Abstract
Study Objectives:
Changes to sleep architecture that occur as a result of the normal aging process may also exacerbate insomnia in older individuals. Therefore, this study assessed the impact of lemborexant compared with placebo and zolpidem tartrate extended release on objective sleep architecture parameters, as measured by polysomnography, in older adults (ages ≥ 55 years) with insomnia disorder from a phase 3 study.
Methods:
Study E2006-G000-304 (SUNRISE 1; NCT02783729) was a global, multicenter, randomized, double-blind, placebo-controlled, active comparator (zolpidem)–controlled, parallel-group study comparing 2 dose levels of lemborexant (5 mg and 10 mg). Sleep architecture was measured using polysomnography. Assessments were collected at baseline during a single-blind placebo run-in and during the first 2 nights and last 2 nights of treatment. Mean values for each sleep stage were based on the 2 consecutive polysomnograms.
Results:
Treatment with lemborexant resulted in significantly greater increases from baseline in total sleep time compared with both placebo and zolpidem. Significant increases from baseline in rapid eye movement sleep and significant decreases from baseline in latency to rapid eye movement sleep were also observed with lemborexant compared with placebo and zolpidem.
Conclusions:
These findings suggest that treatment with lemborexant may address some of the alterations in sleep architecture normally observed in older individuals with insomnia.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Study of the Efficacy and Safety of Lemborexant in Subjects 55 Years and Older With Insomnia Disorder (SUNRISE 1); URL: https://clinicaltrials.gov/ct2/show/NCT02783729; Identifier: NCT02783729.
Citation:
Moline M, Zammit G, Cheng J, Perdomo C, Kumar D, Mayleben D. Comparison of the effect of lemborexant with placebo and zolpidem tartrate extended release on sleep architecture in older adults with insomnia disorder. J Clin Sleep Med. 2021;17(6):1167–1174.
Keywords: lemborexant, insomnia, sleep architecture
BRIEF SUMMARY
Current Knowledge/Study Rationale: The purpose of this study was to provide information to improve on the current treatment paradigm for older patients (ages ≥ 55 years) with insomnia. A comparison of lemborexant with placebo and with zolpidem tartrate extended release provides clinically meaningful information for clinicians treating insomnia.
Study Impact: The results indicate that lemborexant may address some of the changes in sleep architecture that are observed in older individuals with insomnia, with improvement that was sustained over 1 month. These findings provide information on the efficacy of a recently approved treatment option for insomnia in older adults.
INTRODUCTION
Insomnia disorder is characterized by difficulties with sleep onset, sleep maintenance, or early morning awakening sustained over 3 months and is associated with complaints of impaired daytime functioning.1 In patients with insomnia disorder, sleep architecture is often observed to be altered in polysomnographic recordings, including significant reductions of slow wave sleep (stage N3 sleep) and rapid eye movement sleep (stage R sleep) relative to individuals without sleep disturbances.2 Notably, a reduction in stage N3 and stage R sleep may impair memory and attention.3–7 Similar changes to sleep architecture (ie, reductions in stage N3 and stage R sleep and associated increases in nonrapid eye movement [NREM] sleep stages N1 and N2) may occur naturally as a result of the aging process.8 Thus, older adults may be more prone to insomnia.
Cognitive-behavioral therapy is the first recommendation for the treatment of insomnia and has improved sleep in older adults diagnosed with insomnia.9,10 However, when cognitive-behavioral therapy is not effective or is not accessible to the patient, pharmacotherapy may be needed.11,12 Decisions regarding pharmacotherapy should consider a patient’s comorbidities because insomnia has a complex relationship with many other conditions, including an increased risk of type 2 diabetes,13 cardiovascular disease,14 and neurodegenerative disease.15 Commonly prescribed pharmacotherapies for insomnia include benzodiazepines and other nonbenzodiazepine sedative-hypnotics. However, these drugs can have negative effects on sleep architecture. For example, benzodiazepines are known to decrease the time spent in both stage N3 and stage R sleep.16 In a clinical study that included a benzodiazepine and a nonbenzodiazepine γ-aminobutyric acid-ergic agonist, both drugs significantly reduced stage N3 sleep compared with placebo.17 Given that the sleep architecture of patients with insomnia and of older patients often already shows decreased time in stage N3 and stage R sleep,2 these issues indicate significant unmet needs in the treatment of insomnia for older patients.
Dual orexin receptor antagonists (DORAs) are a potential alternative to currently prescribed insomnia treatments. Orexins are neuropeptides with a significant role in sleep-wake transitions and gating wakefulness. DORAs block orexin receptors 1 and 2, resulting in decreased orexin activity.18 The DORA suvorexant has been reported to improve sleep efficiency and total sleep time (TST).19 In a study examining sleep architecture over 3 months of treatment, participants receiving suvorexant showed an increase in the time spent in all sleep stages (ie, improved sleep efficiency), including an increase in the percentage of stage R sleep, compared with participants receiving placebo.20 However, these differences from placebo were the most apparent on the first night of treatment, and the differences of suvorexant treatment vs placebo diminished compared with the first night over the 3 months of the study.
Lemborexant is a new DORA that has recently received approval by the U.S. Food & Drug Administration and the Pharmaceuticals and Medical Devices Agency in Japan and the Health Products and Food Branch of Health Canada for the treatment of insomnia. Two pivotal phase 3 studies, Study E2006-G000-304 (Study 304; SUNRISE 1; NCT02783729) and Study E2006-G000-303 (Study 303; SUNRISE 2; NCT02952820), examined the efficacy and safety of lemborexant in participants with insomnia. In both studies, lemborexant showed improvement in self-reported (sleep diary–based) sleep parameters vs placebo. Study 303, a randomized, double-blind, placebo-controlled (first 6 months [period 1]), global phase 3 study showed greater improvements in self-reported sleep onset and sleep maintenance endpoints with lemborexant compared with placebo over 6 months.21 Study 304, a randomized, double-blind clinical trial of 1,006 participants ages ≥ 55 years with insomnia disorder, showed that lemborexant therapy significantly improved both latency to persistent sleep (time from lights off to the first 20 consecutive epochs [ie, 10 minutes] of nonwakefulness) and sleep maintenance (wake after sleep onset and sleep efficiency) compared with both placebo and zolpidem tartrate extended release therapy, as measured objectively by polysomnography (PSG), over 1 month.22
Here, we present the effects of lemborexant (5 mg, 10 mg) compared with both zolpidem (zolpidem tartrate extended release 6.25 mg) and placebo on sleep architecture, as measured by nocturnal PSG, in participants ages ≥ 55 years with insomnia disorder. This prespecified exploratory objective of Study 304 was to determine whether lemborexant impacts the stages of sleep, as seen with other DORAs (eg, increased stage R sleep and decreased latency to stage R sleep).20
METHODS
Participants
Full details of the methods of Study 304 have been published.22 Study 304 enrolled men ages ≥ 65 years and women ages ≥ 55 years (to capture the increase in insomnia incidence in women in the menopausal age range)23 who met the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) criteria for insomnia disorder, specifically including sleep maintenance difficulties.1 Participants were required to have a self-reported wake after sleep onset ≥ 60 minutes at least 3 nights per week for 3 months or longer, regular time spent in bed (7–9 hours per day), and an Insomnia Severity Index score ≥ 13. Participants also could have difficulties with sleep onset based on the DSM-5 criteria for insomnia disorder.
Exclusion criteria included a current diagnosis of sleep-disordered breathing, periodic limb movement disorder, restless legs syndrome, circadian rhythm sleep disorder, narcolepsy, or exclusionary score on screening instruments to rule out individuals with sleep disorders other than insomnia. The screening instruments consisted of those included in the Sleep Disorders Screening Battery: the Epworth Sleepiness Scale (to exclude individuals with a sleep disorder other than insomnia; a score > 15 was exclusionary), the StopBANG sleep apnea questionnaire (to exclude individuals with sleep apnea; a score ≥ 5 was exclusionary), the International Restless Legs Scale (to exclude individuals with restless legs syndrome; a score ≥ 16 was exclusionary), and a history regarding parasomnias (endorsing a history of parasomnia was exclusionary). Women of childbearing potential and individuals with comorbid medical or psychiatric conditions that would interfere with study assessments or contraindicate administering a sedating drug were also excluded. Prohibited treatments included other treatments for insomnia disorder (drugs or nonpharmacological treatment such as cognitive-behavioral therapy) and any medication that in the opinion of the investigator would cause or exacerbate the participant’s insomnia: A participant had to have discontinued any prohibited medication at least 1 week (or at least 5 half-lives, whichever was longer) before starting the sleep diary.
Study design
This was a global, multicenter, randomized, double-blind, parallel-group study comparing 2 dose levels of lemborexant (5 mg, 10 mg) with placebo and zolpidem.
Participants took part in 2 screening visits and a sleep disorders screening PSG (using bilateral frontal, central, and occipital referential electrode derivations) at the clinical site, after which the investigator or designee confirmed that the participant met the DSM-5 diagnostic criteria for insomnia disorder. During the screening period, a medical, psychiatric, and sleep history interview was conducted and the Sleep Disorders Screening Battery was completed. During the screening and run-in periods, sleep was also assessed using a sleep diary. Additional confirmation of study eligibility was obtained by 2 consecutive nights of baseline PSG after the run-in period. Participants were excluded if they had an apnea-hypopnea index > 15 events/h or periodic limb movements with arousal index > 15 measured by PSG at the second screening visit. Eligible participants were then dispensed placebo and completed an approximately 2-week placebo run-in period and 2 consecutive nights of baseline PSG. After a minimum of 2 nights after the baseline PSGs, the run-in period ended and the baseline period began. Those who continued to meet the eligibility criteria entered the treatment period and were randomized (5:5:5:4 ratio) to receive lemborexant 5 mg, lemborexant 10 mg, zolpidem, or placebo. Paired PSG was conducted on the first 2 nights (nights 1/2) and the last 2 nights (nights 29/30) of the treatment period.
Assessments
Paired PSGs at baseline, nights 1/2 of the treatment period, and nights 29/30 of the treatment period were used to assess change from baseline in minutes for TST (minutes of sleep from sleep onset until terminal awakening), duration of NREM sleep (stages N1, N2, N3) and stage R sleep stages, and stage R sleep latency. Recordings were analyzed in 30-second epochs in accordance with the American Academy of Sleep Medicine manual (The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2)24 by trained PSG scorers blinded to treatment condition. For the scoring of events, an apnea was scored when there was a drop in the peak signal excursion of ≥ 90% of pre-event baseline using an oronasal thermosensor or acceptable alternative and the duration of the drop was ≥ 10 seconds. A hypopnea was scored when there was a drop in the peak signal excursion of ≥ 30% of pre-event baseline using nasal pressure or an acceptable alternative and the duration of the drop was ≥ 10 seconds along with a ≥ 3% drop in oxygen saturation or an association with an arousal. All PSG parameters were obtained separately for each PSG recording and averaged across the pairs of consecutive PSG nights.
Statistical analyses
Sleep architecture parameters were exploratory endpoints in Study 304. Statistical analyses were conducted on the full analysis set, which was defined as all randomized participants who received ≥ 1 dose of the study drug and had ≥ 1 postdose primary efficacy measurement. Changes from baseline in TST and sleep stage duration were analyzed using a mixed-effect model repeated-measurement analysis with the factors of age group, region (North America, Europe), treatment, visit (nights 1/2, nights 29/30), and treatment-by-visit interaction as fixed effects and baseline sleep stage duration or TST as a covariate. Missing values were not imputed and were assumed to be missing at random. P values were based on the mixed-effect model repeated-measurement analysis evaluating the least-squares mean (LSM) treatment difference. Analyses of stage R sleep latency were based on the Wilcoxon rank sum test.
RESULTS
A total of 1,006 participants comprised the full analysis set of Study 304. The majority of participants in all treatment groups completed the study (96.7%, 97.0%, 93.5%, and 95.2% of participants in the lemborexant 10 mg, lemborexant 5 mg, zolpidem, and placebo treatment groups, respectively). Full details of the demographic and baseline characteristics of the study population have been published.22 The median age was 63 years (range, 55–88 years) and the majority of participants were female (869 [86.4%]) and White (727 [72.3%]).
Sleep duration
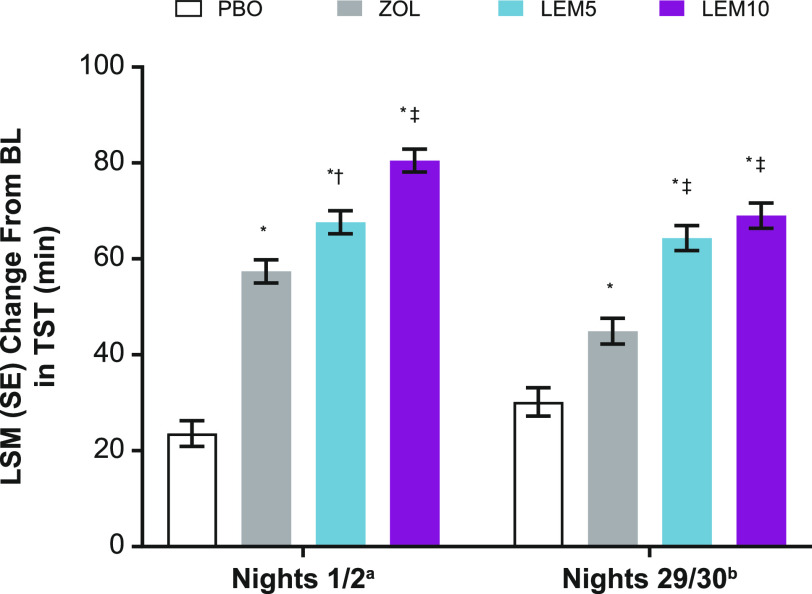
At baseline, all participants had similar TST (Table 1 and Table S1 (95.4KB, pdf) in the supplemental material, which presents the change from baseline for percentage of sleep stages per TST). The LSM (standard error [SE]) change from baseline for TST was significantly greater on nights 1/2 for lemborexant 5 mg (67.6 [2.4] minutes) and lemborexant 10 mg (80.5 [2.4] minutes) compared with placebo (23.6 [2.7] minutes; P < .0001 for all). Significantly greater increases in the LSM (SE) change from baseline were also observed on nights 29/30 for lemborexant 5 mg (64.3 [2.6] minutes) and lemborexant 10 mg (69.0 [2.6] minutes) compared with placebo (30.2 [3.0] minutes; P < .0001 for all).
Table 1.
Baseline sleep stage duration and PSG variables.
| Parameters | PBO (n = 208) | ZOL (n = 263) | LEM5 (n = 266) | LEM10 (n = 269) |
|---|---|---|---|---|
| Total sleep time per time in beda | 330.7 (46.3) | 327.0 (54.9) | 328.0 (54.2) | 325.1 (52.8) |
| Total wake time per time in beda | 149.3 (46.3) | 152.9 (54.8) | 151.8 (53.9) | 154.13 (52.0) |
| Total NREM sleep | 265.5 (39.6) | 261.8 (44.9) | 264.6 (44.6) | 263.5 (44.2) |
| Stage N1 sleep | 35.7 (18.9) | 34.5 (17.8) | 34.6 (19.0) | 38.8 (19.1) |
| Stage N2 sleep | 189.5 (40.9) | 187.4 (41.0) | 185.4 (40.4) | 187.3 (41.3) |
| Stage N3 sleep | 40.3 (26.8) | 39.9 (27.8) | 44.5 (30.9) | 37.4 (26.8) |
| Stage R sleep | 65.2 (19.5) | 65.2 (22.9) | 63.4 (21.3) | 61.6 (20.3) |
| Stage R sleep latency | 99.9 (51.7) | 98.4 (52.4) | 101.4 (54.0) | 100.2 (53.9) |
Values are presented as mean (SD) in minutes. a8 hours. LEM5 = lemborexant 5 mg, LEM10 = lemborexant 10 mg, NREM = nonrapid eye movement sleep, PBO = placebo, PSG = polysomnography, SD = standard deviation, ZOL = zolpidem tartrate extended release 6.25 mg.
On nights 1/2, the LSM (SE) treatment difference in TST was also significantly greater for lemborexant 5 mg (10.3 [3.1] minutes) and lemborexant 10 mg (23.1 [3.1] minutes) compared with zolpidem (P < .001 and P < .0001, respectively). On nights 29/30, the LSM (SE) treatment difference was significantly greater for lemborexant 5 mg (19.4 [3.5] minutes) and lemborexant 10 mg (24.1 [3.5] minutes) compared with zolpidem (P < .0001 for both). At both of these time points, the treatment differences for zolpidem were significantly larger vs placebo (nights 1/2: 33.8 [3.3] minutes, P < .0001; nights 29/30: 14.8 [3.7] minutes, P < .0001; Figure 1).
Figure 1. Change from baseline in TST.
aPBO, n = 208; ZOL, n = 262; LEM5, n = 266; LEM10, n = 269. bPBO, n = 200; ZOL, n = 250; LEM5, n = 260; LEM10, n = 260. *P < .0001 vs PBO; †P < .01 vs ZOL; ‡P < .0001 vs ZOL. BL = baseline, LEM5 = lemborexant 5 mg, LEM10 = lemborexant 10 mg, LSM = least-squares mean, PBO = placebo, SE = standard error, TST = total sleep time, ZOL = zolpidem tartrate extended release 6.25 mg.
NREM sleep
Baseline values for minutes of stage N1 sleep were similar across the treatment groups (Table 1). Neither of the lemborexant-treated groups showed a significantly different change from baseline in stage N1 sleep on nights 1/2 compared with placebo. On nights 1/2, the LSM (SE) change from baseline for minutes of stage N1 sleep was not significantly different for lemborexant 5 mg (3.7 [0.8] minutes) and lemborexant 10 mg (3.5 [0.8] minutes) compared with placebo (1.8 [1.0] minutes; P = .1 for both). On nights 29/30, a significantly greater increase in minutes of stage N1 sleep was seen in both lemborexant treatment groups compared with placebo. The LSM (SE) for minutes of stage N1 sleep was significantly increased for lemborexant 5 mg (6.0 [0.8] minutes) and lemborexant 10 mg (6.6 [0.8] minutes) compared with placebo (0.7 [1.0] minutes; P < .0001 for both).
Both lemborexant treatment groups showed a significantly greater increase in minutes of stage N1 sleep compared with zolpidem at both time points. The LSM (SE) treatment difference on nights 1/2 for minutes of stage N1 sleep was significantly greater for lemborexant 5 mg (3.7 [1.1] minutes) and lemborexant 10 mg (3.5 [1.1] minutes) compared with zolpidem (P < .001 and P < .01, respectively). The LSM treatment difference on nights 29/30 was significantly greater for lemborexant 5 mg (5.5 [1.1] minutes) and lemborexant 10 mg (6.2 [1.1] minutes) compared with zolpidem (P < .0001 for both). The LSM (SE) change from baseline for minutes of stage N1 sleep was not significantly different for zolpidem compared with placebo on nights 1/2 (−0.01 [0.8] minutes; P = .1) or nights 29/30 (0.5 [0.9] minutes; P = .9; Figure 2A).
Figure 2. Change from baseline in all sleep stages.
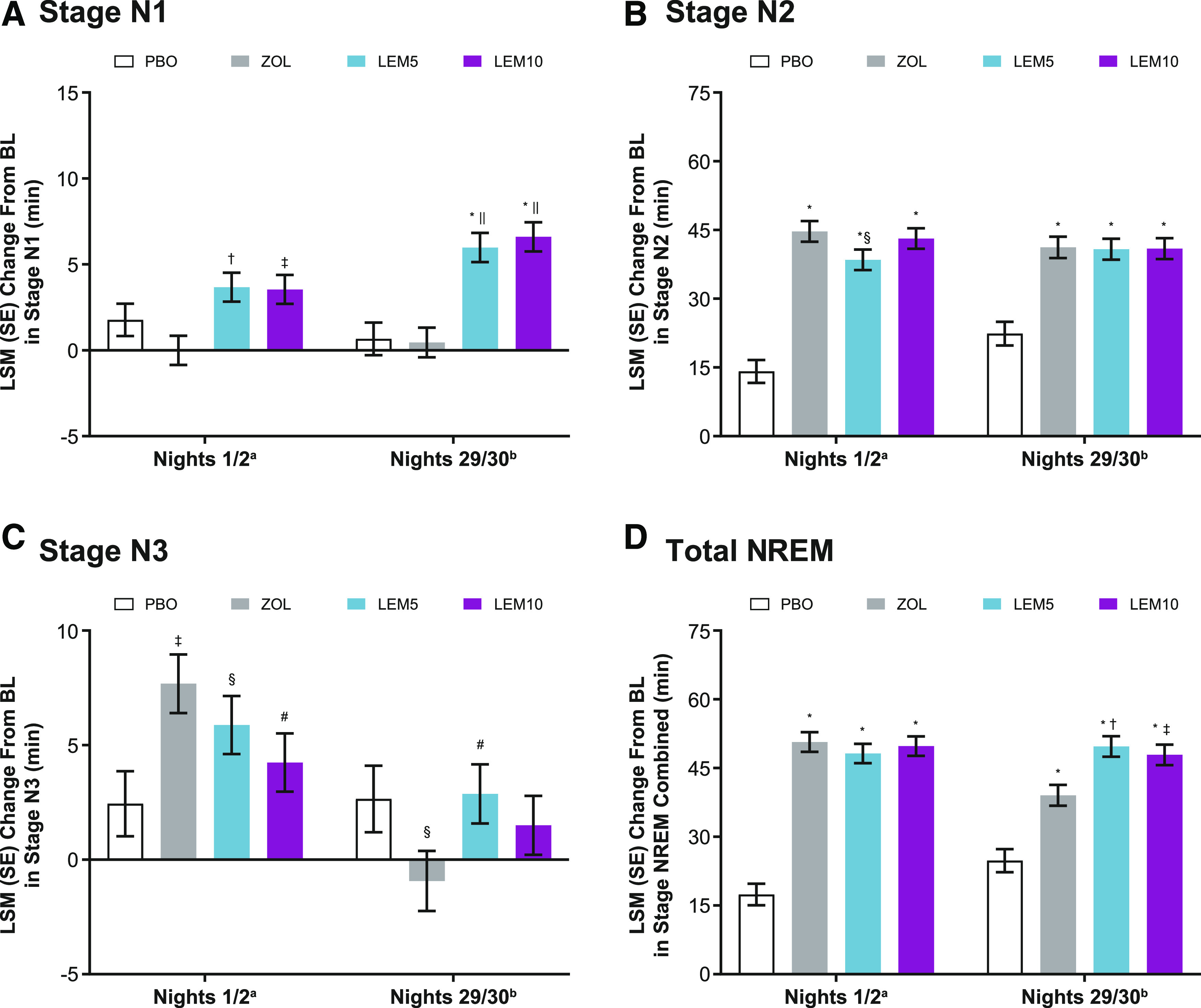
aPBO, n = 208; ZOL, n = 262; LEM5, n = 266; LEM10, n = 269. bPBO, n = 200; ZOL, n = 250; LEM5, n = 260; LEM10, n = 260. (A) *P < .0001 vs PBO; †P < .001, ‡P < .01, ||P < .0001 vs ZOL. (B) *P < .0001 vs PBO; §P < .05 vs ZOL. (C) ‡P < .01, §P < .05 vs PBO; #P < .05 vs ZOL. (D) *P < .0001 vs PBO; †P < .001, ‡P < .01 vs ZOL. P values were based on the mixed-effect model repeated-measurement analysis evaluating the LSM treatment difference for LEM vs PBO, LEM vs ZOL, and ZOL vs PBO. The model included factors of age group, region, treatment, visit (nights 1/2 and nights 29/30), and treatment-by-visit interaction as fixed effects and baseline sleep stage duration as a covariate. BL = baseline, LEM5 = lemborexant 5 mg, LEM10 = lemborexant 10 mg, LSM = least-squares mean, NREM = nonrapid eye movement sleep, PBO = placebo, SE = standard error, ZOL = zolpidem tartrate extended release 6.25 mg.
Baseline values for minutes of stage N2 sleep were similar across treatment groups (Table 1). Both lemborexant treatment groups showed increases in stage N2 sleep compared with placebo. A significant difference in LSM (SE) change from baseline in minutes of stage N2 sleep was observed on nights 1/2 for lemborexant 5 mg (38.5 [2.2] minutes) and lemborexant 10 mg (43.1 [2.2] minutes) compared with placebo (14.1 [2.5] minutes; P < .0001 for both). A significant difference in LSM (SE) change from baseline for minutes of stage N2 sleep was also observed on nights 29/30 for lemborexant 5 mg (40.8 [2.3] minutes) and lemborexant 10 mg (40.9 [2.3] minutes) compared with placebo (22.4 [2.6] minutes; P < .0001 for both).
The LSM (SE) treatment difference on nights 1/2 was also significant for lemborexant 5 mg compared with zolpidem (−6.2 [2.9] minutes; P < .05). No other significant differences for lemborexant 5 mg or lemborexant 10 mg compared with zolpidem were noted.
A significant difference in LSM (SE) change from baseline for minutes of stage N2 sleep was observed for zolpidem compared with placebo on nights 1/2 (44.7 [2.3] minutes; P < .0001) and nights 29/30 (41.2 [2.3] minutes) compared with placebo (22.4 [2.6] minutes; P < .0001; Figure 2B).
Baseline values for minutes of stage N3 sleep were similar across treatment groups (Table 1). A significant difference in LSM (SE) change from baseline in minutes of stage N3 sleep on nights 1/2 was observed for lemborexant 5 mg (5.9 [1.3] minutes) compared with placebo (2.4 [1.4] minutes; P < .05). On nights 29/30, no significant difference was observed for lemborexant 5 mg or lemborexant 10 mg compared with placebo.
The LSM (SE) treatment difference on nights 1/2 was not significant for lemborexant 5 mg vs zolpidem and was significantly less for lemborexant 10 mg vs zolpidem (−3.5 [1.6] minutes; P < .05). The LSM (SE) treatment difference for minutes of stage N3 sleep on nights 29/30 was significantly different for lemborexant 5 mg vs zolpidem (3.8 [1.7] minutes; P < .05), with more minutes of stage N3 sleep with lemborexant 5 mg.
A significant increase from baseline in LSM (SE) for minutes of stage N3 sleep was observed on nights 1/2 for zolpidem (7.7 [1.3] minutes) compared with placebo (2.4 [1.4] minutes; P < .01). On nights 29/30, the LSM (SE) change from baseline was significant for zolpidem (−0.9 [1.3] minutes) compared with placebo (2.7 [1.4] minutes; P < .05), showing a decrease in minutes of stage N3 sleep with zolpidem (Figure 2C).
Baseline values of minutes of total NREM sleep were similar across the treatment groups (Table 1). Both lemborexant treatment groups showed significant increases in total NREM sleep compared with placebo. The LSM (SE) change from baseline for minutes of all NREM sleep combined on nights 1/2 was greater and statistically significant for lemborexant 5 mg (48.2 [2.1] minutes) and lemborexant 10 mg (49.8 [2.1] minutes) compared with placebo (17.4 [2.4] minutes) on nights 1/2 (P < .0001 for both).
The LSM (SE) treatment differences on nights 1/2 for lemborexant 5 mg and lemborexant 10 mg compared with zolpidem were not significant. The LSM (SE) treatment difference for minutes of all NREM sleep combined were significantly greater for lemborexant 5 mg (10.7 [3.0] minutes) and lemborexant 10 mg (8.9 [2.9] minutes) compared with zolpidem on nights 29/30 (P < .001 and P < .01, respectively).
On nights 1/2, the LSM (SE) change from baseline was significantly greater for zolpidem (50.7 [2.1] minutes) vs placebo (17.4 [2.4] minutes; P < .0001). On nights 29/30, the LSM (SE) change from baseline was significantly greater for zolpidem (39.0 [2.3] minutes) compared with placebo (24.8 [2.5] minutes; P < .0001; Figure 2D).
Stage R sleep
Baseline values of minutes of stage R sleep were similar across treatment groups (Table 1). Increased stage R sleep was noted at both time points with both lemborexant doses. The LSM (SE) change from baseline in minutes of stage R sleep on nights 1/2 was greater and statistically significant for lemborexant 5 mg (19.7 [1.3] minutes) and lemborexant 10 mg (31.3 [1.3] minutes) compared with placebo (6.1 [1.5] minutes; P < .0001 for both). On nights 29/30, the LSM (SE) change from baseline was greater and statistically significant for lemborexant 5 mg (14.9 [1.4] minutes) and lemborexant 10 mg (21.7 [1.4] minutes) compared with placebo (5.3 [1.5] minutes; P < .0001).
The LSM (SE) treatment difference on nights 1/2 was significantly greater for lemborexant 5 mg (12.9 [1.7] minutes) and lemborexant 10 mg (24.5 [1.7] minutes) compared with zolpidem (P < .0001 for both). The LSM (SE) treatment difference on nights 29/30 was also significant for lemborexant 5 mg (8.9 [1.8] minutes) and lemborexant 10 mg (15.7 [1.8] minutes) compared with zolpidem (P < .0001 for both). On both nights 1/2 and nights 29/30, there was no significant treatment difference in stage R sleep noted for zolpidem compared with placebo (Figure 3A).
Figure 3. Stage R sleep and stage R sleep latency.
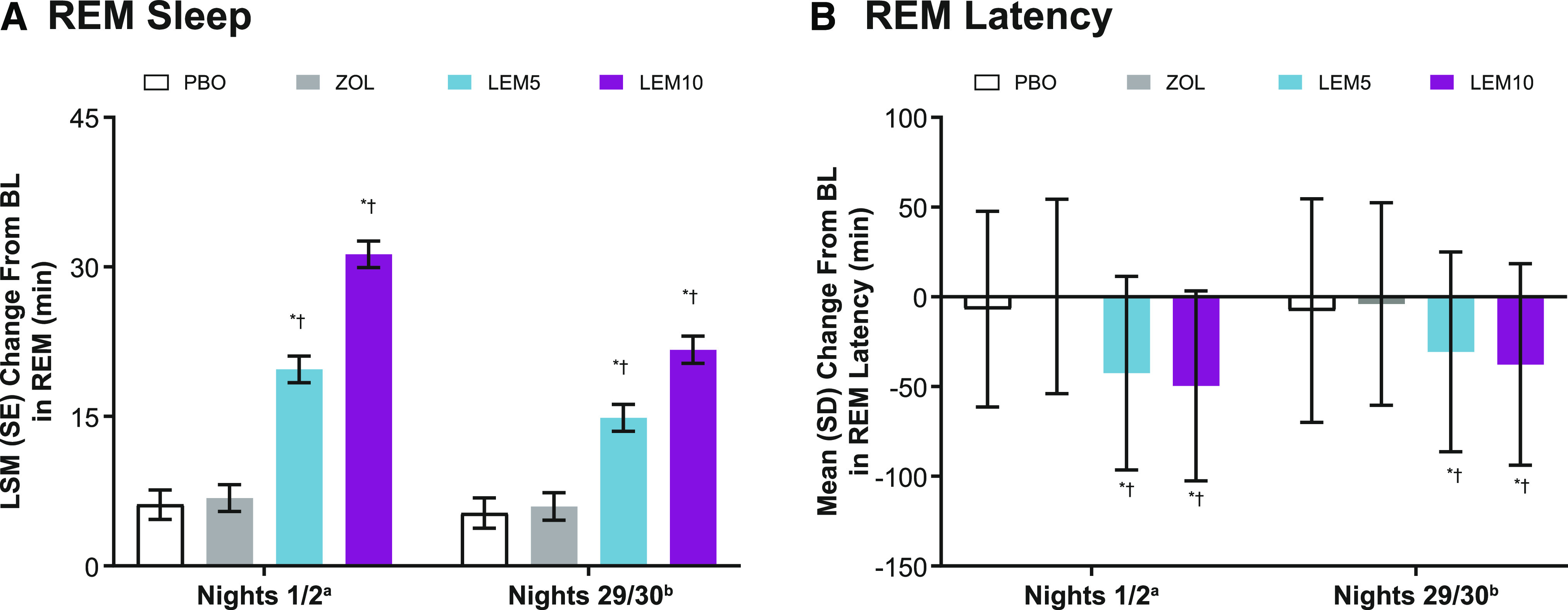
aPBO, n = 208; ZOL, n = 262; LEM5, n = 266; LEM10, n = 269. bPBO, n = 200; ZOL, n = 250; LEM5, n = 260; LEM10, n = 260. (A) *P < .0001 vs PBO; †P < .0001 vs ZOL. P values were based on the mixed-effect model repeated-measurement analysis evaluating the LSM treatment difference for LEM vs PBO, LEM vs ZOL, and ZOL vs PBO. The model included factors of age group, region, treatment, visit (nights 1/2 and nights 29/30), and treatment-by-visit interaction as fixed effects and baseline total stage R sleep duration as a covariate. (B) *P < .0001 vs PBO; †P < .0001 vs ZOL. Stage R sleep latency was defined as the minutes from the first epoch of sleep (N1, N2, or N3 sleep) to the first epoch of stage R sleep. P values were based on the Wilcoxon rank sum test, where treatment differences were estimated using the Hodges-Lehmann estimation. BL = baseline, LEM5 = lemborexant 5 mg, LEM10 = lemborexant 10 mg, LSM = least-squares mean, PBO = placebo, SD = standard deviation, SE = standard error, ZOL = zolpidem tartrate extended release 6.25 mg.
Latency to stage R sleep was observed to be shorter at both time points with lemborexant treatment. The mean (standard deviation) latency to stage R sleep on nights 1/2 was significantly shortened compared with baseline for lemborexant 5 mg (−42.6 [53.9] minutes) and lemborexant 10 mg (−49.6 [52.9] minutes) compared with placebo (−6.9 [54.5] minutes; P < .0001 for both). On nights 29/30, the mean (standard deviation) latency to stage R sleep was also significantly shortened compared with baseline for lemborexant 5 mg (−30.7 [55.7] minutes) and lemborexant 10 mg (−37.7 [56.2] minutes) compared with placebo (−7.7 [62.3] minutes; P < .0001 for both).
Stage R sleep latency was significantly shortened for lemborexant 5 mg (−37.8 minutes) and lemborexant 10 mg (−44.8 minutes) compared with zolpidem (P < .0001 for both) on nights 1/2. In addition, stage R sleep latency on nights 29/30 was significantly shortened for lemborexant 5 mg (−26.8 minutes) and lemborexant 10 mg (−32.3 minutes) compared with zolpidem (P < .0001 for both). Treatment with zolpidem, in contrast, did not have a significant impact on stage R sleep latency on either nights 1/2 or nights 29/30 (Figure 3B).
DISCUSSION
In Study 304, lemborexant 5 mg and lemborexant 10 mg treatments resulted in significantly greater increases from baseline in minutes of TST, total NREM sleep, and stage R sleep and greater decreases in stage R sleep latency compared with placebo on both nights 1/2 and nights 29/30 of treatment. Our findings suggest that lemborexant, similar to other DORAs, impacts stages of sleep.
As expected, based on the mechanism of action (orexin receptor antagonism), lemborexant did increase the minutes of stage R sleep obtained and decreased stage R sleep latency, but not to values that would be considered outside the normal range for individuals in the study’s age range. Some of this increase may have resulted from more continuous sleep with treatment, allowing a putative stage R sleep deficit to be corrected, similar to the stage R rebound experienced by patients with previously untreated obstructive sleep apnea when continuous positive airway pressure treatment is initiated.25 This observation lends further support to previously presented data on lemborexant, from this study and other studies, that showed improved sleep efficiency and data showing that neither stage R nor N3 sleep were suppressed by lemborexant.26
Because lemborexant is a DORA that blocks orexin receptor 2 to a greater extent than orexin receptor 1, the results of this study (increased stage R sleep, reduced latency to stage R sleep, and increased NREM sleep) are not unexpected. These results are consistent with previous research on other DORAs, which found an increase in the percentage of stage R sleep and a reduction in latency to stage R sleep.27 Further, studies have found that the activation of orexin receptor 2, with lesser contribution from orexin receptor 1, suppresses NREM sleep, and both orexin receptor 1 and orexin receptor 2 are involved in the suppression of stage R sleep to a similar degree.27 In contrast, zolpidem, a γ-aminobutyric acid-A receptor modulator, exhibits more widespread inhibition within the sleep-wake system compared with DORAs,28 and this inhibition may be responsible for the modest, though larger, increases in stage N3 sleep found in this study. In addition, because studies have indicated a relationship between stage R sleep and age-related memory impairment, the increased stage R sleep with lemborexant treatment may contribute to providing protection of sleep-dependent memory consolidation in older individuals with insomnia.7,29
There are several limitations to consider regarding the interpretation and general applicability of the results. It is important to note that the improvements in sleep architecture observed with lemborexant, although significant compared with placebo, were small in general and may not be clinically meaningful or noticeable to the patient. The length of the study was 30 days, so the long-term effects of lemborexant on sleep architecture are not currently known. Participation in the study was also limited to individuals ages ≥ 55 years with sleep maintenance complaints. Therefore, these findings cannot be generalized to younger individuals with insomnia. However, this study does provide valuable data on the use of lemborexant in an older population with insomnia. Further, the study had very strict inclusion criteria and thus the results may not be applicable to individuals with less-severe insomnia. A fixed dose (6.25 mg extended release) of zolpidem was used in this study. Although zolpidem is available by prescription at higher doses, 6.25 mg is the initial recommended dose for patients ages ≥ 65 years, and the initial dose for all women, to minimize adverse effects.30 Finally, there was a difference in age requirements for men and women enrolled in this study, which may limit the interpretation of potential sex-based differences. However, the age range was selected to study women in the clinically important menopausal age range (and older) and could also be considered a strength of the study.
In conclusion, these data indicate that treatment with lemborexant may address some of the changes in sleep architecture that are observed in older adults with insomnia. In particular, the increase in TST and stage R sleep with lemborexant treatment observed in the PSG recordings was consistent with the self-reported improvement in sleep maintenance noted with lemborexant compared with placebo in this study and Study 303.21 The changes in sleep architecture observed in Study 304 also persisted through the end of the treatment period, indicating a continued benefit of lemborexant over 1 month for the treatment of insomnia in older adults.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Margaret Moline, Jocelyn Cheng, Carlos Perdomo, and Dinesh Kumar are employees of Eisai Inc. Gary Zammit is an employee and shareholder of Clinilabs Drug Development Corporation; has ownership interest in the Sleep Disorders Institute and Home Sleep and Respiratory Care; has served as a consultant for Eisai Inc., Janssen Pharmaceutical, Purdue, and Takeda; and has served on the speaker’s bureau for Merck. David Mayleben reports receiving grants from Eisai during the conduct of the study and receiving grants from Actelion, Idorsia, Janssen, Jazz, Merck, Novartis, Takeda, and Vanda.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
Medical writing assistance was provided by Maureen Wallace-Nadolski, PhD, of ProScribe – Envision Pharma Group and was funded by Eisai Inc. Envision Pharma Group’s services complied with the international guidelines for Good Publication Practice.
ABBREVIATIONS
- DORA
dual orexin receptor antagonist
- LSM
least-squares mean
- NREM
non-rapid eye movement
- PSG
polysomnography
- SE
standard error
- TST
total sleep time
REFERENCES
- 1. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders . 5th ed. Washington, DC: : American Psychiatric Association; ; 2013. . [Google Scholar]
- 2. Baglioni C , Regen W , Teghen A , et al . Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies . Sleep Med Rev . 2014. ; 18 ( 3 ): 195 – 213 . 10.1016/j.smrv.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 3. Ferrarelli F , Kaskie R , Laxminarayan S , Ramakrishnan S , Reifman J , Germain A . An increase in sleep slow waves predicts better working memory performance in healthy individuals . Neuroimage . 2019. ; 191 : 1 – 9 . 10.1016/j.neuroimage.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 4. Banks S , Dinges DF . Behavioral and physiological consequences of sleep restriction . J Clin Sleep Med . 2007. ; 3 ( 5 ): 519 – 528 . 10.5664/jcsm.26918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y , Liu H , Weed JG , et al . Deficits in attention performance are associated with insufficiency of slow-wave sleep in insomnia . Sleep Med . 2016. ; 24 : 124 – 130 . 10.1016/j.sleep.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 6. Roth T . Slow wave sleep: does it matter? J Clin Sleep Med . 2009. ; 5 ( 2 Suppl ): S4 – S5 . [PMC free article] [PubMed] [Google Scholar]
- 7. Scullin MK , Gao C , Fillmore P , Roberts RL , Pruett N , Bliwise DL . Rapid eye movement sleep mediates age-related decline in prospective memory consolidation . Sleep . 2019. ; 42 ( 6 ): zsz055 . 10.1093/sleep/zsz055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moraes W , Piovezan R , Poyares D , Bittencourt LR , Santos-Silva R , Tufik S . Effects of aging on sleep structure throughout adulthood: a population-based study . Sleep Med . 2014. ; 15 ( 4 ): 401 – 409 . 10.1016/j.sleep.2013.11.791 [DOI] [PubMed] [Google Scholar]
- 9. Haynes J , Talbert M , Fox S , Close E . Cognitive behavioral therapy in the treatment of insomnia . South Med J . 2018. ; 111 ( 2 ): 75 – 80 . 10.14423/SMJ.0000000000000769 [DOI] [PubMed] [Google Scholar]
- 10. Williams J , Roth A , Vatthauer K , McCrae CS . Cognitive behavioral treatment of insomnia . Chest . 2013. ; 143 ( 2 ): 554 – 565 . 10.1378/chest.12-0731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qaseem A , Kansagara D , Forciea MA , Cooke M , Denberg TD ; Clinical Guidelines Committee of the American College of Physicians . Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians . Ann Intern Med . 2016. ; 165 ( 2 ): 125 – 133 . 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 12. Sateia MJ , Buysse DJ , Krystal AD , Neubauer DN , Heald JL . Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med . 2017. ; 13 ( 2 ): 307 – 349 . 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. LeBlanc ES , Smith NX , Nichols GA , Allison MJ , Clarke GN . Insomnia is associated with an increased risk of type 2 diabetes in the clinical setting . BMJ Open Diabetes Res Care . 2018. ; 6 ( 1 ): e000604 . 10.1136/bmjdrc-2018-000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Javaheri S , Redline S . Insomnia and risk of cardiovascular disease . Chest . 2017. ; 152 ( 2 ): 435 – 444 . 10.1016/j.chest.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shamim SA , Warriach ZI , Tariq MA , Rana KF , Malik BH . Insomnia: risk factor for neurodegenerative diseases . Cureus . 2019. ; 11 ( 10 ): e6004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell H , McQueeney M , Bostwick J . Prescription sleep aids for the treatment of insomnia . US Pharm . 2011. ; 36 ( 1 ): 62 – 66 . https://www.uspharmacist.com/article/prescription-sleep-aids-for-the-treatment-of-insomnia [Google Scholar]
- 17. Arbon EL , Knurowska M , Dijk DJ . Randomised clinical trial of the effects of prolonged-release melatonin, temazepam and zolpidem on slow-wave activity during sleep in healthy people . J Psychopharmacol . 2015. ; 29 ( 7 ): 764 – 776 . 10.1177/0269881115581963 [DOI] [PubMed] [Google Scholar]
- 18. Wang C , Wang Q , Ji B , et al . The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases . Front Mol Neurosci . 2018. ; 11 : 220 . 10.3389/fnmol.2018.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herring WJ , Snyder E , Budd K , et al . Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant . Neurology . 2012. ; 79 ( 23 ): 2265 – 2274 . 10.1212/WNL.0b013e31827688ee [DOI] [PubMed] [Google Scholar]
- 20. Snyder E , Ma J , Svetnik V , et al . Effects of suvorexant on sleep architecture and power spectral profile in patients with insomnia: analysis of pooled phase 3 data . Sleep Med . 2016. ; 19 : 93 – 100 . 10.1016/j.sleep.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 21. Kärppä M , Yardley J , Pinner K , et al . Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2 . Sleep . 2020. ; 43 ( 9 ): zsaa123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenberg R , Murphy P , Zammit G , et al . Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial . JAMA Netw Open . 2019. ; 2 ( 12 ): e1918254 . 10.1001/jamanetworkopen.2019.18254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pengo MF , Won CH , Bourjeily G . Sleep in women across the life span . Chest . 2018. ; 154 ( 1 ): 196 – 206 . 10.1016/j.chest.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berry RB , Brooks R , Gamaldo CE , et al .; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. Darien, IL: American Academy of Sleep Medicine; 2015 . [Google Scholar]
- 25. Nigam G , Camacho M , Riaz M . Rapid eye movement (REM) rebound on initial exposure to CPAP therapy: a systematic review and meta-analysis . Sleep Sci Pract . 2017. ; 1 ( 1 ): 13 . 10.1186/s41606-017-0014-7 [DOI] [Google Scholar]
- 26. Murphy P , Moline M , Pinner K , et al . Effects of lemborexant on sleep architecture in subjects with insomnia disorder. Presented at: 30th Annual Meeting of the Associated Professional Sleep Societies, LLC; June 11–16, 2016; Denver, CO . [Google Scholar]
- 27. Mieda M , Hasegawa E , Kisanuki YY , Sinton CM , Yanagisawa M , Sakurai T . Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep . J Neurosci . 2011. ; 31 ( 17 ): 6518 – 6526 . 10.1523/JNEUROSCI.6506-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bettica P , Squassante L , Groeger JA , Gennery B , Winsky-Sommerer R , Dijk DJ . Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia . Neuropsychopharmacology . 2012. ; 37 ( 5 ): 1224 – 1233 . 10.1038/npp.2011.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harand C , Bertran F , Doidy F , et al . How aging affects sleep-dependent memory consolidation? Front Neurol . 2012. ; 3 : 8 . 10.3389/fneur.2012.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ambien. Package insert (zolpidem tartrate tablets). sanofi-aventis U.S. LLC; 2019 . https://www.ambien.com/resources/pdfs/ambien_cr.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.