Abstract
Study Objectives:
To examine the impact of adherence to continuous positive airway pressure (CPAP) therapy on risk of stroke among a nationally representative sample of older adults with obstructive sleep apnea.
Methods:
We performed a retrospective cohort study among Medicare beneficiaries aged ≥ 65 years who were newly diagnosed with obstructive sleep apnea and had initiated CPAP (2009–2013). Monthly indicators of CPAP adherence included charges for machines, masks, or supplies and were summed over a 25-month follow-up to create a CPAP adherence variable. Stroke was modeled as a function of CPAP adherence using generalized estimating equations.
Results:
We found that 5,757 beneficiaries met the inclusion criteria and were included in the final sample. Of these, 407 (7%) experienced stroke. After adjusting for demographic and clinical characteristics, CPAP adherence was associated with a reduced risk of stroke (hazard ratio, 0.98; 95% confidence interval, 0.96–0.99) over 25 months, indicating a 2% reduction in risk of stroke for each month of CPAP adherence. When sensitivity analyses were performed to stratify results by time since the first CPAP charge, the protective effect remained significant for the 12- and 6-month but not the 3-month outcome models.
Conclusions:
In this national analysis of older adult Medicare beneficiaries with obstructive sleep apnea, CPAP adherence was associated with significantly reduced risk of stroke.
Citation:
Wickwire EM, Bailey MD, Somers VK, et al. CPAP adherence is associated with reduced risk for stroke among older adult Medicare beneficiaries with obstructive sleep apnea. J Clin Sleep Med. 2021;17(6):1249–1255.
Keywords: obstructive sleep apnea, stroke, cardiovascular, continuous positive airway pressure, CPAP, treatment, adherence, Medicare, older adults
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is a well-documented risk factor for stroke, including among older adults, but less is known about the potential protective effect of continuous positive airway pressure (CPAP) in reducing stroke in this population. The purpose of the current study was to evaluate the impact of CPAP adherence on the risk of stroke among a national sample of older adult Medicare beneficiaries.
Study Impact: In this national analysis of older adult Medicare beneficiaries with obstructive sleep apnea who initiated CPAP, CPAP adherence was associated with a small but significant reduction in stroke risk even after controlling for multiple demographic and disease comorbidity factors. These results show the importance of CPAP adherence in improving brain health and reducing the risk of stroke among older adults.
INTRODUCTION
Obstructive sleep apnea (OSA) is common and costly in older adults.1 In patients with OSA, the airway becomes partially or fully blocked during sleep, restricting oxygen supply to the brain and resulting in frequent cortical arousals. Untreated OSA increases the risk of cardiovascular diseases,2–5 metabolic syndromes,6–8 depression,9 reduced quality of life,10 and premature death11,12 and dramatically increases health care utilization and costs.13–16 Notably, OSA is a known risk factor for initial and repeat cerebrovascular events.17,18 Stroke is the fifth leading cause of death and disability in the United States and worldwide.19 Indeed, the prevalence of comorbid OSA among patients with stroke ranges from 50% to 70%5,20,21 and is highest among older adults.
The most commonly prescribed and studied treatment for OSA is continuous positive airway pressure (CPAP) therapy. CPAP is associated with improved health outcomes in multiple domains.22 Evidence has shown that CPAP can decrease mortality,23,24 improve cognition,25 and reduce hypertension26 among older adults. Further, among older adults, CPAP is associated with improvements in multiple domains of daytime function, including reduced fatigue, sleepiness, and depression and increased quality of life.27 Despite these benefits, many patients struggle to adjust to the therapy, resulting in suboptimal adherence comparable to adherence to treatment for other chronic diseases.28 In a recent analysis of Medicare beneficiaries newly diagnosed with OSA, 74.9% (n = 2,417) met the 90-day Medicare CPAP adherence criteria, yet only 58.8% of these individuals (n = 1,420; 44% of those who initiated CPAP) maintained possession of the CPAP machine throughout the 13-month rent-to-own period.29 Thus, understanding the impact of CPAP adherence among this population is of paramount importance.
To date, very few studies have evaluated the impact of CPAP adherence on stroke among older adults with OSA.24,30,31 This dearth of data is unfortunate as CPAP could potentially reduce the risk of stroke by reducing coagulability, systemic inflammation, blood pressure, and long-term vascular damage. Martínez-García and colleagues30 conducted a prospective observational study among older adults ages ≥ 65 years (n = 939) and found that compared with individuals without OSA, those with untreated mild-moderate OSA and treated OSA (any severity with CPAP > 4 h/night) were not at increased risk of death because of stroke. Using the same cohort of older adults, Catalan-Serra and colleagues31 reported that CPAP usage (mean adherence = 6.4 h/night) reduced the risk of stroke to levels comparable to those of patients without OSA or with untreated, mild-moderate OSA.
Despite these promising findings regarding CPAP adherence and stroke among older adults, the literature remains mixed. For example, McEvoy and colleagues32 performed a large (n = 2,687), well-publicized randomized clinical trial among middle-aged and older adults with pre-existing coronary or cerebrovascular disease (M = 61.3 years; 81% male) and found no differences between participants randomized to CPAP and control patients (who used sham CPAP) in stroke outcomes. However, in a posthoc propensity score matched analysis, a beneficial effect of CPAP on stroke risk was observed.32 Likewise, Schipper and colleagues33 performed a prospective cohort study among adults (mean age = 53.2 ± 12.3 years) (n = 554) and found no relationship between self-reported CPAP adherence (> 4 h/night) and incidence of stroke (P > .10), although very few events were recorded. Another prospective cohort study conducted among older adults in China (n = 130) found that relative to nonadherence, CPAP adherence (> 4 h/night) was associated with a reduced risk of cardiovascular death (21.6% vs 5.6%; P = .04), but risk reduction for stroke did not reach statistical significance.24 Finally, Parra and colleagues34 performed a relatively small randomized controlled trial (CPAP vs control) among older adults (n = 126) with first-ever stroke. Over 5 years, CPAP did not significantly reduce the recurrence of stroke, although there was a nonsignificant increase in the control group. Stroke is a relatively rare event; thus, many of these prior studies were likely underpowered.
Given the high prevalence of OSA and cerebrovascular events among older adults, the known cerebrovascular risks associated with untreated OSA, and the potential beneficial but uncertain effect of CPAP on these cerebrovascular outcomes, it is somewhat surprising that few studies have evaluated the impact of CPAP adherence on cerebrovascular outcomes specifically among older adults.20 Such insight would have direct clinical and policy relevance. To address this important gap in knowledge, the purpose of this population-level study was to examine the impact of CPAP adherence on cerebrovascular outcomes among a nationally representative sample of older adult Medicare beneficiaries. We hypothesized that CPAP adherence would reduce the risk of cerebrovascular events including stroke.
METHODS
Data source and study population
We conducted a retrospective cohort study among Medicare beneficiaries aged ≥ 65 years and diagnosed with OSA who initiated CPAP using a 5% sample of Medicare administrative data for 2008–2015 obtained from the Centers for Medicare & Medicaid Services Chronic Condition Data Warehouse. The Chronic Condition Data Warehouse data are designed to facilitate research to improve the quality of care by linking beneficiaries across multiple files (eg, drug events, hospitalizations, and outpatient services) and providing extensive documentation on claim types, data use, and access.
OSA was defined as the presence of at least 1 OSA diagnosis between 2009 and 2013 (ie, inpatient or outpatient claims containing International Classification of Diseases, Ninth Revision, Clinical Modification codes 780.51, 327.23, 780.57, or 780.53) and searched for at least 1 CPAP machine charge (ie, Healthcare Common Procedure Coding System code E0601) within 6 months of OSA diagnosis. Note that although the Centers for Medicare & Medicaid Services requires the apnea-hypopnea index to be calculated based on 4% desaturations, previous research has reported a minimal impact of the 4% apnea-hypopnea index rule on OSA diagnoses among older adults.35 We required that no OSA, other sleep diagnoses, or CPAP charges had occurred during the 12 months before the first OSA diagnosis. To ensure the capture of CPAP charges and new stroke events, we required continuous Medicare Parts A, B, and D, with no Medicare Part C (Medicare Advantage) coverage for the 12 months before and the 24 months after the month of the first CPAP charge. Thus, all participants in the cohort survived for 24 months after the first CPAP charge.
CPAP
To facilitate the capture of CPAP adherence, we relied on 2 criteria. The Centers for Medicare & Medicaid Services implemented objective adherence criteria required for reimbursement for CPAP beyond the first 3 months of use (ie, > 4 h of CPAP on 70% of nights, or 21 days in a consecutive 30-day period within the first 90 days of CPAP initiation) in late 2008. Once these criteria are met, CPAP machines are billed over an additional 10 monthly (ie, 13 months total) payments during a “rent-to-own” period. Thus, we utilized the number of CPAP machine charges after the first CPAP charge as a proxy for CPAP adherence. Next, among beneficiaries with 13 CPAP charges, we looked for evidence of continued CPAP use over the following 12 months by searching for Healthcare Common Procedure Coding System codes representing CPAP masks and supplies (E0561, E0562, E0470, E0471, E0472, A7030, A7034, A7044, A7027, A7035, A7036, A7037, A4604, A7038, A7039, A7045, A7046, A7028, A7029, A7031, A7032, A7033, or E1399). At least 1 charge for a CPAP mask or other supply was considered evidence of continued CPAP use in all subsequent months until either the end of follow-up (25 months maximum, counting the month of the first CPAP charge) or the first cerebrovascular event. Finally, we operationalized adherence to CPAP as the number of months of CPAP use. This value either changed monthly with continued CPAP use or remained at a constant value, representing the total number of months with evidence of CPAP use among those who stopped using CPAP. Thus, the adherence variable had a range of 1–25. To ensure temporality between the CPAP adherence variable and stroke events, the adherence variable was lagged by 1 month.
Stroke
Our primary outcome was an ischemic stroke after the first CPAP charge (ie, International Classification of Diseases, Ninth Revision, Clinical Modification codes 436, 435.9, 434.91, 434.11, 434.01, 433.01, 433.11, 433.21, 433.91, 433.81, V12.54, 438.1×, 438.2×, 438.3×, 438.4×, 438.5× 438.8×, or 438.9) and before the end of follow-up. Hemorrhagic stroke was excluded. We used the date of the new stroke event to create a time-varying monthly indicator. We excluded beneficiaries with a history of stroke before the first CPAP charge.
Covariates
The Chronic Condition Data Warehouse contains information on 27 comorbid conditions and 40 other chronic or potentially disabling conditions, with an annual flag for each condition and date of first diagnosis.36 We used the date of first diagnosis to determine whether a condition was present at the first CPAP charge (ie, index date).
Data analysis
We assessed the distribution and frequencies of all variables. We calculated the proportion of beneficiaries who fell into each CPAP adherence category (< 4, 4–12, and > 12 CPAP charges) by outcome status at the end of follow-up and displayed this graphically. We assessed differences in the distribution of demographic and clinical variables by outcomes status using the chi-square goodness-of fit test and the Student t test.
To model the effect of CPAP adherence on the risk of stroke, we used generalized estimating equations with a binary distribution and complementary log-log link (discrete time model).37 Individuals were censored after the first stroke or at the end of the 25-month follow-up. First, we modeled the association between the lagged adherence to the CPAP variable and risk of stroke, adjusting for time since the OSA diagnosis. Next, we added covariates known to be associated with the outcome from a bivariate analysis (Table 1) to the model, keeping those whose P value remained < .05.
Table 1.
Baseline characteristics of Medicare beneficiaries ages ≥ 65 years and diagnosed with OSA 2009–2013 who received ≥ 1 CPAP fills by CV event status (n = 5,757).
| CV Event | No CV Event | P* | |
|---|---|---|---|
| Age, y, mean (SD) | 73.0 (5.9) | 71.7 (5.3) | .001 |
| Sex, n (%) | .08 | ||
| Female | 202 (50) | 2,519 (46) | |
| Male | 205 (50) | 2,932 (55) | |
| Race, n (%) | .14 | ||
| White, non-Hispanic | 351 (86) | 4,784 (89) | |
| Black, non-Hispanic | 32 (8) | 350 (6) | |
| Other | 24 (6) | 272 (5) | |
| Medicaid eligibility, n (%) | 77 (19) | 745 (14) | .006 |
| OREC, n (%) | .13 | ||
| Age | 357 (88) | 4,819 (90) | |
| Other | 50 (12) | 531 (10) | |
| Comorbidities, n (%) | |||
| ADRD | 35 (9) | 260 (5) | .001 |
| Anemia | 209 (51) | 2,186 (41) | < .001 |
| Atrial fibrillation | 101 (25) | 899 (17) | < .001 |
| Cataracts | 289 (71) | 3,218 (60) | < .001 |
| Chronic kidney disease | 115 (28) | 940 (18) | < .001 |
| COPD | 129 (32) | 1,286 (24) | < .001 |
| Depression | 101 (25) | 1,154 (22) | .13 |
| Diabetes | 196 (48) | 2,185 (41) | .004 |
| Gout | 15 (4) | 148 (3) | .28 |
| Heart failure | 180 (44) | 1,400 (26) | < .001 |
| Hyperlipidemia | 362 (89) | 4,413 (82) | < .001 |
| Hypertension | 384 (94) | 4,578 (86) | < .001 |
| Ischemic heart disease | 267 (66) | 2,787 (52) | < .001 |
| Obesity | 46 (11) | 519 (10) | .30 |
| Osteoporosis | 101 (25) | 1,114 (21) | .06 |
| Rheumatoid arthritis | 240 (59) | 2,761 (52) | .004 |
*P value from chi-square goodness-of-fit test or Student t test. ADRD = Alzheimer disease and related dementias, COPD = chronic obstructive pulmonary disease, CPAP = continuous positive airway pressure, CV = cerebrovascular, OREC = original reason for entitlement, OSA = obstructive sleep apnea, SD = standard deviation.
Time was correlated with reduced risk of stroke and with CPAP duration. Thus, we stratified our analyses by time since the first CPAP charge: 3 months, 6 months, 12 months, and 25 months. CPAP duration within each stratum could not be greater than the number of months since the first CPAP charge. Model-building proceeded as described in the above paragraph. We report hazard ratios (HR) and 95% confidence intervals (CI). To determine whether the effect of cumulative CPAP use on the risk of stroke differed among subpopulations, we tested effect modification by dual-eligibility status (ie, Medicare/Medicaid), race, (ie, White non-Hispanic, Black non-Hispanic, and other), and sex by including interaction terms in the regression model one at a time and evaluating the Wald chi-square P value. Statistical significance was set at P < .05.
Analyses were performed with SAS Studio version 3.71 (SAS Institute, Cary, NC). This study was approved by the institutional review board of the University of Maryland, Baltimore.
RESULTS
We identified 5,757 Medicare beneficiaries diagnosed with OSA between 2009 and 2013 who initiated CPAP therapy and met the inclusion criteria. The mean age was 71.8 years (standard deviation, 5.3 years) and 46% were female (Table 1). At the time of the first CPAP charges, the cohort had a high prevalence of comorbidities. For example, the prevalence of diabetes was 41%, that of ischemic heart disease was 53%, and that of hypertension was 86%. Subsequently, 407 (7%) of the overall cohort experienced a stroke during the follow-up period.
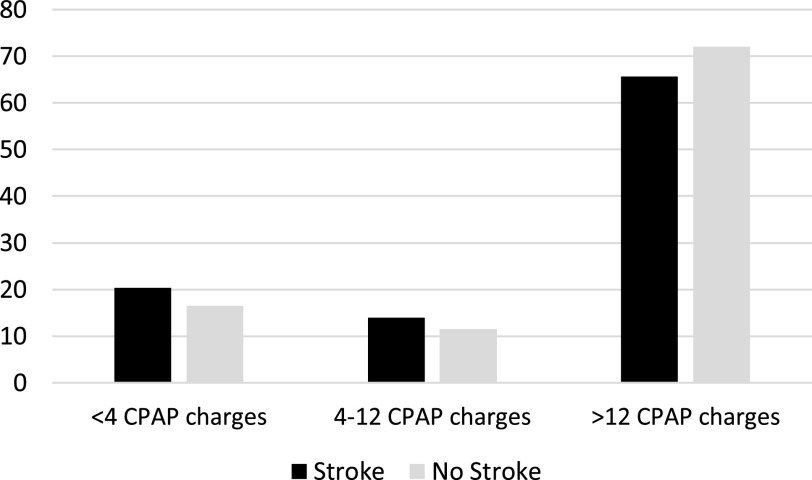
Figure 1 displays the percentage of beneficiaries with and without new stroke events within each CPAP adherence category. Among individuals who did not experience a stroke, 72% had > 12 CPAP charges, whereas only 65.6% of those who experienced a stroke had > 12 CPAP charges. In unadjusted models for the full 25-month follow-up, the lagged duration of CPAP use was associated with a reduction in the risk of stroke (HR, 0.98; 95% CI, 0.96–0.99; Table 2). After adjustment for demographic and clinical characteristics, the lagged duration of CPAP use remained associated with a significant reduction in the risk of stroke (HR, 0.98; 95% CI, 0.97–1.00; P = .02). Thus, for every additional month of CPAP use, an additional 2% reduction in the relative risk of stroke was observed. After adjustment, we observed a similar protective effect of lagged CPAP duration on the risk of stroke at 12 months since the first CPAP charge (HR, 0.96; 95% CI, 0.92–0.99; P = .02) and at 6 months since the first CPAP charge (HR, 0.85; 95% CI, 0.77–0.93; P < .001). At 3 months since the first CPAP charge, we observed a protective effect, but it was not statistically significant (HR, 0.91; 95% CI, 0.76–1.09; P = .29). We observed no effect modification by dual-eligibility status, race, or sex.
Figure 1. Percentage of Medicare beneficiaries with OSA (2009–2013) with and without new stroke events, by CPAP adherence category (n = 5,757).
CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea.
Table 2.
Association between lagged monthly duration of CPAP use and risk of new CV events among Medicare beneficiaries with OSA, stratified by time since first CPAP fill.
| Lagged Monthly Duration of CPAP Use | 25-Month Outcome (407 events) | 12-Month Outcome (246 events) | 6-Month Outcome (138 events) | 3-Month Outcome (99 events) |
|---|---|---|---|---|
| Unadjusted | 0.98 (0.96–0.99) | 0.95 (0.92–0.99) | 0.84 (0.77–0.93) | 0.90 (0.75–1.08) |
| Adjusted* | 0.98 (0.97–1.00)** | 0.96 (0.92–0.99) | 0.85 (0.77–0.93) | 0.91 (0.76–1.09) |
Values are presented as unadjusted and adjusted HRs (95% CI). *25-month outcome model adjusted for cataracts, congestive heart failure, chronic kidney disease, hypertension; 12-month outcome model adjusted for age, congestive heart failure, chronic kidney disease; 6-month outcome model adjusted for age, congestive heart failure; 3-month outcome model adjusted for age, atrial fibrillation, chronic kidney disease. **P = .02. CI = confidence interval, CPAP = continuous positive airway pressure, CV = cerebrovascular, HR = hazard ratio, OSA = obstructive sleep apnea.
DISCUSSION
In this national study of older adult Medicare beneficiaries with OSA, CPAP adherence was associated with a small but significant reduction in the risk of stroke over 25 months. This protective effect of CPAP adherence remained statistically significant even after controlling for demographic characteristics and for medical and psychiatric comorbidities. Not only do these results represent the largest analysis of CPAP adherence and stroke outcomes among older adults to date, but this study also provides a population health perspective highlighting the importance of CPAP adherence as a modifiable factor to improve cerebrovascular health among older adult Medicare beneficiaries.
The most important finding from this study is that each additional month of CPAP adherence was associated with a reduced risk of stroke among older adults, over 6, 12, and 25 months of follow-up. Results remained significant even after controlling for demographic characteristics and medical comorbidities. A lack of significant results at the 3-month follow-up may be explained by 2 factors. First, because the CPAP duration variable was lagged, there were only 2 possible levels of CPAP use at 3 months: 1 month and 2 months. Second, the smaller number of stroke events within 3 months may have reduced power to detect a statistically significant effect, even though the observed effect was in fact protective.
Consistent with a recent analysis of CPAP adherence and cardiovascular disease,38 the present results provide further empirical support for the health benefit of CPAP adherence among older adults. Because of the potentially devastating nature of stroke, even the relatively small reduction in risk observed in this study (ie, 2% reduction per month over 25 months) represents a potentially important clinical and public health priority. Notably, the pattern of protective results remained constant across racial, sex, and socioeconomic subgroups of Medicare beneficiaries. This is the first study to examine the protective effect of CPAP adherence on cerebrovascular risk across health disparity subgroups. A recent study reported that lower socioeconomic status is associated with reduced CPAP adherence among older adults,29 and a majority of studies have found that relative to White individuals, members of racial minority subgroups are less adherent to CPAP.39 The present results highlight the importance of achieving CPAP adherence among all health disparity populations. More broadly, the current findings support the tailoring of evidence-based CPAP adherence interventions (eg, cognitive-behavioral and motivational approaches22) for use in older adults.
Finally, among this national sample of older adults with OSA, the prevalence of comorbid disease was notably high, including comorbid diabetes (41%), ischemic heart disease (53%), and hypertension (86%). By contrast, the prevalence of these conditions was approximately 40% lower (eg, ischemic heart disease ∼31%40) in the overall Chronic Condition Data Warehouse data during the same years.40 These results suggest that OSA comorbidity and multimorbidity warrant further research attention.
Our study possesses strengths. First, unlike many prior studies, our large population sample ensured adequate statistical power to evaluate incident ischemic stroke. Prior studies of CPAP and stroke among older adults have generally been smaller; even the relatively large SAVE trial was underpowered to consider stroke as a stand-alone hard outcome. Second, in addition to being large, our sample was selected from a nationally representative sample of Medicare administrative claims data, ensuring a high degree of generalizability to most older adults in the United States. Third, also related to our sample, the inclusion criteria required not only a new diagnosis of OSA but also the initiation of CPAP therapy, thus ensuring a high degree of specificity for OSA. Finally, we employed a conservative definition of CPAP adherence, assessing both machine charges during the 13-month rent-to-own period and resupply charges during subsequent months of follow-up. To ensure temporality between the adherence measure and stroke events, the CPAP adherence variable was also lagged.
At the same time, our study has limitations. Most important, our administrative methodology precluded the assessment of important potential confounders, such as OSA disease severity, other sleep characteristics, daytime symptoms, and other factors influencing cerebrovascular events and stroke. Second, because we used CPAP machine and resupply charges as a proxy for objective CPAP adherence, we were unable to evaluate the dose-response relationship between hours of nightly CPAP use and stroke outcomes. Even so, our approach to leveraging Medicare claims data to examine the impact of CPAP adherence was based on Medicare policy (90-day adherence criteria requiring physician confirmation of objective device usage and symptomatic improvement) and past literature.29,41 We were not able to adjust for concurrent medications, which could have been confounders if they were associated with both adherence to CPAP and risk of stroke. Finally, we observed important differences in the comorbidity burden associated with CPAP adherence. To address this concern, we controlled for baseline stroke, demographic characteristics, and all disease comorbidities. Despite our best efforts to reduce residual confounding, it is possible that residual confounding persisted between groups.
CONCLUSIONS
This national study represents the largest analysis of the impact of CPAP adherence on cerebrovascular outcomes and stroke among older adults to date. For each month of CPAP use, a reduction in stroke risk was observed. This pattern of results was consistent across all racial, sex, and low–socioeconomic status subgroups of Medicare beneficiaries. Given the massive public health burden associated with cerebrovascular diseases and stroke, multiple stakeholders including patients, payors, policy makers, and health providers should seek to increase awareness and maximize CPAP adherence among older adults with OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was supported by a Strategic Research Award, “Impact of High PAP Adherence on Cardiovascular Outcomes Among Medicare Beneficiaries with Obstructive Sleep Apnea, 2006-2015,” (SRA project title) from the AASM Foundation to the University of Maryland, Baltimore (Principle Investigator: J.S.A.). E.M.W., J.S.A., and S.M.S.’s institution has received research funding from the American Academy of Sleep Medicine Foundation, the U.S. Department of Defense, Merck, and ResMed. EMW has served as a scientific consultant to DayZz, Eisai, Merck, and Purdue and is an equity shareholder in WellTap. The remaining authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Abigail Cirelli for assistance with the literature review.
ABBREVIATIONS
- CI
confidence interval
- CPAP
continuous positive airway pressure
- HR
hazard ratio
- OSA
obstructive sleep apnea
REFERENCES
- 1.Greenberg H, Lakticova V, Scharf SM. Obstructive sleep apnea: clinical features, evaluation, and principles of management. In: Kryger M, Roth T, Dement W, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2016: 1110–1124 [Google Scholar]
- 2. Hung J , Whitford EG , Parsons RW , Hillman DR . Association of sleep apnoea with myocardial infarction in men . Lancet . 1990. ; 336 ( 8710 ): 261 – 264 . 10.1016/0140-6736(90)91799-G [DOI] [PubMed] [Google Scholar]
- 3. Johnson KG , Johnson DC . Frequency of sleep apnea in stroke and TIA patients: a meta-analysis . J Clin Sleep Med. 2010. ; 6 ( 2 ): 131 – 137 . 10.5664/jcsm.27760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee CH , Sethi R , Li R , et al . Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention . Circulation . 2016. ; 133 ( 21 ): 2008 – 2017 . 10.1161/CIRCULATIONAHA.115.019392 [DOI] [PubMed] [Google Scholar]
- 5. Somers VK , White DP , Amin R , et al . Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing . J Am Coll Cardiol. 2008. ; 52 ( 8 ): 686 – 717 . 10.1016/j.jacc.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 6. Punjabi NM , Shahar E , Redline S , Gottlieb DJ , Givelber R , Resnick HE ; Sleep Heart Health Study Investigators . Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study . Am J Epidemiol. 2004. ; 160 ( 6 ): 521 – 530 . 10.1093/aje/kwh261 [DOI] [PubMed] [Google Scholar]
- 7. Reichmuth KJ , Austin D , Skatrud JB , Young T . Association of sleep apnea and type II diabetes: a population-based study . Am J Respir Crit Care Med. 2005. ; 172 ( 12 ): 1590 – 1595 . 10.1164/rccm.200504-637OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drager LF , Lopes HF , Maki-Nunes C , et al . The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome . PLoS One . 2010. ; 5 ( 8 ): e12065 . 10.1371/journal.pone.0012065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peppard PE , Szklo-Coxe M , Hla KM , Young T . Longitudinal association of sleep-related breathing disorder and depression . Arch Intern Med. 2006. ; 166 ( 16 ): 1709 – 1715 . 10.1001/archinte.166.16.1709 [DOI] [PubMed] [Google Scholar]
- 10. Finn L , Young T , Palta M , Fryback DG . Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study . Sleep . 1998. ; 21 ( 7 ): 701 – 706 . [PubMed] [Google Scholar]
- 11. Marshall NS , Wong KK , Cullen SR , Knuiman MW , Grunstein RR . Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort . J Clin Sleep Med. 2014. ; 10 ( 4 ): 355 – 362 . 10.5664/jcsm.3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Punjabi NM , Caffo BS , Goodwin JL , et al . Sleep-disordered breathing and mortality: a prospective cohort study . PLoS Med. 2009. ; 6 ( 8 ): e1000132 . 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kryger MH , Roos L , Delaive K , Walld R , Horrocks J . Utilization of health care services in patients with severe obstructive sleep apnea . Sleep . 1996. ; 19 ( 9 Suppl ): S111 – S116 . 10.1093/sleep/19.suppl_9.S111 [DOI] [PubMed] [Google Scholar]
- 14. Banno K , Ramsey C , Walld R , Kryger MH . Expenditure on health care in obese women with and without sleep apnea . Sleep . 2009. ; 32 ( 2 ): 247 – 252 . 10.1093/sleep/32.2.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost & Sullivan, for the American Academy of Sleep Medicine. Hidden health crisis costing America billions. Underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. Accessed March 7, 2021. https://aasm.org/resources/pdf/sleep-apnea-economic-crisis.pdf
- 16. Wickwire EM , Tom SE , Vadlamani A , et al . Older adult US Medicare beneficiaries with untreated obstructive sleep apnea are heavier users of health care than matched control patients . J Clin Sleep Med. 2020. ; 16 ( 1 ): 81 – 89 . 10.5664/jcsm.8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yaggi HK , Concato J , Kernan WN , Lichtman JH , Brass LM , Mohsenin V . Obstructive sleep apnea as a risk factor for stroke and death . N Engl J Med. 2005. ; 353 ( 19 ): 2034 – 2041 . 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 18. Chan W , Coutts SB , Hanly P . Sleep apnea in patients with transient ischemic attack and minor stroke: opportunity for risk reduction of recurrent stroke? Stroke . 2010. ; 41 ( 12 ): 2973 – 2975 . 10.1161/STROKEAHA.110.596759 [DOI] [PubMed] [Google Scholar]
- 19.Kochanek KD, Murphy SL, Xu JQ, Arias E. Deaths: Final data for 2017, Vol. 68, number 9. National Vital Statistics Reports. Accessed March 7, 2021. https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_09-508.pdf [PubMed] [Google Scholar]
- 20. Collen J , Lettieri C , Wickwire E , Holley A . Obstructive sleep apnea and cardiovascular disease, a story of confounders! Sleep Breath. 2020. ; 24 ( 4 ): 1299 – 1313 . 10.1007/s11325-019-01945-w [DOI] [PubMed] [Google Scholar]
- 21. Davis AP , Billings ME , Longstreth WT Jr , Khot SP . Early diagnosis and treatment of obstructive sleep apnea after stroke: are we neglecting a modifiable stroke risk factor? Neurol Clin Pract. 2013. ; 3 ( 3 ): 192 – 201 . 10.1212/CPJ.0b013e318296f274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wickwire EM , Lettieri CJ , Cairns AA , Collop NA . Maximizing positive airway pressure adherence in adults: a common-sense approach . Chest . 2013. ; 144 ( 2 ): 680 – 693 . 10.1378/chest.12-2681 [DOI] [PubMed] [Google Scholar]
- 23. López-Padilla D , Alonso-Moralejo R , Martínez-García MÁ , De la Torre Carazo S , Díaz de Atauri MJ . Continuous positive airway pressure and survival of very elderly persons with moderate to severe obstructive sleep apnea . Sleep Med. 2016. ; 19 : 23 – 29 . 10.1016/j.sleep.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 24. Ou Q , Chen Y-C , Zhuo S-Q , et al . Continuous positive airway pressure treatment reduces mortality in elderly patients with moderate to severe obstructive severe sleep apnea: a cohort study . PLoS One. 2015. ; 10 ( 6 ): e0127775 . Published correction appears in PLoS One . 2018. ; 13 ( 8 ): e0201923 . 10.1371/journal.pone.0127775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richards KC , Gooneratne N , Dicicco B , et al . CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea . J Am Geriatr Soc. 2019. ; 67 ( 3 ): 558 – 564 . 10.1111/jgs.15758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weaver TE , Chasens ER . Continuous positive airway pressure treatment for sleep apnea in older adults . Sleep Med Rev. 2007. ; 11 ( 2 ): 99 – 111 . 10.1016/j.smrv.2006.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pallansch J , Li Y , Bena J , Wang L , Foldvary-Schaefer N . Patient-reported outcomes in older adults with obstructive sleep apnea treated with continuous positive airway pressure therapy . J Clin Sleep Med. 2018. ; 14 ( 2 ): 215 – 222 . 10.5664/jcsm.6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Platt AB , Kuna ST , Field SH , et al . Adherence to sleep apnea therapy and use of lipid-lowering drugs: a study of the healthy-user effect . Chest . 2010. ; 137 ( 1 ): 102 – 108 . 10.1378/chest.09-0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickwire EM, Jobe SL, Oldstone LM, Scharf SM, Johnson AM, Albrecht JS. Lower socioeconomic status and co-morbid conditions are associated with reduced CPAP adherence use among older adult Medicare beneficiaries with obstructive sleep apnea. Sleep. 2020;. 43(12):zsaa122. 10.1093/sleep/zsaa122 [DOI] [PubMed] [Google Scholar]
- 30. Martínez-García MA , Campos-Rodríguez F , Catalán-Serra P , et al . Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study . Am J Respir Crit Care Med. 2012. ; 186 ( 9 ): 909 – 916 . 10.1164/rccm.201203-0448OC [DOI] [PubMed] [Google Scholar]
- 31. Catalan-Serra P , Campos-Rodriguez F , Reyes-Nuñez N , et al . Increased incidence of stroke, but not coronary heart disease, in elderly patients with sleep apnea . Stroke . 2019. ; 50 ( 2 ): 491 – 494 . 10.1161/STROKEAHA.118.023353 [DOI] [PubMed] [Google Scholar]
- 32. McEvoy RD , Antic NA , Heeley E , et al . SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea . N Engl J Med. 2016. ; 375 ( 10 ): 919 – 931 . 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 33. Schipper MH , Jellema K , Thomassen BJW , Alvarez-Estevez D , Verbraecken J , Rijsman RM . Stroke and other cardiovascular events in patients with obstructive sleep apnea and the effect of continuous positive airway pressure . J Neurol. 2017. ; 264 ( 6 ): 1247 – 1253 . 10.1007/s00415-017-8522-z [DOI] [PubMed] [Google Scholar]
- 34. Parra O , Sánchez-Armengol Á , Capote F , et al . Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial . J Sleep Res. 2015. ; 24 ( 1 ): 47 – 53 . 10.1111/jsr.12181 [DOI] [PubMed] [Google Scholar]
- 35. Korotinsky A , Assefa SZ , Diaz-Abad M , Wickwire EM , Scharf SM . Comparison of American Academy of Sleep Medicine (AASM) versus Center for Medicare and Medicaid Services (CMS) polysomnography (PSG) scoring rules on AHI and eligibility for continuous positive airway pressure (CPAP) treatment . Sleep Breath. 2016. ; 20 ( 4 ): 1169 – 1174 . 10.1007/s11325-016-1327-y [DOI] [PubMed] [Google Scholar]
- 36. Beishuizen SJE , van Munster BC , de Jonghe A , Abu-Hanna A , Buurman BM , de Rooij SE . Distinct cognitive trajectories in the first year after hip fracture . J Am Geriatr Soc. 2017. ; 65 ( 5 ): 1034 – 1042 . 10.1111/jgs.14754 [DOI] [PubMed] [Google Scholar]
- 37. Allison P . Discrete-time methods for the analysis of event histories . Sociol Methodol. 1982. ; 13 : 61 – 98 . 10.2307/270718 [DOI] [Google Scholar]
- 38. Wickwire EM , Bailey MD , Somers VK , et al . CPAP adherence reduces cardiovascular risk among older adults with obstructive sleep apnea [published online ahead of print 2020 Nov 3] . Sleep Breath. 10.1007/s11325-020-02239-2 [DOI] [PubMed] [Google Scholar]
- 39. Wallace DM , Williams NJ , Sawyer AM , et al . Adherence to positive airway pressure treatment among minority populations in the US: a scoping review . Sleep Med Rev. 2018. ; 38 : 56 – 69 . 10.1016/j.smrv.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chronic Conditions Data Warehouse Medicare Administrative Data User Guide. Version 3.6. Accessed March 7, 2021 https://www2.ccwdata.org/documents/10280/19002246/ccw-medicare-data-user-guide.pdf
- 41. Scharf MT , Keenan BT , Pack AI , Kuna ST . Mask refills as a measure of PAP adherence . J Clin Sleep Med. 2017. ; 13 ( 11 ): 1337 – 1344 . 10.5664/jcsm.6810 [DOI] [PMC free article] [PubMed] [Google Scholar]