Abstract
Study Objectives:
The recognition of specific endotypes as drivers of sleep apnea suggests the need of therapies targeting individual mechanisms. Acetazolamide is known to stabilize respiration at high altitude but benefits at sea level are less well understood.
Methods:
All controlled studies of acetazolamide in obstructive sleep apnea and/or central sleep apnea (CSA) were evaluated. The primary outcome was the apnea-hypopnea index.
Results:
Fifteen trials with a total of 256 patients were pooled in our systematic review. Acetazolamide reduced the overall apnea-hypopnea index (mean difference [MD] −15.82, 95% CI: −21.91 to −9.74, P < .00001) in central sleep apnea (MD −22.60, 95% CI: −29.11 to −16.09, P < .00001), but not in obstructive sleep apnea (MD −10.29, 95% CI: −33.34 to 12.77, P = .38). Acetazolamide reduced the respiratory related arousal index (MD −0.82, 95% CI: −1.56 to −0.08, P = .03), improved partial arterial of oxygen (MD 11.62, 95% CI: 9.13–14.11, P < .00001), mean oxygen saturation (MD 1.78, 95% CI: 0.53–3.04, P = .005), total sleep time (MD 25.74, 95% CI: 4.10–47.38, P = .02), N2 sleep (MD 3.34, 95% CI: 0.12–6.56, P = .04) and sleep efficiency (MD 4.83, 95% CI: 0.53–9.13, P = .03).
Conclusions:
Acetazolamide improves the apnea-hypopnea index and several sleep metrics in central sleep apnea. The drug may be of clinical benefit in patients with high loop gain apnea of various etiologies and patterns. The existence of high heterogeneity is an important limitation in applicability of our analysis.
Systematic Review Registration:
Registry: PROSPERO; Name: The effect of acetazolamide in patients with sleep apnea at sea level: a systematic review and meta analysis; URL: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020163316; Identifier: CRD42020163316.
Citation:
Ni Y-N, Yang H, Thomas RJ. The role of acetazolamide in sleep apnea at sea level: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(6):1295–1304.
Keywords: acetazolamide, central sleep apnea, obstructive sleep apnea
INTRODUCTION
Sleep apnea is a disease with intermittent cessation and/or decrease of airflow during sleep. Sleep apnea is associated with adverse cardiovascular and cerebrovascular outcomes and an increased risk of mortality.1–3 Positive airway pressure (PAP), whose main effect is keeping the upper airway open, is regarded as the first line therapy for obstructive sleep apnea (OSA) and is frequently used for treatment of central sleep apnea (CSA), but less effective in the latter one.4,5 Alternative therapies for OSA include oral appliances, surgical enhancement of the upper airway, weight loss, and hypoglossal nerve stimulation.6
Despite widespread use, PAP use is associated with insufficient adherence and variable degrees of residual apnea. In patients with OSA, only about 40–70% patients continue use of PAP for more than 3 months and up to 15–30% of patients may show a residual apnea-hypopnea index (AHI) > 5 events/h after PAP treatment.7–9 One reason for residual apnea is likely persistent high loop gain, leading to instability of respiratory rhythm in nonrapid eye movement sleep.10 Several mechanisms/endotypes that may drive/contribute to the AHI have been recognized, including high loop gain, low arousal threshold, poor muscle responsiveness, and sleep fragmentation.11 Thus, there is a possible role for pharmacological adjuncts that may target high loop gain to improve precision of treatment of sleep apnea syndromes, regardless of the phenotype established by conventional scoring.
Continuous PAP use in CSA is usually associated with substantial residual central apnea.12 Adaptive servo-ventilation is an approved option for CSA, but efficacy is inconsistent,13,14 and sleep quality improvements are inferior to that achieved with CPAP in patients with OSA.15
Acetazolamide (ATZ) has been shown to improve CSA at high altitude.16 Physiological assessments in tightly controlled experimental conditions show that the drug attenuates elevated loop gain and the ventilatory response to arousal in patients with OSA at sea level.17,18 However, its clinical effect on patients with OSA and/or CSA at sea level remains unclear and is not part of any standard practice guideline. We performed this systematic review and meta-analysis to assess if the published literature may support such use.
METHODS
Search strategies
A comprehensive computer search of literature from 1946 to May 2020 was conducted by 2 independent investigators (Y.-N. N. and H.Y.) in PubMed, Medline, Embase, Information Sciences Institute (ISI) Web of Science and Cochrane Central Register of Controlled Trails (CENTRAL) using the keywords of “acetazolamide” or “ATZ” or “Diamox” or “sleep apnea” or “sleep breathing” or “periodic breathing” or “Cheyne-Stokes breathing” without limitations in the publication type or language. The references listed in each identified article were reviewed, and the related articles were manually searched to identify all eligible studies to minimize potential publication bias.
Inclusion and exclusion criteria
Eligible clinical trials were identified based on the following criteria: 1) the participants enrolled in each study included patients with OSA and/or CSA (including those who received treatment but had persistent residual sleep apnea); 2) patients were compared before and after ATZ (pre-post) or divided into an experimental group, in which ATZ was applied, and a control group, in which patients were assigned to receive placebo or another drug; 3) outcomes included but were not limited to standard sleep breathing parameters. We excluded studies if they were performed in animals or in patients under 18 years of age, were conducted at high altitude (defined as a study in patients who ascended ≥ 2,500 m or described as a high-altitude study in the title, abstract, or full manuscript), were designed to assess physiological responses, were published as reviews or case reports, or not undergone peer review.
Study selection
Y.-N. N. and H.Y. performed study selection in 2 phases. First, they screened titles. Second, noncontrolled studies were excluded by screening the abstracts. Third, according to the study inclusion and exclusion criteria listed above, eligible studies were extracted by reviewing full texts. Any discrepancies and doubts were resolved by consensus with a third investigator (R.J.T.).
Data extraction
Data extraction forms including authors, publication year, study design, population, demographic characteristics (age, sex, etc.), type of sleep apnea, comorbidities (heart failure, hypertension, etc.), details about the administration of ATZ (dose and duration), outcome measures, and study results were conducted by the 2 data collectors. A third investigator checked all the information and also resolved discrepancies if there was any disagreement.
Quality assessment
The risk of bias in estimating study outcomes was assessed as low, unclear, or high for each of the following items: sequence generation, allocation sequence concealment, blinding of participants and clinicians, blinding of outcome assessment, incomplete outcome data assessment, and other bias. The overall risk of bias for each included trial was categorized as low if the risk of bias was low in all domains, unclear if the risk of bias was unclear in 1 or more domains and with no judgment of high risk of bias, or high if the risk of bias was high in 1 or more domains. Any divergence was resolved by discussing with a third investigator.
Statistical analysis
Continuous outcomes were analyzed by the inverse variance weighting method and calculated as mean difference (MD) and 95% confidence interval (CI). A Z-value and P value < .05 indicated statistical significance. An initial test for clinical, methodological, and statistical heterogeneities was exploring by using χ2 test. A value of P < .1 and I2 > 50% indicates significance. In view of statistical heterogeneity, random-effects model was applied. We also performed a sensitivity analysis to substitute alternative decisions or ranges of values for decisions that are arbitrary or unclear. All meta-analyses were carried out by using Cochrane systematic review software Review Manager (RevMan; Version 5.3.5; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014) and the results were displayed in Forest plots.
RESULTS
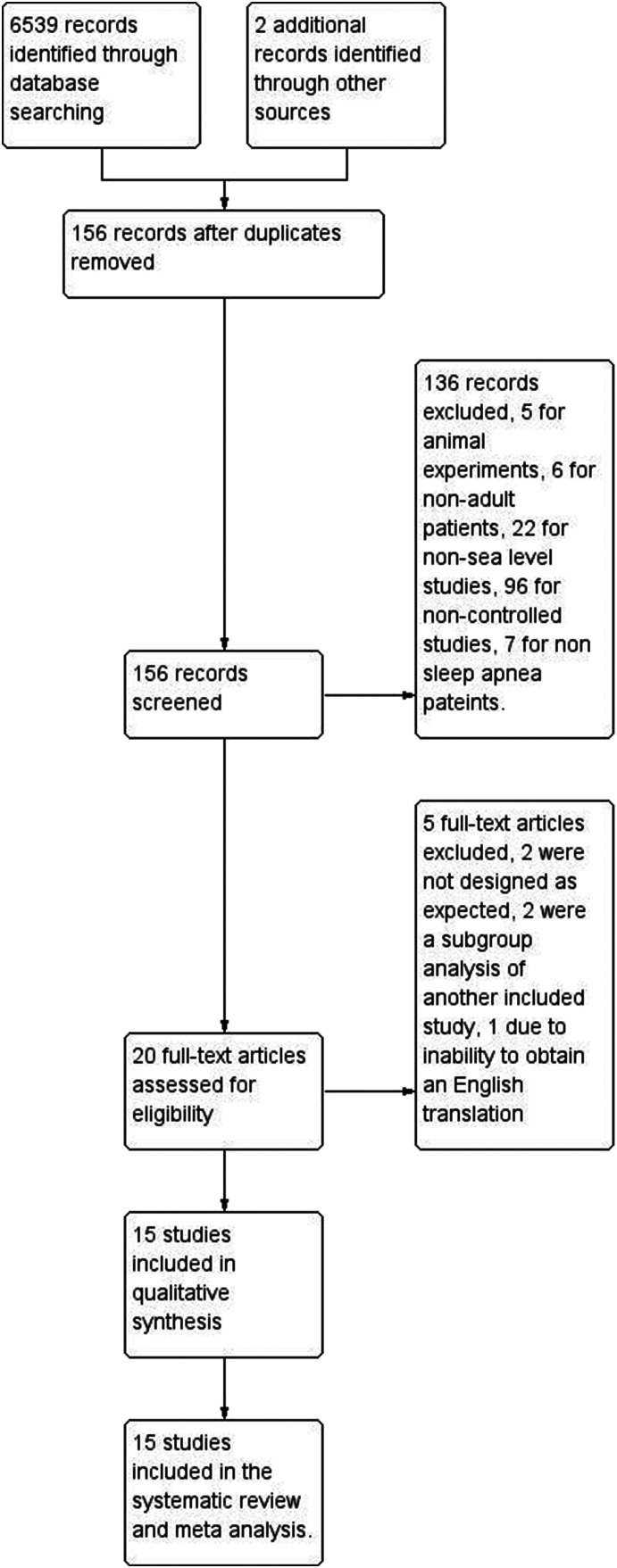
Initially, 6,541 records were identified, of which 6,539 were extracted from electronic databases and 2 were extracted from reference lists review (Figure 1). By screening the titles and abstracts, studies were discarded for duplication (n = 6,385), animal experiments (n = 5), non-adult patients (n = 6), non-sea level studies (n = 22), non-controlled studies (n = 96), and non-apnea patients (n = 7). We searched the full-text articles for the remaining 20 studies, and eventually 15 trials19–32 were enrolled in our final analysis. Two were not designed as expected, as multiple physiological challenges were utilized and could not truly reflect clinical effects,17,18 2 were a subgroup analysis of another included study,33,34 1 due to inability to obtain an English translation.35
Figure 1. Study flow.
Study description
All studies explored the role of ATZ in sleep apnea patients. Nine studies were pre-post studies,19,20,22,23,26–28,30,31 6 studies were randomized crossover studies,21,24,25,29,32,36 among which 1 study included patients using continuous positive airway pressure (CPAP) and was not included in the meta-analysis.21 Among the 14 studies included in the meta-analysis,19,20,22–32 the obstructive apnea index (OAI) was reported in 5 studies,20,24–26,30 and the central apnea index was reported in 6 studies,20,24–26,30,36 and the duration of respiratory events was reported in 4 studies.19,26,27,32 The lowest saturation of oxygen (SaO2) was reported in 7 studies.19,22–24,27,28,32 3 studies reported the mean SaO2,19,29,36 arterial partial carbon dioxide pressure (PaCO2) was reported in 8 studies,20,22,24,27–31 the respiratory related arousal index was reported in 4 studies,24,30,31,36 the slope of the hypercapnic ventilatory response was reported in 7 studies.19,20,22,24,28,30,36 Two studies focused only on patients with OSA,21,32 8 studies only included patients with CSA,20,22,24,25,29–31,36 among which 1 was caused by opioid drugs,25 and 1 was caused by chronic spinal cord injury.36 Three studies included patients with both sleep breathing disorder and heart failure.19,22,24 Table 1 lists the details of each study.
Table 1.
Characteristics of included studies.
| Study | Study Design | Population | Inclusion Criteria | Dose of Acetazolamide | Comparison |
|---|---|---|---|---|---|
| Central | |||||
| DeBacker et al, 199520 | Pre-post study | 14 | CSA | 250 mg orally at night for 1 month | N/A |
| Fontana et al, 201122 | Pre-post study | 12 | Hunter-Cheyne-Stokes breathing with AHI > 15 events/h and heart failure (LVEF < 50%). Patients with NYHA IV, acute coronary syndromes within the previous 6 month, glomerular filtration rate < 30 ml/min or pulmonary disease were excluded | 250 mg twice a day orally for 4 days | N/A |
| Ginter and Sankari, 202036 | Randomized crossover study | 16 | CSA with either chronic spinal cord injury (> 6 months) at or above the T6 level or able-bodied control | 500 mg 2 times a day orally for 3 days | N/A |
| Javaheri, 200624 | Randomized crossover study | 12 | CSA with AHI ≥ 15 events/h, Hunter-Cheyne-Stokes breathing, systolic heart failure with LVEF < 35%, and NYHA II or III | 3.5 mg/kg orally at night for 6 days | Placebo (potassium chloride 30 mEq) |
| Naghan et al, 201925 | Randomized crossover study | 10 | CSA caused by opioid drug | 250 mg orally at night for 6 days | Placebo |
| Ulrich et al, 201529 | Randomized crossover study | 23 | SBD and precapillary pulmonary hypertension or CTEPH, PaO2 ≥ 7.3 kPa during daytime. Patients with predominantly OSA, more than mild lung disease, or concomitant left ventricular disease were excluded | 250 mg twice a day orally for 1 week | Placebo |
| Verbraecken et al, 199830 | Pre-post study | 8 | CSA | 250 mg orally at night for 1 month | N/A |
| White et al, 198231 | Pre-post study | 6 | 5 patients with CSA, 1 with OSA | 250 mg 4 times a day for at least 7 days | N/A |
| Obstructive | |||||
| Eskandari et al, 201821 | Randomized crossover study | 13 | OSA with AHI > 15 events/h, treated systemic hypertension, BMI ≤ 35 kg/m2 and ESS score > 6 | 250 mg orally twice a day for initial 3 days and 3 times a day for the following 2 weeks | AZT + CPAP and CPAP |
| Whyte et al, 198832 | Randomized crossover study | 10 | OSA with AHI ≥ 15 events/h, with at least 2 of the following symptoms: daytime somnolence, loud snoring, unsatisfying nocturnal sleep, or recurrent nocturnal awakening | 250 mg orally twice a day for 1st week and 250 mg 4 times a day for 2nd week | Placebo |
| Unknown or Mixed Type | |||||
| Apostolo et al, 201419 | Pre-post study | 18 | Heart failure with LVEF < 40%, NYHA II or III, PB during exercise | 500 mg intravenous 3 times a day | N/A |
| Inoue et al, 199923 | Pre-post study | 75 | Sleep apnea | Mean of 351.2 mg/day orally for mean 39.5 days | No |
| Sakamoto et al, 199526 | Pre-post study | 20 | Sleep apnea | 250 mg orally for 10 patients, 500 mg orally for 8 patients, and 750 mg orally for 2 patients for 17–115 days | N/A |
| Sharp et al, 198527 | Pre-post study | 10 | 4 patients with OSA, 2 with CSA, 4 with MSA | 500–1,000 mg daily for 4–7 days | N/A |
| Tojima et al, 198828 | Pre-post study | 9 | 8 patients with OSA, 1 with CSA and Hunter-Cheyne-Stokes breathing | 250 mg daily orally for 7–8 days | N/A |
AHI = apnea-hypopnea index, BMI = body mass index, CPAP = continuous positive airway pressure, CSA = central sleep apnea, CTEPH = chronic thromboembolic pulmonary hypertension, ESS = Epworth Sleepiness Scale, LVEF = left ventricular ejection fraction, MSA = mixed sleep apnea, N/A = not available, NYHA = New York Heart Association, OAI = obstructive apnea index, OSA = obstructive sleep apnea, PaO2 = arterial pressure of oxygen, PB = periodic breathing.
A total of 256 patients was pooled from all the included trials in our final systematic review and meta-analysis. Details of baseline characteristics of patients in each enrolled study are shown in Table 2.
Table 2.
Characteristics of patients in included studies.
| Study | Mean Age (years) | Male (%) | BMI | AHI (events/h) | OAHI (events/h) | CAHI (events/h) | Side Effect of Acetazolamide |
|---|---|---|---|---|---|---|---|
| Central | |||||||
| DeBacker et al, 199520 | 47.9 ± 2.8 | 92.9 | 31.5 ± 1.7 | 37.2 ± 6.2 | 3.5 ± 1. 5 (OAI) | 25.5 ± 6.8 (CAI) | Some paresthesia |
| Fontana et al, 201122 | 62 ± 9 | 91.7 | 29 ± 4 | 36 ± 16 | NR | NR | NR |
| Ginter and Sankari, 202036 | Able-bodied: 59.5 ± 11.8; Spinal cord injury: 50.3 ± 12.8 | 81.25 | Able-bodied: 29.2 ± 1.9; Spinal cord injury: 25.8 ± 2.8 | Able-bodied: 21.3 ± 13.8; Spinal cord injury: 27.2 ± 32.0 | Able-bodied: 1.7 ± 2.0 (OAI); Spinal cord injury: 2.8 ± 3.7 (OAI) | Able-bodied: 2.8 ± 4.5 (CAI); Spinal cord injury: 7.3 ± 14.6 (CAI) | NR |
| Javaheri 200624 | 66 ± 6 | 100 | 26.4 ± 4.2 | 55 ± 24 | 1 ± 2 | 44 ± 23 | 8% nocturia |
| Naghan et al, 201925 | 50.2 ± 13.6 | 90 | 29.6 ± 6 | 78.2 ± 39.9 | 1.7 (0.7–19.8)(OAI) | 17.8 (13.3–24.8)(CAI) | NR |
| Ulrich et al, 201529 | 66 (56–71) | 34.8 | 26.6 (25.2–29.3) | 18 (6–40) | NR | NR | NR |
| Verbraecken et al, 199830 | 46 ± 5 | 87.5 | 30 ± 1 | 39 ± 10 | 3 ± 2 | 25 ± 10 | Some paresthesia |
| White et al, 198231 | 57.67 ± 13.63 | 100 | NR | 54 ± 12 (AI) | NR | NR | 33.3% paresthesia and released after dose reduced |
| Obstructive | |||||||
| Eskandari et al, 201821 | 64 ± 7 | 100 | 29 ± 4 | 37 ± 23 | NR | NR | 46% paresthesia, 31% dyspepsia |
| Whyte et al, 198832 | 51.50 ± 11.21 | 80 | NR | 50 ± 26 | NR | NR | 80% paresthesia |
| Unknown and Mixed Type | |||||||
| Apostolo et al, 201419 | 69.1 ± 1.9 | 100 | 24.5 ± 3.33 | 30.8 ± 13.8 | NR | NR | NR |
| Inoue et al, 199923 | 55.2 | 88.0 | 25.6 | 27.1 ± 17.4 | NR | NR | NR |
| Sakamoto et al, 199526 | 54.6 | 100 | NR | 34 ± 23.9 (AI) | NR | NR | 50% numbness,10% pollakiuria and 5% thirst |
| Sharp et al, 198527 | 53.6 ± 10.99 | NR | 33.55 ± 8.81 | OSA: 89 ± 4.9 CSA: 83 ± 35.36 MSA: 64.75 ± 17.39 | NR | NR | NR |
| Tojima et al, 198828 | 58 ± 4 | 55.6 | 29.86 ± 6.15 | 25 ± 6.7 | NR | NR | 22.2% dysesthesia and 11.1% pollakiuria |
AHI = apnea-hypopnea index, AI = apnea index, BMI = body mass index, CSA = central sleep apnea, MSA = mixed sleep apnea, NR = not reported, OAI = obstructive apnea index, OSA = obstructive sleep apnea.
Quality assessment
Quality assessments of the 15 enrolled studies showed no bias in attribution, detection, or reporting in 6 studies, but high bias in selection, performance, and detection was showed in another 9 studies (Figure S1 (455.1KB, pdf) and Figure S2 (455.1KB, pdf) in the supplemental material). No studies were excluded for low quality or dubious decisions in the sensitivity analysis. No publication bias was found (Figure S3 (455.1KB, pdf) ).
Overall effects of ATZ
ATZ reduced the overall AHI (MD −15.82, 95% CI: −21.91 to −9.74, Z = 5.10, P < .00001) (Figure S4 (455.1KB, pdf) ). When divided by different sleep apnea types, ATZ reduced the AHI in patients with CSA (MD −22.60, 95% CI: −29.11 to −16.09, Z = 6.80, P < .00001) and unknown or mixed sleep apnea (MD −8.14, 95% CI: −11.70 to −4.59, Z = 4.49, P < .00001), but not in patients with OSA (MD −10.29, 95% CI: −33.34 to 12.77, Z = 0.87, P = .38) (Figure S5 (455.1KB, pdf) ). The results remained unchanged when divided into those with and without heart failure (Figure S6 (455.1KB, pdf) ).
CSA
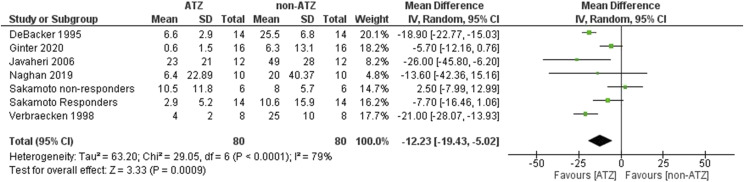
ATZ reduced CSA and the central apnea index was reduced (MD −12.23, 95% CI: −19.43 to −5.02, Z = 3.33, P = .0009). Significant heterogeneity was found in (I2 = 79%, χ2 = 29.05, P < .0001) (Figure 2). The results remained unchanged when divided into those with and without heart failure (Figure S7 (455.1KB, pdf) ).
Figure 2. CAHI.
ATZ = acetazolamide, CAHI = central apnea-hypopnea index, CI = confidence interval, SD = standard deviation.
OSA
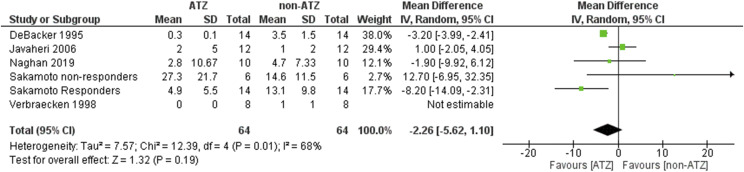
Overall, no significant effect of ATZ in reducing the OAI was found (MD −2.26, 95% CI: −5.62 to 1.10, Z = 1.32, P = .19). ATZ showed a trend of lower the OAI in patients without heart failure (MD −3.64, 95% CI: −7.47 to 0.20, Z = 1.86, P = .06), but statistic significant was not found (Figure S8 (455.1KB, pdf) ). Statistical heterogeneity existed in this analysis (I2 = 68%, χ2 = 12.39, P = .01) (Figure 3). Percentages of change in AHI, OAI, and central apnea index are listed in the Table S1 (455.1KB, pdf) .
Figure 3. OAHI.
ATZ = acetazolamide, OAHI = obstructive apnea-hypopnea index, CI = confidence interval, SD = standard deviation.
Respiratory event duration
No significant effect of ATZ in reducing the duration of respiratory events was found (MD 0.96, 95% CI: −1.32 to 3.24, Z = 0.82, P = .41). Significant heterogeneity existed in this analysis (I2 = 55%, χ2 = 11.08, P = .05) (Figure S9 (455.1KB, pdf) ).
Respiratory related arousal index
ATZ reduced the respiratory related arousal index (MD −0.82, 95% CI: −1.56 to −0.08, Z = 2.17, P = .03). Significant heterogeneity was found (I2 = 57%, χ2 = 6.94, P = .07) (Figure S10 (455.1KB, pdf) ).
PaO2, lowest SaO2, and mean SaO2
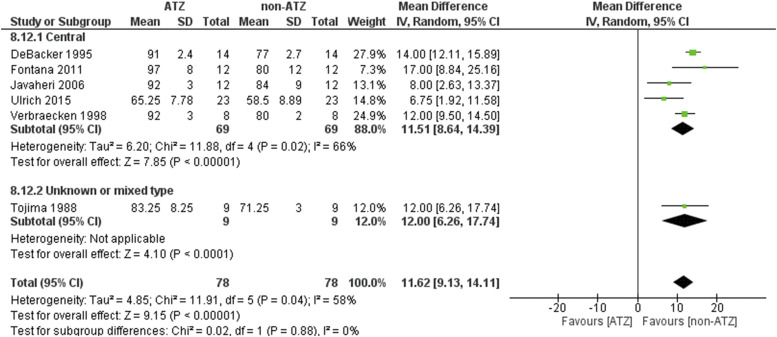
Higher PaO2 was found when patients were treated with ATZ (MD 11.62, 95% CI: 9.13–14.11, Z = 9.15, P < .00001) (Figure 4). High heterogeneity was found in the analysis (I2 = 58%, χ2 = 11.91, P = .04). ATZ did play a role in improving the lowest SaO2 in the overall analysis (MD 2.95, 95% CI: 0.61–5.29, Z = 2.47, P = .01) (Figure S11 (455.1KB, pdf) and Figure S12 (455.1KB, pdf) ). However, no significant role was found in each sleep apnea type. High heterogeneity was found in the analysis (I2 = 52%, χ2 = 16.57, P = .03). ATZ increased the mean SaO2 minimally (MD 1.78, 95% CI 0.53–3.04, Z = 2.78, P = .005) especially in the crossover study (MD 2.15, 95% CI 0.40–3.90, Z = 2.40, P = .02) and patients with CSA (Figure S13 (455.1KB, pdf) and Figure S14 (455.1KB, pdf) )
Figure 4. PaO2.
ATZ = acetazolamide, CI = confidence interval, PaO2 = partial arterial pressure of oxygen, SD = standard deviation.
Sleep architecture
ATZ increased total sleep time (MD 25.74, 95% CI: 4.10–47.38, Z = 2.33, P = .02) (Figure S15 (455.1KB, pdf) ) and improved sleep efficiency (MD 4.83, 95% CI: 0.53–9.13, Z = 2.20, P = .03) (Figure S16 (455.1KB, pdf) ). However, ATZ did not change the percentages of N1 (MD −1.29, 95% CI: −3.10 to 0.53, Z = 1.39, P = .16) (Figure S17 (455.1KB, pdf) ), slow-waveform sleep (MD 0.22, 95% CI: −0.88 to 1.33, Z = 0.40, P = .69) (Figure S18 (455.1KB, pdf) ), and rapid eye movements (Figure S19 (455.1KB, pdf) ) (MD 2.73, 95% CI: −0.28 to 5.75, Z = 1.78, P = .08). The change in N2 (MD 3.34, 95% CI: 0.12–6.56, Z = 2.03, P = .04) was small and clinically meaningless (Figure S20 (455.1KB, pdf) ). High heterogeneity was found in sleep efficiency (I2 = 61%, χ2 = 17.97, P = .01), rapid eye movements (I2 = 86%, χ2 = 28.52, P < .00001) analysis, but not in total sleep time (I2 = 47%, χ2 = 9.47, P = .09), N1 (I2 = 31%, χ2 = 2.90, P = .23), N2 (I2 = 0%, χ2 = 0.98, P = .61), or slow-waveform sleep (I2 = 0%, χ2 = 0.29, P = .87).
Hypercapnic ventilatory response
ATZ increased the hypercapnic ventilatory response (MD 0.70, 95% CI: 0.06–1.35, Z = 2.13, P = .03). Significant statistical heterogeneity was found in this analysis (I2 = 72%, χ2 = 25.36, P = .0007) (Figure S21 (455.1KB, pdf) ).
pH, PaCO2, and HCO−
ATZ lowered blood pH (MD −0.07, 95% CI −0.08 to −0.05, Z = 8.97, P < .00001) (Figure S22 (455.1KB, pdf) ), PaCO2 (MD −4.59, 95% CI: −5.80 to −3.39, Z = 7.46, P < .00001) (Figure S23 (455.1KB, pdf) ), and HCO− (MD −5.65, 95% CI −6.81 to −4.49, Z = 9.54, P < .00001) (Figure S24 (455.1KB, pdf) ). Significant heterogeneity was found in the analysis of pH (I2 = 71%, χ2 = 27.71, P = .0005) and PaCO2 (I2 = 79%, χ2 = 43.79, P < .00001). All the results remained consistent when divided by the type of sleep apnea (Figure S25 (455.1KB, pdf) ).
DISCUSSION
ATZ had beneficial effects in CSA but not OSA. There was improvement in PaO2 and mean SaO2, but no impact on respiratory event duration. No improvement in the lowest SaO2 was found overall. The respiratory related arousal index was reduced and total sleep time and sleep efficiency increased, consistent with improvements in sleep quality. However, slow-wave sleep was not impacted.
ATZ is a drug with complex effects, including powerful carbonic anhydrase inhibition.37 In our included studies, the causes of CSA were mainly heart failure and opioid drugs.25 However, the mechanisms of CSA in patients with heart failure and opioids were different and also the mechanisms of ATZ are different in these 2 types of CSA. It would be expected that the entire spectrum of high loop gain sleep apnea (heart failure, OSA with high loop gain as a driving mechanism, treatment-emergent central) would show a key hyperventilatory feature, that of hypocapnia; opioids induce hypercapnia.
In the patients with heart failure, a model of hyperventilatory CSA increased left atrial/pulmonary capillary wedge pressure,38 and compromised vasodilatory/vasoconstrictive cerebrovascular response to transient hypercapnia/hypocapnia will increase the CO2 response slope sensitivity and narrow the CO2 reserve.38–40 Prolonged circulation time and enhanced carotid chemosensitivity, which increases loop gain, also contributes.10,41 Although ATZ can cause a mild metabolic acidosis and lower PaCO2, the apneic threshold decreases to a larger extent and eupneic PaCO2 also increased.42 Through these mechanisms, the net effect is that the CO2 reserve increases, reducing instability to minor fluctuations in CO2. ATZ increases ventilatory drive and causes mild hyperventilation.43 For a given change in ventilatory volume, the change of PaCO2 is smaller during hyperventilation.42 Thus, the ventilatory response to arousal and plant gain is reduced. The change in PaCO2 to induce instability/apnea is increased by more than 30% and the ventilatory volume required is increased 3-fold when given ATZ.42 The diuretic effect of ATZ44 also improves pulmonary congestion and increases CO2 reserve. Another mechanism for ATZ’s stabilizing effect on ventilatory drive response to hypoxia and hypercapnia could be by reducing chemosensitivity of the peripheral chemoreflex.45,46 The drug can also reduce the slope of hypoxic ventilatory response, in one study from 1.03 to 0.78 (L/min/mm Hg).22 Our analysis found that ATZ increased the ventilatory response change in PaCO2, while Edward’s et al18 showed that the drug reduced the ventilatory response to arousal from sleep. All our included studies measured the hypercapnic response during wake, and thus are not directly comparable to sleep state assessments. Systemic metabolic acidosis, which is a long-term effect of ATZ, can inhibit the hypoxic ventilatory response.47
ATZ may be beneficial in opioid-induced sleep-disordered breathing,25 which is characterized by obstructive and central respiratory abnormality, ataxic breathing, and mild hypercapnia.48 Thus, this is a model of hypoventilatory CSA. Opioids have complex respiratory effects, not only depressing respiration but also disrupting respiratory rhythm. In animal models, experimental instillation of µ-opioid agonists into the pre-Bötzinger complex suppresses ventilation.49,50 Opioids decrease the mean frequency of inspiratory motor nerve output. This type of opioid-induced quantal slowing results from transmission failure of rhythmic drive from preinspiratory neurons to pre-Bötzinger complex inspiratory neurons, depressed below threshold for spontaneous rhythmic activity.51 Genetic deletion of µ-opioid receptors in a glutamatergic pre-Bötzinger complex subpopulation abolishes opioid-mediated depression of inspiratory burst firing by reducing burstlet frequency.52 Activation of μ-opioid receptors in the Kölliker-Fuse nucleus eliminates the postinspiration phase of the respiratory cycle.53 Opioid drugs increase the sensitivity to hypoxia of the peripheral chemoreflex but blunt the sensitivity of the hypercapnic response.54,55 Patients using opioids are exposed to some hypoventilation and elevated PaCO2 during sleep.54 In patients with opioids, the apneic threshold increases by about 6 mm Hg.56 Under this condition, a small change in ventilation will cause a larger change in PaCO2 and interact with the apneic threshold,42 in essence increasing plant gain. The increase in inspiratory drive caused by ATZ through metabolic acidosis and effects on both the central and peripheral chemoreceptors may offset the opioid effects.24 ATZ can reduce fluid overload,57 lower the PaCO2 level, and reduce plant gain,58 all beneficial effects.
A general effect on high loop gain is also suggested by a report on benefits for persistent CPAP treatment associated CSA.59 Our study found that pH and bicarbonate were lower in the ATZ group in both types of sleep apnea. The studies that reported the level of bicarbonate administered ATZ for 7 to 30 days. This indicated that the effect of ATZ in inducing metabolic acidosis might persist for at least 1 month.60 However, more studies should be done to explore whether this effect can persist since renal adaptation could occur.61 The analysis cannot exclude an acute effect of ATZ such as reducing the chemosensitivity of the peripheral chemoreflex. Studies are required to determine the magnitude of peripheral chemoreflex inhibition and impact on AHI.
ATZ was not beneficial for patients with OSA in our analysis. High loop gain may drive part of the AHI in a subset of otherwise labeled patients with OSA, but the likely heterogeneity of pathophysiology possibly did not allow any benefit to show. It is not surprising that ATZ can “change” central to obstructive sleep apnea.27 ATZ improves only high loop gain but not the other endotypes driving sleep apnea.17 Disease drivers frequently coexist, thus the airway is narrow or occluded during the nadir of periodic breathing.62,63 It can be difficult to distinguish OSA and CSA with conventional scoring, thus the studies summarized likely had admixtures of disease beyond that estimated by the scored indices. Some of the included studies did not provide obstructive AHI and central AHI separately, which made it difficult to define the role of ATZ in OSA. ATZ might have a potential role in reducing OAI in patients without heart failure; perhaps it was those with high loop gain who benefitted.
A recent meta-analysis reported that ATZ was effective in both OSA and CSA.64 There are some important differences in the tabulated studies and exclusion criteria. The authors included 28 studies (13 OSA/15 CSA, ATZ = 542 and controls = 553 patients). The AHI reduction was similar in OSA vs CSA, but significantly greater with higher doses (at least up to 500 mg/day). These results differ from ours as 1) physiological studies in OSA patients were included, 2) non-peer-reviewed studies were included, and 3) studies were at both high altitude and sea level, while our study only focused on sleep apnea at sea level. Our results better reflect what may occur when used at sea level.
A response to ATZ was not universal. One included study showed that about 30% of the patients did not respond to ATZ and both CAS and OSA indices increased after ATZ.26 No significant changes were found in arterial blood values such as pH and PaCO2. It is possible that the probability of a response to ATZ is reflected in a change (reduction) of PaCO2 or end-tidal CO2. A different study showed that 54.7% of patients receiving ATZ had no response, and these patients had a higher body mass index (27 ± 6.5 vs 23.8 ± 4.2) and higher AHI (32.5 ± 16.5 vs 20.7 ± 16.5).23 The body mass index association suggests confounding obstructive elements.
Only 1 study compared the results of ATZ and ATZ plus CPAP.21 This study showed that the usage of ATZ plus CPAP was more effective in improving AHI but not SpO2. ATZ did not impact CPAP adherence (usage time: 4.8 ± 2.2 hours when using CPAP vs 5.0 ± 2.0 hours when using CPAP plus ATZ). Five patients used ATZ after uvulopalatopharyngoplasty, and the results showed that ATZ improved the effect of uvulopalatopharyngoplasty.23 These 2 studies suggest that ATZ might have a role as adjunctive therapy, especially if there is residual apnea during therapy for OSA. However, CSA emerging after treatment initiation might not be persistent,65 thus the role for long-term use of ATZ in these patients remains uncertain. Improvements in sleep quality occurred with ATZ—a reduced arousal index and an increase in total sleep time and sleep efficiency. This was likely from an improvement in respiration. ATZ did improve the minimum PaO2 and mean SaO2, but only a trend toward rising nadir SatO2. All the above effects of ATZ were more significant in patients with CSA instead of patients with OSA, unknown, or mixed apnea. This further supports the likelihood that ATZ did benefit CSA, while OSA phenotyping is probably needed to optimize assessment of the drug.
The most common adverse effect of ATZ was paresthesia, whose rates varied from 33.3 to 80%; a dose reduction reduced this effect.31 Other side effects included dyspepsia and increased urination. One study showed that if 2 doses are taken, the effect of ATZ could last for 24 hours.22 A single dose is expected to last a whole night. Thus, 1 report described an acute confusional syndrome in a patient with OSA, possibly from metabolic acidosis induced by ATZ.26 A meta-analysis of adverse events from ATZ had 42 studies and over 1,200 participants.66 The most common side effects, which were dose dependent, were paresthesia, dysgeusia, polyuria, and fatigue.
Three studies reported that ATZ was effective in reducing clinical symptoms of severe sleep apnea, such as snoring, headache, and excessive daytime sleepiness, and improving cognition.26,28,30 However, another study showed no subjective or objective improvements in clinical symptoms.32
Our analysis has some limitations. There were a relatively small number of studies. Most included studies had small sample. Respiratory event scoring standards have changed over the decades and introduced errors in quantification. Significant heterogeneity existed in most of our analysis due to age, body mass index, and AHI, all of which might influence responsiveness to ATZ. Moreover, the administration of ATZ such as dose (varied from 250 mg/day to 1000 mg/day) and length of administration (varied from 3 days to 115 days) were significantly different between studies. Optimal dosing remains undefined, as when used with CPAP, single bedtime 250 mg dosing may be sufficient.60 The studies were not long-term trials, which is an important consideration for sleep apnea treatment.
CONCLUSIONS
ATZ seems effective in partially reducing CSA. Improved phenotyping may be better able to identify patients who may respond. Despite the general consistency of results across the reviewed studies, ready clinical application of our results is limited due to the existence of high heterogeneity.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Division of Pulmonary, Critical Care and Sleep Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA. This study was funded by an American Academy of Sleep Medicine Foundation category-I award to R.J.T. Dr. Thomas is co-inventor and patent holder of the ECG-derived sleep spectrogram, which may be used to phenotype sleep quality and central/complex sleep apnea. The technology is licensed by Beth Israel Deaconess Medical Center to MyCardio, LLC. He is also co-inventor and patent holder of the Positive Airway Pressure Gas Modulator, being developed for treatment of central/complex sleep apnea. He has consulted for Jazz Pharmaceuticals and consults for Guidepoint Global and GLG Councils. He is co-inventor of a licensed auto-CPAP software to for DeVilbiss-Drive. No other authors report conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
Author contributions: Y.-N.N. conceptualized and drafted the manuscript, H.Y. extracted the data, R.J.T. interpreted the results and revised the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ATZ
acetazolamide
- CI
confidence interval
- CPAP
continuous positive airway pressure
- CSA
central sleep apnea
- OAI
obstructive apnea index
- OSA
obstructive sleep apnea
- MD
mean difference
- PaO2
arterial partial oxygen pressure
- PaCO2
arterial partial carbon dioxide pressure
- PAP
positive airway pressure
- SaO2
saturation of oxygen
REFERENCES
- 1.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. 10.1161/01.CIR.99.11.1435 [DOI] [PubMed] [Google Scholar]
- 2. Qie R , Zhang D , Liu L , et al . Obstructive sleep apnea and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of cohort studies . J Diabetes . 2020. ; 12 ( 6 ): 455 – 464 . 10.1111/1753-0407.13017 [DOI] [PubMed] [Google Scholar]
- 3. Punjabi NM , Beamer BA . Alterations in glucose disposal in sleep-disordered breathing . Am J Respir Crit Care Med . 2009. ; 179 ( 3 ): 235 – 240 . 10.1164/rccm.200809-1392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patil SP , Ayappa IA , Caples SM , Kimoff RJ , Patel SR , Harrod CG . Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment . J Clin Sleep Med . 2019. ; 15 ( 2 ): 301 – 334 . 10.5664/jcsm.7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aurora RN , Chowdhuri S , Ramar K , et al . The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses . Sleep . 2012. ; 35 ( 1 ): 17 – 40 . 10.5665/sleep.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waters T . Alternative interventions for obstructive sleep apnea . Cleve Clin J Med . 2019. 86 , 9 , Suppl 1 : 34 – 41 . 10.3949/ccjm.86.s1.06 [DOI] [PubMed] [Google Scholar]
- 7. Bailly S , Daabek N , Jullian-Desayes I , et al . Partial failure of CPAP treatment for sleep apnoea: Analysis of the French national sleep database . Respirology . 2020. ; 25 ( 1 ): 104 – 111 . 10.1111/resp.13650 [DOI] [PubMed] [Google Scholar]
- 8. Riachy M , Najem S , Iskandar M , Choucair J , Ibrahim I , Juvelikian G . Factors predicting CPAP adherence in obstructive sleep apnea syndrome . Sleep Breath . 2017. ; 21 ( 2 ): 295 – 302 . 10.1007/s11325-016-1408-y [DOI] [PubMed] [Google Scholar]
- 9. Baratta F , Pastori D , Bucci T , et al . Long-term prediction of adherence to continuous positive air pressure therapy for the treatment of moderate/severe obstructive sleep apnea syndrome . Sleep Med . 2018. ; 43 : 66 – 70 . 10.1016/j.sleep.2017.09.032 [DOI] [PubMed] [Google Scholar]
- 10. Sands SA , Edwards BA , Kee K , et al . Loop gain as a means to predict a positive airway pressure suppression of Cheyne-Stokes respiration in patients with heart failure . Am J Respir Crit Care Med . 2011. ; 184 ( 9 ): 1067 – 1075 . 10.1164/rccm.201103-0577OC [DOI] [PubMed] [Google Scholar]
- 11. Carberry JC , Amatoury J , Eckert DJ . Personalized management approach for OSA . Chest . 2018. ; 153 ( 3 ): 744 – 755 . 10.1016/j.chest.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 12. Bradley TD , Logan AG , Kimoff RJ , et al .; CANPAP Investigators . Continuous positive airway pressure for central sleep apnea and heart failure . N Engl J Med . 2005. ; 353 ( 19 ): 2025 – 2033 . 10.1056/NEJMoa051001 [DOI] [PubMed] [Google Scholar]
- 13. Cowie MR , Woehrle H , Wegscheider K , et al . Adaptive servo-ventilation for central sleep apnea in systolic heart failure . N Engl J Med . 2015. ; 373 ( 12 ): 1095 – 1105 . 10.1056/NEJMoa1506459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Connor CM , Whellan DJ , Fiuzat M , et al . Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial . J Am Coll Cardiol . 2017. ; 69 ( 12 ): 1577 – 1587 . 10.1016/j.jacc.2017.01.041 [DOI] [PubMed] [Google Scholar]
- 15. Allam JS , Olson EJ , Gay PC , Morgenthaler TI . Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes . Chest . 2007. ; 132 ( 6 ): 1839 – 1846 . 10.1378/chest.07-1715 [DOI] [PubMed] [Google Scholar]
- 16. Latshang TD , Nussbaumer-Ochsner Y , Henn RM , et al . Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial . JAMA . 2012. ; 308 ( 22 ): 2390 – 2398 . 10.1001/jama.2012.94847 [DOI] [PubMed] [Google Scholar]
- 17. Edwards BA , Sands SA , Eckert DJ , et al . Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea . J Physiol . 2012. ; 590 ( 5 ): 1199 – 1211 . 10.1113/jphysiol.2011.223925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards BA , Connolly JG , Campana LM , et al . Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea . Sleep . 2013. ; 36 ( 2 ): 281 – 285 . 10.5665/sleep.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Apostolo A , Agostoni P , Contini M , Antonioli L , Swenson ER . Acetazolamide and inhaled carbon dioxide reduce periodic breathing during exercise in patients with chronic heart failure . J Card Fail . 2014. ; 20 ( 4 ): 278 – 288 . 10.1016/j.cardfail.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 20. DeBacker WA , Verbraecken J , Willemen M , Wittesaele W , DeCock W , Van deHeyning P . Central apnea index decreases after prolonged treatment with acetazolamide . Am J Respir Crit Care Med . 1995. ; 151 ( 1 ): 87 – 91 . 10.1164/ajrccm.151.1.7812578 [DOI] [PubMed] [Google Scholar]
- 21. Eskandari D , Zou D , Grote L , Hoff E , Hedner J . Acetazolamide reduces blood pressure and sleep-disordered breathing in patients with hypertension and obstructive sleep apnea: a randomized controlled trial . J Clin Sleep Med . 2018. ; 14 ( 3 ): 309 – 317 . 10.5664/jcsm.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fontana M , Emdin M , Giannoni A , Iudice G , Baruah R , Passino C . Effect of acetazolamide on chemosensitivity, Cheyne-Stokes respiration, and response to effort in patients with heart failure . Am J Cardiol . 2011. ; 107 ( 11 ): 1675 – 1680 . 10.1016/j.amjcard.2011.01.060 [DOI] [PubMed] [Google Scholar]
- 23. Inoue Y , Takata K , Sakamoto I , Hazama H , Kawahara R . Clinical efficacy and indication of acetazolamide treatment on sleep apnea syndrome . Psychiatry Clin Neurosci . 1999. ; 53 ( 2 ): 321 – 322 . 10.1046/j.1440-1819.1999.00551.x [DOI] [PubMed] [Google Scholar]
- 24. Javaheri S . Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study . Am J Respir Crit Care Med . 2006. ; 173 ( 2 ): 234 – 237 . 10.1164/rccm.200507-1035OC [DOI] [PubMed] [Google Scholar]
- 25. Naghan PA , Raeisi K , Khoundabi B , et al . The effect of acetazolamide on the improvement of central apnea caused by abusing opioid drugs in the clinical trial . Sleep Breath . 2020. ; 24 ( 4 ): 1417 – 1425 . 10.1007/s11325-019-01968-3 . [DOI] [PubMed] [Google Scholar]
- 26. Sakamoto T , Nakazawa Y , Hashizume Y , et al . Effects of acetazolamide on the sleep apnea syndrome and its therapeutic mechanism . Psychiatry Clin Neurosci . 1995. ; 49 ( 1 ): 59 – 64 . 10.1111/j.1440-1819.1995.tb01858.x [DOI] [PubMed] [Google Scholar]
- 27. Sharp JT , Druz WS , D’Souza V , Diamond E . Effect of metabolic acidosis upon sleep apnea . Chest . 1985. ; 87 ( 5 ): 619 – 624 . 10.1378/chest.87.5.619 [DOI] [PubMed] [Google Scholar]
- 28. Tojima H , Kunitomo F , Kimura H , Tatsumi K , Kuriyama T , Honda Y . Effects of acetazolamide in patients with the sleep apnoea syndrome . Thorax . 1988. ; 43 ( 2 ): 113 – 119 . 10.1136/thx.43.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ulrich S , Keusch S , Hildenbrand FF , et al . Effect of nocturnal oxygen and acetazolamide on exercise performance in patients with pre-capillary pulmonary hypertension and sleep-disturbed breathing: randomized, double-blind, cross-over trial . Eur Heart J . 2015. ; 36 ( 10 ): 615 – 623 . 10.1093/eurheartj/eht540 [DOI] [PubMed] [Google Scholar]
- 30. Verbraecken J , Willemen M , De Cock W , Coen E , Van de Heyning P , De Backer W . Central sleep apnea after interrupting longterm acetazolamide therapy . Respir Physiol . 1998. ; 112 ( 1 ): 59 – 70 . 10.1016/S0034-5687(98)00010-3 [DOI] [PubMed] [Google Scholar]
- 31. White DP , Zwillich CW , Pickett CK , Douglas NJ , Findley LJ , Weil JV . Central sleep apnea. Improvement with acetazolamide therapy . Arch Intern Med . 1982. ; 142 ( 10 ): 1816 – 1819 . 10.1001/archinte.1982.00340230056012 [DOI] [PubMed] [Google Scholar]
- 32. Whyte KF , Gould GA , Airlie MA , Shapiro CM , Douglas NJ . Role of protriptyline and acetazolamide in the sleep apnea/hypopnea syndrome . Sleep . 1988. ; 11 ( 5 ): 463 – 472 . 10.1093/sleep/11.5.463 [DOI] [PubMed] [Google Scholar]
- 33. Javaheri S , Sands SA , Edwards BA . Acetazolamide attenuates Hunter-Cheyne-Stokes breathing but augments the hypercapnic ventilatory response in patients with heart failure . Ann Am Thorac Soc . 2014. ; 11 ( 1 ): 80 – 86 . 10.1513/AnnalsATS.201306-201OC [DOI] [PubMed] [Google Scholar]
- 34. Hoff E , Zou D , Schiza S . Carbonic anhydrase, obstructive sleep apnea and hypertension: Effects of intervention . 2020. ; J Sleep Res . 2020. ; 29 ( 2 ): e12956 . 10.1111/jsr.12956 [DOI] [PubMed] [Google Scholar]
- 35. Chin K , Ohi M , Fukui M , Kuriyama T , Hirai M , Kuno K . [Therapy and clinical symptoms in patients with obstructive sleep apnea in Japan] . Nihon Kyobu Shikkan Gakkai Zasshi . 1992. ; 30 ( 2 ): 270 – 277 . [PubMed] [Google Scholar]
- 36. Ginter G , Sankari A , Eshraghi M , et al . Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury . J Appl Physiol 1985 . 2020. ; 128 ( 4 ): 960 – 966 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Preisig PA , Toto RD , Alpern RJ . Carbonic anhydrase inhibitors . Ren Physiol . 1987. ; 10 ( 3-4 ): 136 – 159 . [DOI] [PubMed] [Google Scholar]
- 38. Chenuel BJ , Smith CA , Skatrud JB , Henderson KS , Dempsey JA . Increased propensity for apnea in response to acute elevations in left atrial pressure during sleep in the dog . J Appl Physiol 1985 . 2006. ; 101 ( 1 ): 76 – 83 . [DOI] [PubMed] [Google Scholar]
- 39. Xie A , Skatrud JB , Khayat R , Dempsey JA , Morgan B , Russell D . Cerebrovascular response to carbon dioxide in patients with congestive heart failure . Am J Respir Crit Care Med . 2005. ; 172 ( 3 ): 371 – 378 . 10.1164/rccm.200406-807OC [DOI] [PubMed] [Google Scholar]
- 40. Solin P , Roebuck T , Johns DP , Walters EH , Naughton MT . Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure . Am J Respir Crit Care Med . 2000. ; 162 ( 6 ): 2194 – 2200 . 10.1164/ajrccm.162.6.2002024 [DOI] [PubMed] [Google Scholar]
- 41. Xie A , Skatrud JB , Puleo DS , Rahko PS , Dempsey JA . Apnea-hypopnea threshold for CO2 in patients with congestive heart failure . Am J Respir Crit Care Med . 2002. ; 165 ( 9 ): 1245 – 1250 . 10.1164/rccm.200110-022OC [DOI] [PubMed] [Google Scholar]
- 42. Nakayama H , Smith CA , Rodman JR , Skatrud JB , Dempsey JA . Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep . Am J Respir Crit Care Med . 2002. ; 165 ( 9 ): 1251 – 1260 . 10.1164/rccm.2110041 [DOI] [PubMed] [Google Scholar]
- 43. Sutton JR , Houston CS , Mansell AL , et al . Effect of acetazolamide on hypoxemia during sleep at high altitude . N Engl J Med . 1979. ; 301 ( 24 ): 1329 – 1331 . 10.1056/NEJM197912133012406 [DOI] [PubMed] [Google Scholar]
- 44. Kassamali R , Sica DA . Acetazolamide: a forgotten diuretic agent . Cardiol Rev . 2011. ; 19 ( 6 ): 276 – 278 . 10.1097/CRD.0b013e31822b4939 [DOI] [PubMed] [Google Scholar]
- 45. Swenson ER , Leatham KL , Roach RC , Schoene RB , Mills WJ Jr , Hackett PH . Renal carbonic anhydrase inhibition reduces high altitude sleep periodic breathing . Respir Physiol . 1991. ; 86 ( 3 ): 333 – 343 . 10.1016/0034-5687(91)90104-Q [DOI] [PubMed] [Google Scholar]
- 46. Swenson ER , Hughes JM . Effects of acute and chronic acetazolamide on resting ventilation and ventilatory responses in men . J Appl Physiol 1985 . 1993. ; 74 ( 1 ): 230 – 237 . [DOI] [PubMed] [Google Scholar]
- 47. Teppema LJ , van Dorp EL , Dahan A . Arterial [H+] and the ventilatory response to hypoxia in humans: influence of acetazolamide-induced metabolic acidosis . Am J Physiol Lung Cell Mol Physiol . 2010. ; 298 ( 1 ): L89 – L95 . 10.1152/ajplung.00255.2009 [DOI] [PubMed] [Google Scholar]
- 48. Correa D , Farney RJ , Chung F , Prasad A , Lam D , Wong J . Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations . Anesth Analg . 2015. ; 120 ( 6 ): 1273 – 1285 . 10.1213/ANE.0000000000000672 [DOI] [PubMed] [Google Scholar]
- 49. Montandon G , Qin W , Liu H , Ren J , Greer JJ , Horner RL . PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression . J Neurosci . 2011. ; 31 ( 4 ): 1292 – 1301 . 10.1523/JNEUROSCI.4611-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McKay LC , Janczewski WA , Feldman JL . Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons . Nat Neurosci . 2005. ; 8 ( 9 ): 1142 – 1144 . 10.1038/nn1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mellen NM , Janczewski WA , Bocchiaro CM , Feldman JL . Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation . Neuron . 2003. ; 37 ( 5 ): 821 – 826 . 10.1016/S0896-6273(03)00092-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun X , Thörn Pérez C , Halemani D N , et al . Opioids modulate an emergent rhythmogenic process to depress breathing . eLife . 2019. ; 8 : 8 . 10.7554/eLife.50613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Levitt ES , Abdala AP , Paton JF , Bissonnette JM , Williams JT . μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive . J Physiol . 2015. ; 593 ( 19 ): 4453 – 4469 . 10.1113/JP270822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang D , Teichtahl H , Drummer O , et al . Central sleep apnea in stable methadone maintenance treatment patients . Chest . 2005. ; 128 ( 3 ): 1348 – 1356 . 10.1378/chest.128.3.1348 [DOI] [PubMed] [Google Scholar]
- 55. Teichtahl H , Wang D , Cunnington D , et al . Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients . Chest . 2005. ; 128 ( 3 ): 1339 – 1347 . 10.1378/chest.128.3.1339 [DOI] [PubMed] [Google Scholar]
- 56. Berkenbosch A , Teppema LJ , Olievier CN , Dahan A . Influences of morphine on the ventilatory response to isocapnic hypoxia . Anesthesiology . 1997. ; 86 ( 6 ): 1342 – 1349 . 10.1097/00000542-199706000-00016 [DOI] [PubMed] [Google Scholar]
- 57. Parati G , Revera M , Giuliano A , et al . Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure . Eur Heart J . 2013. ; 34 ( 10 ): 759 – 766 . 10.1093/eurheartj/ehs140 [DOI] [PubMed] [Google Scholar]
- 58. Yumino D , Redolfi S , Ruttanaumpawan P , et al . Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure . Circulation . 2010. ; 121 ( 14 ): 1598 – 1605 . 10.1161/CIRCULATIONAHA.109.902452 [DOI] [PubMed] [Google Scholar]
- 59. Ni YN , Thomas RJ . Acetazolamide for residual apnea and periodic breathing on continuous positive airway pressure therapy . Sleep Med . 2020. ; 71 : 52 – 53 . 10.1016/j.sleep.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 60. Swenson ER . Carbonic anhydrase inhibitors and ventilation: a complex interplay of stimulation and suppression . Eur Respir J . 1998. ; 12 ( 6 ): 1242 – 1247 . 10.1183/09031936.98.12061242 [DOI] [PubMed] [Google Scholar]
- 61. Sartorius OW , Roemmelt JC , Pitts RF , Calhoon D , Miner P . The renal regulation of acid-base balance in man. iv. the nature of the renal compensations in ammonium chloride acidosis . J Clin Invest . 1949. ; 28 ( 3 ): 423 – 439 . 10.1172/JCI102087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Onal E , Burrows DL , Hart RH , Lopata M . Induction of periodic breathing during sleep causes upper airway obstruction in humans . J Appl Physiol 1985 . 1986. ; 61 ( 4 ): 1438 – 1443 . [DOI] [PubMed] [Google Scholar]
- 63. Onal E , Lopata M , O’Connor T . Pathogenesis of apneas in hypersomnia-sleep apnea syndrome . Am Rev Respir Dis . 1982. ; 125 ( 2 ): 167 – 174 . [DOI] [PubMed] [Google Scholar]
- 64. Schmickl CN , Landry SA , Orr JE , et al . Acetazolamide for obstructive and central sleep apnea: a comprehensive systematic review and meta-analysis . Chest . 2020. ; 158 ( 6 ): 2632 – 2645 . 10.1016/j.chest.2020.06.078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Javaheri S , Smith J , Chung E . The prevalence and natural history of complex sleep apnea . J Clin Sleep Med . 2009. ; 5 ( 3 ): 205 – 211 . 10.5664/jcsm.27486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schmickl CN , Owens RL , Orr JE , Edwards BA , Malhotra A . Side effects of acetazolamide: a systematic review and meta-analysis assessing overall risk and dose dependence . BMJ Open Respir Res . 2020. ; 7 ( 1 ): e000557 . 10.1136/bmjresp-2020-000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.