, Vennelle M, Denvir MA, Newby DE, Douglas NJ. Diagnosis of sleep-disordered breathing in patients with chronic heart failure: evaluation of a portable limited sleep study system. J Sleep Res. 2007;16(4):428–435. 10.1111/j.1365-2869.2007.00612.x [DOI] [PubMed] [Google Scholar]
- 30.Rudrappa M, Bollu PC. Cheyne Stokes Respirations. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK448165/. Accessed April 1, 2021. [PubMed] [Google Scholar]
- 31.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141–163. [DOI] [PubMed] [Google Scholar]
- 32.Weinreich G, Armitstead J, Töpfer V, Wang YM, Wang Y, Teschler H. Validation of ApneaLink as screening device for Cheyne-Stokes respiration. Sleep. 2009;32(4):553–557. 10.1093/sleep/32.4.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.