Abstract
Study Objectives:
Acquiring a better comprehension of obstructive sleep apnea physiopathology can contribute to improving patient selection for surgical treatments. We hypothesize that maxillary transverse deficiency restricts the space available for the tongue, leading to upper airway obstruction during sleep. Our primary hypothesis was that maxillary transverse deficiency increases the prevalence of tongue collapse during drug-induced sleep endoscopy (DISE). The secondary hypothesis was that maxillary transverse deficiency will also increase the prevalence of circumferential collapse at the velopharynx. The exploratory hypothesis was that maxillary transverse deficiency is associated with increased obstructive sleep apnea severity. The objectives of this study were to correlate maxillary morphometric measurements with (1) the anatomic level of obstruction during DISE and (2) the apnea-hypopnea index on polysomnography.
Methods:
We made a cross-sectional analysis of patients with obstructive sleep apnea undergoing DISE in search of positive airway pressure alternative treatment. Maxillary measurements were collected from a computed tomography scan (interpremolar distance, intermolar distance [IMD] and sella-nasion A point angle), findings from DISE, and sleep study variables from polysomnography. Correlation between computed tomography, DISE, and polysomnography data was assessed using Pearson’s correlation, and receiver operating characteristic curves were determined for each facial measurement.
Results:
Sixty-nine patients were included in the study. The group with velopharyngeal circumferential collapse had mean IMD = 26.30 mm (25.5–31.45), and the group with anteroposterior collapse had mean IMD = 29.20 mm (26.8–33.10; P = .040). The group with complete tongue-base obstruction had mean interpremolar distance = 26.40 mm (25.1–28) and IMD = 26.30 mm (25.6–28.4), and the group without obstruction had mean interpremolar distance = 28.7 mm (27.2–30; P = .003) and IMD = 34.06 mm (32.1–37; P < .001). The receiver operating characteristic curve determined an IMD cutoff of 29.8 mm for predicting tongue-base obstruction.
Conclusions:
The maxillary transverse deficiency, identified by reduction in interpremolar distance and IMD, predicted the occurrence of complete tongue-base obstruction, complete concentric collapse at the velopharynx, and multilevel obstruction during DISE. We did not find an association between the maxillary measurements and obstructive sleep apnea severity. These associations hold some promise in ultimately supplanting insights previously available only through DISE.
Citation:
Thuler E, Rabelo FAW, Yui M, Tominaga Q, dos Santos V Jr, Arap SS. Correlation between the transverse dimension of the maxilla, upper airway obstructive site, and OSA severity. J Clin Sleep Med. 2021;17(7):1465–1473.
Keywords: OSA, DISE, tongue-base obstruction, maxillary atresia, intermolar distance
BRIEF SUMMARY
Current Knowledge/Study Rationale: Despite previous evidence suggesting a role for maxillary transverse deficiency in obstructive sleep apnea, its pathophysiology has not been completely established. We hypothesized that maxillary transverse deficiency restricts the space available for the tongue at the hard palate, which increases the prevalence of tongue-base collapse and the susceptibility to upper airway obstruction during sleep.
Study Impact: The maxillary transverse deficiency, identified by reductions in interpremolar distance and intermolar distance, predicted the occurrence of tongue-base obstruction, concentric collapse at the velopharynx, and multilevel obstruction during drug-induced sleep endoscopy. These associations hold some promise in ultimately supplanting insights previously available only through drug-induced sleep endoscopy. If these observations are further verified, then the clinical applicability of these measures may improve the outcomes of obstructive sleep apnea surgical procedures.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of breathing interruptions during sleep, despite continued respiratory efforts.1 This condition is attributed to upper airway collapse due to anatomical vulnerability and impaired neuromuscular activity.2 The standard treatment is continuous positive airway pressure, which is capable of keeping the pharyngeal walls sustained, preventing upper airway collapse. However, about 50% of patients are not adherent to this treatment in the long term, which compromises its efficacy.3 The multifactorial etiology of OSA reinforces the importance of differentiating phenotypes and personalizing treatment,4 mainly with regard to surgical patients, whose selection for treatment relies on strict criteria, according to most scientific evidence.5
Impairment of facial development is a determining factor in OSA pathophysiology. A low position of the hyoid bone and the increased length of the pharynx are anatomical findings that have already been associated with the risk of this disease.6 However, most studies were based only on lateral cephalometry and did not analyze the transverse dimension of the face, even though the maxillary transverse deficiency has been previously described in OSA, which means that its pathogenic role has not yet been well established.7 Such facial feature can be properly treated with surgically assisted rapid maxillary expansion (SARME). This procedure has shown promising results in OSA treatment,8,9 despite the transverse measurement of the maxilla remaining relatively unexplored as a potential predictor of OSA treatment outcomes.
The purpose of the current study was to analyze the association between the maxillary transverse dimensions and the anatomical site of upper airway obstruction, the pattern of collapse, and OSA severity. The primary hypothesis was that maxillary transverse deficiency restricts the space available for the tongue, increasing the prevalence of tongue collapse (partial and complete). The secondary hypothesis was that maxillary transverse deficiency will also increase the prevalence of circumferential collapse at the velopharynx. The exploratory hypothesis was that maxillary transverse deficiency is associated with increased OSA severity. We tested these hypotheses by analyzing the correlation between the anatomical measurements of maxillary transverse dimension, the obstructive sites (velum, oropharynx, tongue, epiglottis score) detected during drug-induced sleep endoscopy (DISE), and the sleep study variables from polysomnography.
METHODS
This was a cross-sectional study using chart review data from consecutive patients consulted from January 2017 to January 2019 at an otolaryngology outpatient clinic not specialized in skeletal surgery. Research project was approved by the Research Ethics Board (REB (09821619.5.0000.4561)).
Patients were adults presenting with OSA diagnosed by overnight type 1 polysomnography who were intolerant of continuous positive airway pressure. Patients included also needed to have data available from DISE and facial computed tomography. Patients without all required data who presented with palatine tonsil hypertrophy (grades 3 and 4), lingual tonsil hypertrophy (grades 3 and 4), congenital craniofacial deformity, severe obesity (body mass index > 40), and previous surgical treatment for OSA were excluded.
Polysomnography was scored according to The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (Version 2.0, 2012)10 and OSA was classified by the total apneas and hypopneas per hour of sleep as mild (apnea-hypopnea index [AHI] ≥ 5 and < 15 events/h), moderate (AHI ≥ 15 and < 30 events/h), and severe (AHI ≥ 30 events/h).
DISE was performed in order to detect the upper airway obstructive site and identify the pattern of collapse leading to OSA. All tests were performed with the patient in the supine position by an experienced otolaryngologist, following a protocol able to reproduce the AHI from polysomnography,11 using a propofol target-controlled infusion pump and bispectral index monitoring. The scoring was blinded to other clinical data, according to velum, oropharynx, tongue, epiglottis classification.12,13
Facial computed tomography scans (DICOM format files) were saved and posteriorly analyzed by a trained physician also blinded to the clinical data using the Osirix DICOM Viewer program (Pixmeo SARL, Geneva, Switzerland), which allows real measurements of the structures. The following measures were obtained from the maxilla: the angle between the sella-nasion A point, which indicates the anteroposterior position of the maxilla; interpremolar distance (IPMD), measured in millimeters on the lingual side at the edge of the palatal alveolar bone (Figure 1); and intermolar distance (IMD), measured in millimeters on the lingual side at the edge of the palatal alveolar bone (Figure 2).
Figure 1. Interpremolar distance.
Facial CT scan in coronal (left), axial (center), and sagittal (right) sections, which shows how the interpremolar distance was obtained in the maxilla. CT = computed tomography.
Figure 2. Intermolar distance.
Facial CT scan in sagittal (left), coronal (center), and axial (right) sections, which shows how the intermolar distance (IMD) was obtained in the maxilla. CT = computed tomography.
Although the literature refers to the dental crown as an anatomical reference to measure the transverse dimension of the maxilla, a systematic review has demonstrated that it has been inaccurate and unreliable, compared with skeletal arch width measurements. Computed tomography images were more accurate and reliable, even though a gold standard method is still debated.14 In accord with the objective of this study, to evaluate the skeletal component of the maxillary constriction, the width was measured on the lingual side at the palatal alveolar bone,15 in order to avoid the bias induced by buccolingual teeth inclination, which could camouflage the true transverse deficiency.
Sample size was based on a previous study that had shown a difference of 5 mm between the molars when maxillary transverse measurements were compared in patients with OSA and control patients.7 Assuming a 3-unit (mm) difference between the molars of individuals with mild OSA and those with severe OSA, a sample of 58 individuals was calculated to be sufficient, considering a population standard deviation of 4 mm, a significance level of 5%, and a test power of 80%.
A Mann-Whitney U test and a Kruskal-Wallis test with a Müller-Dunn posttest were applied to compare the groups, whenever it was required. Sample and Pearson correlations were calculated to quantify linear association between continuous variables. Receiver operating characteristic curves were determined for each measurement to show the trade-off between clinical sensitivity and specificity for every possible cutoff. A conditional inference tree, a nonparametric multivariate regression model, was applied to classify the lack of tongue-base collapse from IMD values. We used the partykit library of R 3.5.1 software (https://cran.r-project.org). The significance level was set at 5%.
RESULTS
During the period in which this study was conducted, 146 individuals were evaluated and 69 met all inclusion criteria (12 women [17.4%] and 57 men [82.6%]). Demographic data of the patients are summarized in Table 1. The mean IPMD, IMD, and sella-nasion A point were 26.9 mm (25.4–28.2), 28.50 mm (26.3–32.6), and 83.63° (80.9–85.5), respectively.
Table 1.
Populational descriptive data and DISE findings, according to the VOTE classification.
| Variables | Median | Percentile 25 | Percentile 75 |
|---|---|---|---|
| Age (y) | 41 | 33 | 48 |
| BMI (kg/m2) | 26 | 25 | 28 |
| AHI (events/h) | 18.8 | 10.6 | 30.2 |
| Apnea index (events/h) | 3.9 | 1.0 | 11.2 |
| Hypopnea index (events/h) | 13.4 | 6.3 | 17.2 |
| O2 nadir (%) | 84.6 | 78.0 | 91.2 |
| IPMD (mm) | 26.9 | 25.42 | 28.2 |
| IMD (mm) | 28.5 | 26.3 | 32.6 |
| SNA (degrees) | 83.6 | 80.9 | 85.5 |
| Obstructive Site | Grade | Number | % |
| Velopharynx | 1 | 7 | 10.10 |
| 2 | 62 | 89.90 | |
| Oropharynx | 0 | 2 | 2.90 |
| 1 | 53 | 76.80 | |
| 2 | 14 | 20.30 | |
| Tongue | 0 | 23 | 33.30 |
| 1 | 25 | 36.20 | |
| 2 | 21 | 30.40 | |
| Epiglottis | 0 | 57 | 82.60 |
| 1 | 4 | 5.80 | |
| 2 | 8 | 11.60 |
AHI = apnea-hypopnea index, BMI = body mass index, DISE = drug-induced sleep endoscopy, IMD = intermolar distance, IPMD = interpremolar distance, SNA = sella-nasion A point angle, VOTE = velum, oropharynx, tongue, epiglottis.
Regarding DISE findings, according to velum, oropharynx, tongue, epiglottis classification, 62 individuals (89.9%) presented with complete collapse (grade 2) at the velopharynx, 14 individuals (20.3%) had complete collapse at the oropharynx, and 21 individuals (30.4%) had complete collapse at the tongue base.
The primary collapse of the epiglottis was observed in 8 individuals (11.6%). Multilevel collapse of the Upper Airway (UA) was seen in 62.3% of patients. Mild hypertrophy of the lingual tonsil (grades 1 and 2) was present in 30.4% of individuals, and 69% had no hypertrophy (grade 0) (Table 1).
The statistical analysis of the correlation between the maxillary measurements and DISE findings was made using the intensity (degree) and pattern of collapse at each site according to the velum, oropharynx, tongue, epiglottis classification (Table 2).
Table 2.
Association between maxillary measurements and the collapse detected during DISE.
| Maxillary Measurements | Degree of Collapse (Velopharynx) | Mann-Whitney U Test | |||
|---|---|---|---|---|---|
| Median (mm) | 1 | 2 | |||
| IPMD | 25.9 | 27.3 | 0.306 | ||
| IMO | 29.2 | 28.5 | 0.612 | ||
| SNA angle (°) | 85.85 | 83.46 | 0.116 | ||
| Maxillary Measurements | Pattern of Collapse (Velopharynx) | Mann-Whitney U Test | |||
| Median (mm) | AP | completer circumferential (cc) | |||
| IPMD | 27.0 | 26.2 | 0.416 | ||
| IMO | 29.2 | 26.3 | 0.040 | ||
| SNA angle (°) | 84.03 | 82.67 | 0.583 | ||
| Maxillary Measurements | Degree of Collapse (Oropharynx) | Kruskal-Wallis Test, 0 vs 1 vs 2 | |||
| Median (mm) | 0 | 1 | 2 | ||
| IPMD | 25.15 | 27.00 | 26.75 | 0.758 | |
| IMO | 31.80 | 28.60 | 26.45 | 0.160 | |
| SNA angle (°) | 84.86 | 84.03 | 82.96 | 0.206 | |
| Maxillary Measurements | Degree of Collapse (Tongue Base) | Mann-Whitney U Test | |||
| Median (mm) | 0 | 1 | 2 | ||
| IPMD | 28.7 | 26.5 | 26.4 | 0.003 | |
| IMO | 34.1 | 26.8 | 26.3 | <0.001 | |
| SNA angle (°) | 84.36 | 84.26 | 81.84 | 0.077 | |
| Maxillary Measurements | Degree of Collapse (Epiglottis) | Kruskal-Wallis Test, 0 vs 1 vs 2 | |||
| Median (mm) | 0 | 1 | 2 | ||
| IPMD | 27.5 | 26.3 | 25.9 | 0.242 | |
| IMO | 30.2 | 26.4 | 28.2 | 0.206 | |
| SNA angle (°) | 83.61 | 84.58 | 83.55 | 0.858 | |
DISE = drug-induced sleep endoscopy, IMD = intermolar distance, IPMD = interpremolar distance, SNA = sella-nasion A point angle.
Starting from the velopharynx, we found no association between the facial measurements and the degree of obstruction. However, we found at this site an inverse association between the IMD and the circumferential pattern of collapse, where the group with this pattern had a mean distance of 26.30 mm (25.5–31.45) and the individuals who presented with an anteroposterior pattern presented a distance of 29.20 mm (26.8–33.10; P = .040). The receiver operating characteristic curve (sensitivity × 1-specificity) demonstrated an area under the curve of 0.66 (0.50–0.81), using a cutoff value of 27.5 mm (Figure 3).
Figure 3. Intermolar distance predicting the circumferential pattern of collapse at the velopharynx.
ROC curve (sensitivity × 1-specificity) analyzing the value of intermolar distance using a cutoff value of 27.5 mm in predicting the circumferential pattern of collapse at the velopharynx. AUC = area under the ROC curve, CI = confidence interval, ROC = receiver operating characteristic.
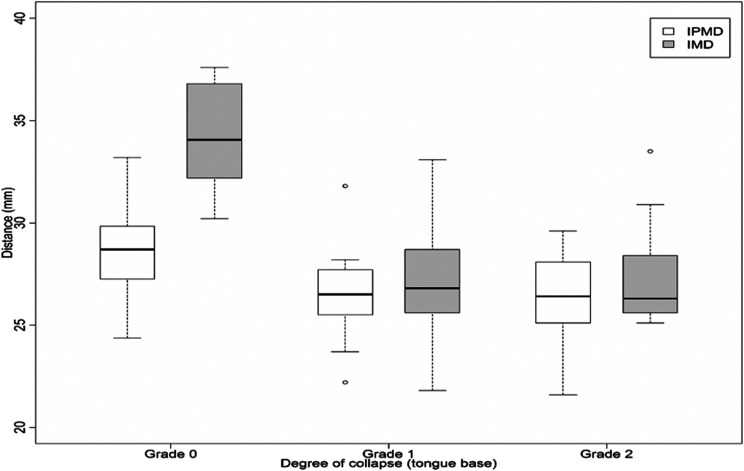
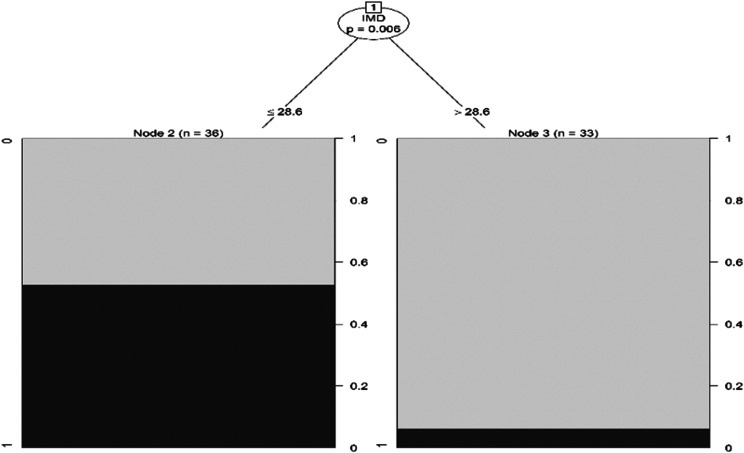
There was also an inverse association between the occurrence of tongue-base collapse and IPMD (P = .003) and IMD (P < .001), such that the group with complete obstruction (grade 2) had lower values (IPMD = 26.40 mm [25.1–28] and IMD = 26.30 mm [25.6–28.4]) than the group without obstruction (IPMD = 28.7 mm [27.2–30] and IMD = 34.06 mm [32.1–37]) (Figure 4). The receiver operating characteristic curve presented an area under the curve of 0.96 (0.91–0.99) based on a cutoff value of 29.8 mm (Figure 5). Moreover, decision tree analysis also demonstrated that this same value of IMD was able to predict the concomitant complete collapse (grade 2) at the tongue base and the velopharynx (Figure 6).
Figure 4. Association between intermolar distance and interpremolar distance and the degree of obstruction.
Boxplot representing the association between intermolar distance and interpremolar distance and the degree of obstruction found at tongue base during DISE (grade of obstruction according to VOTE classification). Mann-Whitney U test was used to analyze the correlation between tongue-base collapse and the facial measurements. DISE = drug-induced sleep endoscopy, grade 0 = no obstruction, grade 1 = partial collapse, grade 2 = complete collapse, IMD = intermolar distance, IPMD = interpremolar distance, VOTE = velum, oropharynx, tongue, epiglottis.
Figure 5. Intermolar distance predicting the absence of tongue-base collapse.
ROC curve (sensitivity × 1 specificity) analyzing the value of IMD in predicting the absence of tongue-base collapse. AUC = area under the ROC curve, CI = confidence interval, IMD = intermolar distance, ROC = receiver operating characteristic.
Figure 6. Intermolar distance prediction of the association between the complete collapse at tongue base and at velopharynx.
Decision tree demonstrating the IMD in the prediction of the association between the complete collapse (VOTE grade 2) at the tongue base and the complete collapse at the velopharynx. The IMD value ≤ 28.9 mm correlates with the concomitant collapse at the tongue base and at the velopharynx. IMD = intermolar distance, VOTE = velum, oropharynx, tongue, epiglottis classification.
We did not find an association between the maxillary measurements and the degree of collapse at the oropharynx, the obstruction caused by the epiglottis, or OSA severity. We made a multivariable analysis (Pearson statistical test) and found no correlation among IPMD, IMD, sex, and age.
DISCUSSION
This is the first study to correlate maxillary transverse deficiency with obstructive sites detected during DISE and OSA severity. The most relevant result in our sample, based on the statistical analysis, was the association between IPMD and IMD with tongue-base obstruction. IMD was also associated with circumferential collapse pattern at the velopharynx. The area under the receiver operating characteristic curve demonstrated that the IMD, at a cutoff value of 29.8 mm, predicted the occurrence of tongue-base obstruction. Circumferential collapse at the velopharynx could be predicted at a cutoff value of 27.5 mm. Multivariable statistical analysis demonstrated that IMD also predicted the occurrence of multilevel obstruction, both at the velopharynx and tongue base simultaneously. Although sex differences were expected, multivariable analysis showed no correlation with IPMD and IMD, which is compatible with another study using linear measurements to evaluate maxillary width.15
It is paramount to highlight that IMD may predict the occurrence of tongue-base obstruction and a circumferential pattern of collapse at the velopharynx, possibly obviating the need for DISE. Since this measure is easily obtainable, its use in clinical practice will facilitate the identification of types of collapse that potentially do not respond to most surgical treatments proposed for OSA. Circumferential collapse at the velopharynx has already been associated with worse clinical outcomes in patients who have undergone pharyngeal surgeries16 and upper airway stimulation treatment.17
Maxillomandibular advancement treats this type of collapse18 by enlarging the anteroposterior dimension of the pharynx. However, in a study using DISE to evaluate patients who have undergone maxillomandibular advancement, tongue-base collapse was not treated effectively.19 The fact that the standard maxillomandibular advancement procedure described for OSA20 did not treat the transverse dimension of the maxilla may explain why tongue-base obstruction persisted in some patients, further emphasizing the strength of the finding. Therefore, the association found between reductions in IMD and both the tongue base and circumferential collapse at the velopharynx may direct patient selection for specific OSA surgical treatment, since most procedures do not enlarge the transverse deficiency of the maxilla.
The development of the transverse deficiency occurs due to multiple factors, including behavioral ones during childhood (eg, mouth breathing, difficulties in breastfeeding, and pacifier use).21 Thus, the reduced effect of tongue pressure over the upper jaw jeopardizes the development of the transverse dimension of the hard palate, which restricts space for the tongue, explaining why the upper airway could be more susceptible to a tongue-driven collapse during sleep.22 For this reason, we encourage caution in proposing hypopharyngeal surgery for patients with OSA, once this facial phenotype is identified. If this type of tongue-base obstruction is not appreciated, then surgical responses may deteriorate, which can also explain why using only DISE to select patients for tongue-base reduction in OSA treatment has not improved surgical outcomes.23
A treatment option for maxillary transverse deficiency is surgical-assisted rapid maxillary expansion, which has already been used with promising results to treat adult patients with OSA.24 Our previous study showed that there was a correlation between an increase in IPMD/IMD and an increase in hypopharyngeal area and the AHI reduction after surgical-assisted maxillary expansion,25 suggesting that both post-surgical improvements were related to increased space for the tongue that improved its position and stability while preventing collapse during sleep. Those results are consistent with our current data and corroborated by other studies aimed at understanding the pathophysiological mechanism of maxillary transverse deficiency in OSA.22,26
We acknowledge that our study has some limitations regarding its reproducibility and external validity. First, we recognize that it is based on a cross-sectional analysis of consecutive patients who were nonobese patients with mild/moderate OSA and who were not continuous positive airway pressure–adherent. DISE also presents variability in sedation protocol, scoring system, and findings interpretation.27,28 Although we used computed tomography images to measure the maxillary transverse deficiency, the lingual face of the alveolar bone is an anatomical reference easy to use in clinical practice, and measurements can be obtained using a dental caliper or a gauge with no need for a computed tomography scan.
Nevertheless, our study establishes a statistically significant association between maxillary transverse deficiency, circumferential collapse at the velopharynx, and tongue-base collapse, providing insight into the interpretation of findings detected during DISE. However, further studies must be carried out to analyze the correlation of these measures with the clinical outcomes of the different treatment options available for OSA, especially pharyngeal surgeries, tongue-base surgeries, and upper airway stimulation, in order to determine the role of these measurements in patient selection and improved surgical success.
CONCLUSIONS
In the group of individuals evaluated in this study, maxillary transverse deficiency, as marked by reductions in IPMD and IMD measured in computed tomography images, predicted the occurrence of tongue-base obstruction, concentric collapse at the velopharynx, and multilevel obstruction discovered during DISE. Even though we have not found an association between maxillary measurements and OSA severity, these associations hold some promise ultimately to supplant the insights previously available only through DISE. If our observations are further verified, then the clinical applicability of measuring reductions in IPMD and IMD may improve the outcomes of OSA surgical procedures.
DISCLOSURE STATEMENT
All authors have read and approved the final manuscript. The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Alan R. Schwartz for his contribution to the final version of this paper. Author contributions: Eric Thuler: study design, data analysis, and manuscript writing; Fábio A.W. Rabelo: data analysis and manuscript review; Mariane Yui: data collection and manuscript review; Quedayr Tominaga: data collection; Vanier dos Santos Jr: data collection; Sergio Samir Arap: study design, data analysis, and manuscript review.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- DISE
drug-induced sleep endoscopy
- IPMD
interpremolar distance
- IMD
intermolar distance
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51(4):313–323. 10.1016/j.pcad.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144–153. 10.1513/pats.200707-114MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokhlesi B, Ayas NT. Cardiovascular events in obstructive sleep apnea—can CPAP therapy save lives? N Engl J Med. 2016;375(10):994–996. 10.1056/NEJMe1609704 [DOI] [PubMed] [Google Scholar]
- 4.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea—new pathways for targeted therapy. Sleep Med Rev. 2018;37:45–59. 10.1016/j.smrv.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Verse T, Dreher A, Heiser C, et al. ENT-specific therapy of obstructive sleep apnoea in adults: a revised version of the previously published German S2e guideline. Sleep Breath. 2016;20(4):1301–1311. 10.1007/s11325-016-1353-9 [DOI] [PubMed] [Google Scholar]
- 6.Neelapu BC, Kharbanda OP, Sardana HK, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79–90. 10.1016/j.smrv.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 7.Seto BH, Gotsopoulos H, Sims MR, Cistulli PA. Maxillary morphology in obstructive sleep apnoea syndrome. Eur J Orthod. 2001;23(6):703–714. 10.1093/ejo/23.6.703 [DOI] [PubMed] [Google Scholar]
- 8.Yoon A, Guilleminault C, Zaghi S, Liu SYC. Distraction osteogenesis maxillary expansion (DOME) for adult obstructive sleep apnea patients with narrow maxilla and nasal floor. Sleep Med. 2020;65:172–176. 10.1016/j.sleep.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 9.Vinha PP, Faria AC, Xavier SP, Christino M, de Mello-Filho FV. Enlargement of the pharynx resulting from surgically assisted rapid maxillary expansion. J Oral Maxillofac Surg. 2016;74(2):369–379. 10.1016/j.joms.2015.06.157 [DOI] [PubMed] [Google Scholar]
- 10.Berry RB, Brooks R, Gamaldo CE, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0. Darien, IL: American Academy of Sleep Medicine; 2012. [Google Scholar]
- 11.Rabelo FAW, Küpper DS, Sander HH, Fernandes RMF, Valera FCP. Polysomnographic evaluation of propofol-induced sleep in patients with respiratory sleep disorders and controls. Laryngoscope. 2013;123(9):2300–2305. 10.1002/lary.23664 [DOI] [PubMed] [Google Scholar]
- 12.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268(8):1233–1236. 10.1007/s00405-011-1633-8 [DOI] [PubMed] [Google Scholar]
- 13.Kezirian E, White D, Malhotra A. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg. 2010;136(4):393–397. 10.1001/archoto.2010.26 [DOI] [PubMed] [Google Scholar]
- 14.Sawchuk D, Currie K, Vich ML, Palomo JM, Flores-Mir C. Diagnostic methods for assessing maxillary skeletal and dental transverse deficiencies: a systematic review. Korean J Orthod. 2016;46(5):331–342. 10.4041/kjod.2016.46.5.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miner RM, Al Qabandi S, Rigali PH, Will LA. Cone-beam computed tomography transverse analysis. Part I: normative data. Am J Orthod Dentofacial Orthop. 2012;142(3):300–307. 10.1016/j.ajodo.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 16.Green KK, Kent DT, D’Agostino MA, et al. Drug-induced sleep endoscopy and surgical outcomes: a multicenter cohort study. Laryngoscope. 2019;129(3):761–770. 10.1002/lary.27655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;9(5):433–438. 10.5664/jcsm.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu SYC, Huon LK, Iwasaki T, et al. Efficacy of maxillomandibular advancement examined with drug-induced sleep endoscopy and computational fluid dynamics airflow modeling. Otolaryngol Head Neck Surg. 2016;154(1):189–195. 10.1177/0194599815611603 [DOI] [PubMed] [Google Scholar]
- 19.Kastoer C, Op de Beeck S, Dom M, et al. Drug-induced sleep endoscopy upper airway collapse patterns and maxillomandibular advancement. Laryngoscope. 2020;130(4):E268–E274. 10.1002/lary.28022 [DOI] [PubMed] [Google Scholar]
- 20.Zaghi S, Holty JEC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg. 2016;142(1):58–66. 10.1001/jamaoto.2015.2678 [DOI] [PubMed] [Google Scholar]
- 21.Guilleminault C, Huang YS. From oral facial dysfunction to dysmorphism and the onset of pediatric OSA. Sleep Med Rev. 2018;40:203–214. 10.1016/j.smrv.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki T, Saitoh I, Takemoto Y, et al. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: a cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2013;143(2):235–245. 10.1016/j.ajodo.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 23.Meraj TS, Muenz DG, Glazer TA, Harvey RS, Spector ME, Hoff PT. Does drug-induced sleep endoscopy predict surgical success in transoral robotic multilevel surgery in obstructive sleep apnea? Laryngoscope. 2017;127(4):971–976. 10.1002/lary.26255 [DOI] [PubMed] [Google Scholar]
- 24.Abdullatif J, Certal V, Zaghi S, et al. Maxillary expansion and maxillomandibular expansion for adult OSA: a systematic review and meta-analysis. J Craniomaxillofac Surg. 2016;44(5):574–578. 10.1016/j.jcms.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Vinha PP, Thuler ER, de Mello-Filho FV. Effects of surgically assisted rapid maxillary expansion on the modification of the pharynx and hard palate and on obstructive sleep apnea, and their correlations. J Cranio-Maxillofacial Surg. 2020;48(4):339–348. 10.1016/j.jcms.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki T, Yoon A, Guilleminault C, Yamasaki Y, Liu SY. How does distraction osteogenesis maxillary expansion (DOME) reduce severity of obstructive sleep apnea? Sleep Breath. 2020;24(1):287–296. 10.1007/s11325-019-01948-7 [DOI] [PubMed] [Google Scholar]
- 27.Spinowitz S, Kim M, Park SY. Patterns of upper airway obstruction on drug-induced sleep endoscopy in patients with sleep-disordered breathing with AHI <5. OTO Open. 2017;1(3): 2473974X17721483. 10.1177/2473974X17721483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vito A, Carrasco Llatas M, Ravesloot MJ, et al. European position paper on drug-induced sleep endoscopy: 2017 update. Clin Otolaryngol. 2018;43(6):1541–1552. 10.1111/coa.13213 [DOI] [PubMed] [Google Scholar]